Abstract
Healthy aging is associated with declines in episodic memory performance that are due in part to deficits in encoding. Emerging results from young adult studies suggest that the neural activity during the time preceding stimulus presentation is sensitive to episodic memory performance. It is unknown whether age-related declines in episodic memory are due solely to changes in the recruitment of processes elicited by stimuli during encoding or also in processes recruited in anticipation of these stimuli. Here, we recorded oscillatory electroencephalography while young and old participants encoded visual and auditory words that were preceded by cues indicating the stimulus modality. Individual differences in alpha oscillatory activity preceding, and following, stimulus onset was predictive of subsequent memory performance similarly across age. Poststimulus theta power correlated positively with episodic memory performance for old but not young adults, potentially reflecting older adults’ tendency to self-generate associations during encoding. Collectively, these results suggest that the preparatory mobilization of neural processes before encoding that benefits episodic memory performance is not affected by age but instead dependent on the individual’s propensity to preemptively mobilize task-specific processes.
Keywords: EEG, Aging, Oscillations, Prestimulus, Long-term memory
1. Introduction
Healthy aging is commonly associated with declines in episodic memory performance (Craik and Rose, 2012; Friedman, 2000; Spencer and Raz, 1995). These declines are due in part to deficits in organization and/or binding of episodic information during encoding (Glisky et al., 2001; Johnson, 1996; Old and Naveh-Benjamin, 2008). Another possible contribution to age-related memory decline is a shift from a proactive to a reactive control strategy (Braver, 2012), which suggests that older adults are less likely to prepare for the upcoming to-be-encoded event than younger adults. In younger adults, preparatory neural activity has been found to reflect successful encoding, for example, prestimulus event related potentials (ERPs) differ according to whether events are subsequently remembered or forgotten (for review: Cohen et al., 2015; Otten et al., 2006), and in some cases, correlate positively with memory performance (Gruber et al., 2013; Guderian et al., 2009; Mackiewicz et al., 2006). Thus, a shift away from engaging in preparatory processes may contribute to episodic memory impairments in older adults.
The subsequent memory effect (SME) is typically measured as the difference in poststimulus neural activity between subsequently forgotten and subsequently remembered events at encoding (Paller et al., 1987; for review: Paller and Wagner, 2002). Neural activity in the preparatory time, or cue-stimulus interval, has also been shown to differ between trials that are subsequently remembered versus forgotten (for review: Cohen et al., 2015). Both prestimulus and poststimulus SMEs have been shown to be influenced by the task characteristics during encoding, such as stimulus modality (Golby et al., 2001; Otten et al., 2006; Park and Rugg, 2010; Wagner et al., 1998), orienting task (Otten and Rugg, 2001; Padovani et al., 2011; Paller et al., 1987), and value or reward for remembering (Adcock et al., 2006; Gruber et al., 2013; Gruber and Otten, 2010). Relatively little has been published concerning pre-SMEs in older adults, but one study suggests that young adults may adjust how they prepare for an upcoming stimulus based on the specific task demands, whereas preparatory effects in older adults may be more general (Koen et al., 2018). Consequently, prestimulus SMEs are thought to reflect, at least in part, preparatory mobilization of material-/task-specific and domain general processes that contribute to memory performance (Adcock et al., 2006; Addante, de Chastelaine and Rugg, 2015; Otten et al., 2006; Xia, Galli and Otten, 2018).
Most prestimulus episodic memory studies have investigated ERPs, but other electrophysiology studies have shown that prestimulus oscillatory activity is sensitive to episodic memory performance (Fell et al., 2011; Gruber et al., 2013; for review: Klimesch, 1999; Merkow et al., 2014; Salari and Rose, 2016). Both the ERP and oscillatory electroencephalography (EEG) response to events reflect changes in oscillatory neural activity that is time-locked to the event, such as stimulus presentation or execution of a response. But unlike ERPs, the oscillatory EEG response also reflects changes in ongoing activity that is not phase-locked to the event (Bastiaansen et al., 2012; Sauseng et al., 2007). Given the variability in the timing and manifestation of pre-SMEs, oscillatory EEG may be more sensitive than ERPs to the detection of preparatory effects. In addition, oscillatory EEG responses are sensitive to underlying functional network dynamics, that may be missed with ERPs, such as the synchronization and desynchronization of specific frequency bands reflecting coupling and uncoupling of networks, respectively (for review: Duzel et al., 2010; Klimesch et al., 2007; Von Stein and Sarnthein, 2000).
Both human and rodent research suggests that synchronous oscillations in the theta frequency band (4–8 Hz) reflect interactions between the hippocampus and cortical areas including the prefrontal cortex, which facilitate long-term memory (Klimesch, 1999; Nyhus and Curran, 2010). Mid-frontal theta power is typically greater for events that are remembered than those that are forgotten during encoding (Hanslmayr et al., 2009; Hsieh and Ranganath, 2014; Staudigl and Hanslmayr, 2013) and retrieval (Addante et al., 2011; Gruber et al., 2013), particularly for events for which contextual associations are recollected (Addante et al., 2011; Gruber et al., 2008). Similar theta effects have been found preceding stimulus onset (Fell et al., 2011; Gruber et al., 2013; Guderian et al., 2009; Klimesch, 1999; Merkow et al., 2014). Because theta increases for remembered events have been shown for various kinds of stimuli and task conditions, it is likely that theta rhythms reflect domain-general operations that contribute to episodic memory (Guderian et al., 2009).
Oscillations in the alpha (8–12 Hz) and beta (14–30 Hz) bands have also been found to relate to memory performance. In contrast to the increases in theta synchrony that contribute to successful encoding and retrieval, alpha and beta desynchronization following stimulus onset have been associated with memory success (for review: Hanslmayr et al., 2012; Sederberg et al., 2003). The alpha frequency band is thought to operate via a release of inhibition such that decreases in power reflect the engagement of the underlying regions, whereas power increases reflect an increase of inhibition (Klimesch, 2012; Klimesch et al., 2007). Although less is known about the functional role of the beta frequency band, decreases in the beta band may reflect a change in the cognitive state (Engel and Fries, 2010), such as the engagement of task related demands. For example, beta desynchronization (decrease in power from a pre-event baseline) over anterior scalp electrodes has been shown during semantic encoding of verbal material but not during other forms of elaborative encoding or shallow encoding tasks (Fellner et al., 2013; Hanslmayr et al., 2009). Intracranial EEG and functional magnetic resonance imaging studies suggest that the left inferior frontal gyrus is a major generator of this beta-encoding effect (Hanslmayr et al., 2011; Sederberg et al., 2003), and transcranial magnetic stimulation evidence suggests that this desynchronization causally contributes to successful encoding (Hanslmayr et al., 2014). Alpha and beta desynchronization is believed to reflect processing within specialized neocortical areas sensitive to presented information (i.e., words, images, sounds, and so forth) (for review: Hanslmayr et al., 2016; Klimesch, 1999, 2012; Klimesch et al., 1999). Consistent with this idea is evidence showing that alpha and beta powers vary topographically during retrieval according to the stimulus and task characteristics at encoding (Khader and Rösler, 2011; Waldhauser et al., 2012; Waldhauser et al., 2016). As recollection is believed to depend, in part, on neural reactivation of sensory information experienced during prior encoding (Rugg et al., 2008), it follows that alpha and beta desynchronization during encoding may reflect processes that ultimately support recollection.
There is limited research on the impact of aging on episodic memory with neural oscillations. EEG evidence from a visuospatial associative encoding task suggests that an age-related reduction in theta synchronization following a to-be-encoded item may contribute to older adults’ memory impairments (Crespo-Garcia et al., 2012). Similarly, MEG evidence suggests that increased stimulus-related theta power preceding encoding predicts relational binding success for the young but not the old in a short-term memory task (Rondina et al., 2016). These results are consistent with findings from short-term memory tasks showing age-related decreases in theta synchronization (Kardos et al., 2014; Karrasch et al., 2004). It is important to note that these studies assessed stimulus-induced changes in oscillatory power relative to baseline but did not compare oscillatory power for successful and unsuccessful memory trials. As discussed previously, the theta frequency band may facilitate communication between the hippocampus and prefrontal cortex. Thus, age-related decreases in theta power during encoding is likely related to decreases in hippocampal activity at encoding (Dennis et al., 2007; Dennis et al., 2008; Dennis and Cabeza, 2008) and reductions in functional connectivity between the hippocampus and the prefrontal cortex (Andrews-Hanna et al., 2014), both of which are associated with worse memory performance. Thus, it remains unclear how aging impacts prestimulus and poststimulus oscillations that reflect encoding success, per se, in episodic memory.
