Abstract
Tissue plasminogen activator (tPA) is a serine protease involved in cleavage of neurotrophic factors. In addition, tPA and neuroserpin can also directly bind to low density lipoprotein receptor-related protein 1 (LRP1), promoting neurogenesis and neurite outgrowth. Given both the cleavage and non-cleavage actions of the fibrinolytic system are crucial in neurological functions, the present study, for the first time, systematically detected the changes of fibrinolytic system factors in rats exposed to chronic unpredictable mild stress (CUMS) or lipopolysaccharide (LPS) and patients with depression. In general, our data demonstrated that both CUMS and LPS reduced tPA but elevated plasminogen activator inhibitor-1 (PAI-1; SERPINE1) mRNA expression. Intriguingly, decreased expression of neuroserpin and LRP1 was also observed in rats exposed to CUMS or LPS. The down-regulated neuroserpin and LRP1 signaling were confirmed by western blotting and immunoflurence data. Likewise, elevated PAI-1 but a significant reduction of neuroserpin and LRP1 mRNA expression were observed in the peripheral blood mononuclear cells (PBMCs) of patients with first-episode depression, and the mRNA levels of PAI-1, neuroserpin and LRP1 were correlated with the Beck Depression inventory (BDI) scores, further strengthening the clinical significance and involvement of the fibrinolytic system in depression. Collectively, the present study demonstrated the alterations of fibrinolytic system in stressed and inflamed brain and in patients with first-episode depression, firstly showing that not only the cleavage actions, but also the non-cleavage actions of the system may play an essential role in the development of depression.
Keywords: tPA, PAI-1, Neuroserpin, LRP1, Depression
1. Introduction
Depression is the most common mental disease characterized by significant depressed mood and anhedonia. The decrease of neural plasticity, neuronal atrophy and abnormal neurogenesis may be implicated in the pathogenesis of depression. Tissue plasminogen activator (tPA) is a highly specific serine proteinase, which is widely expressed not only in endothelial cells but also in neurons and glia of the adult central nervous system (Melchor and Strickland, 2005). Studies revealed that tPA contributed to neuronal cell migration and learning and thus is deemed to play critical roles in the development and plasticity of the nervous system (Teesalu et al., 2004). Recently, neurotrophin hypothesis proposed that neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) have a pivotal role in neuron survival, neurogenesis and synaptic plasticity (Jiang et al., 2017). Several studies have reported the compromised BDNF and NGF signaling in animal models of depression (Filho et al., 2015; Su et al., 2014), and decreased BDNF and NGF serum levels were observed as well in patients with depression (Oglodek et al., 2016). Accumulating evidence suggests that tPA and its inhibitor, PAI-1 (also known as SERPINE1), can mediate the proteolytic cleavage of precursor-BDNF (proBDNF) and precursor-NGF (proNGF) into mature BDNF and NGF (Jiang et al., 2017; Wyganowska-Swiatkowska et al., 2018), which is implicated in depression pathogenesis.
Apart from the cleavage actions, binding of tPA directly to the cell surface receptors, such as LRP1 and annexin II receptor has also been shown to mediate non-proteolytic effects of tPA on neurite outgrowth (Lee et al., 2015). Intriguingly, neuroserpin (SERPINI1), another inhibitor of tPA in the brain, can also promote neurogenesis and neurite outgrowth like tPA, through activation of LRP1, independent of its inhibiting effects of tPA. These data allow us to hypothesize that apart from the involvement in neurotrophic signaling, the non-cleavage actions of tPA and neuroserpin may also play an essential role in depression. Therefore, the present study, for the first time, systematically detected the changes of fibrinolytic system factors by constructing the CUMS model of depression and LPS model of inflammation in rats, and verified our findings in the collected PBMCs from patients with first-episode depression, to provide more evidence to support the key role of fibrinolytic system factors in depression.
2. Materials and methods
2.1. Animals
The single-housed male Sprague-Dawley rats (210–240 g) were randomly divided into four groups with 6 animals each. All rats were maintained under standard laboratory conditions and were allowed to acclimate to the environment for 5 days. All animal use procedures were carried out in accordance with the Regulations of Experimental Animal Administration issued by the State Committee of Science and Technology of the People's Republic of China. Permission for the study was obtained from the Ethics Committee in our university.
2.2. CUMS and LPS procedures and behavioral tests
The CUMS procedure followed our previous study (Jiang et al., 2013). In brief, the CUMS group received random unpredictable stress (including deprivation of food and water, 12/12 h reversed light/dark cycle and light turned on at 7:00 p.m., tilted cage for 24 h, nipped tails for 1 min and loud noise for 20 min) for 4 consecutive weeks. All stressors conducted around 8:00 a.m. Rat received one of these stressors per day randomly and none of the same stressor was applied in 2 consecutive days, whereas the control group for CUMS was left undisturbed.
LPS group rats were intraperitoneally injected with 500 μg/kg of LPS (based on our previous research) (Guo et al., 2016) between 8:00 a.m. and 9:00 a.m. every two days for a total of 7 injections. Likewise, same volume of saline was given intraperitoneally in control group. Twenty-four hours after the last stress or injection, the depressant-like behaviors of rats exposed to CUMS or LPS were evaluated using the forced swimming test (FST) and the sucrose preference test (SPT) (Guo et al., 2016; Tang et al., 2015). Twenty-four hours after the behavioral tests, rats were euthanized and the whole hippocampus and prefrontal cortex tissues were dissected on the ice surface. The left hippocampus and prefrontal cortex tissues were fixed with 4% paraformaldehyde, then embedded in paraffin after dehydrated with 30% sucrose paraformaldehyde, and the right tissues were stored in −80 °C until needed.
