Abstract
Anti-seizure drugs (ASDs) are widely used and known to increase inhibitory tone on neuro-circuits and reduce aberrant synchronous firing in epilepsy. Some ASDs act as agonist at the GABAA receptor. Stiripentol, known to increase GABAA receptor activity as well as the metabolites of GABAA receptor agonists, is often used in the treatment of an epileptic encephalopathy, Dravet syndrome (DS), which is caused by mutations mainly in SCN1A and in other genes such as GABRG2. We have recently generated a Gabrg2+/Q390X knockin mouse model associated with DS in humans. The objective of the study was to explore the effects of stiripentol in DS with GABAA receptor functional deficiency because of the etiology heterogeneity in DS. Monotherapy (stiripentol or Diazepam) and polytherapy (stiripentol and diazepam) treatments were tested in Gabrg2+/Q390X mice challenged with pentylenetetrazol (PTZ) seizure induction in conjunction with video-monitoring synchronized electroencephalogram (EEG) recordings. A combination of stiripentol and diazepam greatly reduced seizure-related events in Gabrg2+/Q390X mice following PTZ administration and increased survival. However, the treatment of stiripentol alone was mostly ineffective in alleviating seizure-related events except that it reduced mortality in PTZ challenged Gabrg2+/Q390X mice. The study suggests that stiripentol could be only used as add-on therapy for DS with GABAA receptor functional deficiency, which is consistent with the most established clinical application of stiripentol. The study highlights the importance of mechanism-based precision treatment for DS considering the highly heterogeneous nature of etiology in DS and the fact that mutations in different genes give rise to the same clinical phenotype.
Keywords: stiripentol, Dravet syndrome, electroencephalogram, epilepsy, GABRG2(Q390X) mutation, benzodiazepine
INTRODUCTION
Epilepsy is a disorder characterized by atypical concurrent firing of neural circuits. Although many incidences of epileptic disorders are the result of a complex summation of genetic elements, single mutations in genes coding for the GABAA receptor have been found to result in epileptic syndromes. For example, genetic mutations in GABRG2 encoding γ2 subunit of the GABAA receptor have been associated with various epilepsy syndromes ((Harkin et al., 2002;Kang et al., 2009). Of the mutations in GABRG2, our focus is on a particular nonsense mutation GABRG2(Q390X) due to its association with more severe forms of epilepsy, Dravet syndrome (DS) — an epileptic encephalopathy that affects about one in 40,000 children under seven years old (Hurst, 1990). The severe symptoms associated with the GABRG2(Q390X) mutation are likely due to the fact that the truncated protein localizes to the endoplasmic reticulum and results in lowered gamma-aminobutyric acid (GABA)-evoked currents, exhibits a dominant-negative effect through the disruption of the normal functioning of wild-type subunits, and dimerizes to form high molecular mass protein complexes, which may cause neuronal dysfunction and contribute to neuronal death (Kang et al., 2015).
Treatments for epilepsy often focus upon anti-seizure drugs (ASDs). Many ASDs increase inhibitory tone to reduce aberrant synchronous neuronal firing. Some ASDs, such as benzodiazepines, act as positive allosteric modulators on GABAA receptors. However, the treatment of epilepsy stemming from a GABRG2 mutation is often not very effective with ASDs such as benzodiazepines alone — given the importance of the proper functioning of the γ2 subunit of the GABAA receptor (Pritchett et al., 1989). With this known, an alternative approach is to use ASDs conjointly. For example, stiripentol is an ASD that is typically co-administered with another anti-convulsant compound as an add-on therapy. It is mainly used because of its metabolic enzyme antagonistic properties although it can also potentiate GABAA receptors. In particular, stiripentol and benzodiazepines act independently at GABAA receptors, and implementing the polytherapy treatment appears to increase the maximum effectiveness compared to either type of drug alone (Fisher, 2011). For the current study, we examined the efficacy of stiripentol alone, diazepam alone (a benzodiazepine), and the co-administration of stiripentol and diazepam in a Gabrg2+/Q390X knockin mouse model via electroencephalogram (EEG) assessment to provide an objective measure of seizure-related events and the effect of stiripentol in DS associated with GABRG2 mutations.
