Abstract
Background
Recent data suggests that the majority of cardiac deaths in patients with heart failure occur in patients with a left ventricular ejection fraction (LVEF) >35%. This study sought to determine the value of guideline based assessment of diastolic dysfunction in predicting all-cause mortality in patients with a first-ever myocardial infarction (MI) with an LVEF >35%.
Methods
A retrospective single centre study involving 383 patients with a first-ever MI (STEMI or NSTEMI) with LVEF >35% was performed. Clinical, angiographic and echocardiographic data were obtained from prospectively maintained institutional databases. Outcomes data were obtained from national death registry. Echocardiography was performed early post-admission for all patients. Significant diastolic dysfunction (DD) was defined was grade 2/3 diastolic dysfunction according to current American Society of Echocardiography/European Association of Cardiovascular Imaging guidelines.
Results
At a median follow up of 2 years, there were 32 deaths. On Cox proportional hazards multivariate analysis incorporating significant clinical variables (age, chronic kidney disease and extent of coronary artery disease), significant DD (HR 2.57, 95%CI 1.16–5.68, p = 0.020) and left ventricular end-diastolic volume index (HR 1.03, 1.04–1.07, p = 0.021) were the only independent echocardiographic predictors of all-cause mortality. Intermodel comparisons using model χ2 and Harrel's-C confirmed incremental value of DD. In the subgroup with LVEF 36–55% (n = 176), significant DD was the only independent echocardiographic predictor (HR 3.56, 95%CI 2.46–9.09, p = 0.006).
Conclusions
The presence of significant DD identifies patients with LVEF >35% following MI who are at a higher risk of all-cause mortality, and who may benefit from further risk stratification and treatment.
Keywords: Diastolic dysfunction, Prognosis, Myocardial infarction
Highlights
-
•
The majority of deaths in heart failure patients occur in patients with LVEF>35%.
-
•
Diastolic dysfunction identifies elevated risk of death in patients with LVEF>35%.
-
•
Diastolic dysfunction thus identifies patients for further risk stratification.
1. Introduction
Whilst left ventricular ejection fraction (LVEF) ≤35% is used as the criterion for implantable cardioverter defibrillator (ICD) implantation in patients with heart failure, recent data suggests that the majority of sudden cardiac death (SCD) in patients with heart failure occurs in patients with either preserved or mild-moderate systolic dysfunction [[1], [2], [3], [4]]. There is currently renewed interest in restratifying the risk of SCD in patients with mild-moderate systolic dysfunction (LVEF ≥35%) with either cardiac magnetic resonance imaging (cMRI) with late gadolinium enhancement (LGE) or electrophysiological studies [[5], [6], [7]]. Recent data have also shown that the aggregate assessment of diastolic dysfunction (DD) utilising the 2016 American Society of Echocardiography/European Association of Cardiovascular Imaging (ASE/EACVI) guideline algorithms was an independent predictor of outcomes [[8], [9], [10], [11], [12], [13]]. The present study sought to define the prognostic value of DD assessed by contemporary ASE/EACVI guidelines in patients with preserved or mild-moderately impaired LVEF (>35%) following a first-ever myocardial infarction (MI) for predicting all-cause mortality. Patients with mild-moderately impaired LVEF (LVEF 36–55%) were studied as a subgroup. The study hypothesis was that significant DD would be a significant predictor of all-cause mortality in this subgroup of patients and thus allow restratification of the risk of SCD in this subgroup.
2. Material and methods
2.1. Study overview
A total of 718 consecutive patients with MI (ST-elevation MI [STEMI] and non-ST-elevation MI [NSTEMI]) between January 2013 and December 2014 at a single tertiary level referral centre were considered for inclusion in this study. Exclusion criteria included previous MI (n = 160), significant mitral valve disease (greater than moderate regurgitation or any stenosis or a prosthetic valve) (n = 32), atrial fibrillation (n = 33), paced rhythm (n = 12), and significant hemodynamic instability (shock, acute pulmonary oedema, requirement for mechanical ventilation, inotropes, intra-aortic balloon pump and those with ventricular tachyarrhythmia) (n = 4), insufficient image quality (n = 16), indeterminate diastolic function (n = 36) as well as LVEF ≤35% (n = 36). All clinical, angiographic and echocardiographic data were obtained from three separate prospectively maintained institutional databases by three groups of investigators blinded to each other's findings. The primary outcome measure was all-cause mortality. Cardiac death was examined as a secondary endpoint. Outcomes data were obtained from state and federal government maintained databases, including the national death registry, where each patient is tracked through a unique Medicare number for hospital admissions and death.