For FST, in brief, each rat was placed individually in a plastic drum (45 cm high × 25 cm in diameter) containing approximately 35 cm of water (24 ± 1 °C). An initial 15 min pretest, followed by a 5-min test 24 h later was examined. Water was changed after every trail. Immobility was judged as floating passively on the water surface, making only slight movements to keep its head above water. Each test session of rats swimming within a 5 min period was videotaped and immobility time was recorded by an experienced observer blind to the experimental design.
Prior to SPT, all rats were housed in individual cages for habituation of 1% sucrose solution for 32 h in two bottles on each side. After 16 h water and food deprivation, 1% sucrose solution and water were replaced in pre-weighed bottles on the cages, and the rats were allowed free access to the two bottles for 1 h. The sucrose solution and water consumption were recorded and the sucrose preference (%) was calculated as the ratio of the volume of sucrose vs total volume of sucrose and water consumed.
2.3. Quantitative real-time PCR analysis
Total RNA was isolated from the hippocampus and prefrontal cortex of rats according to the manufacturer's instructions supplied with the Trizol reagent (Invitrogen, USA). First-strand complementary DNA (cDNA) was synthesized from 1 μg of the total RNA in a 20 μl reaction volume with the FastKing gDNA dispelling RT supermix (TIANGEN). Real-time quantitative PCR was carried out on Bio-rad Cx96 Detection System (Bio-rad, USA) based on the use of SYBR Green (TIANGEN, China) and the expression of the target gene in each sample was tested in triplicate. Expression of the β-actin gene was monitored simultaneously as an internal control to quantify the transcripts of target gene in each sample. Gene-specific primers were listed in Table 1.
Table 1.
Primers of target gene used in the RT-qPCR of rats.
| Gene | Primer sequence | Product size (bp) |
|---|---|---|
| tPA | F: 5′- TGTGCGTCCTGCTGCTTT -3′ R: 5′- TTCCACCCGATTGCCTCT -3′ |
176 |
| PAI-1 | F: 5′-GCTGATGGAGCCTTGAGA-3′ R: 5′- GGAAAGATTTACCAGTGCC -3′ |
157 |
| neuroserpin | F: 5′- ACAATCTTATTCATGGGACG -3′ R: 5′- TTTGCTACTGCTGCCTTT -3′ |
111 |
| LRP1 | F: 5′- CCAACACCCAGCAGAAGA -3′ R: 5′- CAGGCAGATGTCAGAGCAG -3′ |
191 |
| annexin-II | F: 5′- CCAAGTGCCTATGGGTCG -3′ R: 5′- GCTGCGGTTAGTCAGAATGT -3′ |
132 |
| p11 | F: 5′- AGGACCCTCTGGCTGTGGA -3′ R: 5′- TTGGCCTACTTCTTCTGCTTCA -3′ |
154 |
2.4. Protein analysis by western blotting
To confirm the alterations of the factors above in the brain, the protein level was analyzed by Western blotting. The brain tissues of rats were lysed in RIPA buffer, followed by high-speed centrifugation and protein quantification was analyzed using a BCA kit (Solarbio, China). Equivalent proteins (30 μg) were separated by electrophoresis on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to polyvinylidene-fluoride (PVDF) membranes (Solarbio, China). After being blocked at room temperature for 1 h and washed three times in tris-buffered saline with Tween 20 (TBST), the membranes were incubated overnight at 4 °C with primary antibodies against TPA (Abcam, 1:2000 dilution), PAI-1 (Santa Cruz, 1:500 dilution), neuroserpin (Abcam, 1:250 dilution) and LRP-1 (Abcam, 1:20000 dilution). The PVDF membranes were incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 h after being washed three times in TBST. The signals were normalized to β-actin as the internal control. Protein bands were visualized using the ChemiDoc™ XRS + system (Bio-rad, USA), and the integrated densities were quantified using Image Lab software (Bio-rad laboratories, USA).
2.5. Immunofluorescence staining
Brain tissues from the rats of each group were collected and fixed with 4% paraformaldehyde. After the brain tissues were dehydrated with 30% sucrose paraformaldehyde, 16-μm thick brain slices were sectioned using a Leica CM3050 S cryostat. After being washed with 1 × PBS for 30min and blocked with 3% BSA for 30 min, the brain slices were incubated with the primary antibodies against neuroserpin (Abcam, 1:100 dilution) and LRP-1 (Abcam, 1:5000 dilution) overnight at 4 °C. After the sections were washed, primary antibodies were detected with secondary antibodies that were conjugated with Alexa Fluor 488 and/or Alexa Fluor 594 at 37 °C for 1 h. After being stained with 4’, 6-diamidino-2-phenylindole (DAPI, Yeasen), binding was visualized using a confocal microscope (Leica). Four independent experiments were conducted.
2.6. Participants
The study enrolled forty-four unrelated unmedicated Chinese patients with their first-episode depression. All patients were diagnosed with depression strictly according to Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) criteria and were reported no history of other psychiatric disorders such as schizophrenia or mania. Also, depressive symptoms of all patients were also measured by BDI. Forty-six healthy individuals matched pairwise for sex, age, BMI and habits (smoking and alcohol status) were enrolled as a control group (detailed information was displayed in Supplementary Table 1). None of the control participants had any history of central nervous system diseases or any other medical disorders. All the patients and healthy control individuals were recruited from the outpatient clinic of the Jining Psychiatric Hospital of Shandong Province. The study protocol was approved by the Jining Medical University and the ethics committees of all participating hospitals in China. Prior to any study-related procedures, all participants signed a written informed consent.
