Abstract
Background
Pelvic inflammatory disease (PID) is an infectious disease that causes tubal occlusion and other pelvic and abdominal adhesions. The incidence of pelvic inflammatory disease (PID) has increased due to the sexually active status of the young population. This leads to a more serious problem and a larger effect than previously observed. However, there have been few studies on this topic in Asian populations.
Aim
We aimed to evaluate the risk of preterm labor and/or ectopic pregnancy in Taiwanese women following PID.
Design
Using the Taiwan National Health Insurance Database, we designed a retrospective cohort study that included 12- to 55-year-old pregnant women between 2000 and 2010. We selected a 1:3 age-matched control group of non-PID women. The endpoint was any episode of preterm labor or ectopic pregnancy; otherwise, the patients were tracked until 31 December 2010.
Methods
The risk factors for preterm labor or ectopic pregnancy were explored. For cases included from the index date until the end of 2010, we analyzed the risk of incident preterm labor or ectopic pregnancy. With the use of a multivariate Cox proportional hazard regression analysis, we calculated the hazard ratio (HR) with a 95% CI and compared it with that of the control group.
Results
This study examined 30,450 patients with PID and 91,350 controls. During the follow-up period, patients in the PID group were more likely to develop preterm labor or ectopic pregnancy than patients in the control group. The cumulative incidence rates for developing preterm labor were 1.84% (561/30,450 individuals) in patients with PID and 1.63% (1492/91,350 individuals) in patients without PID. On the other hand, the cumulative incidence rate for developing ectopic pregnancy in patients with PID was 0.05% (14/30,450 individuals) but was only 0.04% (33/91,350 individuals) in patients without PID. Compared with those without PID, the patients with PID had a 1.864 times (P<0.001) higher risk of developing preterm labor and a 2.121 times (P = 0.003) higher risk of developing ectopic pregnancy.
Conclusion
Our study provided evidence of an increased risk of preterm labor or ectopic pregnancy in PID patients.
Background
Pelvic inflammatory disease (PID) is an infectious and inflammatory disease of the upper female genital tract, including the uterus, fallopian tubes, and related pelvic organs. PID is a polymicrobial infection typically observed in sexually active females. When microorganisms ascend from the lower genital tract into the upper genital tract, PID gradually develops. The clinical presentation of PID varies in severity, with most patients presenting with mild disease[1]. The diagnosis is sometimes difficult to establish; practical diagnostic methods include a careful history and physical examination (including pelvic examination), laboratory tests (including blood samples and, particularly, a cervical Gram stain or cervical culture result), and sometimes culdocentesis[2–4]. Many women experience a clinically silent spread of infection to the upper genital tract, which results in subclinical PID [5].
In a famous multicenter, randomized clinical trial designed for PID in North America, the PEACH trial, upper genital tract detection of gonorrhea, chlamydia, or endometritis was sufficient to confirm a diagnosis of PID[6]. The PEACH trial results showed that there were no differences in reproductive health outcomes between women with and without endometritis or with upper genital tract infection[7]. However, other studies revealed that a history of pelvic inflammatory disease prior to admission was associated with infertility, preterm labor, chronic pelvic pain and ectopic pregnancy[8–12].
Both obstetricians and gynecologists focus on women's health, and the prevention of pregnancy complications is the main concern. Ectopic pregnancy is a complication of early pregnancy, and preterm labor is a complication that occurs in the second and third trimesters. Getting pregnant and delivering a healthy baby is an important issue for women, families and society. Preterm labor and ectopic pregnancy can have negative consequences on obstetric results and on a woman’s psychological health. Preterm labor and ectopic pregnancy are two independent events, and they have different pathophysiologies. However, both of these conditions share the same risk factor: infection or a previous infectious episode.
Tubal occlusion was found to be diagnosed in 12.8% of patients after one infection, in 35.5% of patients after two infections, and in 75% of patients after three or more infections [13]. Some studies have revealed that the tubal adhesion caused by PID may increase the possibility of ectopic pregnancy [14–19]. At the same time, studies with small sample sizes and single hospital studies have found that both upper and lower genital tract infections, such as PID [20,21] and bacterial vaginosis, are increasingly associated with adverse consequences in obstetrics, such as preterm membrane rupture, preterm labor and preterm birth [20–31]. PID and amnionitis may result in poor outcomes of subsequent pregnancies[32]. However, the effect of PID on ectopic pregnancy and preterm labor has not been studied in recent years or in Taiwanese or Asian populations.
Therefore, we conducted a population-based study utilizing data from a nationwide health insurance database, the Taiwan National Health Insurance Research Database (NHIRD), to examine the risk of developing ectopic pregnancy and preterm labor among patients with PID.
Materials and methods
Data sources
In this study, we used data from the Taiwan National Health Insurance Research Database (NHIRD) to investigate the risk of ectopic pregnancy or preterm labor in patients with PID over a 10-year period. We reviewed records from 2000 to 2010 in the Health Insurance Database, which constitutes a valid representative sample of the total population in Taiwan. The NHIRD has been documenting the medical information of all insured patients since 1995. The national database includes a population of 23.74 million individuals, and it reached 99.6% coverage in 2009[33]. Each small databank for the study was from one million individuals randomly recruited from the NHIRD. The diagnostic and treatment codes in the NHIRD application forms were based on the International Classification of Diseases, Ninth Revision, and Clinical Modification (ICD-9-CM) during the study period. The use of data for our study was permitted by the National Health Research Institute. This study was approved by the Institutional Review Board of Tri-Service General Hospital (IRB No. 2-105-05-082).