METHODS
Mice
Gabrg2+/Q390X heterozygous (het) knockin mice were characterized and used as previously studied (Kang et al., 2015), and their wildtype (wt) littermates were used as controls. We used Gabrg2+/Q390X mice because it represents patient condition. Mice used in the study were bred into C57Bl/6J mice for at least 8 generations and were between 2–4 months old. All procedures were performed in accordance with policies and guidelines set forth by the Vanderbilt University Institutional Animal Care and Use Committee.
EEG recordings and Drug administration
EEG recordings were conducted based on our standard lab protocol (Kang et al., 2015;Warner et al., 2016). Stiripentol was kindly provided by Biocodex (Beauvais, France, 150 mg/kg). Stiripentol was initially dissolved in a 1:10 solution of dimethyl sulfoxide (DMSO) and 0.9 percent saline, then further diluted to a 1:100 concentration of corn oil before administered in animals. The volume injected into each mouse was about 200 μl. Diazepam (0.3mg/kg) and pentylenetetrazol (50mg/kg) were obtained from Sigma-Aldrich (St. Louis, MO). All drugs were administered via intraperitoneal injection (IP). Stiripentol was administered 1 hr (Auvin et al., 2013) while Diazepam was administered 30 min (Warner et al., 2016) respectively before pentylenetetrazol treatment. Mice were routinely recorded for at least 24 hrs for baseline analysis before drug administration and at least 2 hrs after drug administration. For baseline recordings, the first 10 min for each hour were analyzed for 24 hrs while the first 15 min for each hour of the recordings after drug administration were analyzed. In the group with multiple drug treatment, the analysis started after the injection of the last drug. Both behavioral and electrographic seizure activity were monitored.
Statistical analysis
All data was analyzed with GraphPad Prism 5 software. While both male and female mice were used in the study, there were no sex-dependent effects (data not shown), so male and female mice were pooled together in all subsequent analyses. Independent-samples t tests were used for comparisons between genotypes and One sample t test was used when appropriate. A two-way analysis of variance (ANOVA) was used for the comparisons involved two factors and the post hoc test Bonferroni posttest was used for multiple comparisons. All analyses used an alpha level of 0.05 to determine statistical significance. Data were presented as Mean ± SE.
RESULTS
Stiripentol alone increased myoclonic seizures but combination of stiripentol and diazepam reduced seizures in heterozygous Gabrg2+/Q390X mice
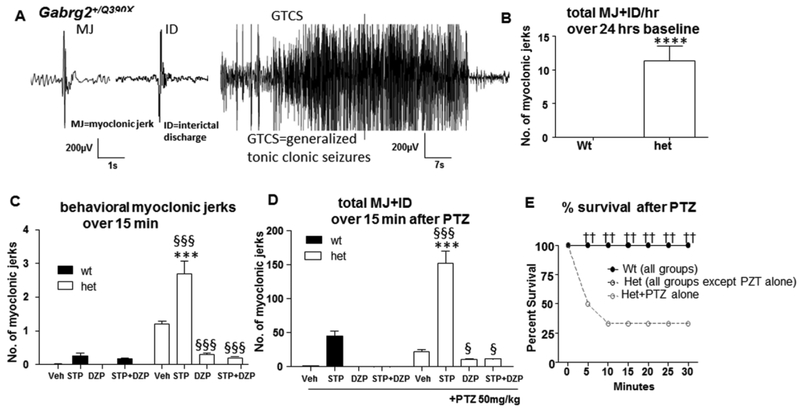
A previous study indicated that Gabrg2+/Q390X mice had increased seizure activities such as increased spontaneous myoclonic jerks (MJ) or myoclonic seizures, interictal discharges and generalized tonic clonic seizures (GTCS), so we wanted to determine the effect of stiripentol on seizure phenotypes in Gabrg2+/Q390X mice. We evaluated the frequency of myoclonic jerks and GTCS in mice at baseline in which there was no injection involved (Figure 1B). We measured the total number of ictal or interictal discharges related to myoclonic seizures with or without overt behavioral manifestations (11.4±2.1, n=18 for het vs 0 for wt, n=15, P<0.0001, t=5.429, df=17) because in some cases, there was similar EEG presentation to those events associated with myoclonic seizures but without noticeable behavioral jerks. Those events were classified as interictal discharges. We evaluated the behavioral myoclonic jerks in mice treated with vehicle (0.9% normal saline, 200μl), stiripentol (150mg/kg) alone, diazepam (0.3mg/kg) alone, or the combination of stiripentol and diazepam (Figure 1C, N=8 for wt vehicle or STP, mice, N=10 for wt DZP, N=7 for STP+DZP; N=11 for het vehicle, STP, DZP and STP+DZP respectively). Contrary to expectation, when only stiripentol was administered, heterozygous mice displayed more myoclonic jerks (2.7±0.38) than