The present study investigated age-related differences in prestimulus and poststimulus neural oscillations associated with episodic memory success at encoding. We had participants encode visually and auditorily presented words with a semantic orienting task, as nonsemantic tasks are less reliable for inducing preparatory processes (Gruber and Otten, 2010; Otten et al., 2006). Audio and visual trials were pseudorandomized, and each trial started with a modality congruent cue. Recognition memory was tested by intermixing previously presented words with new words, and participants responded with an old/new confidence judgment. This paradigm is similar to previous prestimulus subsequent memory studies (for similar studies: Otten et al., 2006; Otten et al., 2010; Park and Rugg, 2010). Overall, we predicted that changes in theta power would be similar across encoding modalities (i.e., domain general), whereas alpha and/or beta power would differ between visual and audio items (i.e., domain specific), in both the prestimulus and poststimulus times. For older adults, specifically, we predicated reduced theta power compared to the young and either no prestimulus SMEs or only domain-general effects.
2. Methods
2.1. Participants
Participants were recruited from the Georgia Institute of Technology and the surrounding community. Thirty-four young adults participated for pay or course credit. Thirty-one older adults participated for pay. All compensation was paid at a rate of $10 per hour for each hour of participation. All participants were right-handed. Participants with neurological conditions, including Alzheimer’s disease, stroke, attention deficit hyperactivity disorder, untreated depression, schizophrenia, and epilepsy were excluded. All participants signed an Institutional Review Board–approved consent form before participation. All older participants completed the Montreal Cognitive Assessment at the beginning of the experimental session to screen out possible mild cognitive impairment (Nasreddine et al., 2005). Participants were excluded if they scored below age-adjusted norms on neuropsychological tests (old: 1), did not complete the experiment (young: 1; old: 4), memory performance was 2.5 standard deviations below the group mean (young: 3; old: 2), or they had less than 12 artifact-free EEG epochs for a condition of interest (young: 6; old: 1). Included participant demographics are presented in Table 1. Older adults had significantly more years of education than younger adults [t(45) = 2.87, p = 0.003].
Table 1.
Participant details
| Demographics | Young | Old |
|---|---|---|
| n (included) | 24 (10 male) | 23 (10 male) |
| Age (y) | 21.37 (3.04) | 67.00 (4.50) |
| MoCA | n. a. | 27.22 (2.19) |
| Education (y) | 14.63 (1.53) | 16.26 (2.34)a |
Mean (SD).
Key: MoCA, Montreal Cognitive Assessment; SD, standard deviation.
Older adults had significantly more years of education than young adults [t(45) = 2.87, p = 0.003].
2.2. Stimuli
A pool of 480 concrete object nouns was used to create the study and test lists. Approximately half of each list consisted of items conceptually bigger or smaller than a standard computer monitor. The nouns were selected from the MRC Psycholinguistic Database (Wilson, 1988) with a written frequency of 10–50 occurrences per million (Kučera and Francis, 1967), a length of 3–12 letters, concrete range of 350–700, and image ability range of 500–700 (Coltheart, 1981). If multiple nouns had the same phonetic representation (e.g., “mail” and “male”), only one was retained. The full stimulus set was checked against the Affective Norms for English Words database, and none were highly arousing for positive or negative valence (Bradley and Lang, 1999). For each participant, the stimulus list was randomized such that each noun had an equal likelihood of being in the study or test list and an equal likelihood of being presented as an auditory or visual item. Auditory stimuli were created with the software program Audacity (http://audacity.sourceforge.net/). All words were recorded by the same female voice and normalized (mean duration = 592 milliseconds (ms); range = 250–1120 ms). All visual presentation occurred on a black background. Visually presented items were displayed in the center of the screen for 590 ms with white letters (Helvetica font, size 36). A white fixation cross was present on the screen at all times except during the period of visual cue and word presentation. The visual cue consisted of the fixation cross turning red for 250 ms, and the auditory cue was a 500 Hz tone presented for 250 ms.
2.3. Procedure
The experiment consisted of three parts: (1) incidental study phase, (2) 30-minute delay, and (3) surprise recognition test. During the delay, participants completed the AX variant of the Continuous Performance Task (Braver et al., 2001) (not reported here). All participants received a short practice before each respective part of the experiment. Practice trials continued for each participant until they fully understood the task. An example of the trial structure, for the study and test period, is presented in Fig. 1. The study period consisted of 4 blocks with 60 trials each. Each block contained an equal number of stimuli from each modality. Trials were pseudorandomized with the requirement that the stimulus modality switch after a maximum of 4 trials. There were equivalent numbers of stay and switch trials in the experiment. Each trial began with a fixation cross randomly jittered between 1300 ms and 1700 ms by intervals of 50 ms. Jitter was included to reduce expectancy-related activity, such as the contingent negative variation before the cue onset. Participants were instructed to use the cue to prepare for the upcoming trial but were given no specific preparation instructions. For each trial, the cue always indicated the upcoming presentation modality of the word. For each word, participants decided if the word’s real-world referent was bigger or smaller than a standard computer monitor. Participants held a small USB number pad with both hands and pressed one button for “yes” and another for “no” using their thumbs. If no response was made within two and a half seconds, the trial continued to the next trial.
Fig. 1.
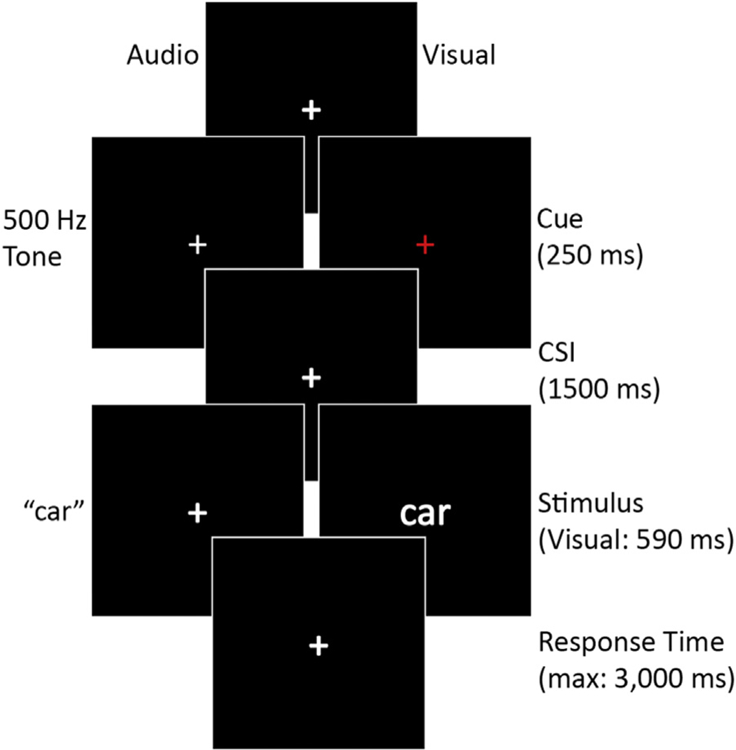
Trial structure and timing for both encoding and retrieval tasks. CSI, cuestimulus interval.