2.7. Human RNA extraction from PBMCs and quantitative real-time PCR
Whole blood was collected by venipuncture into EDTA-treated tubes. For PBMC collection, blood was diluted 1:1 with phosphate buffered saline (PBS), layered on top of Ficoll-Paque Plus (Amersham Biosciences), and centrifuged at 400 g for 30 min at 4 °C. PBMCs were then removed from the plasma-Ficoll interface and rinsed twice with PBS. Total RNA was isolated from PBMCs according to the manufacturer's instructions supplied with the Trizol reagent (Invitrogen, USA). The quality and quantity of total RNA were evaluated using One Drop OD-1000+ (One Drop, China) and the integrity of the total RNA was evaluated in 1% agarose gel. The synthesis of cDNA and the RT-qPCR reaction had been described above and the specific primers of target gene were presented in Table 2.
Table 2.
Primers of target gene used in the RT-qPCR of human.
| Gene | Primer sequence | Product size (bp) |
|---|---|---|
| tPA | F: 5′- GCCTTGTCTCCTTTCTATTCG -3′ R: 5′- GTTGTCGGTGACTGTTCTGTT -3′ |
108 |
| PAI-1 | F: 5′- CCTTGCCCTTGAGTGCTT -3′ R: 5′- TGGCTGGACTTCCTGAGATA -3′ |
118 |
| neuroserpin | F: 5′- GGATTTTGATGCTGCCACTTATC -3′ R: 5′-TTTGGACTTCACTTTCATCATCTTTAG-3′ |
131 |
| LRP1 | F: 5′- CCCAGCAGAAGACGAGTGT -3′ R: 5′- AGCAGGCAGATGTCAGAGC -3′ |
187 |
| annexin-II | F: 5′- TGCTCCAGAACCAACCAG -3′ R: 5′- CTTGCGGAAGTCACCAGA -3′ |
111 |
| p11 | F: 5′- AGACCCTCTGGCTGTGGA -3′ R: 5′- CTACTTCTTTCCCTTCTGCTTC-3′ |
154 |
2.8. Statistical analysis
All data were displayed as mean ± standard error of the mean (SEM). The Student's t-test for unpaired data was used to compare the experiments data between two groups. The demographic and clinical characteristics were analyzed using the non-parametric test. Correlations between fibrinolytic system factor mRNA levels and BDI scores in patients with first-episode depression were calculated and expressed as Spearman's correlation coefficient. The statistical analysis was done using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) and significance levels were considered at p < 0.05.
3. Results
3.1. Depressive-like behaviors in rats exposed to CUMS or LPS
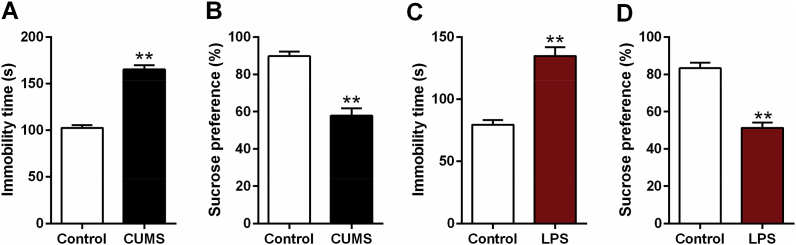
The depressive-like behaviors of rats exposed to CUMS or LPS were tested by FST and SPT. As shown in Fig. 1, the immobility time was significantly longer in model group (CUMS and LPS group) in comparison to the control group (t = −3.549, df = 10, p < 0.01, Fig. 1A; t = −3.559, df = 10, p < 0.01, Fig. 1C; respectively). Compared to that of control group, sucrose preference was significantly reduced in model group (t = 3.637, df = 10, p < 0.01, Fig. 1B; t = 3.987, df = 10, p < 0.01, Fig. 1D; respectively). All of the above tests results suggested a depressive-like behavior in rats induced by CUMS or LPS.
Fig. 1.
Forced swimming and sucrose preference test of depressive-like rats exposed to CUMS or LPS. Data are expressed as means ± SEM (n = 6). *p < 0.05, **p < 0.01 compared to control group.
3.2. Altered fibrinolytic system in rats exposed to CUMS or LPS
To explore the cleavage and non-cleavage actions of fibrinolytic system in depression, we assessed the changes of fibrinolytic system factors in the hippocampus and prefrontal cortex of CUMS and LPS rats.
