Abstract
Tsetse flies (Glossina spp.) are vectors of parasitic trypanosomes, which cause human (HAT) and animal African trypanosomiasis (AAT) in sub-Saharan Africa. In Uganda, Glossina fuscipes fuscipes (Gff) is the main vector of HAT, where it transmits Gambiense disease in the northwest and Rhodesiense disease in central, southeast and western regions. Endosymbionts can influence transmission efficiency of parasites through their insect vectors via conferring a protective effect against the parasite. It is known that the bacterium Spiroplasma is capable of protecting its Drosophila host from infection with a parasitic nematode. This endosymbiont can also impact its host’s population structure via altering host reproductive traits. Here, we used field collections across 26 different Gff sampling sites in northern and western Uganda to investigate the association of Spiroplasma with geographic origin, seasonal conditions, Gff genetic background and sex, and trypanosome infection status. We also investigated the influence of Spiroplasma on Gff vector competence to trypanosome infections under laboratory conditions. Generalized linear models (GLM) showed that Spiroplasma probability was correlated with the geographic origin of Gff host and with the season of collection, with higher prevalence found in flies within the Albert Nile (0.42 vs 0.16) and Achwa River (0.36 vs 0.08) watersheds and with higher prevalence detected in flies collected in the intermediate than wet season. In contrast, there was no significant correlation of Spiroplasma prevalence with Gff host genetic background or sex once geographic origin was accounted for in generalized linear models. Additionally, we found a potential negative correlation of Spiroplasma with trypanosome infection, with only 2% of Spiroplasma infected flies harboring trypanosome co-infections. We also found that in a laboratory line of Gff, parasitic trypanosomes are less likely to colonize the midgut in individuals that harbor Spiroplasma infection. These results indicate that Spiroplasma infections in tsetse may be maintained by not only maternal but also via horizontal transmission routes, and Spiroplasma infections may also have important effects on trypanosome transmission efficiency of the host tsetse. Potential functional effects of Spiroplasma infection in Gff could have impacts on vector control approaches to reduce trypanosome infections.
Author summary
We investigated the association of symbiotic Spiroplasma with the tsetse fly host Glossina fuscipes fuscipes (Gff) to assess if Spiroplasma infections are correlated with Gff genetic background, geography, or season and its interaction with trypanosome parasites. We analyzed distribution and prevalence of Spiroplasma infections across different Gff sampling sites in northern and western Uganda, and found that the symbiont is unevenly distributed and infections have not reached fixation within these sampling sites. We tested for associations with geographic origin of the collections, seasonal environmental conditions at the time of collection, Gff host genetic background and sex, plus trypanosome co-infections. Spiroplasma prevalence was strongly correlated with geographic origin and seasonal environmental conditions. Our parasite infection data suggested a negative correlation of Spiroplasma with trypanosome infection, with only 5 out of 243 flies harboring trypanosome co-infections. We further investigated the influence of Spiroplasma on trypanosome parasite infections in the laboratory. We found that trypanosomes were less likely to establish an infection in Gff individuals that carried Spiroplasma infections. Our results provide new information on host-endosymbiont dynamics in an important human disease vector, and provide evidence that Spiroplasma may confer partial resistance to Gff trypanosome infections. These findings provide preliminary evidence that a symbiont-based control method could be successful in combating tsetse trypanosome transmission to humans and livestock in sub-Saharan Africa.
Introduction
Tsetse flies (Glossina spp.) are vectors of parasitic African trypanosomes that cause human African trypanosomiasis (HAT, commonly referred to as sleeping sickness) and African animal trypanosomiasis (AAT, also known as Nagana in cattle) [1–3]. Several major HAT epidemics in sub-Saharan Africa have occurred during the last century, with the most recent one resulting in over half a million deaths in the 1980s [4–7]. An ambitious campaign led by WHO and international partners has now reduced the prevalence of HAT in west Africa [8] to a threshold considered irrelevant for epidemiological considerations, but millions continue to live at risk of contracting HAT in tsetse inhabited areas [9–11]. Despite calls for elimination of HAT by 2030, there is a lack of effective tools for long-term control of the disease (e.g., vaccines and field-ready diagnostic assays). Furthermore, the presence of animal reservoirs threatens disease elimination efforts going forward, particularly in East Africa (reviewed in [12]) and necessitates the inclusion of vector control applications. Practical interventions [13–15], as well as mathematical models [16–18], suggest that vector control can accelerate efforts for reaching the disease elimination phase. Thus, enhancing the vector-control tool box with effective and affordable methods is a desirable goal. Biological approaches that reduce vector reproduction as well as vector competency have emerged as promising means to reduce disease transmission [19].
Variation in the microbiota associated with tsetse flies can influence their pathogen transmission dynamics [20–24]. Several symbiotic microorganisms have been described from laboratory and field populations of tsetse, including the obligate Wigglesworthia glossinidia, commensal Sodalis glossinidius, parasitic Wolbachia and more recently Spiroplasma [25–31]. A survey of Ugandan Gff revealed that all individuals harbored Wigglesworthia, while Sodalis and Wolbachia associations were sporadic [28,32,33]. Spiroplasma was found in the tsetse species that belong to the Palpalis group (Gff, G. p. palpalis and G. tachinoides), while the tsetse species in the other two subgroups, Morsitans and Fusca, lacked associations with this microbe [31].
The bacterium Spiroplasma confers protection against nematodes, fungi and parasitoid wasps in several insects [34–36]. Spiroplasma also acts as a reproductive parasite that induces a male-killing effect in some arthropod hosts [37–40]. Based on phylogenetic analyses, the Spiroplasma species infecting Gff (both field-caught individuals and one laboratory line) is most closely related to the Citri-Chrysopicola-Mirum (S. insolitum) clade. Certain members of this clade confer protection against parasitoid wasps, nematodes and fungal pathogens in the fruit fly and aphid hosts [31]. In addition, among the Spiroplasma species within this clade are some that are pathogenic to plants and invertebrates and some that exhibit a male killing phenotype in ladybirds, fruit flies, and some butterfly species they infect (reviewed in [41]). Spiroplasma function(s) within the tsetse host remain unknown.
In Uganda, Gff is the main vector of HAT, where it transmits the chronic Gambiense disease caused by Trypanosoma brucei gambiense in the northwest and acute Rhodesiense disease caused by T. b. rhodesiense in the center, southeast and west (reviewed in [42]). It is possible that differences in Gff trypanosome susceptibility (vector competence) among varying geographic regions could be influenced by Spiroplasma, but patterns of Spiroplasma occurrence remain unexplored. Spiroplasma infection success may be influenced by seasonal fluctuations in host Gff population health (fitness) and density. Seasonal changes in the environment can dramatically alter host-endosymbiont dynamics through changes in Gff host physiological status (e.g. hemolymph lipid levels [43,44]) and tsetse population density [45,46]. Collections of Gff in Uganda during different seasons provides the opportunity to test for influence of seasonal fluctuations on Spiroplasma prevalence.
Additionally, patterns of Spiroplasma prevalence are likely influenced by Gff host genetic background, either because of vertical transmission that follows host inheritance patterns, or because of lineage-specific coevolutionary dynamics. Gff in Uganda has high genetic structuring at multiple spatial scales, which provides the opportunity to test for influence of Gff genetic background on Spiroplasma. There are three distinct Gff genetic clusters in the north, west and south of the country, and further population structure that separates the northwest from the northeast with ongoing gene flow between them in an admixture zone [47–49]. For this study, we focus our work on the north and west of Uganda in five watersheds. This sampling included four nuclear (biparentally inherited) genetic backgrounds: the northwest genetic unit (NWGU), the northeast genetic unit (NEGU), the west genetic unit (WGU) and the admixed (ADMX) genetic background intermediate to the NWGU and NEGU [47–49]. This sampling also included three mitochondrial (maternally inherited) genetic backgrounds: a group of related haplotypes associated with the NWGU known at “haplogroup A” (mtA), a group of related haplotypes associated with the NEGU known at “haplogroup B” (mtB), and a group of related haplotypes associated with the WGU known at “haplogroup C” (mtC) [47–49].
In this study, we (i) assessed the infection prevalence of Spiroplasma in Gff among five watersheds in northern and western Uganda, (ii) tested the effect of seasonal environmental variations, host Gff genetic background and sex, and trypanosome co-infections on Spiroplasma prevalence, and (iii) investigated the influence of Spiroplasma infections on Gff trypanosome transmission ability under laboratory conditions. We discuss how our results can elucidate potential functional associations of Spiroplasma with its tsetse host, and the potential applications of this knowledge to disease control.
