Abstract
Background
Childhood antibiotic exposure has important clinically relevant implications. These include disruption to the microbiome, antibiotic resistance, and clinical workload manifesting as treatment ‘failure’.
Aim
To examine the relationship between the number of antibiotic courses prescribed to preschool children for acute respiratory tract infections (RTI), in the preceding year, and subsequent RTIs that failed to respond to antibiotic treatment (‘response failures’).
Design and setting
A cohort study using UK primary care data from the Clinical Practice Research Datalink, 2009 to 2016.
Method
Children aged 12 to 60 months (1 to 5 years) who were prescribed an antibiotic for an acute RTI (upper and lower RTI or otitis media) were included. One random index antibiotic course for RTI per child was selected. Exposure was the number of antibiotic prescriptions for acute RTI up to 12 months before the index antibiotic prescription. The outcome was ‘response failure’ up to 14 days after index antibiotic prescription, defined as: subsequent antibiotic prescription; referral; hospital admission; death; or emergency department attendance within 3 days. The authors used logistic regression models to estimate the odds between antibiotic exposure and response failure.
Results
Out of 114 329 children who were prescribed an antibiotic course for acute RTI, children who received ≥2 antibiotic courses for acute RTIs in the preceding year had greater odds of response failure; one antibiotic course: adjusted odds ratio (OR) 1.03 (95% confidence interval [CI] = 0.88 to 1.21), P = 0.67, n = 230 children; ≥2 antibiotic courses: adjusted OR 1.32 (CI = 1.04 to 1.66), P = 0.02, n = 97.
Conclusion
Childhood antibiotic exposure for acute RTI may be a good predictor for subsequent response failure (but not necessarily because of antibiotic treatment failure). Further research is needed to improve understanding of the mechanisms underlying response failure.
Keywords: antibiotic exposure, children, primary care, treatment failure
INTRODUCTION
Many antibiotic courses that do not benefit children are being prescribed for self-limiting acute respiratory tract infections (RTIs) in the community.1 At least 30% of antibiotics prescribed in outpatient settings in the US, and between 9% and 23% in UK primary care, are unnecessary.2,3 Children <5 years of age are prescribed proportionally more antibiotics for acute RTIs than any other age group.4–7 Antibiotic prescribing varies by country from around one in six children receiving at least one oral antibiotic prescription per year in the Netherlands,4 to over 1 in 2 of all RTIs in children in the US, where this represents about 11.4 million potentially preventable antibiotic prescriptions annually.5
Many children, though, will have had multiple antibiotic courses within their early childhood.8 Such prescribing medicalises self-limiting RTIs in children and goes against recommendations of antibiotic prescribing guidance.9,10 However, in ambulatory care unnecessary exposure to antibiotics is likely to have important clinically relevant implications such as treatment ‘response failure’, mostly in the form of re-consultation and receipt of another antibiotic course.11–13 There is also robust evidence for an association between antibiotic use and resistant bacterial carriage.14,15 Antibiotic use may also disrupt the protective gut and lung microbiomes, and potentially predispose young children to increased susceptibility to certain bacterial and opportunistic pathogens with adverse health outcomes such as higher rates of RTI,16 and subsequent RTIs that may be less responsive to antibiotic treatment.17,18
In this study the authors examined the relationship between the number of antibiotic courses prescribed to preschool children for acute RTIs in the preceding year, and subsequent RTIs that ‘fail’ to adequately respond to antibiotic treatment (response failures).
METHOD
Study design
This observational cohort study used routinely collected primary care data from the Clinical Practice Research Datalink (CPRD) in the UK. The CPRD is a national database of electronic medical records that contains anonymised longitudinal data from over 670 GP clinics across the UK.19 The database includes records of all prescriptions issued, clinical diagnoses, referrals to secondary care, hospitalisations, and investigation results during consultations in primary care. Prescriptions are computer generated and are automatically incorporated into the patient medical record. The data have been extensively validated for pharmaco-epidemiological, clinical, and health service usage research.20–22
How this fits in
| Theoretical predictions about the potential consequences of unnecessary antibiotic use and antibiotic resistance can seem abstract and remote to individuals with common infections in the community. Likewise, primary care clinicians report that they rarely encounter treatment failure because of their prescribing decisions. Yet the authors are aware that unnecessary antibiotic use and resistant infections have worse implications for patients’ illness burden in the community, even for common infections. A subset of the population that is at particular risk of receiving antibiotics unnecessarily is preschool children. The present findings suggest that when children receive more antibiotics for acute respiratory tract infections (RTIs) their likelihood of re-consulting a health professional is affected and increases clinical workload. Children receiving ≥2 antibiotics in the preceding year were most likely to be affected. Incorporating antibiotic exposure data into clinical decision-support systems might prompt clinicians to implement strategies to support a non-antibiotic strategy, for example, informing parents about the anticipated recovery period of common RTIs in children. |
Study population
The authors included children aged 12 to 60 months (1 to 5 years) inclusive, who were prescribed an antibiotic for an acute RTI (upper and lower RTI or otitis media) from January 2009 to September 2016. For the purposes of counting the number of antibiotic courses prescribed for acute RTIs in the preceding year before the start of the study period, data were included from January 2008 to the end of the study date.