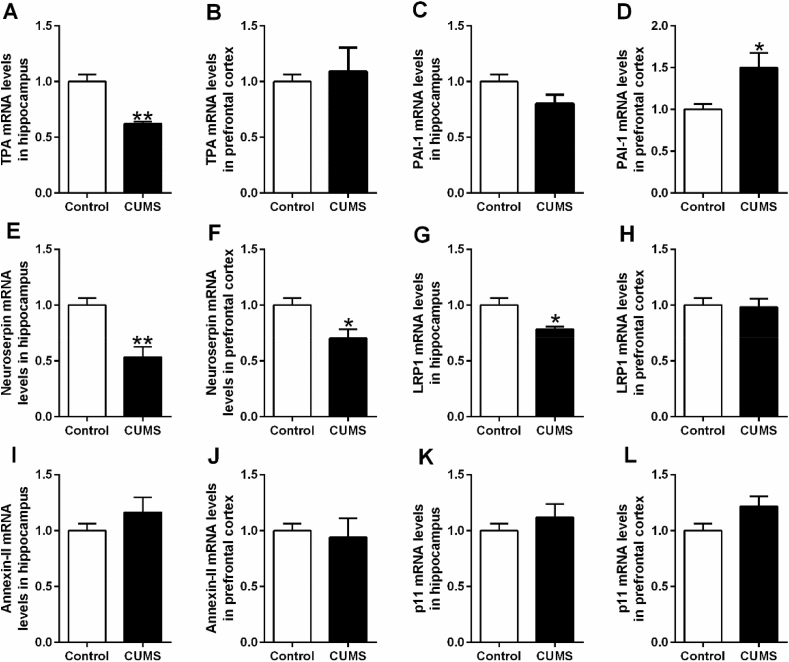
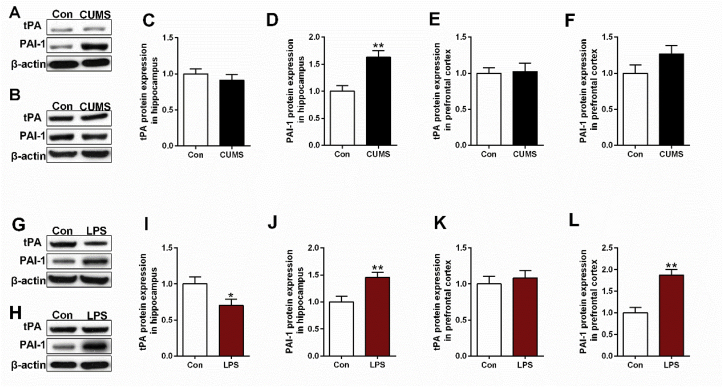
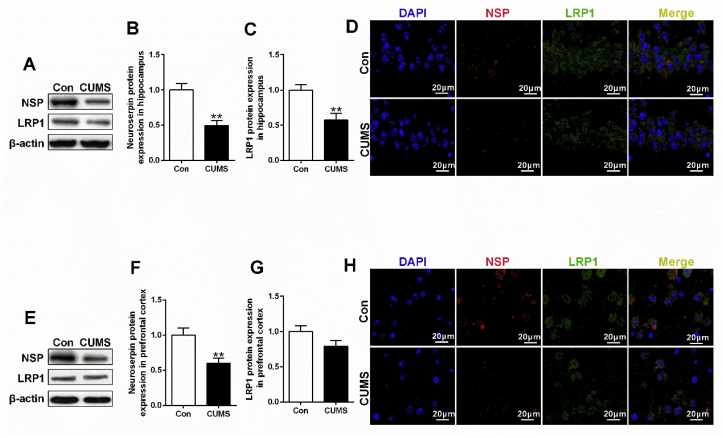
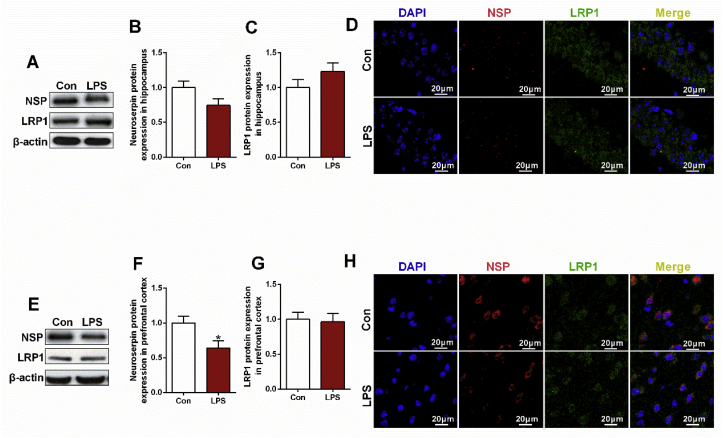
In CUMS rats, significant reduction of tPA and elevation of PAI-1 mRNA expression were observed in hippocampus (t = 6.907, df = 10, p < 0.01, Fig. 2A) and prefrontal cortex (t = −3.126, df = 10, p < 0.05, Fig. 2D) respectively. Intriguingly, decreased mRNA expression in both neuroserpin (hippocampus, t = 4.204, df = 10, p < 0.01, Fig. 2E; prefrontal cortex, t = 2.862, df = 10, p < 0.05, Fig. 2F) and LRP1 (hippocampus, t = 3.158, df = 10, p < 0.05, Fig. 2G) was also observed in CUMS rats. Unexpectedly, CUMS rats exhibited no significant difference of tPA protein expression (Fig. 4C,E), while the up-regulated PAI-1 protein expression was observed in hippocampus (t = −3.964, df = 10, p < 0.01, Fig. 4D) rather than prefrontal cortex (Fig. 4F). Additionally, down-regulated signaling in neuroserpin and LRP1 was confirmed by western blotting (t = 4.287, df = 10, p < 0.01, Fig. 5B; t = 3.178, df = 10, p < 0.01, Fig. 5F; t = 3.434, df = 10, p < 0.01, Fig. 5C) and immunofluorescence data (Fig. 5D,H).
Fig. 2.
The mRNA levels of tPA (A, B), PAI-1 (C, D), neuroserpin (E, F), LRP1 (G, H), annexin-II (I, J) and p11 (K, L) in hippocampus and prefrontal cortex of depressive-like rats exposed to CUMS. The mRNA levels of all factors were detected by RT-PCR. Data are expressed as means ± SEM (n = 6). *p < 0.05, **p < 0.01 compared to control group.
Fig. 4.
Western blotting revealed the changes in tPA and PAI-1 expression in hippocampus (A) and prefrontal cortex (B) of depressive-like rats exposed to CUMS. β-actin is as an internal control. Differences between control and CUMS group has presented in Fig. 4C, D, E, F. Likewise, (G, H) represent western blotting results of tPA and PAI-1 in hippocampus and prefrontal cortex of depressive-like rats exposed to LPS respectively. Differences between control and CUMS group has presented in Fig. 4I, J, K, L. Data are expressed as means ± SEM (n = 6). *p < 0.05, **p < 0.01 compared to control group.
Fig. 5.