Methods
Field sampling
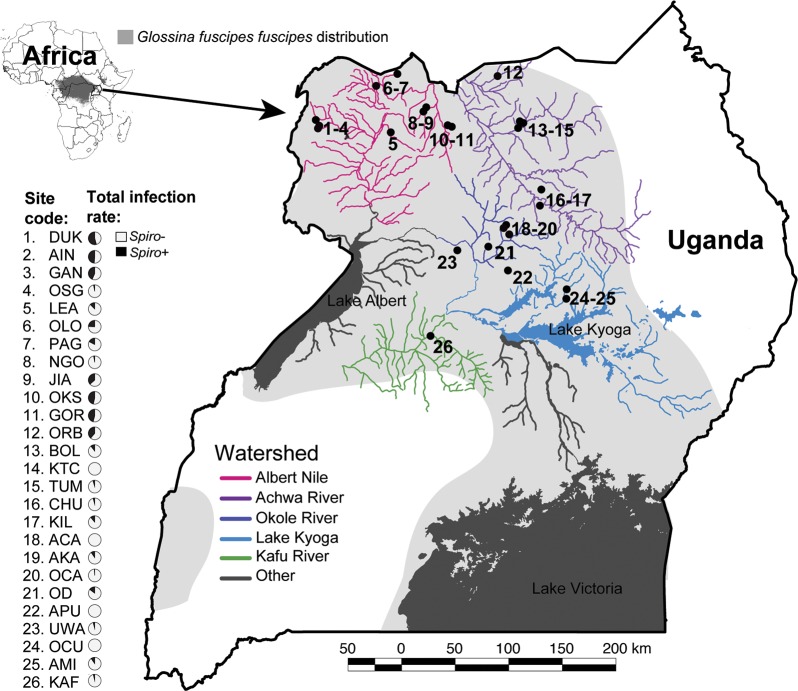
A total of 1415 Gff individuals collected from northern and western Uganda during the period of 2014–2018 (pooled for analysis) across the wet (April—May and August—October), intermediate (June—July), and dry (December—March) seasons were assayed for Spiroplasma infection (S1 Table). Flies were collected on public land using biconical traps. Samples included in this study were chosen to represent a wide range of environmental conditions and Gff backgrounds (Fig 1). The location of each sampling site was placed on a map of Uganda using QGIS v2.12.1 (August 2017; http://qgis.osgeo.org) with free and publicly available data from DIVA-GIS (August 2017; http://www.diva-gis.org). We sampled 11 sites from the Albert Nile watershed (DUK, AIN, GAN, OSG, LEA, OLO, PAG, NGO, JIA, OKS, and GOR), where the majority of Gff belong to the NWGU/mtA genetic background, and a minority belong to the ADMX/mtB genetic background [47–49]. We sampled six sites from the Achwa River watershed (ORB, BOL, KTC, TUM, CHU, and KIL), where Gff belong to a mix of genetic backgrounds including NWGU/mtA, ADMX/mtA, ADMX/mtB, and NEGU/mtB [47–49]. We sampled six sites from the Okole River watershed (ACA, AKA, OCA, OD, APU, and UWA), where Gff hosts belong to a mix of genetic backgrounds including NWGU/mtA, ADMX/mtA, ADMX/mtB, ADMX/mtC, and NEGU/mtB [47–49]. We sampled two sites from the Lake Kyoga watershed (OCU and AMI), where the majority of Gff belong to the NEGU/mtB genetic background, and a minority belong to the ADMX/mtA genetic background [47–49]. Finally, we sampled a single site from the Kafu River watershed (KAF), where the majority of Gff belong to the WGU/mtC genetic background, and a minority belong to the WGU/mtA genetic background [47–49].
Fig 1. Sampling sites and Spiroplasma infection prevalence in northern Uganda.
The map shows the 26 sampling sites and the five watersheds sampled for this study. Light grey shading shows the distribution of Gff in Uganda. The Spiroplasma infection prevalence is shown in pie charts next to each sampling site (gray = Spiroplasma-positive, black = Spiroplasma-negative).
Cloning and sequencing of Spiroplasma 16S rDNA and MLST genes
To assess the Spiroplasma strain infecting the field-collected samples, we cloned and sequenced Spiroplasma-16S rDNA and rpoB (RNA polymerase, subunit beta) from flies from two NGU sampling sites (GAN and GOR). The 16S rDNA touchdown PCR was performed in 20 μL reactions containing 1x GoTaq Green Mastermix (Promega, USA), 0.4 μM of each primer SpirRNAF and SpirRNAR (S2 Table) and 1 μL of template DNA (20-100ng). The PCR profile consisted of an initial denaturation for 3 mins. at 94°C, followed by 8 cycles of 1 min. at 94°C, 1 min. at 63°C, and 1 min. at 72 with a reduction in annealing temperature of 1°C/cycle (63–56°C). This step was followed by 30 cycles of 1 min. at 94°C, 1 min. at 55°C, and 1 min. at 72°C, with a final extension at 72°C for 10 min. Reaction set up for the rpoB-PCR was the same as for 16S rDNA (S2 Table), and the PCR profile consisted of a 3-min. initial denaturation at 94°C, followed by 34 cycles of 90 sec. at 94°C, 90 sec. at 55°C, and 90 sec. at 72°C, with a final extension at 72°C for 10 min. All amplicons were gel purified using the Monarch DNA Gel Extraction Kit (New England Biolabs, USA) and cloned into pGEM-T vector (Promega, USA). In total six 16S rDNA and two rpoB clones were sequenced and analysed using the BLASTN algorithm and finally aligned using Spiroplasma glossinidia data from NCBI as reference.
To test for potential Spiroplasma strain variation among the different Gff sampling sites, we cloned and sequenced the Multi Locus Sequence Analysis (MLST) genes dnaA, fruR, parE and rpoB from two individuals from ACA (Okole River) and OKS (Lake Kyoga) sampling sites. MLST-PCR reactions were performed with the same reaction set up as described above (S2 Table). The parE touchdown PCR was run as described above for 16S rDNA but with 61°C/52°C annealing temperature. A touchdown PCR approach was also used for the fruR locus with 30 sec steps in each cycle and 60°C/52°C annealing temperature. Cloning and sequencing was performed as described above. All sequences were manually edited using Bioedit v7.1.9 [50] and aligned with related sequences available at NCBI using MUSCLE [51].
Spiroplasma, Wolbachia and trypanosome infection status
At the time of collection, sex and wing fray information (all flies were wing fray 2–3, i.e. 4–8 weeks old) was recorded for each fly and midguts were dissected and microscopically analyzed for trypanosome infection status. The dissected guts and reproductive parts were stored in 95% ethanol for further analyses. Genomic DNA was extracted from female and male reproductive parts (RP, n = 1157) as well as from whole bodies (WB, n = 258) using DNeasy Blood and Tissue Kit (Qiagen, Germany). We used flies from Pagirinya (PAG) for which we had DNA available from both the WB and RP tissues to compare the Spiroplasma infection prevalence to rule out potential DNA source bias in the prevalence results. We noted Spiroplasma infection of 18% in WB versus 17% in RP, which is not significantly different (Fisher’s P > 0.999). Consequently, both tissue datasets were pooled for the final infection prevalence analyses. The final sample set was composed of 893 females and 522 males.
Spiroplasma infection prevalence was determined by 16S rDNA touchdown PCR as described above. The presence of Wolbachia was assessed by PCR targeting a single copy membrane protein encoding gene (Wolbachia Surface Protein gene, wsp). The PCR reactions were performed as previously described in [52–54]. All amplified fragments were analysed on 1% agarose gels using Gel Doc EQ quantification analysis software (Bio-Rad, Image Lab Software Version 4.1). We used generalized linear models (GLM) to test for the significance of difference in Spiroplasma infection between Wolbachia-infected and uninfected flies.
Assessment of variation in Spiroplasma density by qPCR
Potential Spiroplasma density differences between individuals and across seasons were tested via quantitative PCR (qPCR) using a CFX96 Real-Time PCR Detection System and iTaq Universal SYBR Green Supermix (Biorad). The relative amount of Spiroplasma was calculated with the 2-(ΔΔCt) method using primers targeting the Spiroplasma RNA polymerase beta gene (rpoB). Values were normalized against the single copy Glossina Peptidoglycan Recognition Protein-LA gene (pgrp-la). All primer sets are listed in S2 Table. The trypanosome infection status of each sample was assessed by microscopic analysis of dissected guts at the time of sample collection in the field.
Mitochondrial DNA (mtDNA) sequencing and microsatellite genotyping
In addition to the samples typed for a previous study [47], we sequenced an additional 161 flies for a 491 bp fragment of mtDNA, and genotyped an additional 131 flies at 16 microsatellite loci. To do this, we extracted total genomic DNA from two legs of individual flies using the Qiagen DNeasy Blood & Tissue kit. For mtDNA sequencing, a 491 bp fragment from the cytochrome oxidase I and II gene (COI, COII) was amplified as described in [47]. PCR amplicons were run on 1% agarose gels, purified and sequenced at the Yale Keck DNA Sequencing facility. New sequences were combined with existing data [47] for a total data set of 490 sequences (S1 Table). For microsatellite analysis, we genotyped flies at 16 microsatellite loci using methods described in [47]. New genotype calls were combined with existing data [47] for a total data set of 558 genotypes at 16 microsatellite loci (S1 Table).
Identifying Gff mtDNA and nuclear genetic backgrounds
To evaluate the potential association between Spiroplasma infection prevalence and the Gff genetic background, we assigned each individual to a single mtDNA haplogroup based on the phylogenetic relationships among haplotypes, and to a single nuclear genetic background based on clustering analysis of microsatellite genotypes.
For mtDNA haplogroup assignment, first evolutionary relationships between the mtDNA haplotypes were assessed by constructing a parsimony-based network using TCS 1.21 [55] as implemented in PopART ([56]; Population Analysis with Reticulate Trees: http://otago.ac.nz). These haplotypes were then grouped by phylogenetic relationship following [48] into three haplogroups, each imperfectly associated with the NWGU, NEGU, and WGU.
For nuclear genetic background assignment, we used a Bayesian clustering analysis in the program STRUCTURE v2.3.4 [57] to group individuals based on their microsatellite genotypes. STRUCTURE assigns individuals into a given number of clusters (K) to maximize Hardy-Weinberg and linkage equilibrium. The program calculates the posterior probability for a range of K and provides a membership coefficient (q-value) of each individual to each cluster. In this analysis, we assessed membership to just three clusters corresponding to the NWGU, NEGU, and WGU. The ADMX does not represent its own cluster, but instead represents a mixed assignment to the NWGU and NEGU. We performed 10 independent runs for K = 3 with a burn-in of 50.000 followed by 250.000 MCMC steps and summarized results across the 10 independent MCMC runs using the software CLUMPAK [58]. Individual flies were assigned to nuclear genetic units based on q-values. Individuals with q-values > 0.8 were assigned to one of the three distinct clusters (NWGU, NEGU or WGU), and individuals with mixed assignment (q-values ranging from 0.2 to 0.8) to the NWGU and NEGU were assigned as “admixed” (ADMX).
Associations of Spiroplasma infection with watershed, season of collection, and host genetic traits
Predictive variables considered included watershed of origin, season of collection, Gff host genetic background (both nuclear and mitochondrial), Gff host sex, and trypanosome co-infection. A challenge in this analysis was that correlation between the geographic origin (specifically watershed) and environmental conditions, as well as Gff genetic background and trypanosome infection status is well established [47–49,59]. We confirmed these correlations among predictive variables and patterns of Spiroplasma infection, we performed a multiple correspondence analysis (MCA).