All patients with MI were considered for an invasive approach unless significant contraindications existed. The default strategy for management of STEMI was primary PCI with 24 h catheterization laboratory activation, and an early invasive approach for NSTEMI, with the aim of angiography/PCI within 24 h of admission for this cohort. All patients were started on evidence based medical therapy for MI including aspirin, dual antiplatelet therapy, statins, angiotensin converting enzyme inhibitors and beta-blockers on admission unless contraindications existed.
2.2. Echocardiography protocol
A comprehensive transthoracic echocardiogram was performed within 24 h of admission for all patients. All echocardiograms were performed on either a General Electric (GE) Vivid E9 machine (Horten, Norway) or a Phillips IE33 machine (Andover, Massachusetts, USA) with tissue Doppler imaging software and a 2.5–5 MHz variable frequency, phased array transthoracic transducer. The echocardiography protocol, performed by experienced clinical sonographers, was in keeping with current guidelines [14]. Of note, whilst the timing of the echocardiogram was standardised to the same time point in the index admission (morning after admission for all patients), the timing of the echocardiogram relative to coronary angiography was different for STEMIs and NSTEMIs due to the different clinical approaches to the timing of revascularization in these two MI subtypes (prior to cardiac catheterization for NSTEMIs and after cardiac catheterization for STEMIs).
Left ventricular systolic function was assessed by LV ejection fraction (LVEF) obtained using biplane method of discs from the apical 4 and 2 chamber views as per current ASE guidelines [14]. DD was assessed on the basis of mitral inflow data, tissue Doppler imaging at the septal and lateral mitral annulus, left atrial size (LAVI) and tricuspid regurgitation velocity (TRV) as per current ASE/EACVI guidelines [15]. Mitral inflow Doppler was obtained by placing a 1 mm pulsed-wave (PW) sample box at the mitral leaflet tips in the apical 4-chamber view at end expiration using a sweep speed of 100 mm/s. Tissue Doppler imaging (TDI) was obtained by placing a 2 mm PW sample box at the septal and lateral mitral annulus (septal and lateral e′). In addition, the average of septal e′ and lateral e′ velocities were calculated (average e′) [15]. E/e′ ratio was calculated using the early mitral inflow E-wave velocity and the average of septal and lateral e′ (E/e′ average). Grades of DD were defined according to the 2016 ASE/EACVI guideline using the algorithm entitled ‘Assessment of diastolic function in patients with depressed LVEF or underlying myocardial disease’ [15]. Grade 3 DD was defined as mitral inflow E/A ratio ≥ 2.0; grade 2 DD was recognized as an E/A ratio of 0.8 to 2.0 (or E/A ratio ≤ 0.8 with E wave >0.5 m/s), and 2 out of 3 of LAVI >34 ml/m2, TRV >2.8 m/s or average E/e′ ratio > 14; and grade 1 DD was recognized as E/A ≤ 0.80 and E wave <0.5 cm/s [15]. Patients not meeting the above criteria for grade 2 were classified as ‘indeterminate’ (when only 2/3 criteria out of LAVI, TRV and average E/e′ were available and one was positive and one negative). Grade 2 and 3 were grouped together into a single group as ‘significant diastolic dysfunction’ for the purposes of this study.
Left atrial size was assessed as follows. LAVI was assessed using a biplane method with an inbuilt disk summation algorithm on the echo machines used in this study as per current ASE guidelines [14]. For LAVI, LA endocardium was traced out in the apical 4 and 2 chamber views at ventricular end systole just prior to mitral valve opening, with the left atrial appendage, the area under the mitral valve annulus and the inflow of the pulmonary veins excluded from the tracing. The height of the LA (h) was divided into 20 segments, with each segment having a height of h/20, and, assuming an oval shape, a major and minor orthogonal diameter (D1 and D2) determined by the inbuilt disk summation algorithm. The total volume was determined using the formula π/4(h)∑(D1)(D2). The calculated volume was indexed to body surface area (BSA) to calculate LAVI.
TRV was obtained from the maximum velocity obtained with continuous wave Doppler echocardiography from complete traces obtained either from the parasternal short axis RV inflow view, parasternal short axis view at the aortic valve level or from the apical 4-chamber view [14]. Color flow mapping was used to align the line of interrogation in line with the regurgitant jet. The use of agitated saline and DEFINITY contrast agent to enhance the TRV signal was at the discretion of the sonographer.