Study definitions
Acute RTIs were defined by ≥1 clinical diagnostic codes (Read codes) relating to acute upper RTIs, for example, rhinosinusitis; lower RTIs, for example, pneumonia and bronchitis; and acute otitis media. Antibiotic prescription included oral therapy with primarily antibacterial activity listed in the British National Formulary for Children (BNFC; Chapter 5.1) licensed for acute RTIs in young children and defined by means of CPRD product codes. There was no minimum antibiotic duration or dose to qualify for inclusion. Drugs with primarily antiviral or antifungal activity and drugs for non-acute RTI, for example, tuberculosis, were excluded. Children who were prescribed an antibiotic at an index consultation that did not have an associated Read code or had an irrelevant (not RTI-related) Read code were excluded from the analysis. Children were also excluded where there was an inadequate follow-up period. All children had to be registered in the CPRD database for at least 14 days after index consultation to allow the outcome to occur and be recorded. Highly specific patient groups in whom specialised long-term antibiotic regimens are recommended for chronic respiratory diseases, for example, tuberculosis, were excluded (code lists for exclusion criteria are available from the authors on request).
Antibiotic exposure
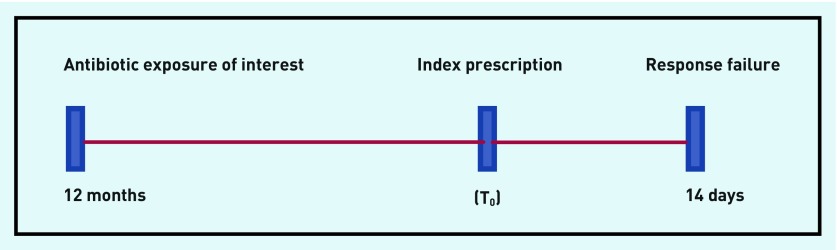
Antibiotic exposure was measured as the number of antibiotic prescriptions for ‘acute RTI’ up to 12 months before the index antibiotic prescription (T0), (Figure 1).
Figure 1.
Antibiotic exposure as the number of antibiotic prescriptions for acute respiratory tract infections (RTIs) up to 12 months before the index prescription (T0).
Antibiotic prescriptions for acute RTI episodes were selected at random. The selection of the random sample of episodes within children was derived using the Stata set seed randomisation command (www.stata.com/manuals13/rsetseed). The index prescription (T0) was defined as the first antibiotic prescription for an acute RTI during the study period to account for multiple individual RTI episodes presenting in each child.
All antibiotic prescriptions associated with acute RTIs up to 12 months before the index prescription were counted.
Covariates for adjustment were as follows (further details of covariates are available from the authors on request):
age (years), at the time of index antibiotic prescription;
sex;
time of year (according to seasons: December to February = winter; March to May = spring; June to August = summer; September to November = autumn);
RTI type (upper, lower, and otitis media);
class of antibiotic (according to the BNFC classification [Table 1]);
presence of ≥1 comorbidities (further details available from the author on request);
childhood vaccination status: diphtheria, tetanus, pertussis, polio and Hib (DTaP/IPV/Hib), and pneumococcal conjugate (PCV);
social deprivation quintiles: based on patient-linked deprivation index score (IMD)23
number of previous response failure episodes during the 12-month period before the index prescription; and
number of consultations for acute RTIs during the 12 months before the index prescription (baseline consultation rate).
Table 1.
Demographic and clinical characteristics of preschool children prescribed antibiotics for an acute RTI in the CPRD searched from 2 January 2009 to 23 September 2016, N = 114 329
| Characteristic | n (%)a |
|---|---|
| Median age, years (IQR) | 2.39 (1.39–3.66) |
|
| |
| Sex, female | 54 379 (47.56) |
|
| |
| Seasonb | |
| Winter | 41 830 (36.59) |
| Spring | 30 110 (26.34) |
| Summer | 15 108 (13.21) |
| Autumn | 27 281 (23.86) |
|
| |
| RTI typec | |
| URTI | 16 048 (14.04) |
| LRTI | 68 634 (60.03) |
| AOM | 28 604 (25.02) |
| URTI and LRTI | 662 (0.58) |
| Other mix | 381 (0.33) |
|
| |
| Comorbidity | 6625 (5.79) |
|
| |
| Antibiotic class | |
| Cephalosporin | 847 (0.74) |
| Macrolides | 11 571 (10.12) |
| Broad-spectrum penicillin (amoxicillin, co-amoxiclav) | 87 723 (76.73) |
| Penicillinase-resistant (flucloxacillin) | 275 (0.24) |
| Penicillin V (phenoxymethylpenicillin) | 13 673 (11.96) |
| Quinolones | 7 (0.01) |
| Trimethoprim-cotrimoxazole | 233 (0.20) |
|
| |
| Vaccination status at index date (complete) | 43 401 (37.96) |
|
| |
| Vaccination status at end of study period (complete)d | 106 803 (93.42) |
|
| |
| Social deprivation quintilese | |
| 1 (least deprived) | 25 125 (21.99) |
| 2 | 23 021 (20.14) |
| 3 | 20 935 (18.32) |
| 4 | 23 186 (20.28) |
| 5 (most deprived) | 21 994 (19.24) |
| Not linked to IMD | 68 (0.06) |
|
| |
| Previous response failuresf in last 12 months | |
| None | 111 281 (97.33) |
| 1 | 3011 (2.63) |
| 2 | 37 (0.03) |
|
| |
| Median previous RTI consultations in last 12 months (IQR) | 4 (2–7) |
|
| |
| Number of children with an RTI-associated antibiotic prescription during the 12 months before the index antibiotic prescription (T0) | |
| None | 95 383 (83.43) |
| 1 | 14 929 (13.06) |
| ≥2 | 4017 (3.51) |
Unless specified otherwise.