We took two approaches to control for correlation among predictive variables with watershed of origin. First, we fit generalized logistic mixed models (GLMM) with the predictive variables of interest (season, Gff host genetic background, sex, and co-infection) as fixed effects with and without watershed of origin as the random effect, and tested the improvement of the models with an analysis of variance (ANOVA) Chi square test. We followed these tests with more complex combinations of predictive variables using watershed as the random effect. GLMM was performed with the ‘glmer’ function in the R [60] package lme4 [61] and fitted using maximum likelihood.
Second, we fit generalized linear models (GLM) one watershed at a time for each predictive variable (season, Gff host nuclear and mtDNA genetic background, sex, and co-infection). Direction and significance of the effect of each predictive variable was assessed with Tukey’s contrasts with p-values (P) obtained from the z distribution and corrected for multiple comparisons using the unconstrained (“free”) adjustment. GLM was performed in R with the multcomp package [62].
Effect of Spiroplasma and trypanosome infection success under laboratory conditions
Effect of Spiroplasma presence on trypanosome infection outcome was also tested in the laboratory by infecting the Gff colony (IAEA, Vienna, Austria), with bloodstream form Trypanosoma brucei brucei (RUMP503) parasites. Pupae from the colony were sent to Yale, and emerging flies were used for parasite infections. This Gff line has been shown to exhibit a heterogeneous Spiroplasma infection prevalence [31]. Following our established protocols [63,64], teneral (newly eclosed) flies were infected by supplementing their first blood meal with 5x106 parasites/ml. All flies that had successfully fed on the infectious blood meal were subsequently maintained on normal blood, which they received every other day. Fourteen days post infection (dpi), the presence of trypanosome infections in the midgut was assessed microscopically plus using a PCR assay. PCR was performed using primers trypalphatubF and trypalphatubR, which target the alpha chain of T. brucei tubulin (S2 Table). The reaction set up and the cycler profile were the same as used for Wolbachia ARM-PCR described above. Spiroplasma presence was assessed in the corresponding reproductive tissue of each fly via PCR assay using the Spiroplasma infection assay described above. We used generalized linear models (GLM) to test for the significance of difference in trypanosome infection between Spiroplasma-infected and uninfected flies.
Results
Spiroplasma infection type from field collections
To assess the Spiroplasma strain infecting Gff sampling sites, we employed 16S rDNA and rpoB sequencing analysis. In samples analyzed from the Okole River and Lake Kyoga watersheds, we found a single strain infection (S1 Fig), which belongs to the Citri-Chrysopicola-Mirum clade and which was also previously identified from a Gff colony [31].
We tested for Spiroplasma infection in 1415 Gff individuals (894 females and 522 males; S1 Table) collected from 26 sampling sites spanning five watersheds (Fig 1). In the northwest region, flies from the Albert Nile watershed (n = 487) had a mean infection rate of 34% (Fig 1 and S1 Table). In the northcentral region, flies from the Achwa River watershed (n = 234) had a mean infection rate of 20%, and the Okole River watershed (n = 389) had a mean infection rate of 5% (Fig 1 and S1 Table). In the northeast region, flies from the Lake Kyoga watershed had relatively low Spiroplasma infection rate, with one of the two sampling sites (AMI, n = 73) having an infection rate of 11%, and the other site (OCU, n = 90) lacking Spiroplasma infection altogether (Fig 1 and S1 Table). In the western region, flies from the Kafu River watershed (n = 142) had an infection rate of 3% (Fig 1 and S1 Table). These results indicate higher Spiroplasma infection in the northwest than in the northeast or west of Uganda, a conclusion that was further supported by tests for association of Spiroplasma with watershed of origin using generalized linear modeling (see discussion of MDS, GLMM, and GLM results below).
Variation in Spiroplasma density
To assess whether potential differences in Spiroplasma infection density could influence our ability to detect the microorganism in the DNA source, we performed qPCR on individuals from the NEGU (AMI) sampled across multiple seasons. We detected varying densities, with the highest Spiroplasma levels observed in the intermediate season (S2 Fig).
Assignment of Gff nuclear and mitochondrial genetic background
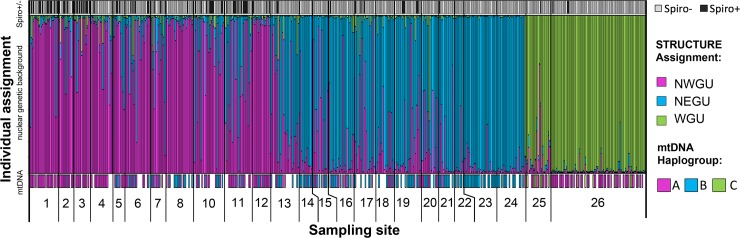
We assigned Gff host nuclear (biparentally inherited) genetic background using STRUCTURE cluster assignments that were based on the microsatellite genotypes. STRUCTURE assignment indicated 195 flies had high (> 0.8) membership probability to the NWGU, 94 to the NEGU, and 109 to the WGU (S1 Table). We found 160 flies with mixed assignment, which we considered members of the ADMX genetic background (S1 Table). Flies assigned to the NWGU had a mean Spiroplasma infection rate of 25%, the ADMX genetic background had a mean infection rate of 15%, the NEGU (n = 94) had a mean infection rate of 2%, and the WGU had a mean infection rate of 4% (Fig 2 and S1 Table).
Fig 2.
Plot of Gff host Spiroplasma infection, STRUCTURE assignment to nuclear genetic background (NWGU, NEGU, and WGU), and mtDNA haplogroup (A, B, and C). Each bar represents a single individual. The top panel shows presence/absence of Spiroplasma of each individual (gray = negative, black = positive). The middle panel shows the STRUCTURE assignment to nuclear genetic background of each individual (pink = northwest genetic unit, blue = northeast genetic unit, green = west genetic unit). The bottom panel shows the mtDNA haplogroup assignment (indicated by colored boxes) to each individual (pink = mtA, blue = mtB, green = mtC, blank = missing data). Numbers below the bar plot correspond to the 26 sampling sites shown on the map in Fig 1.
Although these results suggest higher Spiroplasma infection in the NWGU and ADMX nuclear genetic backgrounds, this pattern was found to driven by correlation between nuclear genetic background and watershed of origin (see discussion of MDS, GLMM, and GLM results below). We assigned Gff host mitochondrial (maternally inherited) genetic background by generating a TCS network of the mtDNA sequences (n = 490). We identified 29 unique mitochondrial haplotypes that grouped into the three previously described major haplogroups: mtA, mtB and mtC (Figs 2 and S4) [47]. 266 flies were assigned to haplogroup A, 209 to haplogroup B, and 15 to haplogroup C (S4 Fig). Flies assigned to mtA had a mean Spirplasma infection rate of 19%, mtB had a mean infection rate of 10%, and mtC had a mean infection rate of 13% (Fig 2 and S1 Table). Although these results suggest higher Spiroplasma infection in the mtA mitochondrial genetic background, this pattern was found to be driven by correlation between mitochondrial genetic background and watershed of origin (see discussion of MDS, GLMM, and GLM results below).
Associations of Spiroplasma infection with watershed of origin, season, and host traits under field collections
The MCA confirmed that there were strong correlations among predictive variables, especially with watershed of origin (S3 Fig). GLMM indicated that watershed of origin and season of collection (wet, intermediate and dry) were the two most important factors influencing Spiroplasma prevalence (Tables 1 and S3 and S4). GLMM of all predictive variables (considered one at a time) were significantly improved by adding watershed as a random effect (ANOVA P ranging from 2.20e-16 to 0.0022, S3 Table). Models exploring multiple predictive variables at a time indicated that season of collection was the only variable that positively influenced the fit of the model (ANOVA P = 3.57e-6, Table 1). Adding random slope or any of the other predictive variables (nuclear or mtDNA genetic background, sex, or trypanosome co-infection) did not significantly improve the model (ANOVA P ranging from 0.2870 to 1.0, S4 Table).
Table 1. Influence of different predictors on the probability of Spiroplasma infection [Pr(Spiro+)] in generalized linear models (GLMM).
Results from generalized linear mixed models (GLMM), where the random effect was watershed (WS) with random intercept (RI), and the fixed effects were season (SN) and/or nuclear genetic background (GB). The table includes the model name, the fixed effect(s), the degrees of freedom (Df), the Akaike information criterion (AIC) with the best model in bold, the Bayesian information criterion (BIC) with the best model in bold, the log likelihood (logLik), the deviance (Dev) of the model, the analysis of variance (ANOVA) used to test for the model’s improvement, the ANOVA Chi square value (ChiSq), the Chi Square degrees of freedom (ChiDf), and the ANOVA p-value. Adding SN was the only fixed effect that significantly improved the model. In contrast, GB did not significantly improve the GLMM. Additionally, changing the order introducing fixed effects or adding random slope also did not improve the GLMM (S3 Table). For all p-values, the level of significance was marked *** if < 0.0001, ** if < 0.001, * if < 0.05, and not marked if > 0.05.