vehicle treated group (1.2±0.08), diazepam (0.3±0.05), or stiripentol/diazepam (0.2±0.03) treatment conditions, as well as when compared to wildtype mice that received only stiripentol (0.25±0.085). We subsequently measured the total number of ictal and interictal discharges with or without behavioral manifestations in each group of mice after pentylenetetrazol (PTZ, 50mg/kg) seizure induction. Myoclonic jerks and related events were significantly greater in mice treated with stiripentol 152.3±18.2, P<0.001) than with diazepam (10.8±1.5; P<0.001), stiripentol/diazepam (11.3±0.98; P<0.001), or pentylenetetrazol alone (22.5±2.4; P<0.001 ) for the heterozygous mice, and when compared to wild-type mice that received only stiripentol (0.25±0.08; P<0.001) (For wt, N=9 mice for vehicle, STP or DZP groups, N=6 mice for STP+DZP; for het, N=8 for vehicle, STP, DZP and STP+DZP respectively, Figure 1D). In both Figure 1C and 1D, mouse genotype and drug treatment were taken as the independent variables. The data indicated that STP increased myoclonic jerks which was reduced by DZP treatment ((P<0.0001 for interaction F(3, 69)=22.19, for drug treatment (F(3, 69)=28.07 as well as for genotype (F(1,69)=73.01)). Similarly, following the administration of pentylenetetrazol, GTCSs were increased in both stiripentol and control groups. However, the total number of GTCSs in the PTZ alone group was hard to evaluate due to the early death of some mice. Thus, the number of GTCSs was not compared (Figure 1D).
Figure 1. The effect of stiripentol on myoclonic ss and survival in Gabrg2+/Q390X knockin mice.
(A) Representative EEG recordings show that a 3-month old Gabrg2+/Q390X knockin mouse had myoclonic jerks (Mj, left panel), interictal discharges (ID, middle panel) which is similar to myoclonic jerks in EEG but without overt behavioral correlation and generalized tonic clonic seizures (GTCS; right panel). (B) The frequency of MJ and ID in the wildtype (wt) or Gabrg2+/Q390X heterozygous (het) mice was measured over a 24-hr session for baseline recording (n=15 for wt and 18 for het, n=15, ****P<0.0001, t=5.429, df=17, One sample t test with test). There was no injection involved in baseline recordings. The total numbers include events with behavioral jerks (MJ) and similar interictal discharges without overt behavioral manifestations (ID). (C) The frequency of behavioral myoclonic jerks in the wt or het mice was measured over the first 15 min of each hour after drug administration. Mice were treated with vehicle (0.9% normal saline, Veh, ip), stiripentol (150mg/kg, STP,ip), diazepam (0.3mg/kg, DZP, ip) alone or a combination of STP and DZP (N=8 for wt vehicle or STP, mice, N=10 for wt DZP, N=7 for STP+DZP; N=11 for het vehicle, STP, DZP and STP+DZP respectively; P<0.0001 for interaction (F(3,69)=22.19; for drug treatment) (F(3,69)=28.07 and for genotype (F(1,69)=73.01)); P<0.001 for both vehicle and STP treated het vs wt, Bonferroni posttests). (D) In pentylenetetrazol (PTZ) treated study, the frequency of total MJ and ID in the wt or het mice was measured over the 15 min after PTZ (50mg/kg, ip) administration (For wt, N=9 mice for vehicle, STP or DZP groups, N=6 mice for STP+DZP; for het, N=8 for vehicle, STP, DZP and STP+DZP respectively, P<0.0001 for interaction (F(3,57)=22.17; drug treatment (F(3,57)=87.17 and genotype (F(1,57)=57.17)); P<0.001 het vs wt for STP+PTZ, Bonferroni posttests. In both C-D, drug treatment and genotype are the independent variables, ***P<0.001 het vs wt with the same drug treated condition; §P<0.05, §§§ P<0.001 vs het vehicle. Two-way ANOVA with Bonferroni posttests. The numbers represent the frequency of events with behavioral jerks and similar interictal discharges without overt behavioral manifestations. (E). Stirpentol alone, as well as in combination with DZP increased survival for Gabrg2+/Q390X knockin mice during pentylenetetrazol (PTZ) seizure induction (N=12–15 for all wildtype and het mice except N=30 mice for het +PTZ group).