Seasons: winter (December to February); spring (March to May); summer (June to August); autumn (September to November).
URTI: acute sinusitis, sore throat, laryngitis, coughs, for example; LRTI: pneumonia, bronchitis, for example; URTI and LRTI: a small proportion of children had both LRTI and URTI Read codes on the same consultation day.
Complete set of specific vaccinations completed by age 5 years.
Index of Multiple Deprivation (IMD) quintile based on patient-linked IMD scores 2010.
Response failure was defined as the earliest occurrence of any of the following within 14 days of the index antibiotic prescription, unless specified otherwise: 1. Prescription of a subsequent antibiotic within 14 days of the initial antibiotic being prescribed to that child; 2. GP record of admission with an infection-related diagnostic code; 3. GP record of death with an infection-related diagnostic code; 4. GP record of referral to an infection-related specialist service; or 5. GP record of an emergency department visit within 3 days of antibiotic initiation. AOM = acute otitis media. CPRD = Clinical Practice Research Datalink. IQR = interquartile range. LRTI = lower respiratory tract infection. RTI = respiratory tract infection. URTI = upper respiratory tract infection.
Outcome
The authors conceptualised the term ‘response failure’ in recognition that, for many acute RTIs, failure may occur for reasons other than the antibiotic treatment itself. For the purposes of this analysis, ‘response failure’ was defined as the earliest occurrence of any of the following:
Prescription of a subsequent antibiotic within 14 days of the index antibiotic prescription (T0) being prescribed to that child;
GP record of hospital admission with an infection-related diagnostic code within 14 days of index antibiotic prescription (T0);
GP record of death with an infection-related diagnostic code within 14 days of index antibiotic prescription (T0);
GP record of referral to an infection-related specialist service within 14 days of index antibiotic prescription (T0); or
GP record of an emergency department visit within 3 days of index antibiotic prescription (T0). The shorter time window likely reflects that the emergency event was related to the illness episode, and acknowledges that carers of young children tend to consult within a shorter period of time if their child’s symptoms do not improve.
Referrals to a specialist service in secondary care were taken from CPRD’s Referral table. This table contains information about who the referral is made to and is linked to a Read code. The researchers matched referrals to secondary care by index consultation dates and selected acute specialties relevant to acute infections with an RTI diagnostic code. Referrals for blood tests and microbiology, such as throat or ear swabs, were excluded. Where it was not clear where the referral was being made to, referrals were based on probable acute RTI-related symptoms, for example, breathlessness. For each index antibiotic prescription (T0), response failure events were derived by searching forward 14 days from T0 for the first indication of response failure as per the definition thereof. For those children who fulfilled one of the criteria of response failure, any RTI-relevant Read code was sought immediately before or after the response failure event.
Statistical methods
Stata (version 13SE) was used to carry out all statistical analyses. Data were summarised as numbers and percentages for categorical variables and means, and standard deviations for continuous variables where appropriate. Two analyses were performed: a multivariable logistic regression analysis on a random index antibiotic course (prescription) for RTI per child (primary analysis) and multivariable logistic regression using a three-level mixed effects model (secondary analysis).
Primary analysis
The primary analysis included one random index antibiotic course (prescription) for RTI per child during the study period. To examine the association between the number of antibiotic courses for acute RTIs in the preceding 12 months and response failure, the researchers calculated unadjusted and adjusted odds ratios (ORs) with 95% confidence intervals (CIs) in relation to the study outcome using multivariable logistic regression.
Secondary analysis
To account for multiple individual RTI episodes presenting in each child nested within each practice, the researchers carried out multivariable logistic regression using a three-level mixed effects model to examine whether GP practice-level, child-level factors, and antibiotic prescriptions were associated with response failure adjusting for covariates.
Sensitivity analyses
Sensitivity analyses were performed, to assess the robustness of the primary analysis, which excluded data from:
index antibiotic prescribing episodes that were preceded by ≥1 antibiotic prescriptions within the last 14 days. These episodes may be response failures rather than index prescriptions based on the present study definition;
index antibiotic prescribing episodes that were preceded by ≥1 antibiotic prescriptions during the 8-week period before the index prescription. Individuals with self-limiting RTIs may continue to experience symptoms such as persistent cough for up to 8 weeks;24,25 and
children who were 12 months of age at the time of index prescription, since these children would only have data from the time the child was registered at the GP surgery and may not have 12 months’ data in the CPRD.
An additional sensitivity analysis was also performed whereby the response failure outcomes were only defined as criteria 2 to 5 of the outcome definition thereby minimising the behavioural aspects of parental consultation and antibiotic prescribing.
Subgroup analysis
Subgroup analysis was performed by RTI type (upper RTI versus lower RTIs versus otitis media).
RESULTS
The researchers found 252 572 preschool children who had a total of 1 546 364 acute RTI consultations (upper and lower RTIs, acute otitis media [AOM]) during the study period. The proportion of children prescribed an antibiotic course during one of these consultations for acute RTI was 45.27% (114 329/252 572). Children had an average of six consultations for RTI over the 92-month study period (0.8 RTI consultations per year).