2.3. Statistical methods
Continuous variables are expressed as mean ± SD and compared using an unpaired t-test if data were normally distributed or the Mann Whitney U test if data were not normally distributed. Categorical variables are presented as percentages and compared with Fisher's exact test. Correlations between factors of interest and outcomes were tested with Cox Proportional Hazards analysis. Factors significant at a level of 0.1 on univariate analysis were considered for inclusion in a multivariable Cox Proportional Hazards analysis. Nested models were constructed to examine the independence and incremental value of DD over significant clinical and angiographic variables for prediction of all-cause mortality. Inter-model comparisons for increase in predictive power were performed by a comparison of the model χ2 (chi squared) at each step by calculating change in overall log-likelihood ratio chi-square. Harrell's C-statistic was also calculated for each model as an analogous overall measure of discrimination for predicting survival. Survival was also expressed using Kaplan Meier Curves, with a log-rank test used to assess for significance between curves. Retrospective power calculations for sample size calculations were determined using sampling survival analysis (logrank test) [16]. A p < 0.05 was considered significant. All statistical analyses were carried out using SPSS version 23 (SPSS Inc., Chicago, Illinois) or with STATA version 15 (StataCorp LLC, Texas, USA).
2.4. Ethical considerations
The study was approved by the institutional Human Research Ethics committee [17].
3. Results
Baseline clinical, angiographic and echocardiographic characteristics of study patients are shown in Table 1. All patients included in the study had complete mitral inflow data (E, A, DT, MV Adur), septal and lateral e′, and LAVI max. TRV was not available or considered incomplete in 132 patients (34.4%). Using the 2016 ASE/EACVI algorithms, 45 patients (12.0%) were classified as having significant DD.
Table 1.
Baseline clinical, angiographic and echocardiographic data.
| Characteristic | N = 383 |
|---|---|
| Age (yrs) | 61 ± 13 |
| Male | 280 (73.1%) |
| Body mass index (kg/m2) | 28.9 ± 5.9 |
| Diabetes | 75 (20.2%) |
| Hypertension | 163 (43.3%) |
| Dyslipidemia | 178 (46.1%) |
| Smoking | 200 (52.2%) |
| Family history IHD | 83 (22.3%) |
| Chronic kidney disease | 39 (10.1%) |
| Stroke/TIA | 31 (8.1%) |
| Angiographic data | |
| LMS | 5 (3.4%) |
| LAD | 146 (38.3%) |
| LCx | 97 (25.2%) |
| RCA | 122 (32.1%) |
| No culprit | 13 (1.1%) |
| Three vessel disease | 50 (13.1%) |
| Management strategy | |
| PCI | 281 (73.1%) |
| CABG | 51 (13.2%) |
| Medical therapy | 47 (12.3%) |
| Discharge medications | |
| Aspirin | 372 (97.1%) |
| Beta blocker | 329 (86.2%) |
| ACE-inhibitor/ARB-blocker | 360 (94.0%) |
| Statin | 368 (96.0%) |
| Dual antiplatelet therapy | 329 (86.2%) |
| LV size and LVEF | |
| Biplane LVEF (%) | 56.1 ± 9.1 |
| Interventricular septum thickness (mm) | 11.2 ± 2.5 |
| LV posterior wall thickness (mm) | 10.0 ± 1.8 |
| LV end diastolic volume index (ml/m2) | 45.3 ± 12.6 |
| LV end systolic volume index (ml) | 21.5 ± 22.1 |
| Diastolic function parameters | |
| Mitral E velocity (cm/s) | 69.6 ± 21.9 |
| Mitral A velocity (cm/s) | 70.4 ± 22.5 |
| E/A ratio | 1.07 ± 0.43 |
| Septal e′ velocity (cm/s) | 6.5 ± 1.9 |
| Lateral e′ velocity (cm/s) | 7.6 ± 3.9 |
| Average e′ velocity (cm/s) | 7.3 ± 2.3 |
| Septal E/e′ | 11.6 ± 5.5 |
| Lateral E/e′ | 8.7 ± 8.7 |
| Average E/e′ | 10.3 ± 5.1 |
| LA maximum volume index (cm3/m2) | 29.8 ± 13.2 |
| Significant DD (grade 2/3) | 45 (12.0%) |
| Right heart parameters | |
| TR peak velocity (m/s) | 2.49 ± 0.47 |
| RA pressure (mmHg) | 5.3 ± 3.3 |
| RV S′ velocity (cm/s) | 18.5 ± 3.5 |
STEMI = ST elevation myocardial infarction; NSTEMI = Non ST elevation myocardial infarction; CAD = coronary artery disease; IHD = ischemic heart disease; RCA = right coronary artery; LAD = left anterior descending; LCx = left circumflex; LMS = left mainstem; PCI = percutaneous coronary intervention; RCA = right coronary artery; SVG = saphenous vein graft; CABG = coronary artery bypass grafting; ACE = angiotensin converter enzyme; LV = left ventricular; BPM = beats per minute; e′ = myocardial early relaxation velocity; IVSd = interventricular septal dimension; LA = left atrial; RA = right atrial; Lat = lateral; LVEF = left ventricular ejection fraction; Mitral E = mitral early inflow wave; Mitral A = mitral late inflow wave; E/A ratio = ratio of mitral E and W inflow velocities; PWd = posterior wall dimension; TR = tricuspid regurgitation; E/e′ ratio = ratio of mitral E wave to e′; mm = millimetre; RV S′ = right ventricular systolic tissue velocity; ml = millilitre; ms = millisecond; cm/s = centimetre per second; DD = diastolic dysfunction.