Study design and sampled participants
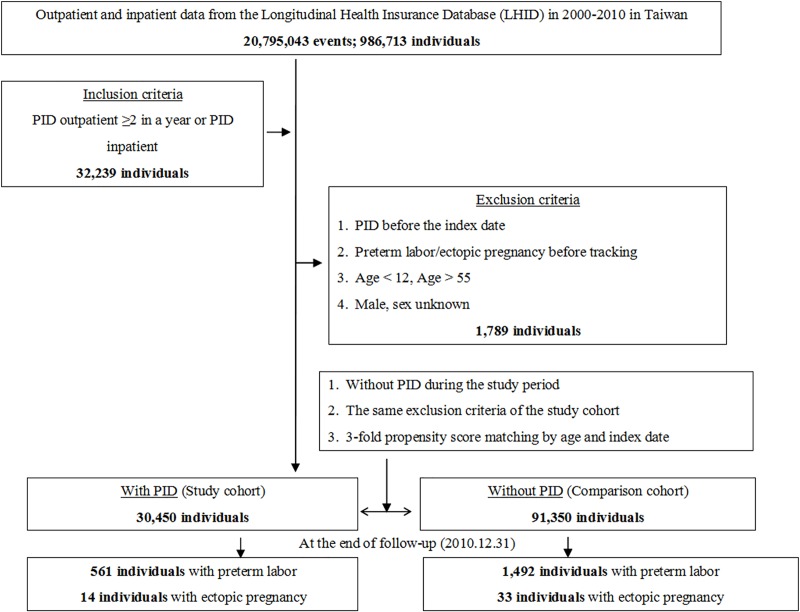
This study utilized a retrospective matched-cohort design. Among the 986,713 individuals recorded from the outpatient and inpatient data from January 1, 2000, to December 31, 2010, 32,239 individuals were diagnosed with PID (ICD-9-CM codes 614.9) prior to the index date. Patients were excluded if they met one of the following criteria: were diagnosed with PID before the index date, had preterm labor or ectopic pregnancy before tracking, were aged <12 years or > 55 years, and were male.
Ultimately, the PID group consisted of 30,450 individuals. The control group had the same exclusion criteria as the case group, but individuals in the control group did not have PID during the study period. The controls were matched 3:1 by index date and age, and the control group included 91,350 individuals. The tracking of the case and control groups continued until December 31, 2010. Tracking ended with the occurrence of preterm labor or ectopic pregnancy. Individuals having had at least two diagnoses of PID (ICD-9-CM code 614.9) according to a gynecologist at a minimum of two visits per year were defined as diagnosed with PID (Fig 1).
Fig 1. The flowchart of study sample selection from the National Health Insurance Research Database in Taiwan.
PID = Pelvic inflammatory disease: ICD-9-CM 614.9. Preterm labor: ICD-9-CM 644. Ectopic pregnancy: ICD-9-CM 633.
Outcome measures
Our study participants were followed from the index date until the onset of preterm labor (ICD-9-CM 644.0–644.9) or ectopic pregnancy (ICD-9-CM 633.0–633.9), until the withdrawal from the National Health Insurance (NHI) program, or until the end of 2010.
Covariates
The covariates included the age group, geographical area of residence, urbanization level of residence (level 1 to 4), number of pregnancies and monthly income. The age groups were categorized as 12–19, 20–29, 30–39, 40–49, or 50–55 years old. The geographical areas of residence were categorized as northern, central, southern, or eastern Taiwan or outlet islands. The urbanization level of residence was defined according to the population and various indicators of the level of development. Level 1 was defined as a population >1,250,000 people and with a specific designation as political, economic, cultural or metropolitan development. Level 2 was defined as a population between 500,000 and 1249,999 people that played an important role in the political system, economy, and culture. Urbanization levels 3 and 4 were defined as a population between 149,999 and 499,999 and <149,999, respectively. The monthly income was categorized into three groups in New Taiwan Dollars [NTD]: <18,000, 18,000 to 34,999, and >35,000. Baseline comorbidities included diabetes mellitus (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401–405), hyperlipidemia (ICD-9-CM code 272), obesity (ICD-9-CM codes 278), heart disease (ICD-9-CM codes 410–429), and chronic kidney disease (CKD) (ICD-9-CM code274.1, 403–404, 440.1, 442.1, 447.3, 572.4, 580–589, 642.1, 646.2).
Statistical analyses
All analyses were performed using SPSS 21 software (SPSS, Inc., Chicago, IL, USA). Chi-square and t tests were used to evaluate the distributions of categorical and continuous variables, respectively.
Differences in the distribution of age, insurance premiums, comorbidities, season, location, urbanization level, level of care between the two groups and between subjects with and without ectopic pregnancy or preterm labor were compared using the chi-square test. Multivariate Cox proportional hazard regression analysis was used to determine the risk of preterm labor and ectopic pregnancy, and the results are presented as hazard ratios (HRs) with 95% confidence intervals (CIs). The preterm labor and/or ectopic pregnancy risk difference between the two groups was estimated using the Kaplan-Meier method along with the log-rank test. The results were considered statistically significant if two-tailed p values were less than 0.05.
Results
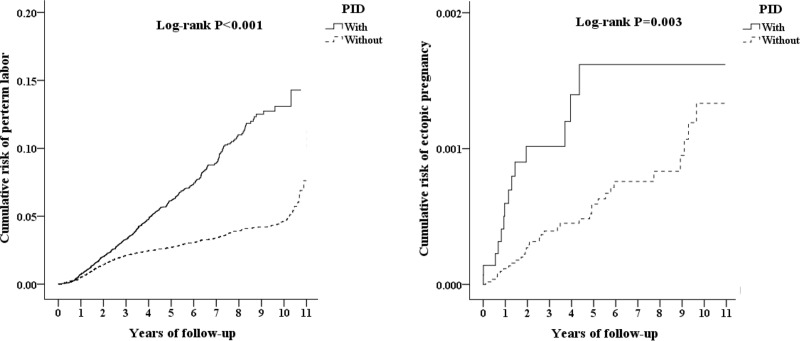
This study examined 30,450 patients with PID and 91,350 controls. Table 1 shows the demographic characteristics of the case and control groups at the end of follow-up. At the 10-year follow-up, patients with a previous PID history had a significantly higher risk of developing preterm labor or ectopic pregnancy than patients without PID. The cumulative incidence rates for developing preterm labor were 1.84% (561/30,450 individuals) in patients with PID and 1.63% (1492/91,350 individuals) in patients without PID. On the other hand, the cumulative incidence rate for developing ectopic pregnancy in patients with PID was 0.05% (14/30,450 individuals) but was only 0.04% (33/91,350 individuals) in patients without PID. The Kaplan-Meier analysis indicated that patients with PID had a significantly higher risk of developing preterm labor or ectopic pregnancy than patients without PID (log-rank test p <0.001; p = 0.003) (Fig 2).