Stiripentol alone or in combination with diazepam increased the rate of survival following pentylenetetrazol administration
Stiripentol alone, diazepam alone, and stiripentol/diazepam all had a 100 percent survival rate following exposure to pentylenetetrazol in both Gabrg2+/Q390X mice and wildtype mice. However, when only pentylenetetrazol was administered, there was only a 33 percent survival rate for the heterozygous mice (10 out of 30 het mice died) (N=12–15 for all wildtype and het mice except N=30 mice for het +PTZ group, Figure 1E).
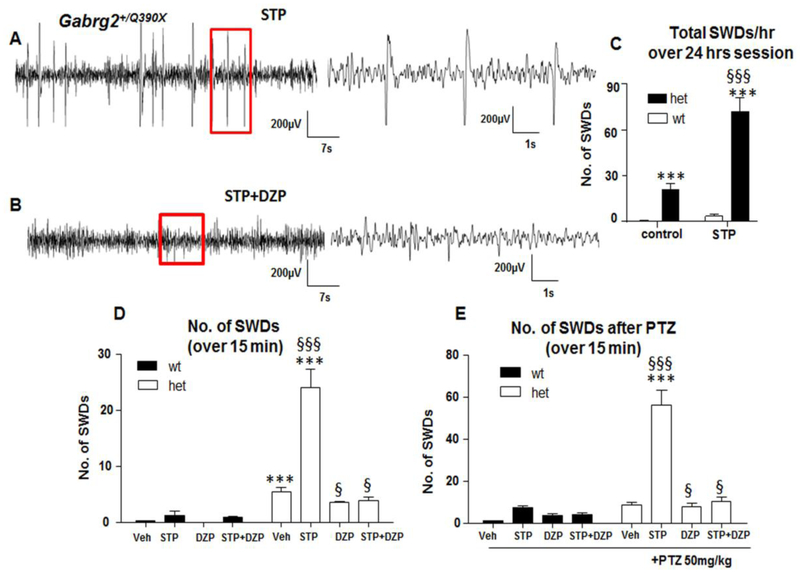
Stiripentol alone increased spike-wave discharges, but combined treatment of stiripentol and diazepam or diazepam alone reduced seizures for heterozygous mice
It is worth noting that there are some interictal discharges induced by stiripentol that are dissimilar to typical ictal discharges associated with myoclonic seizures but were reduced by adding Diazepam (Figure 2A and 2B). These abnormal discharges were classified as spike-wave discharges (SWDs) and the total number was determined regardless of behavioral correlations over a 24-hour period (Figure 2C). Compared to the mice for baseline recordings without any injection (0.65±0.03 for wt and 21.2 ±3.5 for het), the SWDs were increased in the Gabrg2+/Q390X mice treated with stiripentol (3.1±1.3 for wt and 72.4 ±8.6 for het, P<0.001). Genotype and treatment were taken as independent variables. STP increased SWDS in both wildtype and heterozygous mice ((P<0.001 for interaction F (1,43) =39.93; genotype F(1,43) =136.8 and for drug treatment F(1,43)=50.22); N=15 for wt control, N=8 for wt STP; N=18 for het control and 8 for het STP, Figure 1C). Next, the effect of stiripentol, diazepam, or the combination of stiripentol and diazepam was determined in conjunction with PTZ challenge. There are more SWDs observed in the Gabrg2+/Q390X mice in the vehicle treated group (0.31±0.02 for wildtypemice vs 5.4±0.87 for heterozygous mice, P<0.001), which were further increased with stiripentol treatment (het mice +stiripentol) (Figure 2D, for wt, N=9 mice for vehicle, STP or DZP groups, N=6 mice for STP+DZP; for het, N=8 for vehicle, STP, DZP and STP+DZP respectively, P<0.0001 for interaction (F(3,69)=22.49; for drug treatment (F(3,69)=26.33 and for genotype (F(1,69)=73.40)). With the pentylenetetrazol challenge, stiripentol alone increased total SWDs in the Gabrg2+/Q390X mice (56.3±6.9, P<0.001) while Diazepam alone (7.8 ±1.66) or in combination with stiripentol (10.4 ±2.1) reduced the total SWDs ((P<0.0001 for interaction (F(3,57)=22.17; for drug treatment (F(3,57)=87.17 and for genotype (F(1,57)=57.17)). In Figure 2E, P<0.0001 for interaction (F(3,57)=33.53; for drug treatment (F(3,57)=49.53 and for genotype) (F(1,57)=77.76), Figure 2E). Similar to the findings for myoclonic jerks, the data indicated that STP increased SWDs which was reduced by DZP treatment. It is worth noting that the duration of ictal events in each respective group are not significantly different. The duration for myoclonic seizures was about 350 ms while the duration for SWD was about 3 sec across groups. Thus, the main difference of ictal events was the frequency in mice treated with different drugs.
Figure 2. The effect of stiripentol on spike-wave discharges in Gabrg2+/Q390X knockin mice.
(A, B) Representative EEG recordings showing spike-wave discharges (SWDs) in a 3-month old Gabrg2+/Q390X knockin mouse treated with stiripentol alone (STP,150mg/kg, ip) (A) or with stiripentol plus diazepam (DZP,0.3mg/kg, ip) (B). In A and B, boxed regions in the left panel were expanded in the right panel. (C) The occurrence of the SWDs in the wildtype (wt) or Gabrg2+/Q390X heterozygous (het) mice was measured over a 24-hr session for baseline recording without any treatment or with stiripentol (N=15 for wt control, N=8 for wt STP; N=18 for het control and 8 for het STP, ***P<0.001 vs wt in control, §§§ vs het in control, genotype and treatment were taken as independent variables. P<0.001 for interaction F (1,43)=39.93; genotype F(1,43)=136.8 and for treatment F(1,43)=50.22, two-way ANOVA Bonferroni posttests). The first 10 min recordings of each hour were evaluated. (D). The total number of SWDs in the wt or het mice was measured over a 3-hr recording session. The first 15 min of each hour of recordings after drug administration (15 min after administration) was evaluated. Mice were treated with 0.9% normal saline (Vehicle, Veh), stiripentol (150mg/kg, STP, ip), Diazepam (0.3mg/kg, DZP, ip) alone or a combination of STP and DZP ((for wt, N=8 for vehicle and STP, 10 for DZP and 7 for STP+DZP, for het, N=11 for all four conditions. P<0.0001 for interaction (F(3,69)=22.49; drug treatment (F(3,69)=26.33 and genotype (F(1,69)=73.40)). (E). Stirpentol alone increased the SWDs in EEG in Gabrg2+/Q390X knockin mice but DZP alone or in combination with stiripentol reduced the SWDs after a pentylenetetrazol (PTZ, 50mg/kg, ip) seizure induction (for wt, N=9 for vehicle, STP and DZP respectively and N=6 for STP+DZP; for het, N=8 for all four conditions, P<0.0001 for interaction (F(3,57)=33.53; for drug treatment (F(3,57)=49.53 and for genotype) (F(1,57)=77.76)). In D and E, *P<0.05, ***P<0.001 vs wt with the same treated condition; §P<0.05, §§§ P<0.001 vs het vehicle. In C, D, E, drug treatment and genotype are the independent variables.