The testing stage procedure used similar timing as the study phase, with the exception of the judgments made. The test period consisted of all 480 items (240 from the study list and 240 new). Trials were pseudorandomized with the requirement that stimulus modality and old/new status change after a maximum of 4 trials. There were equivalent numbers of stay and switch trials in the experiment. Each block contained an equal number of old visual, old auditory, new visual, and new auditory, along with equal items from each bigger/smaller list. Each studied item presented during test was in the same modality as it was during study. For each item, the participant made an old/new decision with the following response options: “Old High Confidence,” “Old Low Confidence,” “New Low Confidence,” and “New High Confidence.” A fifth “Don’t know” response option was available when participants were unsure of how to respond, to avoid guesses contaminating the other response categories. The trial proceeded one second after the subject response or, if no response, after four and a half seconds. Participants responded by pressing one of five keys on a number pad using the thumbs of both hands. Old and new judgments were counterbalanced between hands across participants.
2.4. Behavioral analysis
Study and test trials were excluded if the participant did not respond or made multiple responses. Trials were also removed if the participant responded faster than 200 ms, as this is roughly 3–4 standard deviations faster than the average response time. Memory accuracy was assessed using Pr (Snodgrass and Corwin, 1988). Pr (Hits–False Alarms) considers an individual subject’s false alarm rate (misclassifying a new item as an old item), which makes the “at chance” rate equal to zero. Due to the subjective nature of the encoding task, accuracy was not assessed, although average subject agreement with predefined (big/small) word lists was 0.848 (SD = 0.114).
2.5. EEG recording
Continuous scalp-recorded EEG data were collected from 32 Ag-AgCl electrodes using an ActiveTwo amplifier system (BioSemi, Amsterdam, Netherlands). Electrode position follows the extended 10–20 system (Nuwer et al., 1999). Electrode positions included the following: AF3, AF4, FC1, FC2, FC5, FC6, FP1, FP2, F7, F3, Fz, F4, F8, C3, Cz, C4, CP1, CP2, CP5, CP6, P7, PO3, PO4, P3, Pz, P4, P8, T7, T8, O1, Oz, and O2. External left and right mastoid electrodes were used for referencing offline. Two electrodes placed superior and inferior to the right eye recorded vertical electrooculogram, and 2 additional electrodes recorded horizontal electrooculogram at the lateral canthi of the left and right eyes. The ActiveTwo system replaces the traditional reference with a Common Mode Sense active electrode and the ground with a Driven Right Leg passive electrode. EEG was sampled at 1024 Hz with 24-bit resolution, and decimation was performed using a 5th order sinc filter to prevent aliasing.
2.6. EEG preprocessing
Offline analysis of the EEG data was done in MATLAB 2015b with the EEGLAB (Delorme et al., 2011; Delorme and Makeig, 2004), ERPLAB (Lopez-Calderon and Luck, 2014), and FieldTrip (Oostenveld et al., 2011) toolboxes. The final frequency range of interest was 2–30 Hz, and our data preprocessing pipeline reduced the sample rate and filtered out frequencies greater than 4 times the top frequency of interest (2 times the Nyquist frequency) on the EEG signal. First, the continuous EEG data were downsampled to 256 Hz with an antialiasing filter cutoff of 0.9 and a transition bandwidth of 0.2, then frequencies below 0.5 Hz (with a transition bandwidth of 0.5 Hz) and above 125 Hz (with a transition bandwidth of 3 Hz) were filtered out. After filtering, the continuous data were referenced to the average of the left and right mastoid electrodes.
To investigate slower frequencies, like theta, longer epochs are needed to account for loss of signal at each end of the epoch due to wavelet decomposition (one-half the wavelet kernel on each end of the epoch). Thus, the continuous data were then epoched from 1500 ms precue to 4000 ms postcue (i.e., 2250 ms poststimulus), but the epoch range of interest was defined as 600 ms precue to 3400 ms postcue (i.e., 1650 ms poststimulus). This epoch interval allowed for the assessment of both prestimulus and poststimulus neural activity. To facilitate both an automated epoch rejection process and an independent component analysis (ICA) used to identify noisy epochs and remove ocular artifacts, each epoch was baselined to the average EEG voltage of the whole epoch.
The automated epoch rejection process was iterated twice on the EEG data and identified epochs in which two or more electrode had extreme voltage shifts. Specifically, the process identified epochs where the voltage range within a 400 ms window (sliding in 100 ms intervals across the epoch) was greater than the 99th percentile of all epoch voltage ranges. Then the process identified epochs where the linear trend slope exceeded the 95th percentile of all epoch ranges with a minimum R2 value of 0.3.
Given the short duration of the visual stimuli (Cue: 250 ms and Stimulus: 590 ms), we excluded epochs in which the participant blinked during cue or stimulus onset. Blinks were identified using the frontal and eye electrodes between −150 and 150 ms from both cue and stimulus (1600–1900 ms) onset. Epochs where the voltage range during 100 ms (sliding in 25 ms intervals) was above the 95th percentile of all epoch voltage ranges within the specified time interval were removed.
After the detection and removal of extreme voltage shifts and blinks, an ICA was run across the epochs, and the components were assessed to identify additional epochs from rejection. Epochs were rejected when the component voltage range was greater than the 99th percentile of all epoch voltage ranges within the 400 ms window (sliding in 100 ms intervals across each epoch) or either the kurtosis or joint probability exceeded 15 standard deviations within the componentor 23 standard deviations of all components for the epoch.
Finally, non–event-related (did not overlap cue or stimulus onset) ocular artifacts (i.e., blinks and horizontal eye movements) were identified by running ICA on the first 20 principle components of the head electrodes for the accepted epochs. The components related to these ocular artifacts were identified by visually inspecting the component time courses with ocular electrodes and investigating the topographic component maps. Once identified, these components were removed (Bell and Sejnowski, 1995; Delorme et al., 2007; Hoffmann and Falkenstein, 2008).
As a last step in EEG preprocessing, each epoch was rebaselined in the time domain to the average activity in the −600 to −100 ms time range (precue onset), for manual inspection. Then, each participant’s data set was inspected for quality and accurate preprocessing. Any remaining epochs containing artifacts, such as muscle activity or saturation, were removed manually. It is important to note that adjusting the EEG voltage baseline in the time domain should not impact the power values returned during conversion into the time-frequency domain, as this equates to adding or subtracting a scalar (Cohen, 2014).
2.7. Time-frequency conversion and processing
After preprocessing the EEG data, each epoch was transformed into a time frequency representation using Morlet wavelets (Percival and Walden, 1993) with 5 cycles in 1 Hz intervals between 2 and 30 Hz. Each time-frequency data point is a product of the weighted sum of the surrounding time and frequency data points; therefore, the center of each wavelet was placed approximately 20 ms apart (50.25 Hz) (Cohen, 2014). Under the specified wavelet parameters at 2 Hz, each returned data point was influenced by +/− 0.4 Hz and +/− 398 ms. At 30 Hz, each returned data point was influenced by +/− 6 Hz and +/− 26.5 ms. Then, individual subject averages were created for each condition and frequency of interest (theta: 3–7 Hz, alpha: 8–12 Hz, and beta: 16–26 Hz) using a 10% trimmed mean (Wilcox and Keselman, 2003).
Given our interest in understanding the neural activity related to the expectation or preparatory period, baseline normalization removes the influence of ongoing neural activity that may influence performance but is not directly related to trial specific preparation. Within each frequency band, on the condition specific averages, a baseline normalization using relative change (Cohen, 2014) was calculated on the average frequency power over the −500 to −200 ms precue time range. As previously stated, each time-frequency data point is a weighted sum of the surrounding time and frequency data points which result in a loss of temporal specificity. Selecting this pre-event baseline accounts for temporal smearing by using a time range that limits the likelihood of post-event activity contaminating the baseline interval (Cohen, 2014).
2.8. Time-frequency analysis
As reported below, high memory performance resulted in a low number of miss trials, specifically in young adults. Many young adult participants had 12 or less artifact-free incorrect old item trials (misses) (young: 8, old: 1). To assess prestimulus and post-stimulus SMEs, we used correctly recognized old items selected with high confidence (HC hits) and a combination of all old items misidentified as new with correctly recognized old items selected with low confidence (forgotten) (for similar approaches: Hanslmayr et al., 2009; Otten and Rugg, 2001). Reported results include the time-range calculated from cue onset and for poststimulus results, the time-range from stimulus onset is presented in parentheses. All analyses are done in sensor space.