Table 1. Characteristics of the study endpoints.
| PID | Total | With | Without | P | |||
|---|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % | |
| Total | 121,800 | 30,450 | 25.00 | 91,350 | 75.00 | ||
| Preterm labor | 2,053 | 1.69 | 561 | 1.84 | 1,492 | 1.63 | 0.014 |
| Ectopic pregnancy | 47 | 0.04 | 14 | 0.05 | 33 | 0.04 | 0.050 |
| Age group (years) | <0.001 | ||||||
| 12–19 | 4,280 | 3.51 | 1,347 | 4.42 | 2,933 | 3.21 | |
| 20–29 | 29,497 | 24.22 | 8,194 | 26.91 | 21,303 | 23.32 | |
| 30–39 | 31,841 | 26.14 | 9,091 | 29.86 | 22,750 | 24.90 | |
| 40–49 | 40,502 | 33.25 | 9,287 | 30.50 | 31,215 | 34.17 | |
| 50–55 | 15,680 | 12.87 | 2,531 | 8.31 | 13,149 | 14.39 | |
| Insurance premium (NT$) | <0.001 | ||||||
| <18,000 | 118,694 | 97.45 | 29,759 | 97.73 | 88,935 | 97.36 | |
| 18,000–34,999 | 2,241 | 1.84 | 530 | 1.74 | 1,711 | 1.87 | |
| ≥35,000 | 865 | 0.71 | 161 | 0.53 | 704 | 0.77 | |
| DM | 4,590 | 3.77 | 951 | 3.12 | 3,639 | 3.98 | <0.001 |
| HTN | 5,030 | 4.13 | 920 | 3.02 | 4,110 | 4.50 | <0.001 |
| Hyperlipidemia | 1,183 | 0.97 | 240 | 0.79 | 943 | 1.03 | <0.001 |
| Obesity | 88 | 0.07 | 24 | 0.08 | 64 | 0.07 | 0.629 |
| Heart disease | 4,694 | 3.85 | 1,226 | 4.03 | 3,468 | 3.80 | 0.073 |
| CKD | 1,734 | 1.42 | 271 | 0.89 | 1,463 | 1.60 | <0.001 |
| Season | <0.001 | ||||||
| Spring | 29,400 | 24.14 | 7,218 | 23.70 | 22,182 | 24.28 | |
| Summer | 31,436 | 25.81 | 7,994 | 26.25 | 23,442 | 25.66 | |
| Autumn | 32,529 | 26.71 | 8,427 | 27.67 | 24,102 | 26.38 | |
| Winter | 28,435 | 23.35 | 6,811 | 22.37 | 21,624 | 23.67 | |
| Location | <0.001 | ||||||
| Northern Taiwan | 52,242 | 42.89 | 13,760 | 45.19 | 38,482 | 42.13 | |
| Central Taiwan | 33,199 | 27.26 | 7,632 | 25.06 | 25,567 | 27.99 | |
| Southern Taiwan | 30,132 | 24.74 | 7,345 | 24.12 | 22,787 | 24.94 | |
| Eastern Taiwan | 5,768 | 4.74 | 1,605 | 5.27 | 4,163 | 4.56 | |
| Outlet islands | 459 | 0.38 | 108 | 0.35 | 351 | 0.38 | |
| Urbanization level | <0.001 | ||||||
| 1 (The highest) | 42,479 | 34.88 | 10,287 | 33.78 | 32,192 | 35.24 | |
| 2 | 52,363 | 42.99 | 13,308 | 43.70 | 39,055 | 42.75 | |
| 3 | 10,778 | 8.85 | 2,728 | 8.96 | 8,050 | 8.81 | |
| 4 (The lowest) | 16,180 | 13.28 | 4,127 | 13.55 | 12,053 | 13.19 | |
| Level of care | <0.001 | ||||||
| Hospital center | 38,369 | 31.50 | 10,535 | 34.60 | 27,834 | 30.47 | |
| Regional hospital | 43,302 | 35.55 | 13,473 | 44.25 | 29,829 | 32.65 | |
| Local hospital | 40,129 | 32.95 | 6,442 | 21.16 | 33,687 | 36.88 | |
P-value (category variable: chi-square/Fisher’s exact test; continuous variable: t-test)
* Abbreviations: DM: Diabetes mellitus, HTN: Hypertension, CKD: Chronic kidney disease
Fig 2. Kaplan-Meier analysis for the cumulative risk of preterm labor/ectopic pregnancy among females aged 12–55 stratified by pelvic inflammatory disease (PID) with the log-rank test.
Left-button events: preterm labor. Right-button events: ectopic pregnancy.
Compared to the controls, patients with PID tended to have lower insurance premiums (97.73% V.S. 97.36%; p<0.001) and had lower rates of DM (3.12% V.S. 3.98%; p<0.001), HTN (3.02% V.S. 4.50%; p<0.001), hyperlipidemia (0.79% V.S. 1.03; p<0.001) and CKD (0.89% V.S. 1.60%; p<0.001). Regarding the season of hospital visits, patients with PID had more frequent visits in summer (26.25% V.S. 25.66%; p<0.001) and autumn (27.67% V.S. 26.38%; p<0.001) than controls. Patients with PID lived more often in less urbanized areas and in northern areas (45.19% V.S. 42.13%) and in eastern Taiwan (5.27% V.S. 4.56%) (P<0.001) than controls. Compared to the controls, more patients with PID were treated in the hospital center (34.60% V.S. 30.47%) and in regional hospitals (44.25% V.S. 32.65%) (P<0.001). Regarding the ages between the two groups, there was a significantly higher percentage of patients between the ages of 12 and 39 in the PID group than in the control group (61.19% V.S. 51.43%; p<0.001) (Table 1).
A Cox regression analysis of the factors associated with the risk of preterm labor and ectopic pregnancy was performed. After adjusting for season, urbanization level of residence, location, number of births and monthly income, the patients with PID had a 1.864 times (P<0.001) higher risk of developing preterm labor and a 2.121 times (P = 0.003) higher risk of developing ectopic pregnancy than patients without PID (Table 2 and S1 Table).