| GLMM | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Random effect | Fixed effect(s) | Df | AIC | BIC | logLik | Dev | ANOVA | ANOVA p-value |
| 1a | WS with RI | None | 2 | 288.1 | 296.0 | -142.1 | 284.2 | NA | NA |
| 2a | WS with RI | SN | 4 | 267.1 | 282.8 | -129.5 | 259.1 | 1a - 2a | 3.57E-06 *** |
| 2b | WS with RI | SN+GB | 7 | 271.6 | 299.1 | -128.8 | 257.6 | 1a - 2b | 0.6899 |
| 3a | WS with RI | GB | 5 | 290.4 | 310.0 | -140.2 | 280.4 | 1a - 3a | 0.287 |
| 3b | WS with RI | GB+SN | 7 | 271.6 | 299.1 | -128.8 | 257.6 | 1a - 3b | 1.13E-05 *** |
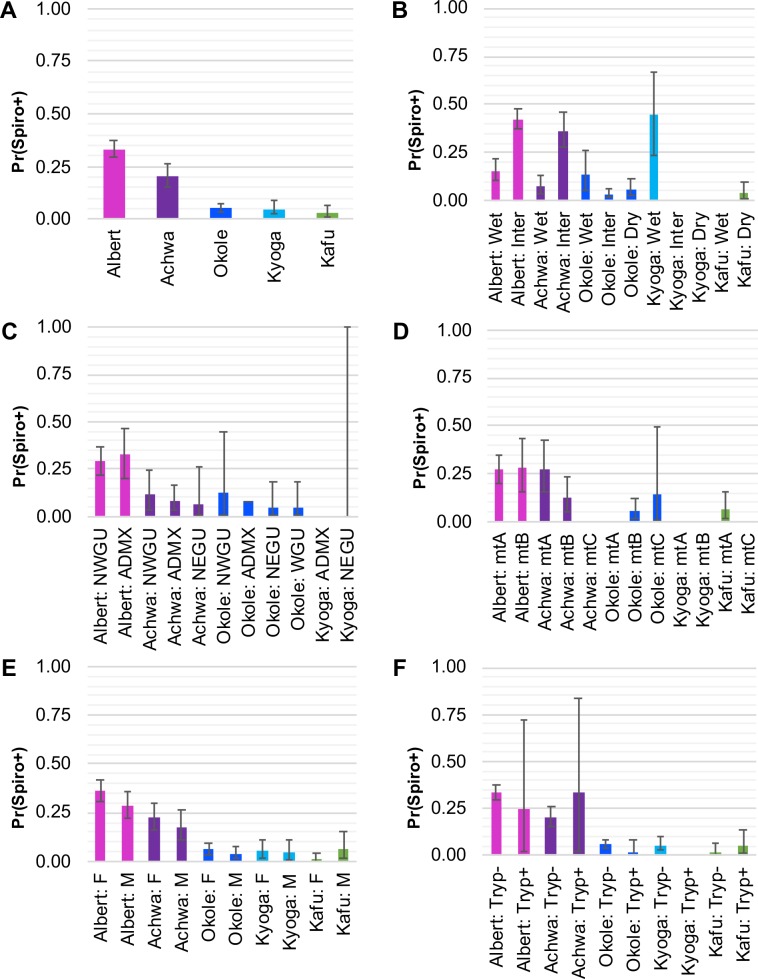
GLM by watershed indicated that flies from the Albert Nile had by far the highest probability of Spiroplasma infection [Pr(Spiro+) = 0.32], followed by flies from the neighboring Achwa River [Pr(Spiro+) = 0.22, Fig 3]. Tukey’s contrasts indicated that the Albert Nile had only somewhat higher Pr(Spiro+) than the Achwa River (Tukey’s contrast P = 0.0015), but that these two watersheds had significantly higher Pr(Spiro+) than any of the other watersheds (Tukey’s contrasts P ranging from 2.00e-16 to 0.0002, Tables 2 and S5).
Fig 3. Probability of Spiroplasma infection by watershed.
Results of the generalized linear models (GLM) by watershed of origin showing estimated probability of Spiroplasma infection [Pr(Spiro+)] with lower 2.5% and upper 97.5% bounds for (A) watershed of origin only, (B) season of collection by watershed, (C) Gff host nuclear genetic background (NWGU = northwest genetic unit, ADMX = admixed, NEGU = northeast genetic unit, WGU = west genetic unit), (D) Gff host mitochondrial genetic background (mtA = haplogroup A, mtB = haplogroup B, mtC = haplogroup C), (E) Gff host sex (F = female, M = male), and (F) trypanosome coinfection (Tryp+ = coinfection, Tryp- = no coinfection). Each bar is labeled with the watershed and predictive variable, and color coded by watershed (pink = Albert Nile, purple = Achwa River, dark blue = Okole River, light blue = Lake Kyoga, green = Kafu River). See S5 Table for details and Tukey’s contrasts that assess significance of the associations.
Table 2. Influence of different predictors on the probability of Spiroplasma infection [Pr(Spiro+)] in generalized linear models (GLM).
Generalized linear model (GLM) by watershed, with season as the predictor. The table includes watershed, season of collection [wet, intermediate (Inter), and dry], GLM estimate of Pr(Spiro+), the lower 2.5% limit and the upper 97.5% limit of the estimate, the Tukey’s contrast (TC) used to test for significant differences in Pr(Spiro+) between seasons, and the TC p-value. The only significant differences in Pr(Spiro+) between seasons was between the wet and the intermediate seasons in the Albert Nile and Achwa River watersheds. Additionally, none of the other predictors (GB, mtDNA haplogroup, trypanosome infection status, or sex) had significant effect on Pr(Sprio) (S5 Table). For all p-values, the level of significance was marked *** if < 0.0001, ** if < 0.001, * if < 0.05, and not marked if > 0.05.
| GLM | ||||||
|---|---|---|---|---|---|---|
| Watershed | Season | Pr(Spiro+) | Lower limit (2.5%) | Upper limit (97.5%) | TC | TC p-value |
| Albert Nile |
Wet | 0.16 | 0.10 | 0.22 | Wet—Inter | 1.160e-07 *** |
| Inter | 0.42 | 0.37 | 0.48 | |||
| Achwa River | Wet | 0.08 | 0.04 | 0.13 | Wet—Inter | 5.380e-06 *** |
| Inter | 0.36 | 0.27 | 0.46 | |||
| Okole River | Wet | 0.13 | 0.05 | 0.26 | Wet—Inter | 0.7777 |
| Inter | 0.03 | 0.02 | 0.06 | Inter—Dry | 0.5577 | |
| Dry | 0.06 | 0.03 | 0.11 | Wet—Dry | 0.0962 | |
| Lake Kyoga | Wet | 0.44 | 0.23 | 0.67 | Wet—Inter | 1 |
| Inter | 0.00 | 0.00 | 1.00 | Inter—Dry | 1 | |
| Dry | 0.00 | 0.00 | 1.00 | Wet—Dry | 1 | |
| Kafu River |
Wet | 0.00 | 0.00 | 1.00 | Wet—Dry | 1 |
| Dry | 0.04 | 0.01 | 0.09 | |||
In addition to watershed of origin, season of collection was strongly associated with Spiroplasma infection. The intermediate season had significantly higher probability of Spiroplasma infection than the wet season, which was especially apparent in the Albert Nile [Pr(Spiro+) = 0.42 vs 0.16)] and Achwa River watersheds [Pr(Spiro+) = 0.36 vs 0.08] (Tukey’s contrasts P = 1.16E-07 and 5.38E-06, respectively; Table 2). There were no other significant differences in Pr(Spiro+) among seasons in any of the other watersheds (Table 2). Additionally, none of the other predictive variables (nuclear or mtDNA genetic background, sex, or trypanosome co-infection) had significant effects on Pr(Spiro+) when analyzed by watershed (Tukey’s contrasts P ranging from 0.0962 to 1.0, S5 Table).
Co-infections of Spiroplasma and Wolbachia symbionts
Previous analysis of Gff from southern and central regions of Uganda also indicated presence of distinct genetic backgrounds associated with these individuals [47,48] as well as the presence of heterogeneous and low-density infections with another endosymbiont, Wolbachia [27, 31]. To investigate for similar patterns in northern Uganda, we analyzed 106 Gff individuals from the Albert Nile (NWGU) and Achwa/Okole River (ADMX) watersheds for the presence of Wolbachia infections (S1 Table). Within this set, 92% of flies were not carrying Wolbachia (98/106). The remaining infected 8% (8/106) were all from Achwa river except one sample. Most Wolbachia-infected flies (6/8) were Spiroplasma-negative, and Wolbachia-negative flies were Spiroplasma-positive and -negative (S1 Table). The generalized mixed models (GLM) indicated no significant correlation between the presence of Wolbachia and the probability of Spiroplasma infection (Tukey’s P = 0.78).
Effects of Spiroplasma on probability of trypanosome coinfection under laboratory conditions
Patterns of Spiroplasma infection from the field collections, although not significant in the GLMM or GLM, indicated a possible correlation between Spiroplasma and trypanosome co-infections. Of the 243 Spiroplasma infected flies identified in the study, only 2% (n = 5) had trypansome co-infection (infection rate among the Spiroplasma-negative flies was 10%; n = 115). To test this correlation under more controlled conditions where we could ensure statistical power, we used a laboratory line of Gff with heterogeneous infection of Spiroplasma [30] to complete a trypanosome infection challenge experiment. Microscopic examination and PCR analysis of challenged individuals (n = 123) revealed a negative correlation between the presence of Spiroplasma and trypanosome co-infection, with 18% of individuals co-infected with both microbes (S+T+; n = 22), 20% infected with only trypanosomes (S-T+; n = 25), 46% infected with only Spiroplasma (S+T-; n = 56), and 16% without infection with either microbe (S-T-; n = 20; Fig 4 and S6 Table). The generalized mixed models (GLM) indicated a significant negative correlation between the presence of Spiroplasma and the probability of trypanosome coinfection (Tukey’s P = 0.0010).
Fig 4. Trypanosome infection experiment using a laboratory Gff line.
