Abstract
The blood-nerve barrier (BNB) formed by tight junction-forming endoneurial microvessels located in the innermost compartment of peripheral nerves, and the perineurium serve to maintain the internal microenvironment required for normal signal transduction. The specific molecular components that define the normal adult human BNB are not fully known. Guided by data derived from the adult human BNB transcriptome, we evaluated the in situ expression of 25 junctional complex, transporter, cell membrane and cytoskeletal proteins in 4 histologically normal adult sural nerves by indirect fluorescent immunohistochemistry to determine proteins specifically expressed by restrictive endoneurial microvascular endothelium. Using Ulex Europaeus Agglutinin-1 expression to detect endothelial cells, we ascertained that the selected proteins were uniformly expressed in ≥ 90% of endoneurial microvessels. P-glycoprotein (also known as ATP binding cassette subfamily B member 1, ABCB1) and solute carrier family 1 member 1 (SLC1A1) demonstrated restricted expression by endoneurial endothelium only, with classic tight junction protein claudin-5 also expressed on fenestrated epineurial macrovessels, and vascular-specific adherens junction protein cadherin-5 also expressed by the perineurium. The expression profiles of the selected proteins provide significant insight into the molecular composition of normal adult peripheral nerves. Further work is required to elucidate the human adult BNB molecular signature in order to better understand its development and devise strategies to restore function in peripheral neuropathies.
Keywords: adapter, endoneurium, excitatory amino acid transporter, intercellular junctions, P-glycoprotein
Introduction
Peripheral nerves and nerve roots are structurally organized into three compartments: the outermost epineurium through which extrinsic blood vessels called the vasa nervorum penetrate to give rise to epineurial arteries and arterioles and receive blood from epineurial venules and veins, the inner perineurium, formed by multiple concentric layers of specialized epithelioid myofibroblasts that form fascicles and surround the innermost endoneurium, which consists of myelinated and unmyelinated axons and capillary-like microvessels. 1-3
Ultrastructural examination of human peripheral nerves has demonstrated that endoneurial microvessels and the innermost layers of the perineurium form electron-dense intercellular tight junctions, while epineurial arteries, arterioles, venules and veins, collectively known as macrovessels, are fenestrated. 3-5 Endoneurial microvessels are in direct communication with circulating blood, and are considered as the blood-nerve barrier (BNB), while the perineurium restricts passive diffusion of interstitial fluid from the epineurium into the endoneurium and vice versa. The restrictive BNB and perineurium serve to maintain the internal endoneurial microenvironment essential for physiological signal transduction by myelinated and unmyelinated axons to and from the central nervous system. 1,2
Guided by in situ immunohistochemical data derived from human peripheral nerves during development and adulthood, as well as data derived from animal models and other restrictive tissue barriers in vitro and in situ, specific molecules have been implicated as essential functional components of the human BNB. Claudin-5 (CLDN5), zona occludens-1 (ZO-1, also known as tight junction protein-1 [TJP1]) and the vascular-specific cadherin-5 (CDH5, also known as vascular endothelial [VE]-cadherin), are commonly cited as molecular components of the specialized BNB junctional complex, with alterations implicated in the pathogenesis of specific peripheral neuropathies. 6-22 However, data has emerged over the past decade on the complexity of specialized intercellular tight and adherens junctional complexes formed by specific claudins and cadherins interacting with adapter molecules such members of the zona occludens subfamily, catenins and other membrane-associated junctional proteins which bind directly or indirectly to cytoskeletal proteins and are responsible for the cellular structural integrity, polarity and the unique biologic functions during normal physiological states. 7,23-27
Our recent work that established the normal adult human BNB transcriptome based on transcripts universally expressed by whole exome sequencing of laser capture microdissected endoneurial microvessels coupled to early- and late-passage primary human endoneurial endothelial cells, with some in situ validation of protein expression on endoneurial endothelium by indirect immunohistochemistry, ascertained 133 transcripts that may be involved in the intercellular junctional complex, as well as 509 transporter transcripts that may be responsible for the influx or efflux of solutes, macromolecules and xenobiotics by the normal adult human BNB. 24 In order to better understand how the human BNB develops, is maintained in health and the functional alterations and adaptations that may occur in disease states such as peripheral neuropathies and traumatic injury, it is essential to determine its specific molecular components prior to elucidating biologically relevant signaling mechanisms.
Guided by our in situ human BNB transcriptome data, we evaluated the expression of 25 proteins in 4 adult sural nerve biopsies with no discernible histopathological evidence of disease by fluorescent light microscopy to determine which proteins are restricted to the tight junction-forming endoneurial microvascular endothelium and more comprehensively characterize the molecular composition of the normal adult human BNB.
Materials and Methods
Indirect fluorescent immunohistochemistry
Four de-identified histologically normal adult sural nerve biopsy specimens (two men and two women, ages 56-65) embedded in Optimum Cutting Temperature® compound and stored at −80°C were utilized for this study. The study was approved by the University of Alabama at Birmingham Institutional Review Board, with an exemption obtained to use archived pathological specimens for research (Protocol Number X140321012). 10 μm-thick axial sections were cut from each sural nerve sample after thermal equilibration at −15°C using a Cryostar Nx50 cryostat (Thermo Fisher Scientific, Waltham, MA). Axial cryostat sections were mounted on Superfrost Plus™ microscope slides (catalog # 4951PLUS4, Thermo Fisher Scientific) and maintained at −20°C prior to processing. Twenty-five proteins, including junctional complex (tight, adherens, gap), specialized transporters, cytoskeletal and cell membrane proteins were selected for investigation, guided by the recently elucidated human BNB transcriptome. 24
Sections were fixed and permeabilized in acetone at −20°C, washed with 1X phosphate-buffered saline (PBS), air dried for 5 minutes, then blocked with 10% normal goat serum (NGS) in 1X PBS for thirty minutes. Without washing, slides from each adult sural nerve were incubated with a specific primary antibody in 2% NGS in 1X PBS for one hour at room temperature. The list of primary antibodies used, their sources and concentrations are shown on Table 1. After washing the slides with 1X PBS, all of the slides were incubated with fluoresceinated Ulex Europaeus Agglutinin-1 (the most sensitive detector of human endothelial cells; a lectin that binds to α-fucose residues on vascular endothelium; 8,28,29 UEA-1 FITC [catalog #L9006, Sigma, 10 μg/mL]) to detect the vascular endothelium, and the following secondary antibodies depending on the host species and immunoglobulin subclass of the primary used, in 2% NGS in 1X PBS for one hour at room temperature in the dark: goat anti-mouse IgG (H + L) Alexa Fluor® 594 conjugate (catalog #A-11005, Life technologies: 4 μg/mL) and goat anti-rabbit IgG (H + L) Alexa Fluor® 594 conjugate (catalog #A-11037, Life technologies: 4 μg/mL). Following washes with 1X PBS, all sections were stained with 0.45 μM 4, 6-diamidino-2-phenylindole (DAPI) for 5 min to detect nuclei and mounted with ProLong® Gold antifade mounting medium (catalog #P36934, Life technologies) prior to image acquisition
Table 1. List of primary antibodies used to characterize normal adult sural nerves.