Table 2. Factors of preterm labor/ectopic pregnancy by using Cox regression.
| Events | Preterm labor | Ectopic pregnancy | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Adjusted HR | 95% CI | 95% CI | P | Adjusted HR | 95% CI | 95% CI | P |
| PID | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.864 | 1.482 | 2.062 | <0.001 | 2.121 | 1.803 | 3.776. | 0.003 |
| Number of births | ||||||||
| 1 | Reference | Reference | ||||||
| ≥2 | 0.993 | 0.482 | 1.795 | 0.513 | 1.024 | 0.589 | 2.131 | 0.330 |
| Age group (years) | ||||||||
| 12–19 | Reference | Reference | ||||||
| 20–29 | 0.607 | 0.462 | 0.797 | <0.001 | 0.343 | 0.079 | 1.484 | 0.152 |
| 30–39 | 0.297 | 0.226 | 0.390 | <0.001 | 0.192 | 0.044 | 0.842 | 0.029 |
| 40–49 | 0.025 | 0.017 | 0.036 | <0.001 | 0.083 | 0.016 | 0.426 | 0.003 |
| 50–55 | 0.000 | - | - | 0.705 | 0.000 | - | - | 0.933 |
| HTN | 1.057 | 1.008 | 1.406 | 0.004 | 1.496 | 0.194 | 11.517 | 0.699 |
| Heart disease | 1.229 | 1.130 | 1.405 | <0.001 | 1.792 | 1.108 | 5.831 | <0.001 |
| CKD | 1.404 | 1.092 | 1.849 | 0.017 | 2.891 | 0.378 | 22.120 | 0.307 |
| Level of care | ||||||||
| Hospital center | 1.275 | 1.069 | 1.626 | 0.010 | 1.883 | 1.669 | 2.126 | <0.001 |
| Regional hospital | 1.194 | 1.026 | 2.576 | 0.038 | 1.475 | 1.324 | 1.644 | <0.001 |
| Local hospital | Reference | Reference | ||||||
HR = hazard ratio, CI = confidence interval, adjusted HR: adjusted variables listed in the table
Adjusted variables: geographical area of residence, urbanization level of residence, monthly income, season, diabetes mellitus, hyperlipidemia, and obesity
Comparing the different age groups, those aged between 12 and 19 years had a significantly higher risk of developing preterm labor than those aged between 20 and 29 (0.607-fold, P<0.001), 30 and 39 (0.297-fold, P<0.001), and 40 and 49 (0.025-fold, P<0.001). Those aged between 12 and 19 years had a significantly higher risk of developing ectopic pregnancy than those aged between 30 and 39 years (0.192-fold, P = 0.029) and between 40 and 49 (0.083-fold, P = 0.003) years old. Those with hypertension (HTN) (P = 0.004), heart disease (P<0.001), and chronic kidney disease (CKD) (P = 0.017) were associated with a higher risk of developing preterm labor than those without these comorbidities. Additionally, those with heart disease (P<0.001) were associated with a higher risk of developing ectopic pregnancy than those without these comorbidities. A higher incidence of preterm labor or ectopic pregnancy development was observed among PID patients who visited the hospital center and regional hospitals than among those who visited local hospitals (Table 2).
The incidence and HR of preterm labor or ectopic pregnancy in populations with or without PID relative to those of the controls are listed in Table 3.
Table 3. Factors of preterm labor/ectopic pregnancy stratified by the variables listed in the table by using Cox regression.
| Events | Preterm labor | Ectopic pregnancy | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PID | With PID | Without PID | With PID vs. Without PID (Reference) | With PID | Without PID | With PID vs. Without PID (Reference) | ||||||||||
| Stratified | Events | Rate | Events | Rate | Adjusted HR | 95% CI | 95% CI | P | Events | Rate | Events | Rate | Adjusted HR | 95% CI | 95% CI | P |
| Total | 561 | 1,208.18 | 1,492 | 532.46 | 1.864 | 1.482 | 2.062 | <0.001 | 14 | 30.15 | 33 | 11.78 | 2.121 | 1.803 | 3.776. | 0.003 |
| Age group (years) | ||||||||||||||||
| 12–19 | 12 | 3,215.26 | 43 | 2,777.33 | 0.969 | 0.878 | 1.069 | 0.264 | 0 | 0 | 2 | 129.18 | 0.000 | - | - | 0.842 |
| 20–29 | 295 | 2,801.93 | 725 | 1,527.55 | 1.535 | 1.391 | 1.694 | <0.001 | 8 | 75.98 | 12 | 25.28 | 2.649 | 1.403 | 5.007 | <0.001 |
| 30–39 | 240 | 1,543.12 | 683 | 788.92 | 1.637 | 1.484 | 1.806 | <0.001 | 5 | 32.15 | 14 | 16.17 | 1.753 | 0.928 | 3.312 | <0.001 |
| 40–49 | 14 | 111.54 | 41 | 66.20 | 1.410 | 1.278 | 1.556 | <0.001 | 1 | 7.97 | 5 | 8.07 | 0.870 | 0.461 | 1.644 | 0.597 |
| 50–55 | 0 | 0 | 0 | 0 | - | - | - | - | 0 | 0 | 0 | 0 | - | - | - | - |
| Insurance premium (NT$) | ||||||||||||||||
| <18,000 | 543 | 1,200.98 | 1,458 | 534.11 | 1.882 | 1.705 | 2.076 | <0.001 | 13 | 28.75 | 30 | 10.99 | 2.307 | 1.221 | 4.359 | <0.001 |
| 18,000–34,999 | 15 | 1,478.78 | 27 | 479.19 | 2.583 | 2.341 | 2.849 | <0.001 | 1 | 98.59 | 2 | 35.50 | 2.449 | 1.296 | 4.627 | <0.001 |
| ≥35,000 | 3 | 1,453.28 | 7 | 438.85 | 2.772 | 2.512 | 3.058 | <0.001 | 0 | 0 | 1 | 62.69 | 0.000 | - | - | 0.994 |
| DM | ||||||||||||||||
| Without | 557 | 1,280.27 | 1,480 | 576.41 | 1.859 | 1.685 | 2.051 | <0.001 | 14 | 32.18 | 33 | 12.85 | 2.207 | 1.169 | 4.171 | <0.001 |
| With | 4 | 136.65 | 12 | 51.18 | 2.234 | 2.025 | 2.465 | <0.001 | 0 | - | 0 | - | - | - | - | - |
| HTN | ||||||||||||||||
| Without | 561 | 1,288.94 | 1,491 | 588.09 | 1.834 | 1.662 | 2.024 | <0.001 | 13 | 29.87 | 33 | 13.02 | 2.023 | 1.071 | 3.823 | <0.001 |