2.9. Significance testing
Significance testing was performed using Monte Carlo permutation tests with temporal and spatial clustering across our 3 frequency bands of interest with the FieldTrip toolbox. Given that each electrode and time point are not independent, we used a nonparametric cluster–based test statistic to control the familywise error rate at a critical alpha level of 0.05 (Blair and Karniski, 1993; Maris and Oostenveld, 2007). The calculation of the cluster-based statistic starts with calculating a t-value for every sample and selecting those samples with above a particular alpha level (in the present study: 0.05). These selected samples were clustered together based on temporal and spatial adjacency. Then the sum of the t-values within each cluster are calculated and used as the test statistic against a calculated null distribution, as described below.
The significance probability of the above test statistic was assessed with the Monte Carlo method. First, a random partition is created by randomly shuffling the condition or group labels and calculating a test statistic on the random partition. This process was repeated 2000 times to create a distribution of test statistics based on random partitions of the data. The proportion of randomized test statistics that fall above the true test statistic is used to calculate the p-value. Only spatiotemporal clusters with a p-value of less than 0.05 (familywise error rate) that were reliable for over 200 ms and had a minimum of 2 neighboring electrodes were considered significant and used for follow-up analyses and quantification (for similar approaches: Addante et al., 2011; Gruber et al., 2013; Hanslmayr et al., 2009; Pastotter et al., 2011; Staudigl et al., 2010).
2.10. Mean level effects
For prestimulus and poststimulus SMEs, this cluster-based method was first applied across modality to investigate a main effect of memory performance (HC hits vs. forgotten). Then, we investigated the interaction between memory performance and modality by comparing the subsequent memory contrast (HC hits vs. forgotten) between the visual and audio modalities. Finally, we investigated the subsequent memory contrast within each modality. These 3 steps were performed across both groups and within each group (i.e., young and old). To directly assess differences in aging, the across modality and within modality subsequent memory contrasts were compared between the young and older adults.
When significant clusters were identified, the average cluster power was calculated and compared between both groups and modalities for each cluster. Reported cluster statistics are based on the differences between the average power within the identified spatiotemporal electrode clusters.
2.11. Correlations
Across-participant correlations were assessed based on memory performance and the subsequent memory contrast within each modality both across and within age groups. Identifying significant clusters was based on the same significance testing method reported previously. For each modality, memory performance was correlated with the power difference in the subsequent memory contrast at each electrode and time point. Only correlations at an alpha level lower than 0.05 were used for spatiotemporal clustering, and cluster significance was assessed with the Monte Carlo method with a familywise error rate of 0.05.
Reported correlation statistics are based on the correlation between memory performance and the subsequent memory contrast within the identified spatiotemporal electrode cluster. Follow-up analyses compared identified correlation coefficients between young and older adults as well as between modalities. These comparisons were performed by converting the correlation coefficients into a Z-score with Fisher’s r to z transformation, and p-values were determined with the difference Z-score. Previous neuroimaging studies have used a similar number of participants in behaviore-brain correlations (Gruber et al., 2013; Guderian et al., 2009; Mackiewicz et al., 2006), 18, 24, and 40, respectively.
3. Results
3.1. Behavioral results
3.1.1. Memory performance
Corrected recognition (Pr), collapsed across confidence, was calculated for both young (visual: M = 0.541, SD = 0.180; audio: M = 0.523, SD = 0.156) and older (visual: M = 0.455, SD = 0.139; audio: M = 0.397, SD = 0.134) adults. Accuracy was assessed with a 2 modality (visual, auditory) X 2 group (young, old) repeated measures ANOVA. The results of the ANOVA revealed a main effect of group [F(1,45) = 6.219, p = 0.016, ] and modality [F(1,45) = 7.535, p = 0.008, ], but no interaction [F(1,45) = 1.976, p = 0.167, ]. These results show that older adults showed worse item recognition than the young and that memory was worse for audio items than visual items across groups.
Given the low trial count for incorrect old items and the use of high confident hits versus a combined forgotten category, we assessed high- and low-confidence Pr. For the high confidence Pr (high confidence hits minus high confidence false alarms) estimates in the young (visual: M = 0.533, SD = 0.165; audio: M = 0.504, SD = 0.152) and older (visual: M = 0.422, SD = 0.151; audio: M = 0.371, SD = 0.141) adults, the results of the high confidence Pr ANOVA revealed the same pattern as the results of the across confidence ANOVA. A main effect of the group [F(1,45) = 8.334, p = 0.006, ] and modality [F(1,45) = 8.132, p = 0.007, ], but no interaction [F(1,45) = 0.670, p = 0.417, ].
For the low confidence Pr (low confidence hits minus low confidence false alarms) estimates in the young (visual: M = 0.009, SD = 0.077; audio: M = 0.018, SD = 0.071) and older (visual: M = 0.032, SD = 0.055; audio: M = 0.026, SD = 0.06) adults, the results of a 2 modality (visual, auditory) × 2 group (young, old) repeated measures ANOVA failed to find significant differences [F(1,45)’s < 0.934, p’s > 0.339]. In addition, a one-sample t-test against chance (zero) on low confidence Pr revealed Pr was not significantly different from chance for young adults [visual: t(23) = 0.551, p = 0.587; audio: t(23) = 1.270, p = 0.217]. For older adults, a one-sample t-test against chance (zero) was significantly above chance for the visual condition [t(22) = 2.811, p = 0.010] and marginally above chance for the audio condition [t(22) = 2.067, p = 0.051].
Consequently, we used high confidence Pr in subsequent correlational analyses. Given the chance and near chance memory performance for low confidence Pr, combining the low confidence hits with the misses should increase the reliability of neural activity related to failed recognition. Response proportions for studied and unstudied items as a function of memory performance are listed in Table 2. The use of the “Don’t Know” response was very low (young: 0.003 [0.004], old: 0.004 [0.009]), and these were excluded from all analyses.
Table 2.
Recognition memory: proportion of responses
| Response |
Old |
New |
||
|---|---|---|---|---|
| Confidence | High | Low | High | Low |
| Young adults | ||||
| Old: visual items | 0.630 (0.160) | 0.167 (0.106) | 0.080 (0.082) | 0.123 (0.078) |
| Old: audio items | 0.609 (0.136) | 0.169 (0.098) | 0.085 (0.085) | 0.137 (0.073) |
| New: visual items | 0.097 (0.066) | 0.158 (0.116) | 0.382 (0.233) | 0.363 (0.177) |
| New: audio items | 0.105 (0.066) | 0.151 (0.095) | 0.371 (0.202) | 0.374 (0.173) |
| Older adults | ||||
| Old: visual items | 0.567 (0.159) | 0.146 (0.107) | 0.163 (0.121) | 0.123 (0.072) |
| Old: audio items | 0.521 (0.158) | 0.166 (0.125) | 0.163 (0.124) | 0.150 (0.086) |
| New: visual items | 0.145 (0.157) | 0.114 (0.098) | 0.478 (0.220) | 0.264 (0.169) |
| New: audio items | 0.150 (0.139) | 0.140 (0.110) | 0.417 (0.223) | 0.293 (0.164) |
Average proportion (standard deviation).
3.1.2. Forgotten condition trial composition
Given that the forgotten condition included both low confident hits and misses, the proportion of low confident hits was assessed with a 2 modality (visual, auditory) x 2 group (young, old) repeated measures ANOVA. The ANOVA results revealed a marginal effect of group [F(1,45) = 3.835, p = 0.056, ], a marginal interaction [F(1,45) = 3.106, p = 0.085, ], and no effect of modality [F(1,45) = 0.499, p = 0.484, ]. Thus, the proportion of low confidence hits in the forgotten category for young adults (visual: 0.447, SD = 0.176; audio: 0.413, SD = 0.15) and older adults (visual: 0.319, SD = 0.207; audio: 0.333, SD = 0.213) was not significantly different.