The primary antibody list to detect selected proteins in normal peripheral nerves based on data obtained from the human BNB transcriptome includes detailed information of the antibodies, including final concentrations used for indirect fluorescent immunohistochemistry.
| Gene Symbol |
Protein Name | Antibody Source |
Catalog Number |
Host/Isotype, Stock Concentration |
Final Concentration |
|---|---|---|---|---|---|
| ABCB1 | P-glycoprotein | Life Technologies | MA5-13854 | Mouse / IgG1, 0.2 mg/mL | 4 μg/mL |
| ACTG1 | Gamma-1 actin | Life Technologies | PA5-13467 | Rabbit / IgG, 2 mg/mL | 40 μg/mL |
| AQP1 | Aquxaporin 1 | Santa Cruz Biotechnology | sc-25287 | Mouse/ IgG1 (kappa light chain), 200 μg/mL | 4 μg/mL |
| CALD1 | Caldesmon 1 | Abcam | ab68878 | Rabbit/ Polyclonal IgG, 0.3 mg/mL | 1 μg/mL |
| CAV1 | Caveolin 1 | Santa Cruz Biotechnology | sc-53564 | Mouse/ IgG2b (kappa light chain), 200 μg/mL | 4 μg/mL |
| CD44 | Cell surface adhesion glycoprotein | Santa Cruz Biotechnology | sc-7297 | Mouse/ IgG1 (kappa light chain), 200 μg/mL | 4 μg/mL |
| CD63 | Tetraspanin family member | Santa Cruz Biotechnology | sc-5275 | Mouse/ IgG2a (kappa light chain), 200 μg/mL | 4 μg/mL |
| CDH5 | Cadherin 5 | Santa Cruz Biotechnology | sc-9989 | Mouse/ IgG1 (kappa light chain), 200 μg/mL | 4 μg/mL |
| CDH6 | Cadherin 6 | Life Technologies | MA1-06305 | Mouse / IgG1, 1 mg/mL | 20 μg/mL |
| CLDN4 | Claudin 4 | Life Technologies | 36-4800 | Rabbit / IgG, 0.25 mg/mL | 2.5 μg/mL |
| CLDN5 | Claudin 5 | Life Technologies | 35-2500 | Mouse / IgG1, 0.5mg/mL | 10 μg/mL |
| CTNNA1 | Alpha-1 catenin | Life Technologies | 13-9700 | Mouse / IgG1 kappa, 0.5 mg/mL | 2 μg/mL |
| ESAM | Endothelial cell adhesion molecule | R&D Systems | MAB4204 | Mouse/ IgG2b, 0.5 mg/mL | 10 μg/mL |
| FLNA | Filamin A | Santa Cruz Biotechnology | sc-17749 | Mouse/ IgG2a (kappa light chain), 200 μg/mL | 4 μg/mL |
| GJA1 | Gap junction protein alpha 1 | Life Technologies | 13-8300 | Mouse / IgG1, 0.5 mg/mL | 10 μg/mL |
| LIN7A | Lin 7A, crumbs cell polarity complex | Life Technologies | PA5-30871 | Rabbit / IgG, 1 mg/mL | 20 μg/mL |
| MPDZ | Multiple PDZ domain crumbs cell polarity complex component | Sigma-Aldrich | HPA020255 | Rabbit/ Polyclonal IgG, 0.1 mg/mL | 2 μg/mL |
| MYO10 | Myosin X | Life Technologies | PA5-55019 | Rabbit / IgG, 0.3 mg/mL | 6 μg/mL |
| PCDH1 | Protocadherin 1 | Life Technologies | PA5-35091 | Rabbit / IgG, 0.47 mg/mL | 9.4 μg/mL |
| SLC1A1 | Glutamate transporter | Cell Signaling Technology | 14501 | Rabbit/IgG, 160 mg/mL | 320 μg/mL |
| SLC16A1 | Monocarboxylate transporter 1 | Santa Cruz Biotechnology | sc-365501 | Mouse/ IgG1 (kappa light chain), 200 μg/mL | 4 μg/mL |
| SLC19A2 | Thiamine transporter | Life Technologies | PA5-53456 | Rabbit / IgG, 0.1 mg/mL | 2 μg/mL |
| TJP1 | Zona occludens-1 | Life Technologies | 61-7300 | Rabbit / IgG, 0.25 mg/mL | 2.5 μg/mL |
| VEZT | Vezatin | Life Technologies | PA5-52115 | Rabbit / IgG, 100 μL, 0.3 mg/mL | 6 μg/mL |
| ZYX | Zyxin | Life Technologies | PA1-25162 | Rabbit / IgG, 13.3 mg/mL | 133 μg/mL |
Image acquisition
Processed slides were viewed and images captured with the 4X, 10X, and 40X objective lenses using a Nikon Eclipse Ci-S Upright epifluorescent microscope with a D5-Qi2 camera (Nikon Instruments Inc., Melville, NY). The Nikon NIS-Elements software was utilized for image processing and co-localization of the specific proteins with endoneurial microvascular endothelial cells that form the BNB using a defined range of “look up table” (LUT) adjustments to reflect observed signal intensities during microscopy in a non-destructive manner to image data, taking into account variations in background signal and fluorescent intensities. LUT values were as follows: DAPI: 4000–8000, UEA-1: 1000–3000, ABCB1: 650–1550, gamma-1 actin (ACTG1): 700–2000, aquaporin 1 (AQP1): 700–2000, caldesmon 1 (CALD1): 600–1500, caveolin 1 (CAV1): 700–3000, cell surface adhesion glycoprotein, CD44: 500–900, tetraspanin family member, CD63: 600–1500, CDH5: 500–800, cadherin-6 (CDH6): 700–1300, claudin-4 (CLDN4): 750–1500, CLDN5: 800–2300, alpha-1 catenin (CTNNA1): 700–1500, endothelial cell adhesion molecule (ESAM): 1100–2200, filamin A (FLNA): 800–4000, gap junction protein alpha 1 (GJA1): 700–2000, crumbs cell polarity complex, LIN7A: 700–1700, multiple PDZ domain crumbs cell polarity complex component (MPDZ): 500–900, myosin X (MYO10): 400–700, protocadherin 1 (PCDH1): 500–800, SLC1A1: 400–700, solute carrier family 16 member 1 (SLC16A1): 700–1500, solute carrier family 19 Member 2 (SLC19A2): 500–700, TJP1: 500–1500, vezatin (VEZT): 500–800 and zyxin (ZYX): 1700–3000.