Decreased levels of neuroserpin and LRP1 in depressive-like rats exposed to CUMS. Western blotting and immunofluorescence staining were used to detect the levels of neuroserpin and LRP1 in hippocampus and prefrontal cortex of depressive-like rats exposed to CUMS. (A, E) Western blotting results of neuroserpin, LRP1 protein levels in hippocampus and prefrontal cortex respectively. (B, C, F, G) Quantification of neuroserpin, LRP1 protein levels in hippocampus and prefrontal cortex respectively. (D, H) Immunofluorescence staining results of neuroserpin and LRP1 in hippocampus and prefrontal cortex respectively. Data are expressed as means ± SEM (n = 6). *p < 0.05, **p < 0.01 compared to control group.
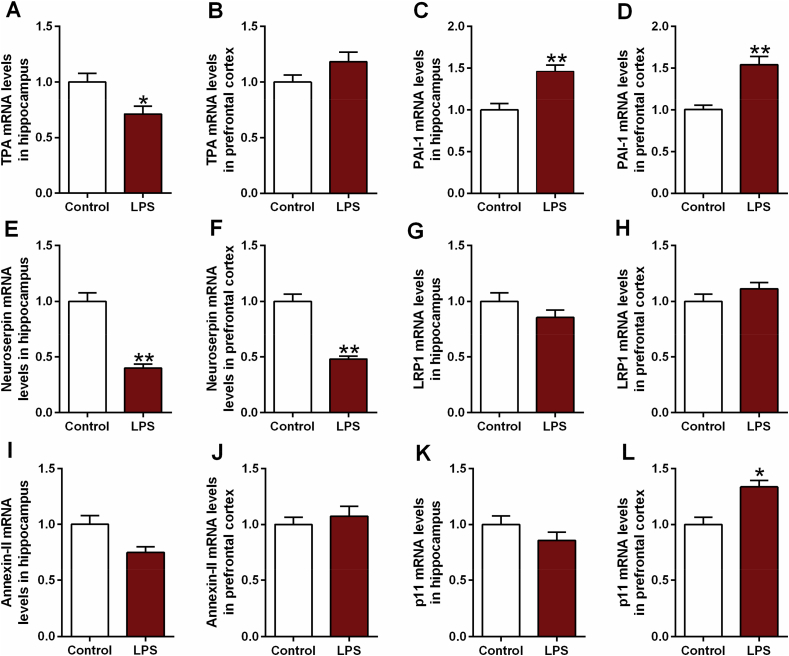
Likewise, LPS rats exhibited a significant reduction of tPA in hippocampus (t = 3.035, df = 10, p < 0.05, Fig. 3A) and elevation of PAI-1 in both hippocampus (t = −4.111, df = 10, p < 0.01, Fig. 3C) and prefrontal cortex (t = −4.042, df = 10, p < 0.01, Fig. 3D). As expected, the reduced tPA (t = 2.298, df = 10, p < 0.05, Fig. 4I) and elevated PAI-1 (t = −3.341, df = 10, p < 0.01, Fig. 4J; t = −4.872, df = 10, p < 0.01, Fig. 4L) was confirmed by western blotting data. Although significant decrease of neuroserpin mRNA was observed in both hippocampus (t = 4.682, df = 10, p < 0.01, Fig. 3E) and prefrontal cortex (t = 4.593, df = 10, p < 0.01, Fig. 3F), the significant difference of LRP1 mRNA expression was failed to be found. According to the western blotting and immunofluorescence data, only significant reduction of neuroserpin was found in the prefrontal cortex (t = 2.567, df = 10, p < 0.05, Fig. 6F). In addition, increased p11 mRNA was observed in LPS rats (t = −2.775, df = 10, p < 0.05, Fig. 3L), while the increase has not been confirmed in p11 protein expression detected by western blotting and immunofluorescence staining.
Fig. 3.
The mRNA levels of tPA (A, B), PAI-1 (C, D), neuroserpin (E, F), LRP1 (G, H), annexin-II (I, J) and p11 (K, L) in hippocampus and prefrontal cortex of depressive-like rats exposed to LPS. The mRNA levels of all factors were detected by RT-PCR. Data are expressed as means ± SEM (n = 6). *p < 0.05, **p < 0.01 compared to control group.
Fig. 6.
Altered levels of neuroserpin and LRP1 in depressive-like rats exposed to LPS. Western blotting and immunofluorescence staining were used to detect the levels of neuroserpin and LRP1 in hippocampus and prefrontal cortex of depressive-like rats exposed to CUMS. (A, E) Western blotting results of neuroserpin, LRP1 protein levels in hippocampus and prefrontal cortex respectively. (B, C, F, G) Quantification of neuroserpin, LRP1 protein levels in hippocampus and prefrontal cortex respectively. (D, H) Immunofluorescence staining results of neuroserpin and LRP1 in hippocampus and prefrontal cortex respectively. Data are expressed as means ± SEM (n = 6). *p < 0.05, **p < 0.01 compared to control group.