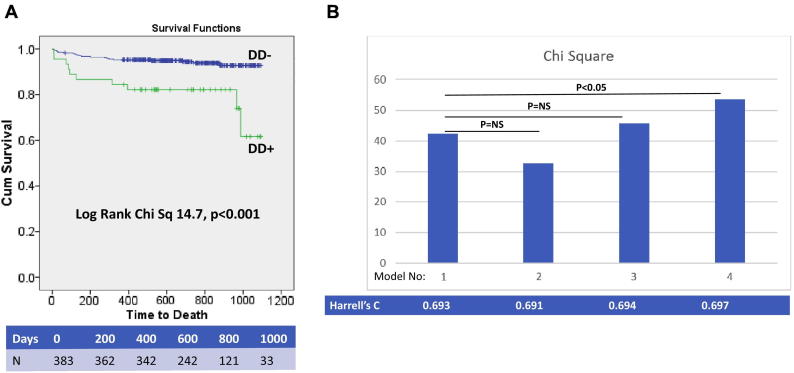
Follow-up data were available for all patients included in the study. There were a total of 32 deaths at a median follow-up of 24 months (maximum follow-up 36 months, minimum follow-up 12 months). Causes of death included MI (n = 7), cardiac arrest (n = 1), sepsis (n = 5), pneumonia (n = 3), cancer (n = 6), gastrointestinal bleeding (n = 3), endstage HF (n = 2), endstage CKD (n = 3) and intracranial haemorrhage (n = 2). Of note, 5 out of 36 patients with LVEF≤35% died during the follow-up period (death rate 13.8% [5/36]), whereas 32 out of 383 patients with LVEF>35% died during the same follow-up period (death rate 8.4% [32/383]), confirming the numerical preponderance of deaths in patients with LVEF>35%. Kaplan Meier analysis for all-cause mortality stratified by presence of guideline assessed DD is shown in Fig. 1A. The results of a Cox proportional hazards univariate analysis for all-cause mortality including clinical, angiographic and echocardiographic variables are shown in Table 2. The major patient subsets at greater risk of adverse events identified on Cox proportional hazards univariate analysis included the elderly, the obese, diabetics, those with chronic kidney disease and those with a greater extent of coronary artery disease. There was also a weak correlation with outcomes in patients not on DAPT (p = 0.049). Of note, LVEF remained a weak predictor of all-cause mortality once all patients with LVEF ≤35% were excluded (p = 0.030). Using nested Cox proportional hazards multivariate models incorporating significant clinical and angiographic variables (age, chronic kidney disease, number of diseased vessels), and separate addition of individual echocardiographic parameters, significant DD and LVEDVI were the only two independent echocardiographic predictors of all-cause mortality, as summarised in Table 3. Intermodel comparisons of the nested Cox showed that the sequential addition of DD and LVEDVI to a model containing significant clinical and angiographic variables resulted in a significant increase in model power (as shown in Fig. 1B). Calculation of Harrel's C confirmed incremental value of DD as a prognostic factor in this subgroup of patients. Of note, body mass index, diabetes, and dual antiplatelet therapy, whilst significant on univariate analysis, were omitted from the final models to avoid model over-fitting by limiting the number of input variables (only the most significant variables selected for the multivariate analysis). However, alternative models with the inclusion of these factors were also constructed and showed similar results, with DD remaining an independent predictor in each analysis.
Fig. 1.
Diastolic Dysfunction and Outcomes: A. Kaplan-Meier Curves for All-Cause Mortality B. Intermodel Comparisons of Model χ2.
A. Cumulative percentage survival free from all-cause mortality stratified by presence of DD; table below Kaplan-Meier curve summarises number of patients at risk during duration of follow-up; B. Nested regression models for all-cause incorporating clinical predictors, left ventricular ejection fraction (LVEF) and diastolic dysfunction. Multivariate clinical model consisted of age, chronic kidney disease, number of diseased vessels, followed by sequential addition of LVEF, LVEDVI, and DD as shown. (DD = diastolic dysfunction; LVEF = left ventricular ejection fraction; LVEDVI = left ventricular end-diastolic volume index).