Co-localization was determined as a yellow or orange color (dependent on relative fluorescent intensity of the selected protein bound to its fluorescent secondary antibody in red) with green-staining endoneurial microvascular endothelial cells (based on UEA-1 FITC intensity). Co-localization with epineurial macrovessels (UEA-1 FITC-positive macrovascular endothelium) was determined as described above. Co-localization of proteins with non-endothelial cells/ tissues within peripheral nerves (UEA-1 FITC-negative) was determined based on the expected morphologic staining profiles guided by prior published experience. 5,25,30-34
Results
Indirect fluorescent immunohistochemistry demonstrated protein expression in ≥ 90% of the endoneurial microvessels identified in the 4 adult sural nerve biopsies (range 90–100%), verifying uniform in situ expression of the selected proteins consistent with the previously published transcriptome data (Table 2). The mean in situ transcript expression levels in laser capture microdissected endoneurial microvessels for each gene studied (based on Fragments Per Kilobase of fragments per Million) have been previously published. 24 Sequencing data have also been published and are freely accessible via the National Center for Biotechnology Information Gene Expression Omnibus (GEO) series accession number GSE107574: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE107574
Table 2. Quantitative expression of selected proteins by normal adult sural nerve endoneurial microvascular endothelium.
Numbers depict the percentages of UEA-1-positive endoneurial microvessels expressing the indicated protein in each complete axial sural nerve cross-section analyzed by indirect fluorescent immunohistochemistry for each normal adult (Sural Nerve 1-4). The selected proteins are expressed in ≥ 90% of endoneurial microvessels with the Sural Nerve 2 demonstrating the most variability in endoneurial microvessel protein expression for unknown reasons. CTNNA1 is the only protein not expressed on 100% of endoneurial microvessels in any of these specimens. These observations suggest some endoneurial endothelial cell heterogeneity that require further investigation.
| Protein Gene Symbol |
Sural Nerve 1 | Sural Nerve 2 | Sural Nerve 3 | Sural Nerve 4 |
|---|---|---|---|---|
| ABCB1 | 100 | 100 | 100 | 100 |
| ACTG1 | 100 | 100 | 100 | 100 |
| AQP1 | 100 | 90 | 100 | 100 |
| CALD1 | 100 | 100 | 100 | 100 |
| CAV1 | 100 | 100 | 100 | 100 |
| CD44 | 100 | 100 | 100 | 100 |
| CD63 | 100 | 100 | 100 | 100 |
| CDH5 | 100 | 93 | 100 | 100 |
| CDH6 | 100 | 97 | 97 | 96 |
| CLDN4 | 100 | 94 | 100 | 100 |
| CLDN5 | 100 | 100 | 100 | 100 |
| CTNNA1 | 94 | 91 | 93 | 94 |
| ESAM | 100 | 100 | 100 | 100 |
| FLNA | 100 | 100 | 100 | 100 |
| GJA1 | 100 | 100 | 100 | 100 |
| LIN7A | 100 | 100 | 100 | 100 |
| MPDZ | 100 | 100 | 100 | 100 |
| MYO10 | 100 | 100 | 100 | 100 |
| PCDH1 | 97 | 91 | 100 | 100 |
| SLC1A1 | 92 | 100 | 97 | 100 |
| SLC16A1 | 96 | 100 | 100 | 100 |
| SLC19A2 | 100 | 100 | 100 | 100 |
| TJP1 | 100 | 100 | 100 | 100 |
| VEZT | 100 | 100 | 100 | 100 |
| ZYX | 100 | 100 | 100 | 100 |
Endoneurial microvessel restricted proteins
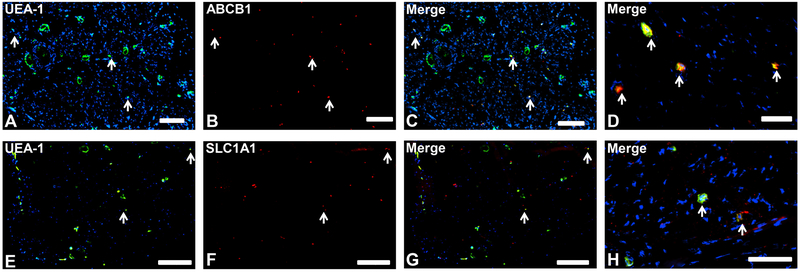
ABCB1 (also known as P-glycoprotein, a major efflux transporter) and SLC1A1 (also known as excitatory amino acid transporter-3 that actively transports glutamate across plasma membranes) 24,35,36 were the only proteins restricted to endoneurial microvessel endothelium (Fig. 1), implying crucial roles in normal human BNB function.
Figure 1. Blood-nerve barrier-restricted proteins.
Representative indirect fluorescent digital photomicrographs of cryostat axial sections of normal adult sural nerves show UEA-1-positive endothelial cells (green; A and E) with proteins ABCB1 and SLC1A1 (red; B and F respectively) demonstrating restricted expression by endoneurial microvascular endothelium on merged images (yellow; C and G respectively), further demonstrated at higher magnification (yellow/orange; D and H respectively). This suggests that these proteins have restricted BNB functions. White arrows demonstrate positively staining endoneurial microvessels. Blue (DAPI) staining indicates nuclei. Scale bar 500 μm for A-C and E-G, and 125 μm for D and H.