3.3. Altered fibrinolytic system in patients with depression
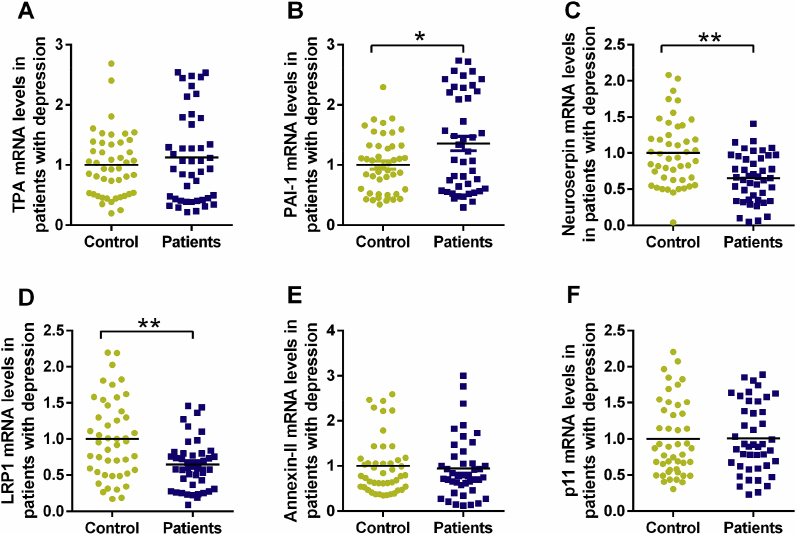
To further verify the alteration of fibrinolytic system factors in rats exposed to CUMS or LPS, the mRNA levels were detected in PBMCs of patients with first-episode depression. As shown in the scatter diagram of Fig. 7, although no statistical difference of tPA mRNA level was found in patients with first-episode depression in comparison to healthy individuals, an apparent elevation of the PAI-1 mRNA level was observed in patients with first-episode depression (t = −2.587, df = 88, p < 0.05, Fig. 7B). In addition, patients with first-episode depression exhibited a pronounced reduction in neuroserpin (t = 3.585, df = 88, p < 0.01, Fig. 7C) and LRP1 (t = 3.065, df = 88, p < 0.01, Fig. 7D) mRNA expression levels relative to healthy individuals. In line with the animal experiments data above, no statistical differences of annexin-II and p11 were found in patients with first-episode depression.
Fig. 7.
The mRNA levels of tPA (A), PAI-1 (B), neuroserpin (C), LRP1 (D), annexin-II (E) and p11 (F) in patients with first-episode depression were presented using scatter-plot diagram. Data are expressed as means ± SEM. *p < 0.05, **p < 0.01 compared to control group.
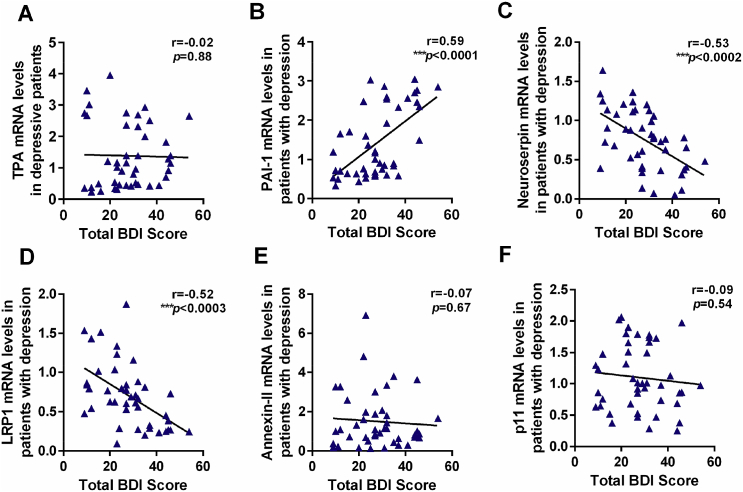
To evaluate the clinical significance of fibrinolytic system in depression, the correlation between the mRNA level of fibrinolytic system factors and BDI scores was assessed. As shown in Fig. 8, the mRNA levels of PAI-1 (r = 0.59, p < 0.001, Fig. 8B), neuroserpin (r = −0.53, p < 0.001, Fig. 8C) and LRP1 (r = −0.52, p < 0.001, Fig. 8D) were correlated with the BDI scores, further strengthening the clinical significance and involvement of the fibrinolytic system in depression.
Fig. 8.
The correlations between mRNA levels of tPA (A), PAI-1 (B), neuroserpin (C), LRP1 (D), annexin-II (E) and p11 (F) and BDI scores in patients with first-episode depression. Data are expressed as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 compared to control group.
4. Discussion
Tissue plasminogen activator (tPA) mediates a number of processes that are pivotal for maintenance of neurological function, including proteolysis of the brain extracellular matrix, degradation of adhesion molecules and activation of neurotrophins, and thus involves in several neuropsychiatric disorders. Apart from the cleavage action of fibrinolytic system, neuroserpin and tPA can directly bind with LRP1 to promote neurogenesis and neurite outgrowth, thereby exert direct neurotrophic actions. Although accumulating evidence suggests that tPA/PAI-1 system is involved in depression through regulating BDNF cleavage (Chen et al., 2017; Jiang et al., 2017), to our knowledge, there is no research focused on the potential link between the non-cleavage actions of fibrinolytic system and pathophysiological mechanisms of depression. It is well known that repeated environmental stress such as chronic unpredictable mild stress, causes the dysfunction of neurons including the decrease of neural plasticity, neuronal atrophy, abnormal neurogenesis and neuroinflammation in multiple brain areas involved in cognitive and emotional functions (Nie et al., 2018), which is thought to induce the depressive-like behaviors, such as anhedonia and depressed mood. Additionally, neuroinflammation can evoke the depressive symptoms through mediating the dysregulation of hypothalamic-pituitary-adrenal (HPA) axis, altering the synaptic plasticity and disturbing neurotransmission, and thus is considered to be tightly related to the development of depression (Grygiel-Gorniak et al., 2019). Therefore, the present study aimed to systematically assess the fibrinolytic system in the brain of rats exposed to chronic stress or LPS. In addition, we also analyzed the mRNA expression of the key factors of fibrinolytic system in the PBMCs from 46 first-episode depression patients and matched controls.