| With | 0 | 0 | 1 | 3.75 | 0.000 | - | - | 0.894 | 1 | 34.37 | 0 | 0.00 | ∞ | - | - | 0.987 |
| Hyperlipidemia | 561 | 1,227.64 | 1,492 | 543.45 | 1.899 | 1.721 | 2.095 | <0.001 | 14 | 30.64 | 33 | 12.02 | 2.257 | 1.195 | 4.265 | 0.012 |
| Obesity | 561 | 1,209.77 | 1,492 | 533.14 | 1.899 | 1.721 | 2.095 | <0.001 | 14 | 30.19 | 33 | 11.79 | 2.257 | 1.195 | 4.265 | 0.012 |
| Heart disease | 557 | 1,272.26 | 1,484 | 563.44 | 1.890 | 1.713 | 2.085 | <0.001 | 14 | 31.98 | 32 | 12.15 | 2.320 | 1.229 | 4.385 | <0.001 |
| CKD | 559 | 1,226.09 | 1,487 | 544.84 | 1.883 | 1.707 | 2.078 | <0.001 | 14 | 30.71 | 32 | 11.72 | 2.309 | 1.222 | 4.363 | <0.001 |
| Season | ||||||||||||||||
| Spring | 128 | 1,279.80 | 368 | 572.93 | 1.869 | 1.694 | 2.062 | <0.001 | 4 | 39.99 | 9 | 14.01 | 2.516 | 1.332 | 4.755 | <0.001 |
| Summer | 153 | 1,275.16 | 379 | 525.13 | 2.032 | 1.842 | 2.242 | <0.001 | 1 | 8.33 | 9 | 12.47 | 0.589 | 0.312 | 1.113 | 0.413 |
| Autumn | 163 | 1,167.24 | 375 | 472.75 | 2.066 | 1.873 | 2.280 | <0.001 | 3 | 21.48 | 8 | 10.09 | 1.878 | 0.994 | 3.549 | 0.058 |
| Winter | 117 | 1,117.57 | 370 | 573.81 | 1.630 | 1.477 | 1.798 | <0.001 | 6 | 57.31 | 7 | 10.86 | 4.654 | 2.464 | 8.795 | <0.001 |
| Urbanization level | ||||||||||||||||
| 1 (The highest) | 178 | 1,242.98 | 489 | 554.34 | 1.877 | 1.701 | 2.070 | <0.001 | 5 | 34.92 | 11 | 12.47 | 2.468 | 1.307 | 4.665 | <0.001 |
| 2 | 269 | 1,286.41 | 684 | 559.40 | 1.925 | 1.744 | 2.123 | <0.001 | 3 | 14.35 | 11 | 9.00 | 1.406 | 0.744 | 2.657 | 0.632 |
| 3 | 51 | 1,079.30 | 112 | 420.19 | 2.150 | 1.948 | 2.372 | <0.001 | 1 | 21.16 | 4 | 15.01 | 1.243 | 0.658 | 2.349 | 0.481 |
| 4 (The lowest) | 63 | 972.66 | 207 | 480.65 | 1.694 | 1.535 | 1.868 | <0.001 | 5 | 77.20 | 7 | 16.25 | 4.187 | 2.217 | 7.912 | <0.001 |
| Level of care | ||||||||||||||||
| Hospital center | 179 | 1,313.51 | 502 | 580.05 | 1.895 | 1.718 | 2.091 | <0.001 | 3 | 22.01 | 5 | 5.78 | 3.359 | 1.779 | 6.348 | <0.001 |
| Regional hospital | 233 | 1,172.03 | 540 | 494.49 | 1.984 | 1.798 | 2.188 | <0.001 | 4 | 20.12 | 16 | 14.65 | 1.211 | 0.641 | 2.288 | 0.682 |
| Local hospital | 149 | 1,152.72 | 450 | 532.79 | 1.811 | 1.641 | 1.998 | <0.001 | 7 | 54.15 | 12 | 14.21 | 3.360 | 1.779 | 6.350 | <0.001 |
PYs = Person-years; Rate: per 105 PYs; Adjusted HR = Adjusted Hazard ratio: Adjusted for the variables listed in Table 3.; CI = Confidence interval
Despite the other factors, patients with a history of PID had HRs for preterm labor ranging from 1.410 (P<0.001) to 2.772 (P<0.001), which were significantly different compared with the values of those without a history of PID. The same status was also noted in the population with ectopic pregnancies; despite the other factors, patients with a history of PID had HRs ranging from 1.753 (P<0.001) to 4.654 (P<0.001), which were significantly different compared with the values of those without a history of PID.
Discussion
Pelvic inflammatory disease (PID) is an inflammatory condition of the female upper genital tract and includes a combination of endometritis, salpingitis, tubo-ovarian abscess, and pelvic peritonitis[34,35]. Approximately forty-one patients with acute pelvic inflammatory disease were evaluated for the coexistence of bacterial vaginosis. Due to the inflammatory process, tubal adhesion and intra-abdominal adhesion are observed after PID[36–41]. PID sometimes progresses to liver capsule inflammation and leads to the development of adhesions and Fitz-Hugh-Curtis syndrome [42–44]. For PID patients, long-term medical treatment, regular follow-up and good compliance are important. However, patients with PID can easily relapse, and it is difficult to achieve complete treatment; poor compliance is often noted in clinical practice [26,45]. The treatment of PID also places a substantial cost burden on the health care system. In developed countries, the annual incidence of PID in women 15 to 39 years of age is approximately 10 to 13 per 1,000 women, with a peak incidence of approximately 20 per 1,000 in women 20 to 24 years of age[46]. Moreover, complications of PID also place an extensive burden on the health care system. Medical costs of treatment have been estimated to be $166 million for chronic pelvic pain, $295 million for ectopic pregnancy, and $360 million for infertility associated with PID in the USA[47]. Preventative PID measures are thought to be more cost-effective [47].
By understanding the association of PID with preterm labor and ectopic pregnancy, assistance and intervention strategies for the clinical prevention of poor outcomes could be provided [19,20,30,48,49]. Some studies revealed that infection could be a precursor to preterm birth or to the premature rupture of membranes. Preexisting infection of the uterine cavity is a predisposing factor of premature membrane rupture, preterm delivery, and amnionitis[23,29,32]. However, there have been no large-scale studies on the relationship between previous PID and preterm labor in Taiwan or other Asian countries.