Primary analysis
Baseline characteristics
A total of 114 329 children were prescribed an antibiotic course for an RTI during the study period. Baseline characteristics of this cohort are shown in Table 1. The median baseline consultation rate for RTI was 4 (interquartile range [IQR] 2–7) in the year previous to the index prescription. Asthma (n = 3134; 47.31%) was the most common comorbidity. One-third of children with a comorbidity had a history of premature birth (n = 2201; 33.22%). Most children had no response failures (n = 111 281; 97.33%) and <3% of children had experienced one or two previous response failures during the 12 months prior to the index RTI consultation.
Antibiotic exposure
A total of 18 946 (16.57%) children received at least one antibiotic prescription for acute RTI in the preceding year (Table 1). The distribution of antibiotic exposure in the year before index prescription was positively skewed with a range of 0 to 12, a median of 0, and only 3.51% being exposed to ≥2 antibiotic courses in the 12 months before index prescription.
Response failure
In the primary analysis, the authors observed 1377 response failures (1.20% of 114 329 children prescribed an antibiotic course for RTI), of which 724 (52.58%) were referrals to an infection-related specialist service in secondary care, 306 (22.22%) were subsequent antibiotic prescriptions, 243 (17.65%) were emergency department visits within 3 days, 103 (7.48%) were hospital admissions, and one death (Table 2).
Table 2.
Antibiotic exposure in the 12 months before index antibiotic prescription (T0) and response failure criteria
| Antibiotic exposure | Response failure, number of children (%) | |||||
|---|---|---|---|---|---|---|
| Referral | Second prescription | Emergency department visit | Hospital admission | Death | Total | |
| None | 494 (68.23) | 268 (87.58) | 209 (86.01) | 78 (75.73) | 1 (100) | 1050 (76.25) |
| 1 | 153 (21.13) | 32 (10.46) | 28 (11.52) | 17 (16.50) | 0 | 230 (16.70) |
| ≥2 antibiotic courses | 77 (10.64) | 6 (1.96) | 6 (2.47) | 8 (7.77) | 0 | 97 (7.04) |
| Total | 724a | 306b | 243c | 103d | 1e | 1377 |
Median days to referral (IQR) = 2 (0–7).
Median days to second prescription (IQR) = 10 (7–13).
Median days to emergency visit (IQR) = 2 (1–7).
Median days to hospital admission (IQR) = 2 (1–9).
Death occurred at day 5. IQR = interquartile range.
Around half of referrals were to paediatric and ear, nose, and throat specialties. Around one-third of referrals occurred on the same day as the index antibiotic prescription. Just under one-quarter of referrals receiving an antibiotic course (23.07%; 167/724) were based on symptoms related to probable acute RTI. The remaining referrals had a linked RTI Read code. The absolute risk of response failure was respectively: referral (0.63%); second prescription (0.27%); emergency department visit (0.21%); hospital admission (0.09%), and death (<0.01%).
Odds of response failure
Children who were prescribed ≥2 antibiotic courses for acute RTI in the preceding year had greater likelihood of response failure after adjusting for covariates; unadjusted odds ratio (OR) 2.22 (95% CI = 1.80 to 2.74), P<0.001; adjusted OR 1.32 (95% CI = 1.04 to 1.66), P = 0.02, n = 97 (Table 3).
Table 3.
Primary analysis: multivariable analysis of the association between RTI-associated antibiotic exposure in the previous year and subsequent response failure for acute RTI, N = 1377 response failures
| Variable | Adjusted odds ratio (95% CI) | P-value |
|---|---|---|
| Number of RTI antibiotic courses in previous year | ||
| None, n= 1050 | 1 (reference) | |
| 1 antibiotic, n= 230 | 1.03 (0.88 to1.21) | 0.67 |
| ≥2 antibiotics, n= 97 | 1.32 (1.04 to 1.66) | 0.02 |
|
| ||
| Age at index prescription (T0) (years) | 0.95 (0.90 to 0.99) | 0.018 |
|
| ||
| Sex | ||
| Male | 1 (reference) | |
| Female | 0.85 (0.76 to 0.95) | 0.003 |
|
| ||
| Seasona | ||
| Winter | 1 (reference) | |
| Spring | 1.09 (0.96 to 1.26) | 0.18 |
| Summer | 1.22 (1.03 to 1.43) | 0.02 |
| Autumn | 1.00 (0.87 to 1.16) | 0.99 |
|
| ||
| RTI typeb | ||
| URTI | 1 (reference) | |
| LRTI | 0.88 (0.75 to 1.04) | 0.13 |
| AOM | 1.05 (0.88 to 1.25) | 0.60 |
| URTI and LRTI | 2.37 (1.48 to 3.79) | <0.001 |
| Other mix | 0.84 (0.31 to 2.27) | 0.73 |
|
| ||
| Comorbidity | ||
| None | 1 (reference) | |
| Present | 1.20 (0.98 to 1.48) | 0.08 |
|
| ||
| Antibiotic drug class | ||
| Cephalosporin | 1 (reference) | |
| Macrolides | 0.77 (0.50 to 1.19) | 0.25 |
| Broad-spectrum penicillin (amoxicillin, co-amoxiclav) | 0.47 (0.30 to 0.71) | 0.001 |
| Penicillinase-resistant (flucloxacillin) | 0.46 (0.14 to 1.54) | 0.21 |
| Penicillin V (phenoxymethylpenicillin) | 0.60 (0.39 to 0.94) | 0.03 |
| Quinolones | 0 (empty) | |
| Trimethoprim-cotrimoxazole | 1.01 (0.41 to 2.53) | 0.98 |
|
| ||
| Number of response failures in previous year | ||
| None | 1 (reference) | |
| 1 | 2.08 (1.67 to 2.58) | <0.001 |
| 2 | 3.93 (1.18 to 13.12) | 0.03 |
|
| ||
| Number of acute RTI consultations in previous year | 1.03 (1.02 to 1.05) | <0.001 |
|
| ||
| Social deprivation | ||
| 1 (least deprived) | 1 (reference) | |
| 2 | 0.96 (0.81 to 1.13) | 0.61 |
| 3 | 1.08 (0.92 to 1.28) | 0.34 |
| 4 | 1.12 (0.95 to 1.32) | 0.17 |
| 5 (most deprived) | 0.99 (0.84 to 1.18) | 0.96 |
|
| ||
| Vaccination status at index prescription (T0) | ||
| Incomplete or none | 1 (reference) | |
| Complete | 1.41 (1.26 to 1.59) | <0.001 |
Seasons: winter (December to February); spring (March to May); summer (June to August); autumn (September to November).