3.1.3. Encoding reaction times
Encoding reaction times by retrieval response are presented in Table 3. In line with the EEG analysis, reaction times were collapsed into high confident hits and forgotten categories and then submitted to a 2 memory (high hits, forgotten) x 2 modality (visual, audio) x 2 group (young, old) ANOVA. The results of the ANOVA only revealed a main effect of modality [F(1,45) = 28.201, p < 0.001, ], all other results, F’s < 2.005, p’s > 0.164. Visual items were faster than audio items (visual: 1187 ms, SD = 254 ms; audio: 1507 ms, SD = 279 ms). Thus, reaction times did not differ between age groups and accuracy.
Table 3.
Recognition memory: encoding reaction times
| Response |
Old |
New |
||
|---|---|---|---|---|
| Confidence | High | Low | High | Low |
| Young adults | ||||
| Visual items | 1139 (259) | 1123 (305) | 1319 (527) | 1179 (339) |
| Audio items | 1451 (294) | 1416 (279) | 1454 (397) | 1429 (392) |
| Older adults | ||||
| Visual items | 1237 (243) | 1178 (294) | 1231 (326) | 1168 (273) |
| Audio items | 1580 (265) | 1500 (342) | 1531 (344) | 1564 (325) |
Average reaction times in milliseconds (standard deviation).
3.1.4. Behavioral summary
In sum, young adults had greater item memory than older adults across modality, and memory was better for visual item compared with audio item across both age groups.
3.2. Time-frequency results
The subsequent memory contrast between high confidence hits and forgotten trials was investigated across and between age groups and modalities. We also correlated these contrasts with the high confidence Pr metric of memory performance. The 3 frequency bands of interest (theta, alpha, and beta) were assessed separately.
3.2.1. Subsequent memory effects
No prestimulus or poststimulus SMEs were found within the theta frequency band, and the cluster analyses did not identify any SMEs that interacted with age or modality.
3.2.1.1. Modality-general poststimulus subsequent memory effects
3.2.1.1.1. Alpha frequency band.
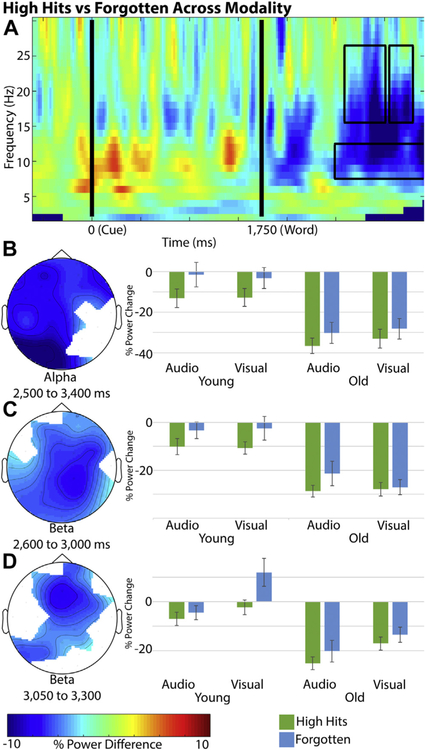
Within the alpha band, a significant cluster was found across modality and within the audio modality. Given the overlapping time and electrode features, we used the cluster found across both modalities for follow-up analyses. The alpha band cluster reflected a significant decrease in power across both modalities and groups for high confident hits compared to forgotten trials across 27 electrodes between 2500 and 3400 ms (750–1650 ms poststimulus) [t(46) = −3.859, p = 0.002]. Further analyses revealed this subsequent memory cluster did not differ by modality [t(46) = −0.445, p = 0.682] or group [t(45) = 0.943, p = 0.338], as seen in Fig. 2B.
Fig. 2.
The heat and topographic maps represent the difference in percent change from baseline between high confident hits and forgotten trials across all participants and modalities. Bar charts are the average percent change from baseline within the identified electrode cluster. Error bars = 1 SEM. (A) The intersecting electrodes from the 3 identified alpha and beta band clusters were averaged together for visualization of the time-time-course in the heat map. (B) Poststimulus alpha power cluster (750–1650 ms poststimulus). (C) Poststimulus beta power cluster (850–1250 ms poststimulus). (D) Poststimulus beta power cluster (1300–1550 ms poststimulus).
3.2.1.1.2. Beta frequency band.
Within the beta band across both groups and modalities, a significant cluster was found across 25 electrodes between 2600 and 3300 ms (850–1550 ms post-stimulus) [t(45) = −0.478,p = 0.001]. Given that the spatiotemporal clustering within each modality identified separate time ranges within the across-modality cluster, we separated the beta effect into early and late time clusters. Across the early cluster, a greater decrease in poststimulus beta power across both modalities and groups for high confidence hits compared with forgotten was found in 28 electrodes between 2600 and 3000 ms (850–1250 ms post-stimulus) [t(46) = −3.393, p = 0.001]. This early cluster did not differ by modality [t(46) = −0.831, p = 0.446] or group [t(45) = 0.834, p = 0.429], as seen in Fig. 2C.
The second or later onsetting beta band effect found a greater decrease in poststimulus beta power across both modalities and groups for high confidence hits compared with forgotten in 18 electrodes between 3050 and 3300 ms (1300–1550 ms poststimulus) [t(46) = −3.250, p = 0.001]. Follow-up analyses showed that the SME did not differ between modality [t(46) = 1.624, p = 0.113] and group [t(45) = −0.875, p = 0.385], but there was a marginally significant modality × group interaction [t(45) = 2.063, p = 0.056]. Further analyses revealed that the late beta SME was larger for young adults compared with older adults in the visual condition [t(45) = 2.20, p = 0.035], but the size of the effect did not differ between the age groups for the audio condition [t(45) = 0.53, p = 0.627], as seen in Fig. 2D.
3.2.2. Correlations between memory performance and the subsequent memory contrast
3.2.2.1. Modality-specific prestimulus alpha across age groups.
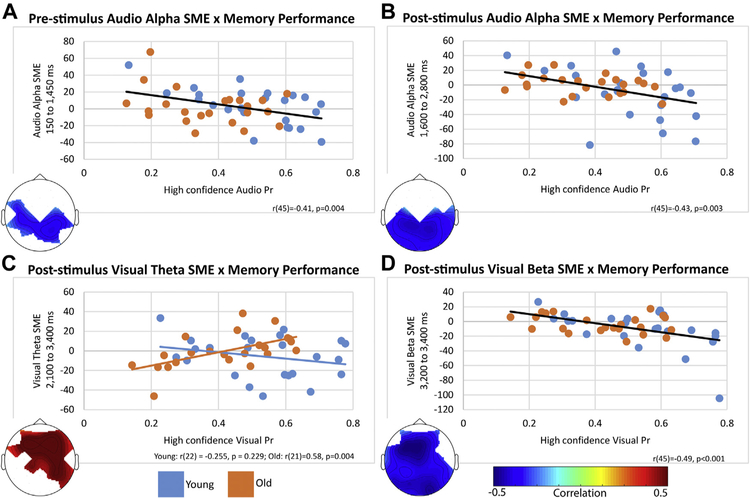
The cluster analysis identified a significant correlation for young adults in the audio condition between the alpha power subsequent memory contrast and high confidence audio Pr across 11 bilateral centroposterior electrodes between 150 and 1450 ms [r(22) = −0.601, p = 0.002]. For older adults, this cluster correlation did not reach significance [r(21) = −0.306, p = 0.156]. However, the correlation coefficients did not differ between young and older adults [Fisher’s r to z difference: p = 0.226], and the correlation remained significant when combining the age groups [r(45) = −0.41, p 0.004], as seen in Fig. 3A.
Fig. 3.
All plots are percent difference in power between high confident hits and forgotten trials by high confidence Pr for the specified modality. (A) Prestimulus audio alpha power. (B) Poststimulus audio alpha power (−150–1050 ms poststimulus). (C) Poststimulus visual theta power correlated with high confidence visual Pr in the old but not the young (350–1650 ms poststimulus). (D) Poststimulus visual beta power (1450–1650 ms poststimulus). Abbreviation: SME, subsequent memory effect.