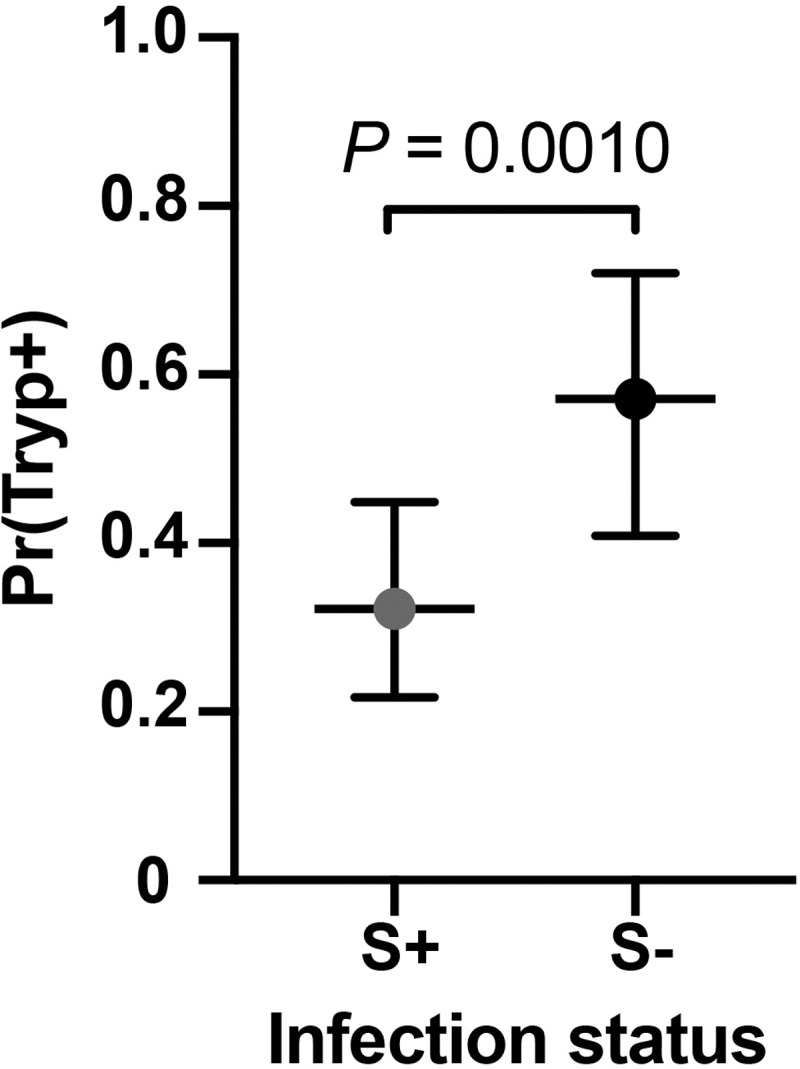
Scatter dot plot shows the percentage of flies 14 days post infection acquisition found to be infected with either Spiroplasma only (S+T-, gray) or with with trypanosome only (S-T+, black). Bars show 95% confidence interval. Infection status was assessed by microscopy (trypanosomes) and PCR (trypanosomes and Spiroplasma). Dotted line shows the median of the data. Abbreviations: S+ Spiroplasma-infected, S- Spiroplasma-uninfected, T+ Trypanosoma-infected, T- Trypanosoma-uninfected.
Discussion
In this study, we performed a spatial and temporal analysis of the prevalence of the endosymbiont Spiroplasma in genetically distinct Gff populations across northern Uganda and assessed the factors that could impact the dynamics of infection with this symbiotic bacterium. The most influential factors that shaped the patchy distribution of Spiroplasma in Gff were the fly’s watershed of origin and the season of collection. The importance of watershed and season suggests that the prevalence of the symbiont is significantly affected by the geographic dispersal of the flies as well as by the changing environmental conditions. The low rate of Spiroplasma and trypanosome co-infections in the field collections, and the negative association of Spiroplasma and with trypanosome infection in a challenge experiment carried out in a laboratory Gff line suggests that Spiroplasma infections may negatively influence the success of parasite infection outcome.
Geographic origin and seasonality
We found that flies residing in geographically separated watersheds have significantly different Spiroplasma infection prevalences (Figs 1 and S3). Gff is a riparian species that inhabits low bushes or forests at the margins of rivers, lakes or temporarily flooded scrub land. These water connections may influence dispersal patterns by limiting dispersal between the different watersheds. Limited dispersal would minimize contact among flies from different watersheds. This could suggest that horizontal transfer occurs between flies within the same watersheds, or that there is environmental acquisition of Spiroplasma in Gff. However, it remains to be elucidated whether flies encounter Spiroplasma from the environment or during feeding. Interestingly, although tsetse are strict blood feeders on vertebrate hosts, a recent study has indicated that they are capable of feeding on water with or without sugar when deprived of a blood meal [65]. This feeding behavior would allow Gff to acquire Spiroplasma from the environment, and could account for the strong association of Spiroplasma with watershed of origin, and lack of correlation with other host genetic background. Finally, transfer of Spiroplasma via ectoparasites could account for different infection frequencies. Although there are no ectoparasites described yet, which are associated with tsetse flies, this route of transmission cannot be excluded.
In addition to geographic origin, the season of collection was an important factor shaping the Gff-Spiroplasma association in Uganda. We found that the intermediate season (June—July) was correlated with higher Spiroplasma infection prevalence than either the wet (April—May and August—October) or dry (December—March) seasons (Table 1 and Fig 3). This might be because the environmental conditions during the wet and dry seasons restrict the availability of animal hosts for tsetse blood feeding. This could indicate that the intermediate season represents optimal foraging conditions for Gff. In fact, Spiroplasma survival in Drosophila is dependent on the availability of hemolymph lipids [43,44]. Hence, during nutritionally optimal times, maintenance of the symbiont may be less costly for the host than during a period of compromised fitness, and thus higher prevalence and density is observed during these periods [43,66]. Other symbionts capable of triggering host phenotypes, such as male-killing, are also affected by host fitness. The persistence of Wolbachia, for example, is negatively affected by host fitness via temperature as well as by other stress factors (e.g. [66–68]). Thus, the more stressful conditions of the dry season may reduce the overall fitness of the fly and consequently the Spiroplasma infection densities. This scenario is supported by our finding of different Spiroplasma densities in flies analyzed across the three sampling seasons (Figs 2 and S2). Varying Spiroplasma densities can also influence the transmission efficiency of the symbiont from mother to tsetse’s intrauterine progeny. Furthermore, if Spiroplasma is horizontally transferred via the environment, the climatic conditions can also impact the abundance of Spiroplasma for acquisition by Gff. Higher Spiroplasma density in the environment during the intermediate season could facilitate their acquisition by the tsetse host. In support of this theory, free-living bacterial communities that reside in aquatic systems are strongly affected by environmental factors, such as pH [69,70] and seasonality [71]. A more recent study has highlighted the significant effect of seasonality-related changes in soil bacterial communities [72]. Hence, the changing environmental conditions that occur between the dry, wet, and intermediate season might impact Spiroplasma density and consequently the infection density. However, seasonality alone cannot explain the differences of the symbiont differences but other parameters such as e.g. temperature variations should be considered.
Host genetic nuclear and mitochondrial background
Genetically variable Gff populations residing within the Gambiense and Rhodesiense HAT belts could influence the inheritable microbiota composition, which in turn could have important implications in transmission dynamics and vector control outcomes [73–75]. In that context, we evaluated the potential correlation between host nuclear genetic differences and Spiroplasma infection prevalence across the 26 Gff sampling sites. However, we found that after accounting for watershed of origin in the GLMM model, Spiroplasma infection was not significantly influenced by Gff host nuclear or mitochondrial genetic background (Figs 2 and 3 and S5 Table). Lack of association of with host genetic background provides further support for the idea that Spiroplasma may be acquired by horizontal transfer between flies within the same watersheds, or through contact with other sources of the bacteria in the environment.
Spiroplasma and Wolbachia
In Drosophila, Spiroplasma can either coexist with the endosymbiotic Wolbachia without little or no impact on each other [76], or negatively affect Wolbachia presence. Goto and coworkers showed that the presence of Spiroplasma suppresses Wolbachia infection density in D. melanogaster [77]. Interestingly, while the tsetse species in the Morsitans subgroup, such as G. morsitans, harbor Wolbachia infections, they lack Spiroplasma associations [31]. In contrast, the tsetse species in the Palpalis subgroup, such as Gff and G. palpalis, harbor Spiroplasma infections, but lack Wolbachia associations [31]. Spiroplasma might be negatively affected by the presence of Wolbachia infection in the species within the Morsitans subgroup [31]. As such, the high titer of Wolbachia noted in G. morsitans reproductive organs could suppress the establishment of Spiroplasma infections. Our prior and current studies with distinct Gff populations in Uganda also indicate a potential negative influence for the two endosymbiont infections [28]. Our previous finding reported Wolbachia infections across 18 Gff sampling sites from the Northcentral, West and South of Uganda [28]. In this study we detected 8% Wolbachia infections in samples analyzed from the Achwa River watershed, which is the geographic area with highest Spiroplasma infection prevalence (S1 Table). However, 6/8 Wolbachia-positive samples were negative for Spiroplasma, and hence the idea of reciprocal exclusion of both entities remains a possibility although the GLM did not suggest a significant correlation between the two bacterial entities. We had also noted that the Wolbachia density in the reproductive organs of Gff is unusually low, and that Spiroplasma densities can vary across seasons, thus rendering co-infection detections technically challenging, particularly when using whole body DNA, as was the case with a number of flies analyzed in this study.
It also remains to be elucidated if the presence of viral microorganisms plays a role for the infection dynamics of Spiroplasma in Ugandan Gff populations. The salivary gland hypertrophy virus (SGHV), a common virus of Glossina, is inversely correlated with Wolbachia infection prevalence in Gff in Uganda [28]. Hence, the correlation between Spiroplasma and SGHV remains to be tested.
Role of Spiroplasma association with Gff physiology
Reproductive influences, such as male killing, have been associated with maternally inherited symbionts, including Spiroplasma, where the symbiont drives its own dispersal by selectively killing male embryos in ladybirds, fruit flies and certain butterfly species (reviewed in [41]). As we observed both Spiroplasma-infected males and females and there was also no sex bias in the offspring of the laboratory-reared Gff, this bacterium likely does not confer a male killing trait to Gff. Pairwise comparison suggested a slightly higher infection prevalence in females, but such a slight difference is unlikely to be the result of a male killing phenotype.