URTI: acute sinusitis, sore throat, laryngitis, coughs, for example, LRTI: pneumonia, bronchitis, for example. URTI and LRTI: two separate RTI Read codes for antibiotic prescribing event on same day. AOM = acute otitis media. IQR = interquartile range. LRTI = lower respiratory tract infection. RTI = respiratory tract infection. URTI = upper respiratory tract infection.
Children who had one antibiotic course during the previous year also had greater odds of response failure, but this was no longer statistically significant after adjustment; unadjusted OR 1.41 (95% CI = 1.22 to 1.62), P <0.001; adjusted OR 1.03 (95% CI = 0.88 to 1.21), P = 0.67, n = 230.
Secondary analysis
Incorporating multiple RTI episodes in each child, there were 190 290 acute RTI episodes in 114 329 children from 380 GP practices during the study period. Baseline characteristics of this cohort are available from the authors on request.
Response failure
There were 3709 response failures included in the secondary analysis. The median number of response failures per practice was 8 (IQR 4–14) during the study period. Subsequent antibiotic prescriptions (1757, 47.37%) was the top criterion for response failure, followed by referrals to an infection-related specialist service in secondary care (1359, 36.64%); emergency department visits within 3 days (426, 11.49%); hospital admissions (166, 4.48%); and one death.
Odds of response failure
When accounting for multiple individual RTI episodes presenting in each child nested within each general practice, secondary analysis supported the findings from the primary analysis. Greater odds of response failure were observed in children who had one antibiotic course during the previous year; one antibiotic course: unadjusted odds ratio (OR) 1.62 (95% CI = 1.50 to 1.75), P<0.001; adjusted OR 1.16 (95% CI = 1.07 to 1.27), P<0.001, n = 1029. The likelihood of response failure was greater for children who had ≥2 antibiotic courses during the previous year: unadjusted odds ratio (OR) 2.92 (95% CI = 2.68 to 3.17), P<0.001; adjusted OR 1.92 (95% CI = 1.75 to 2.10), P<0.001, n = 820. Data showing values from response failure and odds of response failure are available from the authors on request.
Sensitivity analyses
When excluding RTI-associated antibiotic prescribing episodes that were preceded by ≥1 antibiotic courses within the last 14 days, the association between ≥2 antibiotic courses and response failure was of borderline statistical significance: one antibiotic course: adjusted OR 1.00 (95% CI = 0.85 to 1.18), P = 0.98, n = 217; ≥2 antibiotic courses: OR 1.28 (95% CI = 1.01 to 1.61), P = 0.046, n = 90.
When excluding antibiotic prescribing episodes that were preceded by ≥1 antibiotic prescription during the 8-week period before the index prescription, a statistically significant association was not demonstrated between children receiving ≥2 antibiotic courses and response failure: ≥2 antibiotic courses: OR 1.13 (95% CI = 0.86 to 1.47), P = 0.38, n = 66.
Excluding children who were 12 months of age at the time of index prescription (14 438 excluded; 12.62%) did not change the overall results: one antibiotic course: adjusted OR 1.03 (95% CI = 0.87 to 1.22), P = 0.75, n = 215; ≥2 antibiotics courses: OR 1.29 (95% CI = 1.01 to 1.64), P = 0.04, n = 95.
By excluding children where the first criterion of the present study outcome was used, that is, prescription of a subsequent antibiotic within 14 days of the initial antibiotic being prescribed to that child; n = 306 excluded, the authors observed that the prescription of one antibiotic course was associated with significantly increased risk of response failure; adjusted OR 1.21 (95% CI = 1.02 to 1.44), P = 0.03, n = 198, as was prescription of ≥2 antibiotics; OR 1.54 (95% CI = 1.20 to 1.98), P = 0.001, n = 91.
Data showing these results from sensitivity analysis are available from the authors on request.