Follow-up analyses found this cluster was not significant in the visual condition for the young [r(22) = −0.08, p = 0.709], the old [r(21) = 0.276, p = 0.202], or across groups [r(45) = 0.094, p = 0.528]. The correlation coefficients between the audio and visual modalities were significantly different for the young [p = 0.046] and across all participants [p = 0.015] but only marginal for the old [p = 0.058].
3.2.2.2. Modality-specific poststimulus activity across age groups
3.2.2.2.1. Alpha frequency band.
The cluster analysis identified a significant correlation across all participants in the audio condition between the alpha power subsequent memory contrast and high confidence audio Pr in 17 posterior electrodes between 1600 and 2800 ms (−150–1050 ms poststimulus) [r(45) = −0.43, p = 0.003], as seen in Fig. 3B. Investigating each age groups separately revealed that the correlation remained significant in the young [r(22) = −0.442, p = 0.03] but failed to reach significance in the old [r(21) = −0.335, p = 0.119], although the correlation coefficients were not significantly different [p = 0.684].
Follow-up analyses found this cluster was not significant in the visual condition for the young [r(22) = −0.132, p = 0.538], the old [r(21) = −0.279, p = 0.197], or across groups [r(45) = 0.02, p = 0.895]. The correlation coefficients between the audio and visual modalities were significantly different across all participants [p = 0.024] and the older adults [p = 0.045] but not for the young adults [p = 0.268].
3.2.2.2.2. Beta frequency band.
The cluster analysis identified a significant correlation across all participants in the visual condition between the beta power subsequent memory contrast and high confidence visual Pr in 26 widespread electrodes between 3200 and 3400 ms (1450–1650 ms poststimulus) [r(45) = −0.49, p < 0.001], as seen in Fig. 3D. Investigating each age groups separately revealed that the correlation remained significant in the young [r(22) = −0.567, p = 0.004] but failed to reach significance in the old [r(21) = −0.295, p = 0.172], although the correlation coefficients were not significantly different [p = 0.277].
Follow-up analyses found this cluster was not significant in the audio condition for the young [r(22) = −0.051, p = 0.814], the old [r(21) = −0.191, p = 0.383], or across groups [r(45) = −0.036, p 0.81]. The correlation coefficients between the audio and visual modalities were significantly different across all participants [p = 0.019] and marginally different in the young adults [p = 0.055] but not in the older adults [p = 0.726].
3.2.2.3. Modality-specific group differences in poststimulus theta.
The cluster analysis identified a significant correlation for older adults in the visual condition between the theta power subsequent memory contrast and high confidence visual Pr across 16 frontocentral electrodes between 2100 and 3400 ms (350–1650 ms poststimulus) [r(21) = 0.58, p = 0.004]. For young adults, this cluster correlation was not significant [r(22) = −0.255, p = 0.229], and the correlation coefficients were significantly different from each other [p = 0.003], as seen in Fig. 3C. In addition, the correlation was not significant across both groups [r(45) = 0.041, p = 0.783].
Follow-up analyses found this cluster was not significant in the audio condition for the young [r(22) = −0.134, p = 0.532], the old [r(21) = 0.027, p = 0.901], or across groups [r(45) = −0.056, p = 0.708]. The correlation coefficients between the audio and visual modalities were significantly different for the old [p = 0.044] but not the young [p = 0.683] or across all participants [p = 0.649].
3.2.3. Time-frequency summary
The time-frequency analyses found that greater posterior alpha desynchronization correlated with higher memory performance for audio trials across all participants in both the prestimulus and poststimulus time intervals. In addition, on average power, widespread poststimulus alpha power desynchronization was greater for remembered compared with forgotten trials across both modalities and age groups. Within the beta frequency band, widespread poststimulus beta power desynchronization was greater for remembered compared with forgotten items across both modalities and age groups. In addition, a late onsetting widespread beta desynchronization correlated with higher memory performance for visually presented items across age groups.
We found an age group difference within the theta frequency band. For older adults, greater poststimulus theta synchronization correlated with higher memory performance for visually presented item. No relationship was found between theta power and memory performance for young adults.
4. Discussion
Given accumulating research showing that the time before a to-be-remembered event influences the successful encoding of an event, we investigated neural oscillations both before (i.e., cue-stimulus interval) and after (poststimulus interval) the to-be-remembered event during encoding. We investigated the extent to which age-related episodic memory impairments could be attributed to domain-specific and domain-general processes that support encoding both before and after stimulus onset. Behaviorally, we found young adults remembered more events than the older adults, and that memory was worse for audio items compared to visual items in both age groups. Before stimulus, greater alpha desynchronization in the subsequent memory contrast correlated with higher audio memory performance across both young and older adults. After stimulus, high confident hit items had greater alpha and beta desynchronization than forgotten items across age groups, but the effect was marginally larger in the young. In addition, greater poststimulus alpha in the subsequent memory contrast correlated with higher audio memory performance across age groups. For the beta frequency band, greater poststimulus power in the subsequent memory contrast correlated with higher visual memory performance. Finally, for older adults only, greater theta synchronization in the subsequent memory contrast correlated with higher visual memory performance. These results and their implications are discussed below.
4.1. Behavioral results
Across age groups, memory accuracy for auditorily presented words was lower than that for visually presented words. This was not expected as a previous study using a design similar to our own showed no difference in memory performance between these stimulus modalities (Otten et al., 2010). Anecdotally, some participants, particularly older adults, commented that some auditory stimuli were more difficult to perceive than visual stimuli. One possible explanation for this difficulty is that the auditory stimuli were recorded in a female voice and aging is associated with loss of hearing for higher frequencies (Ferrand, 2002). If the auditory stimuli had been recorded in a male voice, with a deeper voice, we may have seen better memory performance for auditory items. Indeed, in the study by Otten et al., (2010), words were recorded in a male voice (Otten et al., 2010). Interestingly, we suspect that the perceived difficulty difference between visual and auditory trials may have contributed to at least some of the modality differences in the EEG oscillatory patterns, discussed below. Future research modulating stimulus quality within a single presentation modality would help directly answer this question.
4.2. Prestimulus memory effect is sensitive to modality but not age
As discussed in Section 1, we wanted to determine whether anticipatory engagement of domain-specific perceptual and/or domain-general encoding processes contribute to subsequent memory accuracy and the extent to which aging impacts this engagement. For auditorily presented words only, greater prestimulus alpha desynchronization for subsequent hits than forgotten items over centroposterior scalp sites was predictive of better memory accuracy for auditory events across the age groups. Both alpha and beta desynchronization is believed to reflect the active engagement of specialized neocortical areas sensitive to processing or maintaining the perceived materials (for reviews: Engel and Fries, 2010; Hanslmayr et al., 2016). Furthermore, anticipation of auditory stimuli has been associated with alpha oscillations over similar centroposterior scalp sites (Mazaheri et al., 2014). We suggest that the prestimulus memory effect observed here reflects the early activation of cortical areas that supports successful encoding of auditory events. These results are consistent with attention studies in which prestimulus cues facilitate early engagement of the domain-specific cortical areas engaged by the stimuli (for review: Arnal and Giraud, 2012; Driver and Frith, 2000; Foxe and Snyder, 2011; Luck et al., 1997). The pattern of centroposterior alpha desynchronization persisted through the stimulus period supporting the idea that early engagement of the processes that support subsequent speech perception supports later memory performance for these events. An alternative and not-mutually exclusive interpretation is that alpha desynchronization in the present study reflects the engagement of general attentional processes (for review: Klimesch, 2012). Given the lower performance of the audio items in both groups, higher levels of attention may have been required to perform well on the audio trials. The lack of age group differences was unexpected, given that previous research has shown older adults shift away from using proactive control (or preparatory process) during cognitive control tasks (for review: Braver, 2012). But it is not unfounded, as healthy older adults have been shown to flexibly shift between proactive and reactive control based on the specific task demands (Braver et al., 2009). Thus, regardless of age, individuals who mobilized preparatory processes during audio trials showed higher performance.