Table 2.
Univariate cox proportional hazard analysis to identify significant predictors for all-cause mortality.
| Variable | Hazard ratio | 95%CI | p-Value |
|---|---|---|---|
| Clinical/angiographic variables | |||
| Age | 1.05 | 1.02–1.09 | 0.001 |
| Male | 1.78 | 0.68–4.66 | 0.243 |
| BMI | 1.05 | 1.03–1.09 | 0.038 |
| Smoking | 1.96 | 0.93–4.13 | 0.074 |
| Hypertension | 1.76 | 0.86–3.63 | 0.124 |
| Dyslipidemia | 1.13 | 0.52–2.22 | 0.734 |
| Diabetes | 2.90 | 1.38–6.06 | 0.005 |
| Chronic kidney disease | 6.59 | 3.13–13.89 | <0.001 |
| Stroke/TIA | 1.54 | 0.88–4.12 | 0.212 |
| Post-PCI TIMI flow | 1.43 | 0.64–3.12 | 0.332 |
| No of diseased vessels | 2.08 | 1.40–3.10 | <0.001 |
| Infarct type (STEMI) | 1.53 | 0.62–3.75 | 0.347 |
| LV and RV size and function | |||
| LVEF | 0.96 | 0.92–0.99 | 0.030 |
| LVEDVi | 1.03 | 1.01–1.05 | 0.009 |
| LVESVi | 1.02 | 0.99–1.02 | 0.611 |
| LVMI | 1.77 | 1.06–2.71 | 0.049 |
| RV S′ | 1.04 | 0.90–1.12 | 0.938 |
| Guideline recommended diastolic parameters | |||
| E/A > 2 | 1.36 | 0.17–10.0 | 0.759 |
| Average E/e′ > 14 | 3.41 | 1.59–7.28 | 0.002 |
| TR Velocity > 2.8 m/s | 1.76 | 0.92–3.14 | 0.155 |
| LAVI >34 ml/m2 | 3.22 | 1.57–6.67 | 0.001 |
| Significant (grade 2/3) DD | 3.97 | 1.86–8.47 | <0.001 |
| Discharge medications | |||
| Aspirin | 0.74 | 0.22–3.12 | 0.718 |
| Beta-blocker | 1.04 | 0.61–2.19 | 0.927 |
| ACE-inhibitor/ARB blocker | 0.55 | 0.25–1.25 | 0.076 |
| Statin | 0.90 | 0.24–3.23 | 0.562 |
| Dual antiplatelet therapy | 0.54 | 0.29–0.99 | 0.049 |
MACE = major adverse cardiovascular events; BMI = body mass index; STEMI = ST elevation MI; BMI = body mass index; LV = left ventricular; LVEF = left ventricular ejection fraction; LVEDVi = left ventricular end diastolic volume index; LVESVi = left ventricular end systolic volume index; E/A = ratio of mitral E wave to A wave; E/e′ = ratio of mitral E wave to e′; TR = tricuspid regurgitation; LAVI = left atrial volume index; DD2016 = diastolic dysfunction by 2016 guidelines; DD2009 = diastolic dysfunction by 2009 guidelines.
Table 3.
Nested cox proportional hazards models to identify independent predictors of all-cause mortality.
| MACE | Univariate analysis |
Multivariate analysis |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
|||||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | 1.05(1.02–1.09) | 0.001 | 1.02(0.99–1.06) | 0.202 | 1.03(0.99–1.07) | 0.16 | 1.02(0.99–1.06) | 0.176 | 1.01(0.98–1.05) | 0.425 |
| CKD | 6.59(3.13–13.89) | <0.001 | 3.97(1.72–9.13) | 0.001 | 2.95(1.08–8.02) | 0.034 | 3.90(1.69–9.01) | 0.001 | 3.95(1.70–9.14) | 0.001 |
| No of vessel diseased | 2.08(1.40–3.10) | <0.001 | 1.74(1.15–2.63) | 0.009 | 1.62(1.00–2.61) | 0.05 | 1.65(1.09–2,49) | 0.018 | 1.72(1.13–2.60) | 0.011 |
| LVEDVI | 1.03(1.01–1.05) | 0.009 | 1.03(1.04–1.07) | 0.021 | ||||||
| LVEF | 0.96(0.92–0.99) | 0.03 | 0.96(0.92–1.02) | 0.06 | ||||||
| DD | 3.97(1.86–8.47) | <0.001 | 2.57(1.16–5.68) | 0.02 | ||||||
| Model Chi Sq | NA | 42.3 | 32.5 | 45.6 | 53.55 | |||||
CKD = chronic kidney disease; LVEDVI = left ventricular end diastolic volume index; LVEF = left ventricular ejection fraction; DD = diastolic dsyfunction.