Subgroup analysis
Children who received ≥2 antibiotic courses specifically with a lower RTI (n = 68 634) in the preceding 12 months were more likely to have response failure; one antibiotic course: OR 1.13 (95% CI = 0.92 to 1.39), P = 0.25; ≥2 antibiotic courses: OR 1.60 (95% CI = 1.19 to 2.16), P = 0.002. A statistically significant association was not observed for the other four categories of acute RTIs coded in the CPRD (data available from the authors on request).
DISCUSSION
Summary
Children <5 years of age who received ≥2 antibiotic courses in the previous year for acute RTI had greater likelihood of response failure for subsequent acute RTIs compared with children who had received none. The findings presented here are supported after accounting for multiple individual RTI episodes in children nested within clinics. In the primary analysis, referrals to a specialist service in secondary care accounted for around half of response failures and one-third of these occurred on the same day as the antibiotic prescription. Further studies are needed in which the reason for referral can be accurately elucidated.
Strengths and limitations
In the absence of routine microbiological sampling for acute RTIs in primary care, the estimates for response failure give a pragmatic, clinically relevant outcome measure that patients and clinicians in the community can relate to. A strength of this study is the scale and quality of primary care data used and therefore the results are likely to be generalisable to similar paediatric populations. The authors’ analysis adjusted for baseline consultation rate and previous response failures. A methodological strength is that the main findings are supported by the secondary analysis, where the authors have considered multiple RTI episodes in each child nested in GP practices over the study period. In addition, greater ORs were observed in the sensitivity analysis excluding children where the first criterion of the present study outcome was used, that is, antibiotic prescriptions, adjusted OR 1.32 (95% CI = 1.04 to 1.66) versus OR 1.54 (95% CI = 1.20 to 1.98). This finding supports the primary analysis suggesting that patient demand for antibiotics is unlikely to be driving this association or perhaps that a child with a history of frequent antibiotic use may prompt the clinician to suspect an anatomical or immunity-related issue and seek specialist opinion. The absolute increase in the likelihood of response failure between non-exposed and exposed preschool children is relatively low (0.3%, calculated estimate based on an adjusted OR of 1.32, that is, 0.94% [1050/111 281]*1.32).
There are important limitations to this study. The true aetiology of these acute RTIs is not known. Therefore, evidence of antibiotic non-response may relate to suboptimal diagnosis and inappropriate treatment and medicalisation of self-limiting RTIs in children rather than lack of treatment effectiveness. These data cannot report the relative contribution of bacterial resistance or answer the question of causality of the antibiotic prescription. Only children with acute lower RTI treated with antibiotics were associated with response failure and may reflect illness severity. The study findings, therefore, may have identified children who are prone to infections (but without a relevant comorbidity) without identifying the association with number of antibiotic prescriptions. There was no association when excluding antibiotic prescribing patterns for the 8 weeks before the index prescription and might contradict the antibiotic–microbiome theory.
In the primary analysis, referral to a specialist service accounted for most response failure. The authors acknowledge that the reason for referral may include other issues incidental to the acute presentation. Available data were unable to distinguish between urgent referrals and a routine specialist referral for an ongoing or unrelated RTI-associated problem. Likewise, the researchers were not able to reliably exclude children on prophylactic antibiotics, for example, flucloxacillin for cystic fibrosis. However, the number of children on prophylactic antibiotics is likely to be small in such a large cohort. Within the present cohort study of nearly 8 years, only 725/252 572 children (0.29%) were prescribed flucloxacillin, of which only three children experienced a response failure (further details available from the authors on request). Lastly, though adjustments for important covariates were made, it was not possible to account for confounding due to illness severity from routinely collected data.
Comparison with existing literature
The present findings are consistent with a recent study from the US examining treatment failure rates within 14 days of over 30 000 children (aged 6 months to 12 years) with acute RTIs where around 3% required a new prescription for a systemic antibiotic. Through 30 days, 8.2% of children experienced treatment failure.13 The former study did not specifically look at other clinically defined criteria, for example, hospital admission, or antibiotic exposure in the previous year. Previous population-based studies in the UK found that around 1 in 10 patients of all ages prescribed antibiotics for an acute RTI (upper and lower RTIs, AOM) in the community experience an antibiotic treatment failure.11,26 Close to 95% of these antibiotic treatment failures were in the form of re-consultation and receipt of a different antibiotic prescription within 30 days of their initial prescription. These previous studies found that the highest failure rates were for lower RTIs, which is similar to the present findings. However, these studies did not focus on preschool children. The authors surmise that lower rates of failure found in the present study relate to a number of factors including: a different outcome definition for failure (14 days instead of 30 days); other studies analysing both inpatients, outpatients, urgent care settings; prescription drug claims including injectable or intravenous antibiotics; and/or a focus on all children as opposed to preschool children.