DISCUSSION
The present study indicates that the polytherapy treatment of stiripentol and diazepam greatly attenuates seizure-related events in Gabrg2+/Q390X knockin, a specific Dravet syndrome mouse. These findings are consistent with other studies suggesting the advantages of co-administering stiripentol with a benzodiazepine ((Fisher, 2011;Inoue and Ohtsuka, 2015)), and we are the first to report the effect of stiripentol in Gabrg2+/Q390X mice. Stiripentol binds at a site on the α3 subunit that is specifically different from the benzodiazepine binding site, thus offering synergistic effects when stiripentol and a benzodiazepine are paired together (Chiron and Dulac, 2011). However, when stiripentol was administered alone or in conjunction with pentylenetetrazol, the ictal and interictal discharges associated with the Gabrg2+/Q390X knockin mice were significantly enhanced. Our findings appear to be contradictory to other reports, which noted improved seizure control using only stiripentol as monotherapy (Cao et al., 2012;Chiron et al., 2000). For example, in a different Dravet syndrome mouse model Scn1a+/R1407X, stiripentol alone has protective effects against hypothermia-induced seizures in mice at 1 month old but not in mice at 5 months old. It is possible that differential pathophysiology underlying each Dravet syndrome mouse model may contribute to the differential effect of stiripentol seen in each animal model. The main pathology of Scn1a+/R1407X Dravet syndrome mouse is likely impaired GABAergic interneuronal activity, thus leading to reduced presynaptic GABA release while the main pathophysiology of Gabrg2+/Q390X Dravet syndrome mouse is reduced postsynaptic GABAA receptor expression and channel function. Both pathologies in the two different conditions would give rise to a very similar clinical phenotype such as Dravet syndrome. Nevertheless, the effect of stiripentol for monotherapy merits further investigation as this information would be very valuable for clinical application but can not be tested directly in patients.
The mechanism of action for stiripentol still remains unclear, but it is reported to enhance the release of GABA as well as increase GABAA receptor-mediated transmission (Quilichini et al., 2006). A key difference between the present study and other studies is the use of the Gabrg2+/Q390X knockin mice. In Gabrg2+/Q390X knockin mice, the nonsense mutation is known for producing nonfunctional properties at the γ2 subunit (Kang et al., 2013), and it is possible there is altered GABAergic signaling, such as compensatory increase of GABA due to the presence of Gabrg2(Q390X) mutation, but this requires further elucidation. Given the importance of a properly functioning γ2 subunit at the GABAA receptor (Pritchett et al., 1989), the mechanism of action for stiripentol may have been altered to produce a different overall effect (i.e., enhancing spike-wave discharges) in Gabrg2+/Q390X mice. However, this idea needs to be further explored by comparing DS models with different genetic mutations and thorough characterization of each model at the molecular level. It is worth noting that this study didn’t use power spectrum analysis (Giordano et al., 2015), it is possible that some features went unnoticed during manual EEG scoring by which only a portion of data was sampled. Nevertheless, this study highlights the importance of achieving a mechanistic understanding and treatment for each group of mutations in different genes.
In conclusion, while not as beneficial when administered independently, the co-administration of stiripentol and diazepam reversed different types of seizures typically experienced in the Gabrg2+/Q390X mice. Importantly, stiripentol alone increased survival during pentylenetetrazol seizure induction. This suggests that increased SWDs are not necessarily correlated with a higher mortality in epilepsy at least in genetic epilepsy with GABRG2 deficiency based on this mouse model. Perhaps, the SWDs associated with STP in this particular disease model do not have the same effect as GTCSs that are associated with sudden unexpected death in epilepsy (SUDEP). The differences identified in this model to stiripentol treatment may be due to the pathogenic effect from GABRG2(Q390X) mutation (Kang et al., 2015), which may not be extrapolated to other Dravet syndrome mouse models. In summary, the findings from this study suggest that stiripentol may be beneficial for DS associated with GABRG2 mutations when used in adjunct therapy. The findings also highlight the importance of understanding the etiologic heterogeneity for DS and mechanism-based precision treatment.
Highlights.