With respect to the individual parameters incorporated in the novel 2016 guideline algorithms, only average E/e′ > 14 and LAVI >34 mls/m2 were significantly associated with all-cause mortality on Cox proportional hazards univariate analysis as listed in Table 3. In a Cox proportional hazards multivariate analysis incorporating significant clinical predictors, neither LAVI >34 mls/m2 (HR 1.60, 95%CI 0.72–3.58, p = 0.245) nor average E/e′ > 14 (HR 1.35, 95%CI 0.56–3.25, p = 0.501) remained independent predictors. For completeness, the independent prognostic value of TRV (>2.8 m/s) and E/A (>2) were both were shown to be non-significant in multivariate models.
With respect to the secondary endpoint of cardiac death (n = 10 events), DD had a significant association with outcomes on univariate Cox proportional hazards analysis (HR 5.00, 95%CI 1.41–7.9, p = 0.013). In a multivariate Cox proportional hazards analysis incorporating significant clinical predictors (age, CKD, number of diseased vessels), significant DD remained an independent predictor of outcomes (HR 3.96, 95%CI 1.06–6.92).
For the subgroup of patients with LVEF 36–55% (n = 176), there were 23 patients with significant DD and a total of 20 deaths at a median follow up of 2 years, with a corresponding death rate of 11.3% (20/176). On Cox proportional hazards univariate analysis, significant DD (HR 5.00, 95%CI 1.79–13.9, p = 0.002) was a significant predictor of all-cause mortality, as were the following individual diastolic parameters: LAVI>34 mls/m2 (HR 4.01, 95%CI 1.45–11.11, p = 0.007) and average E/e′ > 14 (HR 3.87, 95%CI 1.99–16.01, p < 0.001). On Cox proportional hazards multivariate analysis incorporating significant clinical and angiographic variables (age, number of diseased vessels), significant DD was the only significant echocardiographic variable for prediction of all-cause mortality (HR 3.56, 95%CI 2.46–9.09, p = 0.006).
The adequacy of the sample size selected for this study was tested via retrospective power analyses. Retrospective survival power analyses (sample size calculations) based on current sample (n = 383) and number of events observed in the study found that when using the 2016 DD guidelines, the minimal required sample sizes for acceptable Type I (α; two sided) and Type II (β; 1-power) error probability was n = 227 when assessing all-cause mortality (α = 0.01, β = 0.01).
4. Discussion
The novel finding of this study is that significant DD as assessed by contemporary ASE/EACVI guidelines was an independent predictor of all-cause mortality in patients with preserved or mild-moderately reduced LVEF (LVEF > 35%) following a first-ever MI. In the subgroup of patients with LVEF 36–55%, significant DD was the only independent echocardiographic predictor of all-cause mortality. Importantly, LVEF did not remain an independent predictor of outcomes in this subset of patients. The clinical significance of this finding is that it may allow restratification of the risk of all-cause mortality in patients with LVEF >35% following MI, and identify patients who may need further investigation and treatment.
LVEF ≤35% has become embedded in clinical practice as the echocardiographic criterion for implantable cardioverter-defibrillator implantation based on the results of seminal studies on the prevention of SCD with ICDs [4]. Furthermore, specific heart failure therapies such as mineralocorticoid receptor antagonists and cardiac resynchronisation therapy are generally reserved for patients with a moderate to severe reduction in LVEF as per current guidelines. However, the value of LVEF in patients with LVEF >35% is limited [5,6]. Other novel echocardiographic indices, apart from diastolic dysfunction, that have the potential to allow further risk stratification include global longitudinal strain, left atrial strain, diastolic strain rate and mechanical dispersion [[18], [19], [20]]. However, these strain based parameters require additional sonographer and cardiologist expertise as well as more advanced equipment and software, whereas the assessment of DD forms a part of the standard echocardiogram in most echocardiographic laboratories.
The major limitation of early assessment of LVEF is that is confounded by myocardial stunning. However studies have shown that the risk of death is highest in the first thirty days post MI, and the evolving literature appears to favour early risk stratification [6]. Moreover, patients with MI are 4–5 times more likely than patients with non-ischaemic LV dysfunction to have SCD [6]. For this reason, early risk stratification with echocardiography is desirable in patients with MI, and the value of early assessment of LVEF has been studied previously in randomised controlled trials [21,22]. Importantly, whilst LVEF early after MI is confounded by stunning, diastolic function assessed early after MI is a powerful marker of prognosis after MI and may not be confounded by myocardial stunning, which represents an advantage [[10], [11], [12]].