Importantly, the underlying reasons for response failure events are complex and multifactorial. Possible explanations include that these failures are more to do with parents’ healthcare-seeking behaviour and that they are unaware of the normal duration of common RTI illness.27 Prescriber factors include the clinician’s inclination to prescribe an antibiotic because of diagnostic uncertainty coupled with concern that the infection might progress.28 Other factors may also include that antibiotic use in young children disturbs the fragile microbiome with subsequent increased susceptibility to infection,29 or that antibiotic-resistant infections could have a role to play in leading to response failures.30
Implications for research and practice
Previous antibiotic exposure for acute RTI in children may give an indication for subsequent failure, but not necessarily because of antibiotic treatment failure. The present findings suggest that when children receive more antibiotics their likelihood of re-consulting a health professional is affected and increases clinical workload, even though the majority of RTIs in children are viral, self-limiting, and would not be expected to have benefited from antibiotic treatment. Indirectly, this points to the importance of appropriate safety netting and informing parents, or carers, of the natural recovery period of common RTIs in children.24
Incorporating these exposure data into clinical decision-support systems will prompt primary care clinicians to implement strategies to support non-antibiotic strategies, such as informing parents about the anticipated recovery period of common RTIs in children.31 This is especially true where the child does not have clinical evidence of an infection that obviously requires treatment with antibiotics. Indirectly, this can help curb expectations for antibiotics,32 and facilitate better shared decision making during consultations based on tangible outcomes that parents, or carers, and clinicians can relate to.33
Better-quality indicators are needed for common infections to improve the appropriateness of antibiotic prescribing and reduce overall antibiotic prescribing.34 A recent UK database study found almost one-third of antibiotic prescriptions could not be linked to a clinically informative condition.35 Novel quality indicators should centre around acute RTIs, where the majority of unnecessary antibiotic prescribing occurs, and focus on the diagnostic process leading to a diagnosis and the decision to prescribe an antibiotic, rather than simply the choice of antibiotic.36
Routinely collected primary care data in their current format are unable to tease out the intricacies of everyday prescribing decisions. Large-scale observational studies in primary care settings are therefore needed to improve understanding of the mechanisms underlying response failure, how these may be addressed to minimise unnecessary antibiotic prescribing and use, and to determine the proportion of clinical response failures for common infections that are indeed attributable to antibiotic resistance and microbiome disruption.
Acknowledgments
The authors are grateful to Constantinos Koshiaris for helpful suggestions, critical reading of the manuscript, and statistical advice.
Funding
This research was funded by the Scientific Foundation Board of the Royal College of General Practitioners (grant reference: SFB 2015-33). The funding source was not involved in study design, data collection, analysis or interpretation, report writing, or submission for publication. The early use of Antibiotics for at Risk Children with Influenza (ARCHIE) research programme is funded by the National Institute for Health Research’s (NIHR) Applied Research Programme. This publication summarises independent research funded by the NIHR under its Programme Grants for Applied Research Programme (grant reference: RP-PG-1210-12012). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. Oliver van Hecke’s salary was funded by the ARCHIE research programme grant.
Ethical approval
The study protocol was reviewed by the Independent Scientific Advisory Committee (ISAC) and approved on 14 October 2016 (ISAC Protocol 16_162R).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Pouwels KB, Dolk FCK, Smith DRM, et al. Actual versus ‘ideal’ antibiotic prescribing for common conditions in English primary care. J Antimicrob Chemother. 2018;73(Suppl 2):19–26. doi: 10.1093/jac/dkx502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA. 2016;315(17):1864–1873. doi: 10.1001/jama.2016.4151. [DOI] [PubMed] [Google Scholar]
- 3.Smieszek T, Pouwels KB, Dolk FCK, et al. Potential for reducing inappropriate antibiotic prescribing in English primary care. J Antimicrob Chemother. 2018;73(Suppl 2):ii36–ii43. doi: 10.1093/jac/dkx500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Bont EG, van Loo IH, Dukers-Muijrers NH, et al. Oral and topical antibiotic prescriptions for children in general practice. Arch Dis Child. 2013;98(3):228–231. doi: 10.1136/archdischild-2012-303134. [DOI] [PubMed] [Google Scholar]
- 5.Kronman MP, Zhou C, Mangione-Smith R. Bacterial prevalence and antimicrobial prescribing trends for acute respiratory tract infections. Pediatrics. 2014;134(4):e956–e965. doi: 10.1542/peds.2014-0605. [DOI] [PubMed] [Google Scholar]
- 6.Holstiege J, Schink T, Molokhia M, et al. Systemic antibiotic prescribing to paediatric outpatients in 5 European countries: a population-based cohort study. BMC Pediatr. 2014;14:174. doi: 10.1186/1471-2431-14-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60(9):1308–1316. doi: 10.1093/cid/civ076. [DOI] [PubMed] [Google Scholar]
- 8.Hay AD, Heron J, Ness A, ALSPAC study team. The prevalence of symptoms and consultations in pre-school children in the Avon Longitudinal Study of Parents and Children (ALSPAC): a prospective cohort study. Fam Pract. 2005;22(4):367–374. doi: 10.1093/fampra/cmi035. [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Health and Care Excellence . Respiratory tract infections (self-limiting): prescribing antibiotics CG69. London: NICE; 2008. https://www.nice.org.uk/guidance/cg69 (accessed 16 Jul 2019) [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention . Antibiotic prescribing and use in doctor’s offices. Pediatric treatment recommendations. CDC; 2017. https://www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/pediatric-treatment-rec.html (accessed 16 Jul 2019) [Google Scholar]