The role of salpingitis in the recurrence of ectopic pregnancy was studied in a historical cohort of 2,501 women who had undergone laparoscopic examination for acute salpingitis. The study concluded that salpingitis was a risk factor for first ectopic pregnancy[9]. However, there have been no large-scale studies of the relationship between previous PID and ectopic pregnancy in Taiwan or other Asian countries.
This nationwide, population-based study is a large-scale study that investigates the association of PID with ectopic pregnancy and preterm labor. We confirmed that PID is a significant risk factor for ectopic pregnancy and preterm labor. The PID population is at higher risk of ectopic pregnancy and preterm labor compared to the general population. Among PID patients, patients aged 12–19 years have a higher risk of developing ectopic pregnancy and preterm labor than other age groups. Some infectious diseases are pandemic diseases with obvious seasonal characteristics, and outbreaks occur periodically. The results showed that there was no significant difference between seasons. PID is not a pandemic disease.
In addition, patients with hypertension, heart disease, and CKD have an approximately 1.057 to 1.404 times higher risk of preterm labor. Therefore, hypertension, heart disease, and CKD are contributing factors to the development of preterm labor in patients with PID. People with poor health or hygiene problems may have more serious problems or consequences. Patients with hypertension, heart disease, and CKD have the potential for high-risk pregnancies with other complications. With the development of preeclampsia, eclampsia, general edema, or severe dyspnea complicated by heart disease and CKD, delivery is considered an important treatment, and preterm delivery may occur. From the analysis of related factors, we know that women of a young age are at the highest risk of PID. Sexual risk behavior is a critical problem in adolescents. Adolescents engaging in early and unsafe sexual activities represent a high-risk population for infection with human immunodeficiency virus, other sexually transmitted diseases, and unplanned pregnancy[50]. Adolescents who experience PID are highly likely to experience adverse reproductive health outcomes [51,52]. This outcome is an important issue in the prevention of PID[51–53]. The prevention of pelvic inflammatory disease, including comprehensive sex education, the promotion of condom use, and the provision of condoms, is a cornerstone in the prevention of sexually transmitted infection globally[54]. Our results showed that PID had a more prominent role in preterm labor and ectopic pregnancy than other factors; therefore, we consider PID to be a potential risk factor for preterm labor or ectopic pregnancy. Further study of PID should focus on improving disease detection, implementing cheap and effective treatments and, specifically, understanding the pathophysiology.
The strengths of our study include its population-based design, the use of well-established cohort data with a large sample size and the extended follow-up period used to identify PID as a risk factor for developing preterm labor and ectopic pregnancy. Nevertheless, there are still some limitations of this study. First, although the coding of the NHIRD has been validated for some diseases, no reports are available regarding the coding severity of PID. The infectious pathogens and microbiology involved in PID were also unable to be retrieved via the databank. At the same time, the effect on the severity of preterm labor or the site of ectopic pregnancy could not be analyzed. Second, the NHIRD registry was not able to provide detailed information regarding the laboratory results, health-related lifestyle or past history of the patients, such as smoking status, body mass index, gynecological history and family history, some of which can increase the risk of preterm or ectopic pregnancy. Third, the incidence of PID may be underestimated because patients without a hospital visit or a diagnosis of subclinical pelvic inflammatory disease[5] could not be identified from the Taiwan NHI data set. Fourth, the study was based on outpatient and inpatient data, which may not represent the general population (S2 Table).
Conclusions
This study demonstrated that PID is a significant and independent risk factor for preterm labor and ectopic pregnancy. Compared with those without PID disease, patients with PID history had a 1.864 times (P<0.001) higher risk of developing preterm labor and a 2.121 times (P = 0.003) higher risk of developing ectopic pregnancy. The effect of disease progression on the development of preterm labor and/or ectopic pregnancy needs to be further elucidated in future studies. The results from this study indicate that clinical doctors need to perform a cautious assessment of PID patients with pregnancy problems.
Supporting information
(DOCX)
(DOCX)
Data Availability
Data are available from the Taiwan National Health Insurance Research Database (NHIRD). Researchers who meet the criteria for access to confidential data may make data access requests here: https://www.mohw.gov.tw/mp-2.html.
Funding Statement
This study was funded by Tri-Service General Hospital Research Foundation (TSGH- C107-004 and TSGH- C108-003). The funder has no role in the design, concept, data collection and interpretation, analysis, drafting or other process in this paper.