The clinical value of achieving further risk stratification from standard echocardiographic parameters in patients with LVEF >35% is that it identifies patients who may benefit from further investigation to refine the risk of SCD, including holter monitoring, electrophysiological studies, performance of signal averaged electrocardiography, or further evaluation with contrast enhanced cardiac MRI for presence of LGE [6]. Whilst a comprehensive review of these modalities is beyond the scope of this discussion, further investigation using the combination of these modalities with echocardiography may help to identify patients with LVEF >35% who may benefit from ICD implantation to prevent SCD [5]. “In the wider context, the prognostic value of significant diastolic dysfunction has been studied extensively in a broad range of clinical scenarios, including following MI, and has been shown to be a robust prognostic marker [[8], [9], [10], [11], [12], [13],23]. In this study, its prognostic value in patients with mild-moderate systolic dysfunction following MI is highlighted.”
4.1. Limitations
This study has a number of limitations. The subgroup of patients with a LVEF >35% represented a moderate sample size. A significant proportion of patients with potentially grade 2 diastolic dysfunction were classified as ‘indeterminate’ and had to be excluded. Measurement of systolic function in the early phase following MI may underestimate LVEF due to myocardial stunning. Data on novel diastolic parameters such as global longitudinal strain, left atrial strain and early diastolic strain rate, which have been correlated with outcomes following MI previously, were not available. Whilst the timing of the echocardiogram relative to coronary angiography was different for STEMIs and NSTEMIs, this was felt to be unavoidable due to the different clinical approaches to angiography in these two MI subtypes. Data on baseline cancer and depression were not systematically collected and thus represents a limitation of the dataset used for this study. Finally, data on biomarkers such as BNP and NT pro-BNP were not available.
4.2. Conclusions
This study demonstrates that significant DD as assessed by contemporary ASE/EACVI guidelines was an independent predictor of all-cause mortality in patients with preserved or mild-moderately reduced LVEF (LVEF >35%) in patients following a first-ever MI. The clinical significance of this finding is that it may allow restratification of the risk of all-cause mortality in patients with LVEF >35% following MI, and identify patients who may benefit from further investigation and treatment.
Abbreviations
- ASE
American Society of Echocardiography
- DD
Diastolic dysfunction
- EAE
European Association of Echocardiography
- EACVI
European Association of Cardiovascular Imaging
- LAVI
Left atrial volume index
- LV
Left ventricular
- LVEF
Left ventricular ejection fraction
- MACE
Major adverse cardiovascular events
- MI
Myocardial infarction
- PCI
Percutaneous coronary intervention
Acknowledgments
Acknowledgements
The contribution of James Armstrong and Kym Smith for performing the echocardiographic measurements, Jo-anne Sippel for the database management, and Drs Christopher Hammett, Peter Stewart and Rajesh Shetty for access to the catheterization data, is gratefully acknowledged.
Declaration of competing interest
None.
Sources of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Makikallio T.H., Barthel P., Schneider R. Prediction of sudden cardiac death after acute myocardial infarction: role of Holter monitoring in the modern treatment era. Eur. Heart J. 2005;26:762–769. doi: 10.1093/eurheartj/ehi188. [DOI] [PubMed] [Google Scholar]
- 2.Narayanan K., Reinier K., Uy-Evanado A. Frequency and determinants of implantable cardioverter defibrillator deployment among primary prevention candidates with subsequent sudden cardiac arrest in the community. Circulation. 2013;128:1733–1738. doi: 10.1161/CIRCULATIONAHA.113.002539. [DOI] [PubMed] [Google Scholar]
- 3.de Vreede-Swagemakers J.J., Gorgels P., Dubois-Arbouw W.I. Out-of-hospital cardiac arrest in the 1990's: a population-based study in the Maastricht area on incidence, characteristics and survival. J. Am. Coll. Cardiol. 1997;30:1500–1505. doi: 10.1016/s0735-1097(97)00355-0. [DOI] [PubMed] [Google Scholar]
- 4.Epstein A.E., DiMarco J.P., Ellenbogen K.A. ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2013. 2012;27:283–352. doi: 10.1161/CIR.0b013e318276ce9b. [DOI] [PubMed] [Google Scholar]