It is not immediately clear why prestimulus memory effects were found for audio and not for visually presented words. Based on previous ERP evidence, we had predicted that similar prestimulus memory effects would be observed for both modalities (Otten et al., 2006, 2010). One possible explanation may relate to the unexpected difficulty confound between the modalities. As discussed earlier, memory performance was worse for auditory than for visual items. Thus, the prestimulus activity pattern observed here together with the greater difficulty for the audio trials may suggest that anticipatory activation of speech processing areas was observed at least in part because these trials were more difficult to encode. One could argue that the centroposterior alpha desynchronization SME was not observed for visual trials not because it is auditory-related but because visual trials were easier to encode and placed lower demands on early mobilization of top-down attention. Although we cannot rule out this possibility, the lack of relationship between the magnitude of this effect and memory accuracy for visual trials, such that even low performing individuals showed no visual pre-SME, speaks against it. Another nonmutually exclusive possibility is that the prestimulus alpha effect for auditory trials reflects greater motivation (Gruber et al., 2013; Gruber and Otten, 2010) to attend the upcoming audio stimulus. As suggested previously, some participants found the audio items perceptually more difficult than the visual items. Collectively, these results support the idea that prestimulus memory effects are affected by numerous factors that may influence preparation including perceived difficulty (Speer et al., 2003), reward (Gruber et al., 2013; Gruber and Otten, 2010) and the particular demands of the subsequent task (Otten et al., 2006; Padovani et al., 2011).
4.3. Poststimulus memory effects are sensitive to modality and age
Consistent with previous studies, widespread desynchronization in the alpha and beta frequency range was greater for subsequent hits than forgotten trials (Hanslmayr et al., 2009; Klimesch et al., 1996; Minarik et al., 2018) between 900 and 1600 ms after stimulus onset. This SME was additionally insensitive to modality and age. The spatial distribution of this effect and the fact that it was similar across stimulus modalities suggest that it is likely supported by multiple neural generators and reflects several underlying processes that facilitate learning, including semantic elaboration and visual imagery. Specifically, given the verbal nature of the stimuli and the elaborative orienting task (“is this bigger than a monitor?”), it seems likely that semantic elaboration is one contributor to the SME effect. Similar SMEs in a similar time range within the beta band have been observed for verbal stimuli encoded in a deep but not a shallow orienting task (Hanslmayr et al., 2009). Transcranial magnetic stimulation evidence suggests that this desynchronization causally contributes to successful encoding (Hanslmayr et al., 2014). Previous EEG evidence showing greater beta desynchronization for real than pseudo words, and intracranial data linking this activity to the left inferior frontal gyrus, suggest a role for beta oscillations in semantic processing (Hanslmayr et al., 2011). The similar spatial distribution and time course of the alpha SME shown here is consistent with data showing that alpha desynchronization may also support semantic processing (for review: Klimesch, 1999). Collectively, these findings together with the present results are consistent with the well-established idea that elaborative encoding facilitates episodic memory success (for review: Craik and Lockhart, 1972) and that older adults can effectively use this strategy to support episodic memory when instructed to do so (Naveh-Benjamin et al., 2007). However, the beta desynchronization SME was somewhat reduced over frontal sites for old relative to young adults, particularly for visual trials late in the encoding epoch (1250–1550 ms). One possible explanation is that abbreviated semantic elaboration contributes to poorer encoding in older adults. Another possibility is that slight differences in the forgotten trial composition could have influenced the marginal beta effect. Given the paucity of data investigating age-related changes in oscillatory EEG, future studies manipulating the orienting task demands will be necessary to draw more definitive conclusions regarding these group differences.
In addition to the modality invariant poststimulus SMEs described previously, there were also modality-specific correlations between poststimulus activity and subsequent memory accuracy. A widespread beta desynchronization was greater for subsequent confident hits than forgotten visually presented words and predictive of better memory accuracy across age. This correlation was observed relatively late during encoding and overlapped both spatially and temporally with the average SME, described previously, that was modality and age invariant. Thus, although this correlation was specific to the visual modality, the cognitive operations underlying this effect also support successful encoding for the auditory modality. We believe it is most likely that individuals who engaged in continued semantic elaboration and visual imagery to make size judgments were more likely to remember visually presented words. As discussed previously, the anticipatory engagement of perceptual processes for auditorily presented words before stimulus onset and continuing through the stimulus period may have reduced the need for protracted encoding. Future studies manipulating stimulus modality together with other factors that influence engagement of prestimulus activity, including reward (Gruber et al., 2013), will be helpful in evaluating this possibility.
Contrary to what one might predict based on some prior evidence (Crespo-Garcia et al., 2012; Rondina et al., 2016), there was no evidence that subsequent memory activity in the theta band was reduced by age. In fact, older, but not young adults, showed greater mid-frontal theta power for subsequent confident hits than forgotten visual trials that were predictive of greater memory accuracy. We had predicted that this effect would be observed both preceding and following stimulus onset, consistent with previous findings in young adults (Addante et al., 2011; Fell et al., 2011; Gruber et al., 2008, 2013; Guderian et al., 2009; Hanslmayr et al., 2009; Staudigl and Hanslmayr, 2013). Furthermore, the correlation between the magnitude of the theta SME and memory accuracy that we observed for older adults has been shown for young adults in previous studies (Gruber et al., 2013). When considering these findings, it is important to discuss differences between the memory tasks and memory measures cross studies. In the present study, we compared activity between words subsequently recognized with high confidence with those recognized with low confidence or not recognized. Although events that are recollected are typically associated with high confidence judgments, it is also possible for events recognized on the basis of familiarity, for which no episodic details are recollected, to be based on high confidence (Yonelinas, 1994). As we did not direct participants to only respond with high confidence if they could recover specific episodic details or assess memory for objective details (i.e., source, context), we cannot be certain that our EEG contrast nor our estimate of memory accuracy is sensitive to recollection, exclusively.
Previous studies showing midfrontal theta band SMEs have often assessed memory directly for episodic details including object-location associations (Rondina et al., 2016), subjective recollection (Gruber et al., 2008), or source memory accuracy (Addante et al., 2011). These results have been taken as consistent with computational models suggesting that theta oscillations, generated by the hippocampus, facilitate episodic memory via functional interactions between the hippocampus and the cortex (Duzel et al., 2010; Hasselmo and Eichenbaum, 2005; Nyhus and Curran, 2010) and with the well-known critical role of the hippocampus in memory for episodic details (for review: Eichenbaum et al., 2007). Although it is unlikely that the theta SME measured at the scalp directly reflects hippocampal oscillations, due to its subcortical location, the long-range hippocampal-cortical theta interaction effects would be measurable (for review: Hsieh and Ranganath, 2014; Nyhus and Curran, 2010).
An alternative interpretation of increased theta power for older adults is that those who were reliant on reactive control processes were more successful at encoding the information. Older adults are prone to shift to reactive control strategies1 (Braver, 2012; Braver et al., 2005) and reactive control has been shown to increase frontal theta power and frontoparietal connectivity (for review: Cavanagh and Frank, 2014; Cooper et al., 2015). Given that theta synchronization is generally associated with the working memory control processes involved in numerous cognitive processes (for review: Hanslmayr and Staudigl, 2014), and without an assessment of proactive or reactive control within the task, the influence of reactive control on theta power is speculative. However, if reactive control was benefiting older adults, then we would have expected to find a negative prestimulus (i.e., proactive) effect. However, we should note using proactive and reactive control processes are not mutually exclusive, and a shift in control strategy is not necessarily reflective of worse performance (for review: Braver, 2012; Braver et al., 2001, 2009, 2005). We believe the most likely explanation for the lack of theta SME before or after stimulus onset for young adults may be a consequence of the manner in which we assessed memory success.