We also evaluated the potential role of Spiroplasma on tsetse’s immune physiology by measuring the correlation between trypanosome and Spiroplasma infection status. Of the 1415 samples analyzed, we found only five with trypanosome and Spiroplasma co-infections. Although not significant in the GLMM or GLM, this negative results could have been caused by lack of statistical power in the field collected data. To further address the question of a similar protective effect, we performed trypanosome infection experiments using a colonized Gff line that displays heterogeneous Spiroplasma infection prevalence [31]. Our finding that infections with Spiroplasma alone were more frequent than co-infections with Tbb (56% vs. 18%, respectively; S4 Table) indicates a negative correlation between the presence of the symbiont and the parasite, and suggests that both entities negatively impact each other’s fitness. In accordance with investigations in Drosophila [78,79], Spiroplasma infections may confer physiological traits that protect its tsetse host from being colonized by trypanosome infections. In different hosts, Spiroplasma induces a protective effect against nematodes, fungi and parasitoid wasps [35,36,78]. It remains to be elucidated, whether a potential protection in Gff against parasite infections results from niche competition of both microbes within the host, or by expression of certain Spiroplasma-derived molecules, which block trypanosomes. Alternatively, it has been shown that infections with certain Wolbachia strains confer an immune enhancement phenotype to their Drosophila and mosquito hosts, hence increasing the resistance to other pathogens [80; also reviewed in 81]. Such an immune enhancement, which affect the trypanosome transmission success in Spiroplasma infected Gffs however, is possible but rather unlikely given the low infection frequency of Wolbachia (12%) in the tested flies.
The heterogeneous Spiroplasma infection prevalence in the Gff line that we used in the parasite challenge experiment (Fig 4) might result from imperfect maternal transmission to progeny and may similarly be influencing the infection prevalence noted in natural populations. Tsetse are viviparous and the mother supports the development of her progeny in an intrauterine environment. Endosymbiotic Wigglesworthia and Sodalis are maternally acquired by the progeny in female milk secretions during the lactation process, while Wolbachia is transovarially transmitted. Spiroplasma infections in the gonads suggest that this endosymbiont is also transovarially transmitted, although transmission through milk secretions remains to be investigated. While tsetse females remain fecund throughout their entire life (and can produce 8–10 progeny), the transmission of Spiroplasma from mother to her intrauterine progeny however may be more efficient during the early gonotrophic cycles and decrease over the course of female’s reproductive lifespan. Such a scenario could explain why we observed that only 50–60% of colony flies are infected with Spiroplasma. We will further address this question by testing the efficiency of symbiont transmission from mother to each of her offspring in a follow-up study by developing single lines from each pregnant female. Such an imperfect transmission efficiency can also influence the heterogeneous infection prevalence we noted in field populations.
In conclusion, the infection prevalence of Spiroplasma in Gff populations in northern Uganda is significantly correlated with different watersheds of origin and seasonal environmental conditions. These associations indicate that seasonal fluctuations and other transmission modes than strictly vertically are drivers of Spiroplasma acquisition in Gff in Uganda.
We further demonstrate that colonized Gff are less likely to establish trypanosome parasite infections when carrying Spiroplasma infections, which is of particular interest in the context of alternative vector control approaches to control trypanosome infections.
Supporting information
Spiroplasma strains from the Albert Nile watershed (OKS) and the Okole River watershed (ACA) are identical to each other and highly conserved in comparison to the Spiroplasma present in two individuals screened from a Gff laboratory line (KX159379_sGfus_BL, KX159380_sGfus_SL) and one Uganda field sample (KX159381_sGfus_U350). These sequences were recently published in [31]. The tree was calculated in CLC using Jukes-Cantor as distance measure with 1000 replicates, and rooted against the outgroup Spiroplasma culicicola. Scale is 0.3 substitutions per site.
(TIF)
The graph shows the relative density of Spiroplasma tested via 16S rDNA in Gff from AMI population (Lake Kyoga) across wet, dry and intermediate season. Spiroplasma levels are higher in intermediate and wet season compared to the dry season.
(TIF)
Dimensions 1 and 2 (accounting for 31.7% and 15.6% of the variation) are plotted. Populations are depicted by triangles. Association with trypanosomes and Spiroplasma is shown as open triangles (infected and uninfected). Further associations shown in the plot are the host mtDNA genetic background (mtA, mtB, mtC), the nuclear genetic background (NWGU, ADMX, NEGU, WGU), the season (Dry, Inter, Wet), and the watershed of origin (Albert Nile, Achwa River, Okole River, Lake Kyoga, Kafu River).
(TIF)
The figure shows the TCS network of the COI gene and the proportion of infected individuals within the mtDNA haplogroups A, B, and C. Haplogroup A is associated with the NWGU, haplogroup B is associated with the NEGU, and haplogroup C is associated with the WGU. Red represents individuals with Spiroplasma infection while black represents the uninfected ones. The circle size represents the number of individuals sharing a haplotype; small black nodes represent inferred haplotypes; dashes between haplotypes represent a single mutational step. The haplotype network was generated using the TCS method [55] available in POPART [56].
(TIF)
The table on the main tab (‘Individuals’) includes information by individual: Fly ID, sampling site code, sampling site name, district, latitude, longitude, watershed of origin, year of collection, month of collection, season of collection, sex, the tissue used to screen for Spiroplasma (RP = reproductive, WB = whole body), the mitochondrial haplogroup assigned (mtA, mtB, mtC), the STRUCTURE assigned nuclear genetic background (NWGU, ADMX, NEGU, WGU), the PCR-based Spiroplasma and Wolbachia infection status, the visually-based trypanosome infection status, and the source of the genetic data, with individuals that were genotyped for this study indicated as “this study”. A summary of numbers (totals, females, males, Spiroplasma infection) per sampling site and per watershed is included in a separate tab of the same file labeled ‘Sampling Sites’.
(XLSX)
Sequence of primers for non-quantitative plus quantitative PCR is provided in this list.
(DOCX)
Output from generalized linear mixed models (GLMM) with one predictive variable at a time (with and without watershed as a random effect).
(XLSX)
Output from generalized linear mixed model (GLMM) with multiple predictive variables.
(XLSX)
Output from generalized linear models (GLM) by watershed.
(XLSX)
The table summarizes numbers of analyzed flies upon challenge with Tbb and their infection status: S+T+, S+T-, S-T+, S-T-. All flies were analyzed 14 days post infection. Infection status by individual is given in an extra sheet (by individual). The GLM sheet includes the details if the GLM, which was run to analyze the significance of the difference between groups. Abbreviations: Tbb Trypanosoma brucei brucei. S+ Spiroplasma-infected, S- Spiroplasma-uninfected, T+ Tbb-infected, T-Tbb-uninfected.
(XLSX)
Acknowledgments
We thank the Gulu University field team for sampling Gff in Uganda. We also thank Andrew G. Parker and Adly M.M. Abd-Alla from the Insect Pest Control Laboratory, Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, Vienna, Austria for providing Gff flies. We thank Jillian Armstrong and Fatos Karadeniz for their help with DNA extractions and PCR analyses.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by NIH awards R0AI139525 (SA), U01A11I5648 (SA), R01AI068932 (SA, GC), grant D43TW007391 from the Fogarty International Center (SA), by the Ambrose Monell Foundation (SA), and by grant GR028389 from the Bill and Melinda Gates Foundation (SA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bruce D. Preliminary report on the tsetse fly disease or nagana in Zululand. Durban: Bennett and Davis; 1895. [Google Scholar]
- 2.Kleine FK. Weitere wissenschaftliche Beobachtungen über die Entwicklung von Trypanosomen in Glossinen. Dtsch Med Wochenschr. 1909;35: 924–925. [Google Scholar]
- 3.Cox FEG. History of sleeping sickness (African trypanosomiasis) Infect Dis Clin N Am. 2004;18: 231–245. [DOI] [PubMed] [Google Scholar]
- 4.Barret M. The rise and fall of sleeping sickness. The Lancet. 2006;367: 1377–8. [DOI] [PubMed] [Google Scholar]
- 5.Brun R, Blum J, Chappuis F, Burri C. Human African trypanosomiasis. The Lancet. 2010;375: 148–159. [DOI] [PubMed] [Google Scholar]
- 6.Headrick DR. Sleeping Sickness Epidemics and Colonial Responses in East and Central Africa, 1900–1940. PLOS Negl Trop Dis. 2014;8: e2772 10.1371/journal.pntd.0002772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aksoy S, Buscher P, Lehane M, Solano P, Van Den Abbeele J. Human African trypanosomiasis control: Achievements and challenges. PLOS Negl Trop Dis. 2017;11: e0005454 10.1371/journal.pntd.0005454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco JR, Simarro PP, Diarra A, Ruiz-Postigo JA, Jannin JG. The journey towards elimination of gambiense human African trypanosomiasis: not far, nor easy. Parasitology. 2014;141: 748–60. 10.1017/S0031182013002102 [DOI] [PubMed] [Google Scholar]
- 9.Kristjanson PM, Swallow BM, Rowlands GJ, Kruska RL, De Leeuw PN. Measuring the costs of African animal trypanosomosis, the potential benefits of control and returns to research. Agricultural Systems. 1999;59: 79–98. [Google Scholar]