Restrictive junctional complex and specialized transporter proteins
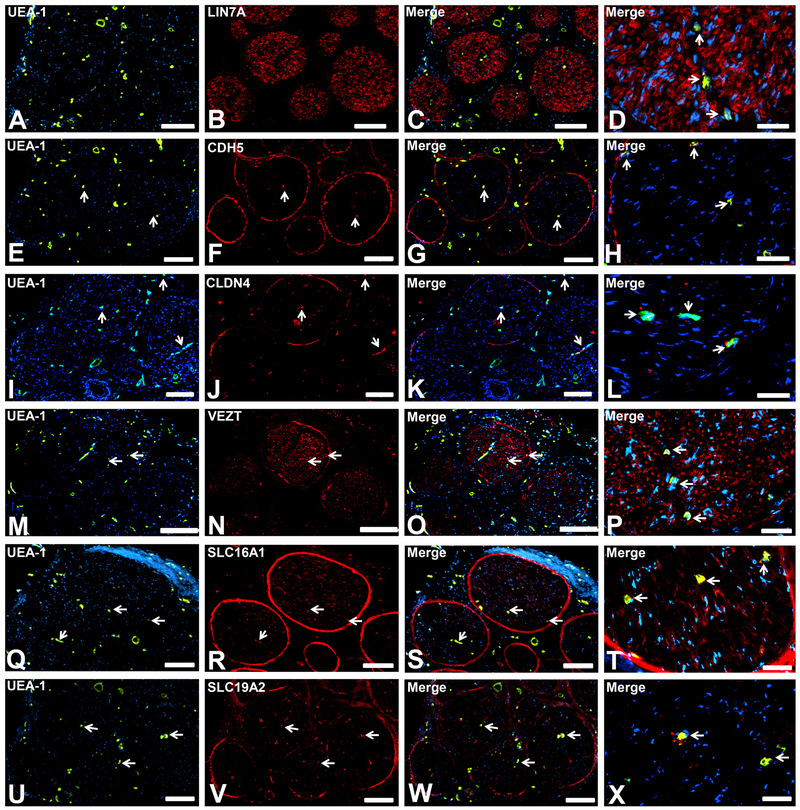
LIN7A (a major determinant of cell polarity and asymmetric membrane channel and receptor distribution, Fig. 2A-D) was expressed by endoneurial endothelium and large diameter axons, implying an important restrictive structural role at the BNB that is also biologically relevant in axons. CDH5 (adherens junction protein, Fig. 2E-H) and CLDN4 (tight junction protein, Fig. 2I-L) were expressed by endoneurial endothelium and the perineurium only, implying important roles in forming specialized restrictive intercellular junctional complexes. VEZT (adherens junction transmembrane protein, Fig. 2M-P) demonstrated expression by the BNB, perineurium and axons. This suggests a role in the restrictive intercellular junctional complex, as well as the structural integrity of axons in peripheral nerves. SLC16A1 (monocarboxylate transporter-1, a known lactate transporter, Fig. 2Q-T) and SLC19A2 (thiamine [vitamin B1] transporter, Fig. 2U-X) were both expressed by endoneurial endothelium and the perineurium, as well as Schwann cells (SLC16A1) or leukocytes (SLC19A2), implying specialized BNB transporter functions shared with other cell types. Alternatively, these cells may utilize lactate, thiamine or both as essential nutrients required for normal biological functions.
Figure 2. Restrictive junctional complex and transporter proteins.
Representative indirect fluorescent digital photomicrographs of cryostat axial sections of normal adult sural nerves show UEA-1-positive endothelial cells (green; A, E, I, M, Q, U) with expression of LIN7A, CDH5, CLDN4, VEZT, SLC16A1 and SLC19A2 (red; B, F, J, N, R, V respectively) restricted to endoneurial microvessels shown in the merged images at lower and higher magnifications (yellow/ orange). LIN7A expression by endoneurial microvessels is apparent only at higher magnification (D). These proteins are expressed by the restrictive BNB and shared with other selected cell types suggesting specialized roles in the restrictive junctional complex formation (LIN7A, CDH5, CLDN4 and VEZT) or specialized nutrient transporters (SLC16A1 and SLC19A2) within normal adult peripheral nerves, as determined by known morphological profiles in situ. White arrows demonstrate positively staining endoneurial microvascular endothelium. Blue (DAPI) staining indicates nuclei. Scale bar 500 μm for A-C, E-G, I-K, M-O, Q-S and U-W, and 125 μm for D, H, L, P, T and X.
Vascular-specific endothelial cell proteins
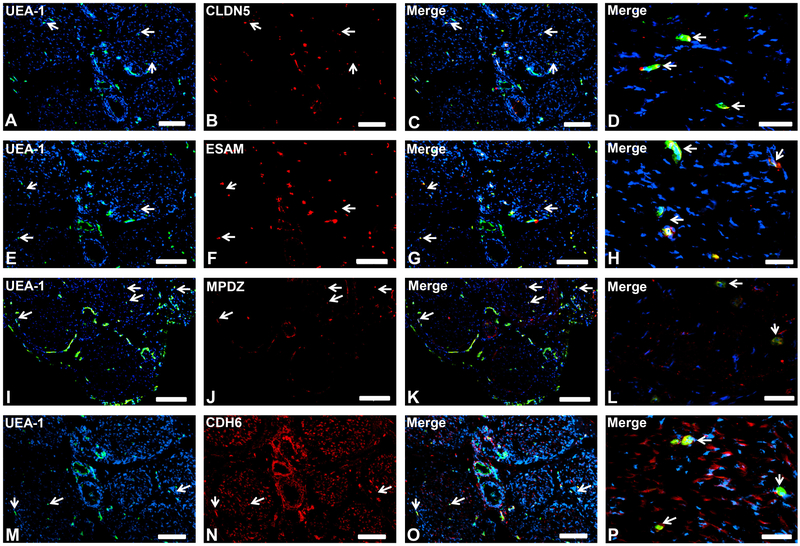
CLDN5, ESAM, MPDZ and CDH6 were expressed by both BNB-forming endoneurial microvessel and fenestrated epineurial macrovessel endothelium (Fig. 3), implying that these proteins are vascular-specific endothelial cell markers that do not confer tight-junction restrictive barrier properties in normal human adult nerves, but may serve as important membrane structural components. CDH6, a member of the cadherin superfamily that serves as a major component of adherens junctions, was also expressed on large diameter axons (Fig. 3I-L), suggesting a structural role in axonal integrity.
Figure 3. Endothelial cell-specific proteins.
Representative indirect fluorescent digital photomicrographs of cryostat axial sections of normal adult sural nerves show UEA-1-positive endothelial cells (green; A, E, I, M) with expression of CLDN5, ESAM, MPDZ and CDH6 (red; B, F, J, N respectively) restricted to endoneurial microvessels and fenestrated epineurial macrovessels shown in the merged images at lower and higher magnifications (yellow/ orange). CDH6 is also expressed by large diameter axons (M-P). Expression of these proteins by both endoneurial microvascular and epineurial macrovascular endothelium suggests endothelial-specific, non-restrictive barrier functions at the normal adult human BNB. White arrows demonstrate positively staining endoneurial microvessels. Blue (DAPI) staining indicates nuclei. Scale bar 500 μm for A-C, E-G, I-K, M-O, and 125 μm for D, H, L and P.