References
- 1.Banikarim C, Chacko MR. Pelvic inflammatory disease in adolescents; 2005. Elsevier; pp. 175–180. [DOI] [PubMed] [Google Scholar]
- 2.Eschenbach DA (1980) Epidemiology and diagnosis of acute pelvic inflammatory disease. Obstetrics & Gynecology 55: 142S–152S. [DOI] [PubMed] [Google Scholar]
- 3.Brunham RC, Gottlieb SL, Paavonen J (2015) Pelvic inflammatory disease. New England Journal of Medicine 372: 2039–2048. 10.1056/NEJMra1411426 [DOI] [PubMed] [Google Scholar]
- 4.Bugg CW, Taira T, Zaurova M (2016) Pelvic inflammatory disease: diagnosis and treatment in the emergency department [digest]. Emerg Med Pract 18: S1–S2. [PubMed] [Google Scholar]
- 5.Wiesenfeld HC, Hillier SL, Meyn LA, Amortegui AJ, Sweet RL (2012) Subclinical pelvic inflammatory disease and infertility. Obstetrics & Gynecology 120: 37–43. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell C, Prabhu M (2013) Pelvic inflammatory disease: current concepts in pathogenesis, diagnosis and treatment. Infectious Disease Clinics 27: 793–809. 10.1016/j.idc.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haggerty CL, Ness RB, Amortegui A, Hendrix SL, Hillier SL, et al. (2003) Endometritis does not predict reproductive morbidity after pelvic inflammatory disease. American journal of obstetrics and gynecology 188: 141–148. 10.1067/mob.2003.87 [DOI] [PubMed] [Google Scholar]
- 8.Safrin S, Schachter J, Dahrouge D, Sweet RL (1992) Long-term sequelae of acute pelvic inflammatory disease: a retrospective cohort study. American journal of obstetrics and gynecology 166: 1300–1305. 10.1016/s0002-9378(11)90626-7 [DOI] [PubMed] [Google Scholar]
- 9.Joesoef MR, Westrom L, Reynolds G, Marchbanks P, Cates W (1991) Recurrence of ectopic pregnancy: the role of salpingitis. American journal of obstetrics and gynecology 165: 46–50. 10.1016/0002-9378(91)90221-c [DOI] [PubMed] [Google Scholar]
- 10.Bjartling C, Osser S, Persson K (2000) The frequency of salpingitis and ectopic pregnancy as epidemiologic markers of Chlamydia trachomatis. Acta Obstet Gynecol Scand 79: 123–128. [DOI] [PubMed] [Google Scholar]
- 11.Shaaban OM, Youssef AE, Khodry MM, Mostafa SA (2013) Vaginal douching by women with vulvovaginitis and relation to reproductive health hazards. BMC Womens Health 13: 23 10.1186/1472-6874-13-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenz JD, Dillard JP (2018) Pathogenesis of Neisseria gonorrhoeae and the Host Defense in Ascending Infections of Human Fallopian Tube. Front Immunol 9: 2710 10.3389/fimmu.2018.02710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weström L (1975) Effect of acute pelvic inflammatory disease on fertility. American journal of obstetrics and gynecology 121: 707–713. 10.1016/0002-9378(75)90477-9 [DOI] [PubMed] [Google Scholar]
- 14.Rekart ML, Gilbert M, Meza R, Kim PH, Chang M, et al. (2013) Chlamydia public health programs and the epidemiology of pelvic inflammatory disease and ectopic pregnancy. J Infect Dis 207: 30–38. 10.1093/infdis/jis644 [DOI] [PubMed] [Google Scholar]
- 15.Ngui R, Ravindran S, Ong DB, Chow TK, Low KP, et al. (2014) Enterobius vermicularis salpingitis seen in the setting of ectopic pregnancy in a Malaysian patient. J Clin Microbiol 52: 3468–3470. 10.1128/JCM.01191-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cumming DC, Honore LH, Scott JZ, Williams KE (1988) Microscopic evidence of silent inflammation in grossly normal fallopian tubes with ectopic pregnancy. Int J Fertil 33: 324–328. [PubMed] [Google Scholar]
- 17.Rosen Y, Kim B (1974) Tubal gestation associated with Schistosoma mansoni salpingitis. Obstet Gynecol 43: 413–417. [PubMed] [Google Scholar]
- 18.Li C, Meng CX, Sun LL, Zhao WH, Zhang M, et al. (2015) Reduced prevalence of chronic tubal inflammation in tubal pregnancies after levonorgestrel emergency contraception failure. Pharmacoepidemiol Drug Saf 24: 548–554. 10.1002/pds.3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assouni Mindjah YA, Essiben F, Foumane P, Dohbit JS, Mboudou ET (2018) Risk factors for ectopic pregnancy in a population of Cameroonian women: A case-control study. PLoS One 13: e0207699 10.1371/journal.pone.0207699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knudtson EJ, Shellhaas C, Stephens JA, Senokozlieff M, Ye H, et al. (2007) The association of chronic endometritis with preterm birth. Am J Obstet Gynecol 196: 337 e331–334. [DOI] [PubMed] [Google Scholar]
- 21.Pitegoff JG, Cathro DM (1986) Chlamydial infections and other sexually transmitted diseases in adolescent pregnancy. Semin Adolesc Med 2: 215–229. [PubMed] [Google Scholar]
- 22.Adinkra P, Lamont RF (2000) Adverse obstetric sequelae of bacterial vaginosis. Hosp Med 61: 475–477. [DOI] [PubMed] [Google Scholar]
- 23.Ismail MA, Pridjian G, Hibbard JU, Harth C, Moawad AA (1992) Significance of positive cervical cultures for Chlamydia trachomatis in patients with preterm premature rupture of membranes. Am J Perinatol 9: 368–370. 10.1055/s-2007-999266 [DOI] [PubMed] [Google Scholar]
- 24.Locksmith G, Duff P (2001) Infection, antibiotics, and preterm delivery. Semin Perinatol 25: 295–309. [DOI] [PubMed] [Google Scholar]
- 25.Romero R, Espinoza J, Mazor M (2004) Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil Steril 82: 799–804. 10.1016/j.fertnstert.2004.05.076 [DOI] [PubMed] [Google Scholar]
- 26.Koumans EH, Kendrick JS, Group CDCBVW (2001) Preventing adverse sequelae of bacterial vaginosis: a public health program and research agenda. Sex Transm Dis 28: 292–297. [DOI] [PubMed] [Google Scholar]
- 27.Reddy UM, Rice MM, Grobman WA, Bailit JL, Wapner RJ, et al. (2015) Serious maternal complications after early preterm delivery (24–33 weeks' gestation). Am J Obstet Gynecol 213: 538 e531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thinkhamrop J, Hofmeyr GJ, Adetoro O, Lumbiganon P, Ota E (2015) Antibiotic prophylaxis during the second and third trimester to reduce adverse pregnancy outcomes and morbidity. Cochrane Database Syst Rev 1: CD002250 10.1002/14651858.CD002250.pub2 [DOI] [PubMed] [Google Scholar]