The Gabrg2+/Q390X knockin mice associated with DS had increased seizures and death. (=85 characters)
Stiripentol alone was ineffective in suppressing seizures but increased survival. (=82 characters)
Add-on of stiripentol to diazepam was beneficial in Gabrg2+/Q390X mice. (=79 characters)
Stiripentol can be used as adjunct therapy in DS with GABRG2 deficiency. (=85 characters)
The response to stiripentol may be different based on different genetic mutations. (=84 characters)
ACKNOWLEDGEMENTS
The authors are thankful for the use of the Murine Neurobehavioral Core at the Vanderbilt University Medical Center to generate the data. All the experimental procedures were approved by Vanderbilt University Division of Animal Care. We are grateful to Dr. David Mott in University of South Carolina for his advice on dilution and application of stiripentol. Special thanks to Jeffrey Song for proofreading the manuscript.The study was supported by research grants from Biocodex (Beauvais, France) and from the National Institutes of Health (NINDS R01 082635) to KJQ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
None of the authors have any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- Auvin S, Lecointe C, Dupuis N, Desnous B, Lebon S, Gressens P, Dournaud P, 2013. Stiripentol exhibits higher anticonvulsant properties in the immature than in the mature rat brain. Epilepsia 54, 2082–2090. [DOI] [PubMed] [Google Scholar]
- Cao D, Ohtani H, Ogiwara I, Ohtani S, Takahashi Y, Yamakawa K, Inoue Y, 2012. Efficacy of stiripentol in hyperthermia-induced seizures in a mouse model of Dravet syndrome. Epilepsia 53, 1140–1145. [DOI] [PubMed] [Google Scholar]
- Chiron C, Dulac O, 2011. The pharmacologic treatment of Dravet syndrome. Epilepsia 52 Suppl 2, 72–75. [DOI] [PubMed] [Google Scholar]
- Chiron C, Marchand MC, Tran A, Rey E, d’Athis P, Vincent J, Dulac O, Pons G, 2000. Stiripentol in severe myoclonic epilepsy in infancy: a randomised placebo-controlled syndrome-dedicated trial. STICLO study group. Lancet 356, 1638–1642. [DOI] [PubMed] [Google Scholar]
- Fisher JL, 2011. Interactions between modulators of the GABA(A) receptor: Stiripentol and benzodiazepines. Eur J Pharmacol 654, 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano C, Vinet J, Curia G, Biagini G, 2015. Repeated 6-Hz Corneal Stimulation Progressively Increases FosB/DeltaFosB Levels in the Lateral Amygdala and Induces Seizure Generalization to the Hippocampus. Plos One 10, e0141221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkin LA, Bowser DN, Dibbens LM, Singh R, Phillips F, Wallace RH, Richards MC, Williams DA, Mulley JC, Berkovic SF, Scheffer IE, Petrou S, 2002. Truncation of the GABA(A)-receptor gamma2 subunit in a family with generalized epilepsy with febrile seizures plus. Am J Hum Genet 70, 530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst DL, 1990. Epidemiology of severe myoclonic epilepsy of infancy. Epilepsia 31, 397–400. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Ohtsuka Y, 2015. Long-term safety and efficacy of stiripentol for the treatment of Dravet syndrome: A multicenter, open-label study in Japan. Epilepsy Res 113, 90–97. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Shen W, Macdonald RL, 2009. The GABRG2 mutation, Q351X, associated with generalized epilepsy with febrile seizures plus, has both loss of function and dominant-negative suppression. J Neurosci 29, 2845–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JQ, Shen W, Macdonald RL, 2013. Trafficking-deficient mutant GABRG2 subunit amount may modify epilepsy phenotype. Ann Neurol 74, 547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JQ, Shen W, Zhou C, Xu D, Macdonald RL, 2015. The human epilepsy mutation GABRG2(Q390X) causes chronic subunit accumulation and neurodegeneration. Nat Neurosci 18, 988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH, 1989. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature 338, 582–585. [DOI] [PubMed] [Google Scholar]
- Quilichini PP, Chiron C, Ben-Ari Y, Gozlan H, 2006. Stiripentol, a putative antiepileptic drug, enhances the duration of opening of GABA-A receptor channels. Epilepsia 47, 704–716. [DOI] [PubMed] [Google Scholar]
- Warner TA, Shen W, Huang X, Liu Z, Macdonald RL, Kang JQ, 2016. DIfferential molecular and behavioral alterations in mouse models of GABRG2 haploinsufficiency versus dominant negative mutations associated with human epilepsy. Hum Mol Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]