Specialized vascular endothelial and epithelial cell proteins
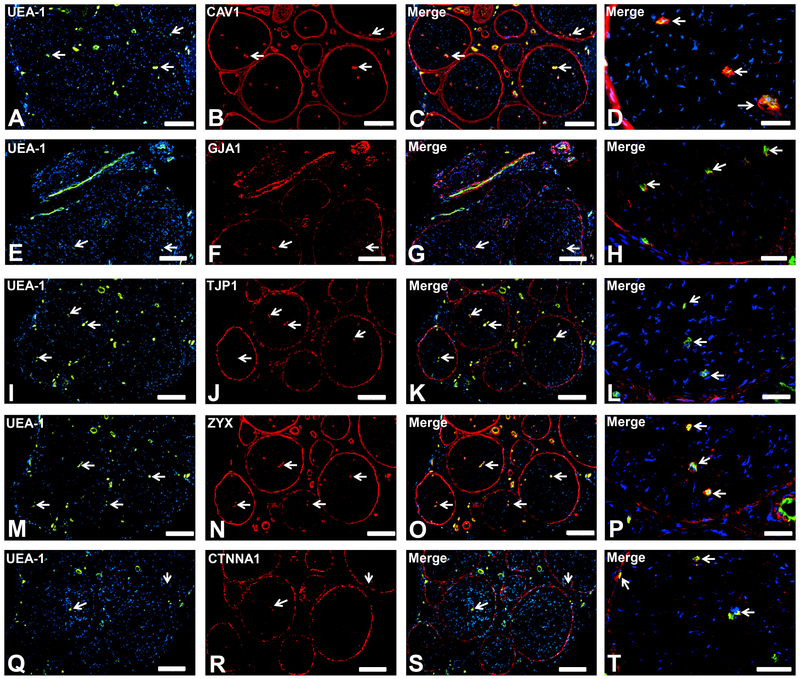
CAV1, GJA1, TJP1, ZYX and CTNNA1 were expressed by endoneurial microvessels, epineurial macrovessels and the perineurium (Fig. 4), implying that these proteins can be considered as vascular endothelial and specialized epithelial cell markers in peripheral nerves. CAV1 and GJA1 could serve as mediators of caveolae-dependent transcytosis and intercellular molecular transport respectively while TJP1, ZYX and CTNNA1 serve as important membrane-associated adapter proteins needed for the structural integrity of the intercellular junctional complex in restrictive (BNB and perineurium) and nonrestrictive (endoneurial macrovessels) biological interfaces in normal adult peripheral nerves.
Figure 4. Endothelial and specialized epithelial-specific proteins.
Representative indirect fluorescent digital photomicrographs of cryostat axial sections of normal adult sural nerves show UEA-1-positive endothelial cells (green; A, E, I, M, Q) with expression of CAV1, GJA1, TJP1, ZYX and CTNNA1 (red; B, F, J, N, R respectively) restricted to endoneurial microvessels, fenestrated epineurial macrovessels and the perineurium, shown in the merged images at lower and higher magnifications (yellow/ orange for endothelial cells). Expression of these proteins by both microvascular and macrovascular endothelial cells and the perineurium suggests non-restrictive barrier, but specialized endothelial and epithelial cell functions in the normal adult human peripheral nerves. White arrows demonstrate positively staining endoneurial microvessels. Blue (DAPI) staining indicates nuclei. Scale bar 500 μm for A-C, E-G, I-K, M-O, and Q-S and 125 μm for D, H, L, P and T.
Membrane and cytoskeletal proteins
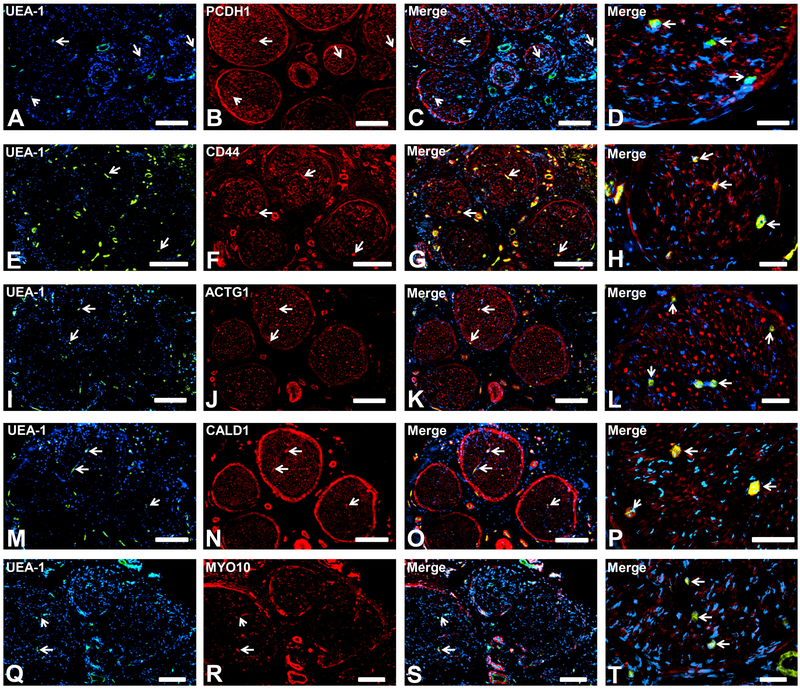
PCDH1 (protocadherin-1, a member of the subfamily of cadherin-associated molecules with high expression in neural tissues) 37 was expressed by the BNB, as well as epineurial macrovessels, perineurium and Schwann cells (Fig. 5A-D), implying an important role in maintaining membrane integrity in peripheral nerves. CD44 (Fig. 5E-H), ACTG1 (Fig. 5I-L), CALD1 (Fig. 5M-P) and MYO10 (Fig. 5Q-T) were expressed by the BNB, epineurial macrovessels, perineurium and axons, implying general roles in maintaining membrane and cytoskeletal integrity in peripheral nerves. The pattern of MYO10 expression also suggests a structural role in clusters of unmyelinated axons, also known as Remak bundles, while the other proteins were detected in large diameter myelinated axons.
Figure 5. Peripheral nerve cell-specific structural proteins.
Representative indirect fluorescent digital photomicrographs of cryostat axial sections of normal adult sural nerves show UEA-1-positive endothelial cells (green; A, E, I, M, Q) with expression of PCDH1, CD44, ACTG1, CALD1 and MYO10 (red; B, F, J, N, R respectively) on endoneurial microvessels, fenestrated epineurial macrovessels, perineurium and Schwann cells (PCDH1) and axons (CD44, ACTG1, CALD1 and MYO10), shown in the merged images at lower and higher magnifications (yellow/ orange for endothelial cells). Expression of these proteins by these cell types implies roles in maintaining the structural integrity of peripheral nerve specific cells in normal adult nerves. White arrows demonstrate positively staining endoneurial microvessels. Blue (DAPI) staining indicates nuclei. Scale bar 500 μm for A-C, E-G, I-K, M-O, and Q-S and 125 μm for D, H, L, P and T.