- 10.Simarro PP, Cecchi G, Franco JR, Paone M, Diarra A, Ruiz-Postigo JA, et al. Estimating and Mapping the Population at Risk of Sleeping Sickness. PLOS Negl Trop D. 2012; 6: e1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cecchi G, Paone M, Argilés Herrero R, Vreysen MJB, Mattioli RC. Developing a continental atlas of the distribution and trypanosomal infection of tsetse flies (Glossina species). Parasit vectors. 2015; 8: 284 10.1186/s13071-015-0898-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Büscher P, Bart JM, Boelaert M, Bucheton B, Cecchi G, Chitnis N, et al. Informal Expert Group on Gambiense HAT Reservoirs: Do cryptic reservoirs threaten gambiense-sleeping sickness elimination? Trends Parasitol. 2018;34: 197–207. 10.1016/j.pt.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solano P, Torr SJ, Lehane MJ. Is vector control needed to eliminate gambiense human African trypanosomiasis? Front Cell Inect Microbiol. 2013;3: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Courtin F, Camara M, Rayaisse JB, Kagbadouno M, Dama E, Camara O, et al. Reducing human-tsetse contact significantly enhances the efficacy of sleeping sickness active screening campaigns: A promising result in the context of elimination. PLOS Negl Trop Dis. 2015;9: e0003727 10.1371/journal.pntd.0003727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehane M, Alfaroukh I, Bucheton B, Camara M, Harris A, Kaba D, et al. Tsetse control and the elimination of Gambian sleeping sickness. PLOS Negl Trop Dis 10 2016;e0004437 10.1371/journal.pntd.0004437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis S, Aksoy S, Galvani A. A global sensitivity analysis for African sleeping sickness. Parasitology. 2011;138: 516–26. 10.1017/S0031182010001496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rock KS, Stone CM, Hastings IM, Keeling MJ, Torr SJ, Chitnis N. Mathematical models of human african trypanosomiasis epidemiology. Adv Parasitol. 2015;87: 53–133. 10.1016/bs.apar.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 18.Gilbert JA, Medlock J, Townsend JP, Aksoy S, Ndeffo Mbah M, Galvani AP. Determinants of human African trypanosomiasis elimination via paratransgenesis. PLOS Negl Trop Dis. 2016;10: e0004465 10.1371/journal.pntd.0004465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kariithi HM, Meki IK, Schneider DI, De Vooght L, Khamis FM, Geiger A, et al. Enhancing vector refractoriness to trypanosome infection: achievements, challenges and persepectives. BMC Microbiol. 2018;18(Suppl 1): 179 10.1186/s12866-018-1280-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLOS Pathog. 2008;4: e1000098 10.1371/journal.ppat.1000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong Y, Manfredini F, Dimopoulos G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLOS Pathog. 2009;5: e1000423 10.1371/journal.ppat.1000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss B, Aksoy S. Microbiome influences on insect host vector competence. Trends Parasitol. 2011;27: 514–22. 10.1016/j.pt.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cirimotich CM, Ramirez JL, Dimopoulos G. Native microbiota shape insect vector competence for human pathogens. Cell Host Microbe. 2011;10: 307–310. 10.1016/j.chom.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narasimhan S, Rajeevan N, Liu L, Zhao YO, Heisig J, Pan J, et al. Gut microbiota of the tick vector Ixodes scapularis modulate colonization of the Lyme disease spirochete. Cell Host Microbe. 2014;15: 58–71. 10.1016/j.chom.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aksoy S. Wigglesworthia gen. nov. and Wigglesworthia glossinidia sp. nov., taxa consisting of the mycetocyte-associated, primary endosymbiont of tsetse flies. Int J Syst Bacteriol. 1995; 45: 848–851. 10.1099/00207713-45-4-848 [DOI] [PubMed] [Google Scholar]
- 26.Dale C, Maudlin I. Sodalis gen. nov. and Sodalis glossinidius sp. nov., a microaerophilic secondary endosymbiont of the tsetse fly Glossina morsitans morsitans. Int J Syst Bacteriol. 1999;49: 267–75. 10.1099/00207713-49-1-267 [DOI] [PubMed] [Google Scholar]
- 27.Cheng Q, Ruel TD, Zhou W, Moloo SK, Majiwa P, O'Neill SL, et al. Tissue distribution and prevalence of Wolbachia infections in tsetse flies. Med Vet Entomol. 2000;14: 44–50. [DOI] [PubMed] [Google Scholar]
- 28.Alam U, Hyseni C, Symula RE, Brelsfoard C, Wu Y, Kruglov O, et al. Implications of microfauna-host interactions for trypanosome transmission dynamics in Glossina fuscipes fuscipes in Uganda. Appl Environ Microbiol. 2012; 78: 4627–4637. 10.1128/AEM.00806-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Weiss BL, Aksoy S. Tsetse fly microbiota: form and function. Front Cell Infect MIcrobiol. 2013;3: 69 10.3389/fcimb.2013.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss BL, Wang J, Maltz MA, Wu Y, Aksoy S. Trypanosome infection establishment in the tsetse fly gut is influenced by microbiome-regulated host immune barriers. PLOS Pathog. 2013;9: e1003318 10.1371/journal.ppat.1003318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doudoumis V, Blow F, Saridaki A, Augustinos A, Dyer NA, Goodhead I, et al. Challenging the Wigglesworthia, Sodalis, Wolbachia symbiosis dogma in tsetse flies: Spiroplasma is present in both laboratory and natural populations. Sci Rep. 2017;7: 4699 10.1038/s41598-017-04740-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Symula RE, Alam U, Brelsfoard C, Wu Y, Echodu R, Okedi LM, et al. Wolbachia association with the tsetse fly, Glossina fuscipes fuscipes, reveals high levels of genetic diversity and complex evolutionary dynamics. BMC Evol Biol. 2013;13: 31 10.1186/1471-2148-13-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aksoy E, Telleria EL, Echodu R, Wu Y, Okedi LM, Weiss BL, et al. Analysis of multiple tsetse fly populations in Uganda reveals limited diversity and species-specific gut microbiota. Appl Environ Microbiol. 2014;80: 4301–12. 10.1128/AEM.00079-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaenike J, Stahlhut JK, Boelio LM, Unckless RL. Association between Wolbachia and Spiroplasma within Drosophila neotestacea: an emerging symbiotic mutualism? Mol Ecol. 2010;19: 414–25. 10.1111/j.1365-294X.2009.04448.x [DOI] [PubMed] [Google Scholar]
- 35.Xie J, Vilchez I, Mateos M. Spiroplasma bacteria enhance survival of Drosophila hydei attacked by the parasitic wasp Leptopilina heterotoma. PLOS One. 2010;5: e12149 10.1371/journal.pone.0012149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Łukasik P, Guo H, van Asch M, Ferrari J, Godfray HC. Protection against a fungal pathogen conferred by the aphid facultative endosymbionts Rickettsia and Spiroplasma is expressed in multiple host genotypes and species and is not influenced by co-infection with another symbiont. J Evol Biol. 2013;26: 2654–61. 10.1111/jeb.12260 [DOI] [PubMed] [Google Scholar]
- 37.Jiggins FM, Hurst GD, Jiggins CD, v d Schulenburg JH, Majerus ME. The butterfly Danaus chrysippus is infected by a male-killing Spiroplasma bacterium. Parasitology. 2000; 120(Pt 5): 439–46. [DOI] [PubMed] [Google Scholar]
- 38.Goodacre SL, Martin OY, Thomas CFG, Hewitt GM. Wolbachia and other endosymbiont infections in spiders. Mol Ecol. 2006;15: 517–527. 10.1111/j.1365-294X.2005.02802.x [DOI] [PubMed] [Google Scholar]
- 39.Tinsley MC, Majerus MEN. A new male-killing parasitism: Spiroplasma bacteria infect the ladybird beetle Anisosticta novemdecimpunctata (Coleoptera: Coccinellidae). Parasitology. 2006;132(Pt 6): 757–765. 10.1017/S0031182005009789 [DOI] [PubMed] [Google Scholar]
- 40.Duron O, Bouchon D, Boutin SSS, Boutin S, Bellamy L, Zhou L, et al. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008;6: 27 10.1186/1741-7007-6-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larracuente AM, Meller VH. Host-symbiont interactions: male-killers exposed. Curr Biol. 2016; 26(10): R429–31. 10.1016/j.cub.2016.03.057 [DOI] [PubMed] [Google Scholar]
- 42.Aksoy S, Caccone A, Galvani AP, Okedi LM. Glossina fuscipes populations provide insights for human African trypanosomiasis transmission in Uganda.Trends Parasitol. 2013. August;29: 394–406. 10.1016/j.pt.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paredes JC, Herren JK, Schüpfer F, Lemaitre B. The role of lipid competition for endosymbiont-mediated protection against parasitoid wasps in Drosophila. MBio. 2016;7: e01006–16. 10.1128/mBio.01006-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reynolds KT, Thomson LJ, Hoffmann AA. The effects of host age, host nuclear background and temperature on phenotypic effects of the virulent Wolbachia strain popcorn in Drosophila melanogaster. Genetics. 2013;164: 1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cano J, Descalzo MA, Ndong-Mabale N, Ndong-Asumu P, Bobuakasi L, Nzambo-Ondo S, et al. Predicted distribution and movement of Glossina palpalis palpalis (Diptera: Glossinidae) in the wet and dry seasons in the Kogo trypanosomiasis focus (Equatorial Guinea). J Vector Ecol. 2007;32:218–25. [DOI] [PubMed] [Google Scholar]
- 46.Cano J, Descalzo MA, Ndong-Mabale N, Ndongo-Asumu P, Bobuakasi L, Buatiché JN, et al. Spatial and temporal variability of the Glossina palpalis palpalis population in the Mbini focus (Equatorial Guinea). Int J Health Geogr. 2007;6:36 10.1186/1476-072X-6-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Opiro R, Saarman NP, Echodu R, Opiyo EA, Dion K, alyard A,et al. Genetic diversity and population structure of the tsetse fly Glossina fuscipes fuscipes (Diptera: Glossinidae) in Northern Uganda: Implications for vector control. PLOS Negl Trop Dis. 2017;11: e0005485 10.1371/journal.pntd.0005485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beadell JS, Hyseni C, Abila PP, Azabo R, Enyaru JC, Ouma JO, et al. Phylogeography and population structure of Glossina fuscipes fuscipes in Uganda: Implications for control of tsetse. PLOS Negl Trop Dis. 2010;4: e0005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saarman NP, Opiro R, Hyseni C, Echudo R, Opiyo EA, Dion K, et al. The population genomics of multiple tsetse fly (Glossina fuscipes fuscipes) admixture zones in Uganda. Mol Ecol. 2019;28: 66–85. 10.1111/mec.14957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall T. BioEdit: An important software for molecular biology. GERF Bulletin of Biosciences. 2011;2: 60–61. [Google Scholar]
- 51.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32: 1792–7. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller WJ, Ehrman L, Schneider DI. Infectious speciation revisited: impact of symbiont-depletion on female fitness and mating behavior of Drosophila paulistorum. PLOS Pathog. 2010;6: e1001214 10.1371/journal.ppat.1001214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riegler M, Sidhu M, Miller WJ, O'Neill SL. Evidence for a global Wolbachia replacement in Drosophila melanogaster. Curr Biol. 2015;15: 1428–33. [DOI] [PubMed] [Google Scholar]