- 11.Currie CJ, Berni E, Jenkins-Jones S, et al. Antibiotic treatment failure in four common infections in UK primary care 1991–2012: longitudinal analysis. BMJ. 2014;349:g5493. doi: 10.1136/bmj.g5493. [DOI] [PubMed] [Google Scholar]
- 12.McGrath LJ, Becker-Dreps S, Pate V, Brookhart MA. Trends in antibiotic treatment of acute otitis media and treatment failure in children, 2000–2011. PLoS One. 2013;8(12):e81210. doi: 10.1371/journal.pone.0081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerber JS, Ross RK, Bryan M, et al. Association of broad-vs narrow-spectrum antibiotics with treatment failure, adverse events, and quality of life in children with acute respiratory tract infections. JAMA. 2017;318(23):2325–2336. doi: 10.1001/jama.2017.18715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 15.Goossens H, Ferech M, Vander Stichele R, et al. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459):579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 16.Biesbroek G, Tsivtsivadze E, Sanders EA, et al. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am J Respir Crit Care Med. 2014;190(11):1283–1292. doi: 10.1164/rccm.201407-1240OC. [DOI] [PubMed] [Google Scholar]
- 17.Johnson CL, Versalovic J. The human microbiome and its potential importance to pediatrics. Pediatrics. 2012;129(5):950–960. doi: 10.1542/peds.2011-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doan T, Arzika AM, Ray KJ, et al. Gut microbial diversity in antibiotic-naive children after systemic antibiotic exposure: a randomized controlled trial. Clin Infect Dis. 2017;64(9):1147–1153. doi: 10.1093/cid/cix141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Int J Epidemiol. 2015;44(3):827–836. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hobbs FD, Bankhead C, Mukhtar T, et al. Clinical workload in UK primary care: a retrospective analysis of 100 million consultations in England, 2007–14. Lancet. 2016;387(10035):2323–2330. doi: 10.1016/S0140-6736(16)00620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Velde RY, Wyers CE, Teesselink E, et al. Trends in oral anti-osteoporosis drug prescription in the United Kingdom between 1990 and 2012: variation by age, sex, geographic location and ethnicity. Bone. 2017;94:50–55. doi: 10.1016/j.bone.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong D, Ashworth M, Dregan A, White P. The relationship between prior antimicrobial prescription and meningitis: a case-control study. Br J Gen Pract. 2016. [DOI] [PMC free article] [PubMed]
- 23.Ministry of Housing, Communities & Local Government. Official statistics. English indices of deprivation 2010. 2011. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2010 (accessed 19 Jul 2019)
- 24.Thompson M, Vodicka TA, Blair PS, et al. Duration of symptoms of respiratory tract infections in children: systematic review. BMJ. 2013;347:f7027. doi: 10.1136/bmj.f7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shields MD, Thavagnanam S. The difficult coughing child: prolonged acute cough in children. Cough. 2013;9(1):11. doi: 10.1186/1745-9974-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berni E, Scott LA, Jenkins-Jones S, et al. Non-response to antibiotic treatment in adolescents for four common infections in UK primary care 1991–2012: a retrospective, longitudinal study. Antibiotics (Basel) 2016;5(3) doi: 10.3390/antibiotics5030025. pii: E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coxeter PD, Mar CD, Hoffmann TC. Parents’ expectations and experiences of antibiotics for acute respiratory infections in primary care. Ann Fam Med. 2017;15(2):149–154. doi: 10.1370/afm.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fletcher-Lartey S, Yee M, Gaarslev C, et al. Why do general practitioners prescribe antibiotics for upper respiratory tract infections to meet patient expectations: a mixed methods study. BMJ Open. 2016;6(10):e012244. doi: 10.1136/bmjopen-2016-012244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosch AA, de Steenhuijsen Piters WA, van Houten MA, et al. Maturation of the infant respiratory microbiota, environmental drivers and health consequences: a prospective cohort study. Am J Respir Crit Care Med. 2017;196(12):1582–1590. doi: 10.1164/rccm.201703-0554OC. [DOI] [PubMed] [Google Scholar]
- 30.Van Hecke O, Wang K, Lee JJ, et al. The implications of antibiotic resistance for patients’ recovery from common infections in the community: a systematic review and meta-analysis. Clin Infect Dis. 2017;65(3):371–382. doi: 10.1093/cid/cix233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tonkin-Crine S, Yardley L, Little P. Antibiotic prescribing for acute respiratory tract infections in primary care: a systematic review and meta-ethnography. J Antimicrob Chemother. 2011;66(10):2215–2223. doi: 10.1093/jac/dkr279. [DOI] [PubMed] [Google Scholar]
- 32.Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci. 2012;7:37. doi: 10.1186/1748-5908-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coxeter P, Del Mar CB, McGregor L, et al. Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care. Cochrane Database Syst Rev. 2015;11:CD010907. doi: 10.1002/14651858.CD010907.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NHS England Quality premium guidance 2017–19. 2017. https://www.england.nhs.uk/resources/resources-for-ccgs/ccg-out-tool/ccg-ois/qual-prem/. (accessed 11 Jan 2019)
- 35.Dolk FCK, Pouwels KB, Smith DRM, et al. Antibiotics in primary care in England: which antibiotics are prescribed and for which conditions? J Antimicrob Chemother. 2018;73(Suppl 2):ii2–ii10. doi: 10.1093/jac/dkx504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saust LT, Monrad RN, Hansen MP, et al. Quality assessment of diagnosis and antibiotic treatment of infectious diseases in primary care: a systematic review of quality indicators. Scand J Prim Health Care. 2016;34(3):258–266. doi: 10.1080/02813432.2016.1207143. [DOI] [PMC free article] [PubMed] [Google Scholar]