Non-specific cellular function proteins
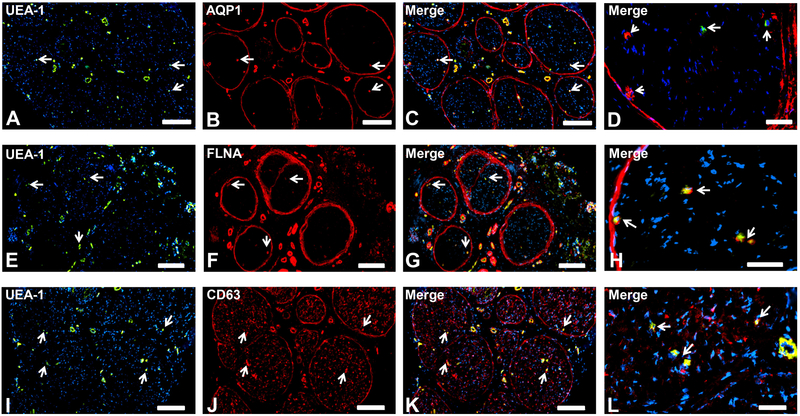
AQP1, FLNA and CD63 (Fig. 6) were expressed by the BNB, epineurial macrovessels, perineurium and pericytes, implying general roles in normal cellular function within adult peripheral nerves, with CD63 also expressed by Schwann cells.
Figure 6. Generalized cellular proteins.
Representative indirect fluorescent digital photomicrographs of cryostat axial sections of normal adult sural nerves show UEA-1-positive endothelium (green; A,E, I) with proteins AQP1, FLNA and CD63 (red; B, F, J respectively) demonstrating expression by micro- and macrovascular endothelial cells, the perineurium and pericytes on merged images at lower and higher magnifications (yellow/orange for endothelial cells). AQP1-negative endoneurial microvessels with retained pericyte expression (white arrowheads; D) are seen in a single normal adult sural nerve (Sural Nerve 2, see Table 2). CD63 is also expressed by Schwann cells (I-L). White arrows demonstrate positively staining endoneurial microvessels. Blue (DAPI) staining indicates nuclei. Scale bar 500 μm for A-C, E-G and I-K, and 125 μm for D, H and L.
Discussion
This study provides an attempt to elucidate the essential molecular components of the normal human adult BNB in situ, guided by prior transcriptome data, with particular focus on the intercellular junctional complex that confers specialized restrictive barrier properties. We determined that ABCB1 and SLC1A1 were the only proteins selectively expressed by endoneurial microvascular endothelium, implying that these transporters are essential components of the adult human BNB required for endoneurial homeostasis. The expression of LIN7A, CDH5, CLDN4 and VEZT by the BNB and perineurium suggest that these molecules are essential components of specialized restrictive intercellular junctional complexes in healthy adult peripheral nerves, while SLC16A1 and SLC19A2 are essential specialized nutrient transporters required for normal adult peripheral nerve function. Our data imply that CDH5 is not vascular endothelial-specific in human peripheral nerves, but is an important component of adherens junctions in specialized restrictive barriers. CLDN4 requires further evaluation as a specific component of restrictive tight junctions in human peripheral nerves based on this study.
CLDN5, a molecule commonly implicated as a critical molecular component of vascular endothelial tight junctions, 8,9,13,17,38 was also expressed by fenestrated epineurial macrovessels, as previously published in pathologic human sural nerve biopsies.18 This suggests that CLDN5, along with ESAM, MPDZ and CDH6, are molecular markers of vascular endothelial cell membranes in normal adult peripheral nerves and are not associated with the restrictive intercellular junctional barrier functions. This is in contrast to inferences made about human BNB function in vitro and in situ. 13,18,20 In support of our hypothesis that CLDN5 is not an essential component of the restrictive BNB, our recently published study evaluating the role of glial-derived neurotrophic factor (GDNF)-mediated restoration of murine sciatic nerve endoneurial microvessel large macromolecular (horseradish peroxidase) impermeability following non-transecting crush injury in normal, heterozygous and tamoxifen-inducible conditional GDNF knockout mice failed to demonstrate a relationship between CLDN5 expression and loss or restoration of BNB function. 25
In that study, CLDN5 expression was retained on all sciatic nerve endoneurial microvessels shortly after crush injury (similar to the Sham surgery control nerves) at a time when vessels were horseradish peroxidase-permeable. CLDN5 expression was unchanged during BNB recovery (complete by 14 days post-injury) in mice with normal upregulated GDNF expression (wildtype and heterozygous mice), with no differences seen in the age-and sex-matched conditional GDNF knockout mice with abrogated GDNF expression and delayed BNB recovery. 25 These observations were also consistent with a previously published in vitro human BNB study that did not conclusively demonstrate upregulation of CLDN5 or tyrosine phosphorylation of CLDN5 associated with GDNF-mediated restoration of restrictive barrier properties following injury. 7
TJP1 is also commonly cited as an essential adapter molecule associated with vascular endothelial tight junctions. 8,9,23,38 Our data demonstrated expression by fenestrated epineurial macrovascular endothelial cells and the perineurium, in addition to endoneurial microvascular endothelial cells. This suggests that this molecule, as well as CAV1, GJA1, ZYX and CTNNA1 are essential structural components of vascular endothelial and specialized epithelial cell membranes required for normal adult peripheral nerve function, and do not directly confer restrictive barrier properties. PCDH1, a member of the protocadherin family of proteins that interact with cadherins, 37 was also expressed on these vascular endothelial and perineurial cells, as well as Schwann cells (which also form autotypic junctions), 22 suggesting expression in peripheral nerves at restrictive and nonrestrictive intercellular membranes.
Our previous in vitro human BNB study demonstrated low hydraulic conductivity consistent with the notion that the BNB tightly regulates water flux in and out of the endoneurium in order to strictly maintain ionic concentrations essential for physiologic action potential conduction. 39 Based on this study, AQP1 may serve as a generalized channel for water transport in multiple peripheral nerve cell types required for normal metabolism and physiology, rather than a regulator of water influx or efflux across the BNB and perineurium.
This study provides essential new information on the molecular composition of human endoneurial vascular endothelium with some insight into specific transporters and junctional complex proteins that potentially contribute to forming the restrictive BNB, guided by data derived from the human BNB transcriptome. It is important to recognize that most of the proteins studied are expressed outside of the BNB in peripheral nerves, and that “classic” markers of vascular endothelial tight and adherens junctions, CLDN5 and CDH5, were not restricted to human adult endoneurial microvascular endothelium in situ, emphasizing the need for further study to elucidate the specific molecular components of the uniquely restrictive human adult BNB intercellular junctional complex and its specialized transporters. Guided by the extensive transcript data present in the human BNB transcriptome,24 comparative in situ studies with the human blood-brain barrier are feasible so as to ascertain unique human BNB characteristics.