The CUMS or LPS-induced longer immobility time and the reduced sucrose preference during FST and SPT reflected despair and anhedonia like-behaviors, respectively, indicating that the depressive-like behaviors were successfully induced in this study. Although both CUMS and LPS markedly reduced tPA mRNA expression in the hippocampus, the significant decrease of tPA protein level was only observed in the LPS treated rats. However, CUMS and LPS both enhanced PAI-1 mRNA and protein status in the prefrontal cortex, and increased PAI-1 expression was also observed in the hippocampus of LPS treated rats. In accordance, higher mRNA level of PAI-1 in the PBMCs of patients with first-episode depression was found compared with healthy control individuals, whereas tPA level failed to appear a significant difference. The inhibited cleavage actions of tPA and thereby suppressed BDNF maturation were widely reported in animals exposed to chronic stress (Zhang et al., 2018; Zhu et al., 2017). Recently, our previous study also suggested that sustained inflammatory process also markedly inhibits BDNF biotransformation to its mature form via tPA/PAI-1 system. In support, previous research showed elevated PAI-1 status in the brain, cerebrospinal fluid (CSF) and serum of chronic stress exposed rats, and in the serum of drug-naive depression patients (Gao et al., 2018; Jiang et al., 2016). These findings and our data suggest that the stress or LPS-induced PAI-1 expression, and thereby decreased tPA activity, but not tPA expression itself, are more likely account for the compromised BDNF cleavage in either the inflamed or stressed brain found in previous studies and our research.
Notwithstanding previous studies are mainly focused on the cleavage action of tPA, tPA and NSP can directly bind with the cell surface receptor, such as LRP1 and annexin-II, resulting in neurite growth and neurogenesis, which are independent of their cleavage actions. There is now mounting evidence that the binding of tPA to LRP1 can lead to the up-regulation of matrix metalloproteinase-9 (MMP-9), a protease which can promote the proteolysis of extracellular matrix (ECM) components contributing to either neuronal migration or axonal growth (Zhang et al., 2009). Additionally, a study on the regulation of macrophage activation and innate immunity by tPA showed that LRP1 is necessary for tPA to inhibit the inflammatory response to LPS (Mantuano et al., 2017). Interestingly, there is also evidence that neuroserpin may regulate neuronal function through pathways independent of its inhibitory activity, suggesting that neuroserpin may also have a function beyond the regulation of tPA (Lee et al., 2008, 2012). As we all know, the cell surface receptor LRP1 not only functions as a clearance receptor, it may also function as a signaling receptor in a variety of cellular reactions (Kruithof and Dunoyer-Geindre, 2014). Thus, it was supposed to regulate the activity of proteases and protease inhibitors and is pivotal for neuronal development and synaptic function, including spine density and synaptic integrity in hippocampal neurons (Herz and Bock, 2002; Makarova et al., 2003). Surprisingly, neuroserpin can also bind the LRP1 rather than depend on tPA to trigger the neurite outgrowth effects (Wu et al., 2010). This is further supported by studies showing that LRP1 can modulate the neuroserpin activity, suggesting a key role of neuroserpin in neurological disorders through a non-inhibitory mechanism (Lee et al., 2012). Although the reduced tPA was only observed in LPS rats, there was nonetheless an apparent decrease of neuroserpin and LRP1 in rats exposed to CUMS or LPS. As expected, the down-regulated neuroserpin and LRP1 signaling were confirmed by western blotting and immunoflurence data, indicating their co-expression in vivo. Moreover, the current study found that low neuroserpin and LRP1 levels were negatively correlated with BDI scores of patients with first-episode depression. To our knowledge, our study firstly reported an association between clinical severity in first-episode depression and expression of the neuroserpin and LRP1 gene, indicating that reduced neuroserpin and LRP1 may play a vital role in the development of depression.
5. Conclusion
Collectively, the present study firstly evaluated fibrinolytic system in stressed and inflamed brain and in patients with first-episode depression. Our data showed that both chronic stress exposure and sustained immune activation can induce PAI-1 expression, thereby suppressing tPA cleavage activity, resulting in compromised maturation of neurotrophic factors, such as BDNF. On the other hand, the direct neurotrophic actions of tPA and neuroserpin through LRP1 receptor might be also suppressed, providing the first evidence for the involvement of both cleavage and non-cleavage actions of fibrinolytic system in the development of depression.
Author contributions
Pei Jiang designed the study; Gongying Li and Xueyuan Zhou were responsible for the collection of blood samples and acquisition and interpretation of clinical data of patients in the study; Dan Chen and Lei Chen completed the animal models construction; Ruili Dang, Mengqi Yang and Wenxiu Han performed the experiments; Wenxiu Han interpreted the data and drafted the manuscript; Pengfei Xu and Yujin Guo revised the manuscript critically for important intellectual content. All authors approved the final version of the manuscript.
Declarations of interest
None.
Funding sources
This work was supported by the National Natural Science Foundation of China (81602846, 81571334, 81703625) and Taishan Scholar Program of Shandong Province (tsqn201812159).
Acknowledgements
We would like to thank all participants for their contributions and all organizations that funded our research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2019.100188.
Contributor Information
Wenxiu Han, Email: ppheqq_cool@126.com.
Ruili Dang, Email: rosydang@126.com.
Pengfei Xu, Email: pengfeixucsu@outlook.com.
Gongying Li, Email: Ligongying2005@126.com.
Xueyuan Zhou, Email: xueyuan_zhou@qq.com.
Lei Chen, Email: chenlei_8090@163.com.
Yujin Guo, Email: guoyujin99@126.com.
Mengqi Yang, Email: mengqiqiyang@163.com.
Dan Chen, Email: bmdmdd@163.com.