- 29.Toth M, Witkin SS, Ledger W, Thaler H (1988) The role of infection in the etiology of preterm birth. Obstet Gynecol 71: 723–726. [PubMed] [Google Scholar]
- 30.Andrews WW, Goldenberg RL, Hauth JC, Cliver SP, Conner M, et al. (2005) Endometrial microbial colonization and plasma cell endometritis after spontaneous or indicated preterm versus term delivery. Am J Obstet Gynecol 193: 739–745. 10.1016/j.ajog.2005.02.128 [DOI] [PubMed] [Google Scholar]
- 31.Ma JS, Mei X, Niu YX, Li QG, Jiang XF (2016) Risk Factors and Adverse Pregnancy Outcomes of Succenturiate Placenta: A Case-Control Study. J Reprod Med 61: 139–144. [PubMed] [Google Scholar]
- 32.Toth M, Chaudhry A, Ledger WJ, Witkin SS (1993) Pregnancy outcome following pelvic infection. Infect Dis Obstet Gynecol 1: 12–15. 10.1155/S1064744993000043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng T-M (2009) Taiwan’s National Health Insurance system: high value for the dollar. Six Countries, Six Reform Models—the Healthcare Reform Experience of Israel, the Netherlands, New Zealand, Singapore, Switzerland and Taiwan Hackensack, NJ: World Scientific: 171–204. [Google Scholar]
- 34.Wiesenfeld HC, Sweet RL, Ness RB, Krohn MA, Amortegui AJ, et al. (2005) Comparison of acute and subclinical pelvic inflammatory disease. Sexually transmitted diseases 32: 400–405. [DOI] [PubMed] [Google Scholar]
- 35.Jennings LK, Krywko DM (2018) Pelvic Inflammatory Disease (PID). StatPearls. Treasure Island (FL). [PubMed] [Google Scholar]
- 36.Westrom L (1995) Effect of pelvic inflammatory disease on fertility. Venereology 8: 219–222. [PubMed] [Google Scholar]
- 37.Savel'eva GM, Boginskaia LN, Breusenko VG, Tangieva ZS, Shtyrov SV, et al. (1995) [The prevention of the adhesive process after surgical interventions in gynecological patients in the reproductive period]. Akush Ginekol (Mosk): 36–39. [PubMed] [Google Scholar]
- 38.Hillis SD, Joesoef R, Marchbanks PA, Wasserheit JN, Cates W Jr., et al. (1993) Delayed care of pelvic inflammatory disease as a risk factor for impaired fertility. Am J Obstet Gynecol 168: 1503–1509. 10.1016/s0002-9378(11)90790-x [DOI] [PubMed] [Google Scholar]
- 39.Dubuisson JB, Aubriot FX, Vacher-Lavenu MC, Pichard C, Henrion R (1987) [Chronic salpingitis and extra-uterine pregnancy. Results of the histologic study of 215 tubal pregnancies]. J Gynecol Obstet Biol Reprod (Paris) 16: 27–31. [PubMed] [Google Scholar]
- 40.Jurstrand M, Jensen JS, Magnuson A, Kamwendo F, Fredlund H (2007) A serological study of the role of Mycoplasma genitalium in pelvic inflammatory disease and ectopic pregnancy. Sex Transm Infect 83: 319–323. 10.1136/sti.2007.024752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rutgers JK, Lawrence WD (2014) A small organ takes center stage: selected topics in fallopian tube pathology. Int J Gynecol Pathol 33: 385–392. 10.1097/PGP.0000000000000143 [DOI] [PubMed] [Google Scholar]
- 42.Wang S-P, Eschenbach DA, Holmes KK, Wager G, Grayston JT (1980) Chlamydia trachomatis infection in Fitz-Hugh-Curtis syndrome. American journal of obstetrics and gynecology 138: 1034–1038. 10.1016/0002-9378(80)91103-5 [DOI] [PubMed] [Google Scholar]
- 43.Peter NG, Clark LR, Jaeger JR (2004) Fitz-Hugh-Curtis syndrome: a diagnosis to consider in women with right upper quadrant pain. Cleveland Clinic journal of medicine 71: 233–241. [DOI] [PubMed] [Google Scholar]
- 44.Kimball MW, Knee S (1970) Gonococcal perihepatitis in a male: The Fitz-Hugh-Curtis syndrome. New England Journal of Medicine 282: 1082–1084. 10.1056/NEJM197005072821908 [DOI] [PubMed] [Google Scholar]
- 45.Morris M, Nicoll A, Simms I, Wilson J, Catchpole M (2001) Bacterial vaginosis: a public health review. BJOG 108: 439–450. [DOI] [PubMed] [Google Scholar]
- 46.Weström L (1980) Incidence, prevalence, and trends of acute pelvic inflammatory disease and its consequences in industrialized countries. American Journal of Obstetrics & Gynecology 138: 880–892. [DOI] [PubMed] [Google Scholar]
- 47.Rein DB, Kassler WJ, Irwin KL, Rabiee L (2000) Direct medical cost of pelvic inflammatory disease and its sequelae: decreasing, but still substantial. Obstetrics & Gynecology 95: 397–402. [DOI] [PubMed] [Google Scholar]
- 48.Espinoza J, Erez O, Romero R (2006) Preconceptional antibiotic treatment to prevent preterm birth in women with a previous preterm delivery. Am J Obstet Gynecol 194: 630–637. 10.1016/j.ajog.2005.11.050 [DOI] [PubMed] [Google Scholar]
- 49.Tita AT, Cliver SP, Goepfert AR, Conner M, Goldenberg RL, et al. (2007) Clinical trial of interconceptional antibiotics to prevent preterm birth: subgroup analyses and possible adverse antibiotic-microbial interaction. Am J Obstet Gynecol 197: 367 e361–366. [DOI] [PubMed] [Google Scholar]
- 50.Kotchick BA, Shaffer A, Miller KS, Forehand R (2001) Adolescent sexual risk behavior: A multi-system perspective. Clinical psychology review 21: 493–519. [DOI] [PubMed] [Google Scholar]
- 51.Houck CD, Barker D, Rizzo C, Hancock E, Norton A, et al. (2014) Sexting and sexual behavior in at-risk adolescents. Pediatrics 133: e276–e282. 10.1542/peds.2013-1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goller JL, De Livera AM, Guy RJ, Low N, Donovan B, et al. (2018) Rates of pelvic inflammatory disease and ectopic pregnancy in Australia, 2009–2014: ecological analysis of hospital data. Sex Transm Infect 94: 534–541. 10.1136/sextrans-2017-053423 [DOI] [PubMed] [Google Scholar]
- 53.Twenge JM, Sherman RA, Wells BE (2015) Changes in American adults’ sexual behavior and attitudes, 1972–2012. Archives of Sexual Behavior 44: 2273–2285. 10.1007/s10508-015-0540-2 [DOI] [PubMed] [Google Scholar]
- 54.Workowski KA, Bolan GA (2015) Sexually transmitted diseases treatment guidelines (2015). Reproductive Endocrinology: 51–56. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
Data are available from the Taiwan National Health Insurance Research Database (NHIRD). Researchers who meet the criteria for access to confidential data may make data access requests here: https://www.mohw.gov.tw/mp-2.html.