An in situ molecular characterization study using immunohistochemistry is limited by tissue quality and its preservation, method of fixation, relative antigen expression and antibody specificity. Uniform molecular expression in ≥ 90% of detected endoneurial microvessels in 4 histologically normal adult sural nerve biopsy specimens provides a high degree of confidence on the anatomical localization of the chosen proteins at the BNB. Molecular expression of specific proteins by the perineurium, epineurial macrovessels, axons, Schwann cells, pericytes and leukocytes was ascertained based on the typical anatomical localization and appearance of these microstructures or cells in normal peripheral nerves, rather than specific co-localization studies as the purpose of the study was primarily to determine the molecular composition of the normal adult human BNB in situ. Further verification would be needed before performing in vitro or in vivo functional studies in animal models, taking into account interspecies vascular endothelial cell heterogeneity. 40-45
This study does not determine the precise subcellular protein localization in human endoneurial microvascular endothelial cells. Immunogold electron microscopy and super resolution microscopy 46-49 provide avenues to more precisely determine the in situ subcellular localization of specific molecules restricted to the human BNB, particularly components of the restrictive intercellular junctional complex such as CLDN4, or determine the luminal or abluminal localization of transporters such as SLC1A1 prior to functional studies. This is an important future direction. Due to junctional dysregulation in cultured endothelial cells and the molecular and phenotypic heterogeneity between microvascular endothelial cells from different tissues and species, 8,24,40-45 careful selection of highly conserved intercellular junctional complex components and transporters is needed to determine the signaling mechanisms implicated in human BNB formation during development, maintenance of function in normal healthy physiologic states, and ascertain the alterations and adaptations that occur in disease states such as peripheral neuropathy of different etiologies, as well as traumatic peripheral nerve injury.
Acknowledgements
Special thanks for the technical staff of the Shin J Oh Muscle and Nerve Histopathology Laboratory, University of Alabama at Birmingham for the initial processing and cryopreservation of the human sural nerve biopsies.
Funding Statement
Research in the Neuromuscular Immunopathology Research Laboratory was supported by the National Institutes of Health (NIH) under Grant R01 NS075212 [2012–2017] and institutional funds from the University of Alabama at Birmingham. The funding sources had no involvement in the conduct of the research, manuscript preparation, data collection/ analyses or decision to submit this work for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest
E.E.U. has a non-exclusive commercial license (held by Baylor Licensing Group) for simian virus-40 large T-antigen immortalized human endoneurial endothelial cells and has received royalties from Springer Science + Business Media for an edited book on laboratory protocols that describes a flow-dependent in vitro human BNB leukocyte trafficking assay. X.O. and C.D. declare that they have no competing interests.
References
- 1.Olsson Y Microenvironment of the peripheral nervous system under normal and pathological conditions. Crit Rev Neurobiol. 1990;5(3):265–311. [PubMed] [Google Scholar]
- 2.Mizisin AP, Weerasuriya A. Homeostatic regulation of the endoneurial microenvironment during development, aging and in response to trauma, disease and toxic insult. Acta neuropathologica. 2011;121(3):291–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reina MA, Lopez A, Villanueva MC, de Andres JA, Leon GI. [Morphology of peripheral nerves, their sheaths, and their vascularization]. Rev Esp Anestesiol Reanim. 2000;47(10):464–475. [PubMed] [Google Scholar]
- 4.Reina MA, Lopez A, Villanueva MC, De Andres JA, Maches F. [The blood-nerve barrier in peripheral nerves]. Rev Esp Anestesiol Reanim. 2003;50(2):80–86. [PubMed] [Google Scholar]
- 5.Dong C, Palladino SP, Helton ES, Ubogu EE. The pathogenic relevance of alphaM-integrin in Guillain-Barre syndrome. Acta neuropathologica. 2016;132(5):739–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pummi KP, Heape AM, Grenman RA, Peltonen JT, Peltonen SA. Tight junction proteins ZO-1, occludin, and claudins in developing and adult human perineurium. J Histochem Cytochem. 2004;52(8):1037–1046. [DOI] [PubMed] [Google Scholar]
- 7.Yosef N, Ubogu EE. GDNF restores human blood-nerve barrier function via RET tyrosine kinase-mediated cytoskeletal reorganization. Microvasc Res. 2012;83(3):298–310. [DOI] [PubMed] [Google Scholar]
- 8.Yosef N, Xia RH, Ubogu EE. Development and characterization of a novel human in vitro blood-nerve barrier model using primary endoneurial endothelial cells. Journal of neuropathology and experimental neurology. 2010;69(1):82–97. [DOI] [PubMed] [Google Scholar]
- 9.Abe M, Sano Y, Maeda T, et al. Establishment and characterization of human peripheral nerve microvascular endothelial cell lines: a new in vitro blood-nerve barrier (BNB) model. Cell Struct Funct. 2012;37(2):89–100. [DOI] [PubMed] [Google Scholar]
- 10.Orte C, Lawrenson JG, Finn TM, Reid AR, Allt G. A comparison of blood-brain barrier and blood-nerve barrier endothelial cell markers. Anat Embryol (Berl). 1999;199(6):509–517. [DOI] [PubMed] [Google Scholar]
- 11.Sano Y, Shimizu F, Nakayama H, et al. Endothelial cells constituting blood-nerve barrier have highly specialized characteristics as barrier-forming cells. Cell Struct Funct. 2007;32(2):139–147. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu F, Sano Y, Abe MA, et al. Peripheral nerve pericytes modify the blood-nerve barrier function and tight junctional molecules through the secretion of various soluble factors. J Cell Physiol. 2011;226(1):255–266. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu F, Sano Y, Saito K, et al. Pericyte-derived glial cell line-derived neurotrophic factor increase the expression of claudin-5 in the blood-brain barrier and the blood-nerve barrier. Neurochem Res. 2012;37(2):401–409. [DOI] [PubMed] [Google Scholar]
- 14.Ubogu EE. The molecular and biophysical characterization of the human blood-nerve barrier: current concepts. J Vasc Res. 2013;50(4):289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannini C, Dyck PJ. Ultrastructural morphometric abnormalities of sural nerve endoneurial microvessels in diabetes mellitus. Annals of neurology. 1994;36(3):408–415. [DOI] [PubMed] [Google Scholar]