Pei Jiang, Email: jiangpeicsu@sina.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Chen S. Combined serum levels of multiple proteins in tPA-BDNF pathway may aid the diagnosis of five mental disorders. Sci. Rep. 2017;7:6871. doi: 10.1038/s41598-017-06832-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filho C.B. Chronic unpredictable mild stress decreases BDNF and NGF levels and Na(+),K(+)-ATPase activity in the hippocampus and prefrontal cortex of mice: antidepressant effect of chrysin. Neuroscience. 2015;289:367–380. doi: 10.1016/j.neuroscience.2014.12.048. [DOI] [PubMed] [Google Scholar]
- Gao H. Anti-depressant-like effect of atractylenolide I in a mouse model of depression induced by chronic unpredictable mild stress. Exp Ther Med. 2018;15:1574–1579. doi: 10.3892/etm.2017.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygiel-Gorniak B. Cytokine secretion and the risk of depression development in patients with connective tissue diseases. Psychiatry Clin. Neurosci. 2019;73:302–316. doi: 10.1111/pcn.12826. [DOI] [PubMed] [Google Scholar]
- Guo Y. Quantitative profiling of neurotransmitter abnormalities in the hippocampus of rats treated with lipopolysaccharide: focusing on kynurenine pathway and implications for depression. J. Neuroimmunol. 2016;295–296:41–46. doi: 10.1016/j.jneuroim.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Herz J., Bock H.H. Lipoprotein receptors in the nervous system. Annu. Rev. Biochem. 2002;71:405–434. doi: 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- Jiang H. The serum protein levels of the tPA-BDNF pathway are implicated in depression and antidepressant treatment. Transl. Psychiatry. 2017;7 doi: 10.1038/tp.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H. Plasminogen activator inhibitor-1 in depression: results from animal and clinical studies. Sci. Rep. 2016;6:30464. doi: 10.1038/srep30464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P. Stress and vitamin D: altered vitamin D metabolism in both the hippocampus and myocardium of chronic unpredictable mild stress exposed rats. Psychoneuroendocrinology. 2013;38:2091–2098. doi: 10.1016/j.psyneuen.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Kruithof E.K., Dunoyer-Geindre S. Human tissue-type plasminogen activator. Thromb. Haemost. 2014;112:243–254. doi: 10.1160/TH13-06-0517. [DOI] [PubMed] [Google Scholar]
- Lee T.W. Neuroserpin regulates N-cadherin-mediated cell adhesion independently of its activity as an inhibitor of tissue plasminogen activator. J. Neurosci. Res. 2008;86:1243–1253. doi: 10.1002/jnr.21592. [DOI] [PubMed] [Google Scholar]
- Lee T.W. The serine protease inhibitor neuroserpin regulates the growth and maturation of hippocampal neurons through a non-inhibitory mechanism. J. Neurochem. 2012;121:561–574. doi: 10.1111/j.1471-4159.2011.07639.x. [DOI] [PubMed] [Google Scholar]
- Lee T.W. Physiological and pathological roles of tissue plasminogen activator and its inhibitor neuroserpin in the nervous system. Front. Cell. Neurosci. 2015;9:396. doi: 10.3389/fncel.2015.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova A. The low density lipoprotein receptor-related protein modulates protease activity in the brain by mediating the cellular internalization of both neuroserpin and neuroserpin-tissue-type plasminogen activator complexes. J. Biol. Chem. 2003;278:50250–50258. doi: 10.1074/jbc.M309150200. [DOI] [PubMed] [Google Scholar]
- Mantuano E. Tissue-type plasminogen activator regulates macrophage activation and innate immunity. Blood. 2017;130:1364–1374. doi: 10.1182/blood-2017-04-780205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchor J.P., Strickland S. Tissue plasminogen activator in central nervous system physiology and pathology. Thromb. Haemost. 2005;93:655–660. doi: 10.1160/TH04-12-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X. The innate immune receptors TLR2/4 mediate repeated social defeat stress-induced social avoidance through prefrontal microglial activation. Neuron. 2018;99:464–479 e7. doi: 10.1016/j.neuron.2018.06.035. [DOI] [PubMed] [Google Scholar]
- Oglodek E.A. Melatonin and neurotrophins NT-3, BDNF, NGF in patients with varying levels of depression severity. Pharmacol. Rep. 2016;68:945–951. doi: 10.1016/j.pharep.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Su G.Y. Antidepressant-like effects of Xiaochaihutang in a rat model of chronic unpredictable mild stress. J. Ethnopharmacol. 2014;152:217–226. doi: 10.1016/j.jep.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Tang M. Antidepressant-like effect of n-3 PUFAs in CUMS rats: role of tPA/PAI-1 system. Physiol. Behav. 2015;139:210–215. doi: 10.1016/j.physbeh.2014.11.054. [DOI] [PubMed] [Google Scholar]
- Teesalu T. Tissue plasminogen activator and neuroserpin are widely expressed in the human central nervous system. Thromb. Haemost. 2004;92:358–368. doi: 10.1160/TH02-12-0310. [DOI] [PubMed] [Google Scholar]
- Wu J. Neuroserpin protects neurons from ischemia-induced plasmin-mediated cell death independently of tissue-type plasminogen activator inhibition. Am. J. Pathol. 2010;177:2576–2584. doi: 10.2353/ajpath.2010.100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyganowska-Swiatkowska M. Can EGCG alleviate symptoms of down syndrome by altering proteolytic activity? Int. J. Mol. Sci. 2018;19:E248. doi: 10.3390/ijms19010248. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. The low-density lipoprotein receptor-related protein 1 mediates tissue-type plasminogen activator-induced microglial activation in the ischemic brain. Am. J. Pathol. 2009;174:586–594. doi: 10.2353/ajpath.2009.080661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F. Ketamine ameliorates depressive-like behaviors by tPA-mediated conversion of proBDNF to mBDNF in the hippocampus of stressed rats. Psychiatry Res. 2018;269:646–651. doi: 10.1016/j.psychres.2018.08.075. [DOI] [PubMed] [Google Scholar]
- Zhu Y. Kai-Xin-San series formulae alleviate depressive-like behaviors on chronic mild stressed mice via regulating neurotrophic factor system on hippocampus. Sci. Rep. 2017;7:1467. doi: 10.1038/s41598-017-01561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.