- 5.Selvanayagam J.B., Hartshorne T., Billot L. Cardiovascular magnetic resonance-GUIDEd management of mild to moderate left ventricular systolic dysfunction (CMR GUIDE): study protocol for a randomized controlled trial. Ann Nonivasive Electro. 2017;22 doi: 10.1111/anec.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaman S., Kovoor P. Sudden cardiac death early after myocardial infarction: pathogenesis, risk stratification, and primary prevention. Circulation. 2014;129:2426–2435. doi: 10.1161/CIRCULATIONAHA.113.007497. [DOI] [PubMed] [Google Scholar]
- 7.Zaman S., Taylor A.J., Stiles M., Chow C., Kovoor P. Programmed ventricular stimulation to risk stratify for early cardioverter-defibrillator implantation to prevent tachyarrhythmias following acute myocardial infarction (PROTECT-ICD): trial protocol, background and significance. Heart Lung Circ. 2016;25:1055–1062. doi: 10.1016/j.hlc.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Sato K., Negishi K., Cremer P.C. Reliability of updated left ventricular diastolic function recommendations in predicting elevated left ventricular filling pressure and prognosis. Am. Heart J. 2017;189:28–39. doi: 10.1016/j.ahj.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Sanchis A.R., Falces C., Poyatos S., Vidal B., Sitges M. Differential clinical implications of current recommendations for the evaluation of left ventricular diastolic function by echocardiography. J. Am. Soc. Echocardiogr. 2018;31:1203–1208. doi: 10.1016/j.echo.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Prasad S.B., Guppy-Coles K.B., Stanton T. Diastolic dysfunction assessed using contemporary guidelines and prognosis following myocardial infarction. J. Am. Soc. Echocardiogr. 2018;31:1127–1136. doi: 10.1016/j.echo.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Hillis G.S., Moller J.E., Pellikka P.A. Noninvasive estimation of left ventricular filling pressure by E/e′ is a powerful predictor of survival after acute myocardial infarction. J. Am. Coll. Cardiol. 2004;43:360–367. doi: 10.1016/j.jacc.2003.07.044. [DOI] [PubMed] [Google Scholar]
- 12.Moller J.E., Whalley G.A., Dini F.L. Independent prognostic importance of a restrictive left ventricular filling pattern after myocardial infarction: an individual patient meta-analysis: Meta-Analysis Research Group in Echocardiography acute myocardial infarction. Circulation. 2008;117:2591–2598. doi: 10.1161/CIRCULATIONAHA.107.738625. [DOI] [PubMed] [Google Scholar]
- 13.Moller J.E., Hillis G.S., Oh J.K. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107:2207–2212. doi: 10.1161/01.CIR.0000066318.21784.43. [DOI] [PubMed] [Google Scholar]
- 14.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Nagueh S.F., Smiseth O.A., Appleton C.P. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Machin D, Campbell MJ, Tan SB, Tan SH. Sample Size Tables for Clinical Studies. 209. 3rd ed. Chichester: Wiley-Blackwell.
- 17.Shewan L.G., Rosano G.M.C., Henein M.Y., Coats A.J.S. A statement on ethical standards in publishing scientific articles in the International Journal of Cardiology family of journals. Int. J. Cardiol. 2014;170:253–254. [Google Scholar]
- 18.Stanton T., Leano R., Marwick T.H. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–364. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 19.Singh A., Addetia K., Maffessanti F., Mor-Avi V., Lang R.M. LA strain for categorization of LV diastolic dysfunction. JACC Cardiovasc. Imaging. 2017;10:735–743. doi: 10.1016/j.jcmg.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ersboll M., Andersen M.J., Valeur N. Early diastolic strain rate in relation to systolic and diastolic function and prognosis in acute myocardial infarction: a two-dimensional speckle-tracking study. Circ Cardiovasc Imaging. 2014;2:356–364. doi: 10.1093/eurheartj/eht179. [DOI] [PubMed] [Google Scholar]
- 21.Hohnloser SH Kuck K.H., Dorian P. DINAMIT Investigators. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N. Engl. J. Med. 2004;351:2481–2488. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 22.Steinbeck G Andresen D., Seidl K. IRIS Investigators. Defibrillator implantation early after myocardial infarction. N. Engl. J. Med. 2009;361:1427–1436. doi: 10.1056/NEJMoa0901889. [DOI] [PubMed] [Google Scholar]
- 23.Gharacholou S.M., Scott C.G., Takahashi P.Y., Nkomo V.T., McCully R.B., Fine N.M., Pellikka P.A. Left ventricular diastolic function and long-term outcomes in patients with normal exercise echocardiographic findings. Am. J. Cardiol. 2013;112(2):200–207. doi: 10.1016/j.amjcard.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]