- 16.Hadden RD, Gregson NA, Gold R, Smith KJ, Hughes RA. Accumulation of immunoglobulin across the ‘blood-nerve barrier’ in spinal roots in adoptive transfer experimental autoimmune neuritis. Neuropathology and applied neurobiology. 2002;28(6):489–497. [DOI] [PubMed] [Google Scholar]
- 17.Hirakawa H, Okajima S, Nagaoka T, Takamatsu T, Oyamada M. Loss and recovery of the blood-nerve barrier in the rat sciatic nerve after crush injury are associated with expression of intercellular junctional proteins. Exp Cell Res. 2003;284(2):196–210. [DOI] [PubMed] [Google Scholar]
- 18.Kanda T, Numata Y, Mizusawa H. Chronic inflammatory demyelinating polyneuropathy: decreased claudin-5 and relocated ZO-1. Journal of neurology, neurosurgery, and psychiatry. 2004;75(5):765–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omura K, Ohbayashi M, Sano M, Omura T, Hasegawa T, Nagano A. The recovery of blood-nerve barrier in crush nerve injury--a quantitative analysis utilizing immunohistochemistry. Brain Res. 2004;1001(1–2):13–21. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu F, Sawai S, Sano Y, et al. Severity and patterns of blood-nerve barrier breakdown in patients with chronic inflammatory demyelinating polyradiculoneuropathy: correlations with clinical subtypes. PloS one. 2014;9(8):e104205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanda T Biology of the blood-nerve barrier and its alteration in immune mediated neuropathies. Journal of neurology, neurosurgery, and psychiatry. 2013;84(2):208–212. [DOI] [PubMed] [Google Scholar]
- 22.Peltonen S, Alanne M, Peltonen J. Barriers of the peripheral nerve. Tissue Barriers. 2013;1(3):e24956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochimica et biophysica acta. 2008;1778(3):660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palladino SP, Helton ES, Jain P, et al. The Human Blood-Nerve Barrier Transcriptome. Sci Rep. 2017;7(1):17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong C, Helton ES, Zhou P, et al. Glial-derived neurotrophic factor is essential for blood-nerve barrier functional recovery in an experimental murine model of traumatic peripheral neuropathy. Tissue Barriers. 2018;6(2):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17(9):564–580. [DOI] [PubMed] [Google Scholar]
- 27.Sluysmans S, Vasileva E, Spadaro D, Shah J, Rouaud F, Citi S. The role of apical cell-cell junctions and associated cytoskeleton in mechanotransduction. Biol Cell. 2017;109(4):139–161. [DOI] [PubMed] [Google Scholar]
- 28.Holthofer H, Virtanen I, Kariniemi AL, Hormia M, Linder E, Miettinen A. Ulex europaeus I lectin as a marker for vascular endothelium in human tissues. Laboratory investigation; a journal of technical methods and pathology. 1982;47(1):60–66. [PubMed] [Google Scholar]
- 29.Mojsilovic-Petrovic J, Nesic M, Pen A, Zhang W, Stanimirovic D. Development of rapid staining protocols for laser-capture microdissection of brain vessels from human and rat coupled to gene expression analyses. J Neurosci Methods. 2004;133(1–2):39–48. [DOI] [PubMed] [Google Scholar]
- 30.Ubogu EE. Inflammatory neuropathies: pathology, molecular markers and targets for specific therapeutic intervention. Acta neuropathologica. 2015;130(4):445–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ubogu EE, Yosef N, Xia RH, Sheikh KA. Behavioral, electrophysiological, and histopathological characterization of a severe murine chronic demyelinating polyneuritis model. Journal of the peripheral nervous system : JPNS. 2012;17(1):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia RH, Yosef N, Ubogu EE. Clinical, electrophysiological and pathologic correlations in a severe murine experimental autoimmune neuritis model of Guillain-Barre syndrome. Journal of neuroimmunology. 2010;219(1–2):54–63. [DOI] [PubMed] [Google Scholar]
- 33.Xia RH, Yosef N, Ubogu EE. Selective expression and cellular localization of pro-inflammatory chemokine ligand/receptor pairs in the sciatic nerves of a severe murine experimental autoimmune neuritis model of Guillain-Barre syndrome. Neuropathology and applied neurobiology. 2010;36(5):388–398. [DOI] [PubMed] [Google Scholar]
- 34.Yuan F, Yosef N, Lakshmana Reddy C, et al. CCR2 gene deletion and pharmacologic blockade ameliorate a severe murine experimental autoimmune neuritis model of Guillain-Barre syndrome. PloS one. 2014;9(3):e90463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahringer A, Fricker G. ABC transporters at the blood-brain barrier. Expert Opin Drug Metab Toxicol. 2016;12(5):499–508. [DOI] [PubMed] [Google Scholar]
- 36.Nalecz KA. Solute Carriers in the Blood-Brain Barier: Safety in Abundance. Neurochem Res. 2017;42(3):795–809. [DOI] [PubMed] [Google Scholar]
- 37.Yagi T Clustered protocadherin family. Dev Growth Differ. 2008;50 Suppl 1:S131–140. [DOI] [PubMed] [Google Scholar]
- 38.Reinhold AK, Rittner HL. Barrier function in the peripheral and central nervous system-a review. Pflugers Arch. 2017;469(1):123–134. [DOI] [PubMed] [Google Scholar]
- 39.Helton ES, Palladino S, Ubogu EE. A novel method for measuring hydraulic conductivity at the human blood-nerve barrier in vitro. Microvasc Res. 2017;109:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circulation research. 2007;100(2):158–173. [DOI] [PubMed] [Google Scholar]
- 41.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circulation research. 2007;100(2):174–190. [DOI] [PubMed] [Google Scholar]
- 42.Murphy HS, Bakopoulos N, Dame MK, Varani J, Ward PA. Heterogeneity of vascular endothelial cells: differences in susceptibility to neutrophil-mediated injury. Microvasc Res. 1998;56(3):203–211. [DOI] [PubMed] [Google Scholar]
- 43.Nolan DJ, Ginsberg M, Israely E, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26(2):204–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Potente M, Makinen T. Vascular heterogeneity and specialization in development and disease. Nat Rev Mol Cell Biol. 2017. [DOI] [PubMed] [Google Scholar]
- 45.Yano K, Gale D, Massberg S, et al. Phenotypic heterogeneity is an evolutionarily conserved feature of the endothelium. Blood. 2007;109(2):613–615. [DOI] [PubMed] [Google Scholar]
- 46.Zobel K, Hansen U, Galla HJ. Blood-brain barrier properties in vitro depend on composition and assembly of endogenous extracellular matrices. Cell Tissue Res. 2016;365(2):233–245. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe S, Jorgensen EM. Visualizing proteins in electron micrographs at nanometer resolution. Methods Cell Biol. 2012;111:283–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bartle EI, Rao TC, Urner TM, Mattheyses AL. Bridging the gap: Super-resolution microscopy of epithelial cell junctions. Tissue Barriers. 2018;6(1):e1404189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sigal YM, Zhou R, Zhuang X. Visualizing and discovering cellular structures with super-resolution microscopy. Science. 2018;361(6405):880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]