- 54.Schneider DI, Klasson L, Lind AE, Miller WJ. More than fishing in the dark: PCR of a dispersed sequence produces simple but ultrasensitive Wolbachia detection. BMC Microbiol. 2014;14: 121 10.1186/1471-2180-14-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clement M, Posada D, Crandall KA. TCS: a computer program to estimate gene genealogies. Mol Ecol. 2000;9: 1657–9. [DOI] [PubMed] [Google Scholar]
- 56.Leigh JW, Bryant D. PopART: Full-feature software for haplotype network construction. Methods Ecol Evol. 2015;6: 1110–1116. [Google Scholar]
- 57.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kopelman NM, Mayzel J, Jakobsson M, Rosenberg NA, Mayrose I. Clumpak: a program for identifying clustering modes and packaging population structure inferences across K. Mol Ecol Resour. 2015;5: 1179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Echodu R, Sistrom M, Hyseni C, Enyaru J, Okedi L, Aksoy S, et al. Genetically distinct Glossina fuscipes fuscipes populations in the Lake Kyoga region of Uganda and its relevance for human African trypanosomiasis. Biomed Res Int. 2013;213: 614721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.R Core Team. R: A language and environment for statistical computing. 2018; Vienna, Austria: R Foundation for Statistical Computing; Available from http://www.r-project.org/. [Google Scholar]
- 61.Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effect models using lme4. Journal of Statistical Software. 2015;67: 1–48. [Google Scholar]
- 62.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometrical Journal. 2008;50: 346–363. 10.1002/bimj.200810425 [DOI] [PubMed] [Google Scholar]
- 63.Welburn SC, Maudlin I. The nature of the teneral state in Glossina and its role in the acquisition of trypanosome infection in tsetse. Ann Trop Med Parasitol. 1992;86: 529–36. 10.1080/00034983.1992.11812703 [DOI] [PubMed] [Google Scholar]
- 64.Vigneron A, Aksoy E, Weiss BL, Bing X, Zhao X, Awuoche EO, et al. A fine-tuned vector-parasite dialogue in tsetse's cardia determines peritrophic matrix integrity and trypanosome transmission success PLOS Pathog. 2018;14: e1006972 10.1371/journal.ppat.1006972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Solano P, Salou E, Rayaisse JB, Ravel S, Gimonneau G, Traore I, Bouyer J. Do tsetse flies only feed on blood? Infect Genet Evol. 2015;36: 184–189. 10.1016/j.meegid.2015.09.016 [DOI] [PubMed] [Google Scholar]
- 66.Herren JK, Paredes JC, Schüpfer F, Arafah K, Bulet P, Lemaitre B. Insect endosymbiont proliferation is limited by lipid availability. Elife. 2014;3: e02964 10.7554/eLife.02964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ross PA, Hoffmann AA. Continued Susceptibility of the wMel Wolbachia infection in Aedes aegypti to heat stress following field deployment and selection. Insects. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Truitt AM, Kapun M, Kaur R, Miller WJ. Wolbachia modifies thermal preference in Drosophila melanogaster. Environ Microbiol. 2018. July 3 10.1111/1462-2920.14347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fierer N, Jackson RB. The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci U S A. 2006;103: 626–31. 10.1073/pnas.0507535103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chu H, Fierer N, Lauber CL, Caporaso JG, Knight R, Grogan P. Soil bacterial diversity in the Arctic is not fundamentally different from that found in other biomes. Environ Microbiol. 2011;12: 2998–3006. [DOI] [PubMed] [Google Scholar]
- 71.Gilbert JA, Field D, Swift P, Newbold L, Oliver A, Smyth T, et al. The seasonal structure of microbial communities in the Western English Channel. Environ Microbiol. 2009;11: 3132–9. 10.1111/j.1462-2920.2009.02017.x [DOI] [PubMed] [Google Scholar]
- 72.Buscardo E, Geml J, Schmidt SK, Freitas H, da Cunha HB, Nagy L. Spatio-temporal dynamics of soil bacterial communities as a function of Amazon forest phenology.Sci Rep. 2018;8: 4382 10.1038/s41598-018-22380-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moloo SK, Kabata JM, Waweru F, Gooding RH. Selection of susceptible and refractory lines of Glossina morsitans centralis for Trypanosoma congolense infection and their susceptibility to different pathogenic Trypanosoma species. Med Vet Entomol. 1998;12: 391–8. [DOI] [PubMed] [Google Scholar]
- 74.Geiger A, Ravel S, Mateille T, Janelle J, Patrel D, Cuny G, et al. Vector competence of Glossina palpalis gambiensis for Trypanosoma brucei s.l. and genetic diversity of the symbiont Sodalis glossinidius. Mol Biol Evol. 2006;24: 102–9. 10.1093/molbev/msl135 [DOI] [PubMed] [Google Scholar]
- 75.Pais R, Lohs C, Wu Y, Wang J, Aksoy S. The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly. Appl Environ Microbiol. 2008;74: 5965–74. 10.1128/AEM.00741-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shokal U, Yadav S, Atri J, Accetta J, Kenney E, Banks K, et al. Katakam A, Jaenike J, Eleftherianos I. Effects of co-occurring Wolbachia and Spiroplasma endosymbionts on the Drosophila immune response against insect pathogenic and non-pathogenic bacteria. BMC Microbiol. 2016;16: 16 10.1186/s12866-016-0634-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goto S, Anbutsu H, Fukatsu T. Asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts coexisting i the same insect host. Appl Environ Microbiol. 2006;72: 4805–10. 10.1128/AEM.00416-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hamilton PT, Peng F, Boulanger MJ, Perlman SJ. A ribosome-inactivating protein in a Drosophila defensive symbiont. Proc Natl Acad Sci U S A. 2016;113: 350–5. 10.1073/pnas.1518648113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ballinger MJ, Perlman SJ. Generality of toxins in defensive symbiosis: Ribosome-inactivating proteins and defense against parasitic wasps in Drosophila. PLOS Pathog. 2017;13: e1006431 10.1371/journal.ppat.1006431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martinez J, Longdon B, Bauer S, Chan YS, Miller WJ, Bourtzis K, et al. Symbionts commonly provide broad spectrum resistance to viruses in insects: a comparative analysis of Wolbachia strains. PLOS Pathog. 2014;10: e1004369 10.1371/journal.ppat.1004369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnson KN. The impact of Wolbachia on virus infection in mosquitoes. Viruses. 2015;7: 5705–17. 10.3390/v7112903 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spiroplasma strains from the Albert Nile watershed (OKS) and the Okole River watershed (ACA) are identical to each other and highly conserved in comparison to the Spiroplasma present in two individuals screened from a Gff laboratory line (KX159379_sGfus_BL, KX159380_sGfus_SL) and one Uganda field sample (KX159381_sGfus_U350). These sequences were recently published in [31]. The tree was calculated in CLC using Jukes-Cantor as distance measure with 1000 replicates, and rooted against the outgroup Spiroplasma culicicola. Scale is 0.3 substitutions per site.
(TIF)
The graph shows the relative density of Spiroplasma tested via 16S rDNA in Gff from AMI population (Lake Kyoga) across wet, dry and intermediate season. Spiroplasma levels are higher in intermediate and wet season compared to the dry season.
(TIF)
Dimensions 1 and 2 (accounting for 31.7% and 15.6% of the variation) are plotted. Populations are depicted by triangles. Association with trypanosomes and Spiroplasma is shown as open triangles (infected and uninfected). Further associations shown in the plot are the host mtDNA genetic background (mtA, mtB, mtC), the nuclear genetic background (NWGU, ADMX, NEGU, WGU), the season (Dry, Inter, Wet), and the watershed of origin (Albert Nile, Achwa River, Okole River, Lake Kyoga, Kafu River).
(TIF)
The figure shows the TCS network of the COI gene and the proportion of infected individuals within the mtDNA haplogroups A, B, and C. Haplogroup A is associated with the NWGU, haplogroup B is associated with the NEGU, and haplogroup C is associated with the WGU. Red represents individuals with Spiroplasma infection while black represents the uninfected ones. The circle size represents the number of individuals sharing a haplotype; small black nodes represent inferred haplotypes; dashes between haplotypes represent a single mutational step. The haplotype network was generated using the TCS method [55] available in POPART [56].
(TIF)
The table on the main tab (‘Individuals’) includes information by individual: Fly ID, sampling site code, sampling site name, district, latitude, longitude, watershed of origin, year of collection, month of collection, season of collection, sex, the tissue used to screen for Spiroplasma (RP = reproductive, WB = whole body), the mitochondrial haplogroup assigned (mtA, mtB, mtC), the STRUCTURE assigned nuclear genetic background (NWGU, ADMX, NEGU, WGU), the PCR-based Spiroplasma and Wolbachia infection status, the visually-based trypanosome infection status, and the source of the genetic data, with individuals that were genotyped for this study indicated as “this study”. A summary of numbers (totals, females, males, Spiroplasma infection) per sampling site and per watershed is included in a separate tab of the same file labeled ‘Sampling Sites’.
(XLSX)
Sequence of primers for non-quantitative plus quantitative PCR is provided in this list.
(DOCX)
Output from generalized linear mixed models (GLMM) with one predictive variable at a time (with and without watershed as a random effect).
(XLSX)
Output from generalized linear mixed model (GLMM) with multiple predictive variables.
(XLSX)
Output from generalized linear models (GLM) by watershed.
(XLSX)
The table summarizes numbers of analyzed flies upon challenge with Tbb and their infection status: S+T+, S+T-, S-T+, S-T-. All flies were analyzed 14 days post infection. Infection status by individual is given in an extra sheet (by individual). The GLM sheet includes the details if the GLM, which was run to analyze the significance of the difference between groups. Abbreviations: Tbb Trypanosoma brucei brucei. S+ Spiroplasma-infected, S- Spiroplasma-uninfected, T+ Tbb-infected, T-Tbb-uninfected.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.