If the memory measure used in the present study was not particularly sensitive to the processes supported by mid-frontal theta synchrony, what might explain the beneficial impact of theta synchronization on subsequent memory accuracy for older adults? One possible explanation is that older adults either generated a greater number of episodic associations during encoding and/or used these associations to support their item recognition decisions to a greater extent than young adults. Such an explanation is consistent with the previously observed discrepancy between age-related declines in objective and subjective tests of recollection (Ciaramelli and Ghetti, 2007; Duarte et al., 2006; Duarte et al., 2008; Mark and Rugg, 1998). Specifically, age-related declines in source or context memory are common despite relatively intact subjective reports of recollection, such as age equivalency in “remember” that are often rich in detail (Gallo et al., 2011). Subjective recollection can be based on less differentiated information or self-generated associations (i.e., thoughts, feelings) that are not typically assessed. By contrast, objective recollection requires participants to successfully bind experimental associations (e.g., spatial location, color, and modality) to studied materials and recover those specific associations during retrieval. The greater dependence of objective recollection than subjective recollection on executive functioning, which is disrupted by age, contributes to older adults’ disproportionate impairment for objective memory tests (Duarte et al., 2006, 2008). If older adults in the present study based their item recognition decisions on recollection of thoughts and feelings associated with the stimuli, which they are more likely to self-generate than young adults (Carstensen and Turk-Charles, 1994; Comblain et al., 2004; Hashtroudi et al., 1990; Kensinger et al., 2014; Leshikar et al., 2015), then the theta synchrony effect may be unsurprising. We predict that if we had assessed recollection objectively, young adults, to a greater extent than older adults, would show the positive association between theta synchrony and memory accuracy that was shown here for older adults. An interesting future study would be to compare the relationship between the oscillatory activity patterns observed here and subjective and objective recollection memory tests. Importantly, the present pattern of results supports the idea that hippocampal integrity and/or hippocampal-cortical communication is not necessarily negatively impacted by age (Duarte et al., 2008; Dulas and Duarte, 2012; Morcom et al., 2007).
5. Conclusion
Our results are consistent with the idea that brain activity preceding to-be-encoded events, similar to that engaged during encoding, is beneficial for subsequent memory performance and that this anticipatory activity can be spared by age. Oscillatory patterns of activity were largely similar across age, as consistent with data showing that older adults can successfully encode when given effective encoding tasks. A greater reliance on theta oscillations for old than young adults may reflect older adults’ greater tendency to self-generate associations that may support their episodic memory accuracy when tasks are not constrained to objective experimental associations. We further suggest that the use and manifestation of preparatory processes is dependent on the specific task demands and individual differences in using preparation. In the current task, these demands are likely to include information about the orienting task (i.e., size judgment), stimulus presentation (i.e., visual or audio), the level of engagement (i.e., attention), and other learned information (e.g., difficulty). Future research that focuses on separating the contribution of these information components may aid in understanding how preparation influences successful encoding. For example, including a neutral trial condition would help control for general attentional processes. Another interesting manipulation would be to include multiple task and stimulus conditions, where the cue could indicate both task and stimulus or a single dimension. Given that informative cues with higher levels of information would reduce ambiguity during stimulus presentation, older adults may benefit to a greater extent than younger adults by using the advanced information and engaging in preparatory processes. Regardless of the exact process, prestimulus neural activity carries information related to successful long-term memory encoding in both young and older adults.
Acknowledgements
This study was supported by the National Science Foundation under Grant Number BCS-1125683. This research was also supported in part by a NIA Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant, Grant #5T32AG000175.
Footnotes
Disclosure
The authors report no conflicts of interest.
Behavioral results from the AX-CPT filler task found response patterns suggestive of proactive control in both young and older adults. In addition, the error rates and standardized reaction times did not differ between age groups.
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD, 2006. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron 50, 507–517. [DOI] [PubMed] [Google Scholar]
- Addante RJ, de Chastelaine M, Rugg MD, 2015. Pre-stimulus neural activity predicts successful encoding of inter-item associations. Neuroimage 105, 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addante RJ, Watrous AJ, Yonelinas AP, Ekstrom AD, Ranganath C, 2011. Prestimulus theta activity predicts correct source memory retrieval. Proc. Natl. Acad. Sci. U S A 108, 10702–10707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Saxe R, Yarkoni T, 2014. Contributions of episodic retrieval and mentalizing to autobiographical thought: evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. Neuroimage 91, 324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnal LH, Giraud A-L, 2012. Cortical oscillations and sensory predictions. Trends Cogn. Sci 16, 390–398. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M, Mazaheri A, Jensen O, 2012. Beyond ERPs: oscillatory neuronal dynamics. In: The Oxford Handbook of Event-Related Potential Components Oxford University Press, New York, NY, pp. 31–50. [Google Scholar]
- Bell AJ, Sejnowski TJ, 1995. An information-maximization approach to blind separation and blind deconvolution. Neural Comput 7, 1129–1159. [DOI] [PubMed] [Google Scholar]
- Blair RC, Karniski W, 1993. An alternative method for significance testing of waveform difference potentials. Psychophysiology 30, 518–524. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ, 1999. Affective Norms for English Words (ANEW): Instruction Manual and Affective Ratings. Vol. 30, No. 1, pp. 25–36. Technical Report C-1, the Center for Research in Psychophysiology, University of Florida. [Google Scholar]
- Braver TS, 2012. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn. Sci 16, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Keys BA, Carter CS, Cohen JD, Kaye JA, Reed BR, 2001. Context processing in older adults: evidence for a theory relating cognitive control to neurobiology in healthy aging. J. Exp. Psychol. Gen 130, 746–763. [PubMed] [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM, 2009. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc. Natl. Acad. Sci. U S A 106, 7351–7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Satpute AB, Rush BK, Racine CA, Barch DM, 2005. Context processing and context maintenance in healthy aging and early stage dementia of the Alzheimer’s type. Psychol. Aging 20, 33–46. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Turk-Charles S, 1994. The salience of emotion across the adult life span. Psychol. Aging 9, 259. [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, 2014. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci 18, 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramelli E, Ghetti S, 2007. What are confabulators’ memories made of? A study of subjective and objective measures of recollection in confabulation. Neuropsychologia 45, 1489–1500. [DOI] [PubMed] [Google Scholar]
- Cohen MX, 2014. Analyzing Neural Time Series Data: Theory and Practice The MIT Press, Cambridge, Massachusetts. [Google Scholar]
- Cohen N, Pell L, Edelson MG, Ben-Yakov A, Pine A, Dudai Y, 2015. Periencoding predictors of memory encoding and consolidation. Neurosci. Biobehav. Rev 50, 128–142. [DOI] [PubMed] [Google Scholar]
- Coltheart M, 1981. The MRC psycholinguistic database. Q. J. Exp. Psychol 33 (Sec A), 497–505. [Google Scholar]
- Comblain C, D’Argembeau A, Van der Linden M, Aldenhoff L, 2004. The effect of ageing on the recollection of emotional and neutral pictures. Memory 12, 673–684. [DOI] [PubMed] [Google Scholar]
- Cooper PS, Wong AS, Fulham WR, Thienel R, Mansfield E, Michie PT, Karayanidis F, 2015. Theta frontoparietal connectivity associated with proactive and reactive cognitive control processes. Neuroimage 108, 354–363. [DOI] [PubMed] [Google Scholar]
- Craik FI, Lockhart RS, 1972. Levels of processing: a framework for memory research. J. Verbal Learn. Verbal Behav 11, 671–684. [Google Scholar]
- Craik FI, Rose NS, 2012. Memory encoding and aging: a neurocognitive perspective. Neurosci. Biobehav Rev 36, 1729–1739. [DOI] [PubMed] [Google Scholar]
- Crespo-Garcia M, Cantero JL, Atienza M, 2012. Effects of semantic relatedness on age-related associative memory deficits: the role of theta oscillations. Neuroimage 61, 1235–1248. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S, 2004. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21. [DOI] [PubMed] [Google Scholar]
- Delorme A, Mullen T, Kothe C, Akalin Acar Z, Bigdely-Shamlo N, Vankov A, Makeig S, 2011. EEGLAB, SIFT, NFT, BCILAB, and ERICA: new tools for advanced EEG processing. Comput. Intell. Neurosci 2011, 130714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Sejnowski T, Makeig S, 2007. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage 34, 1443–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R, 2008. Neuroimaging of healthy cognitive aging. In: Craik FIM, Salthouse TA (Eds.), The Handbook of Aging and Cognition, third ed. Psychology Press, New York, pp. 1–54. [Google Scholar]
- Dennis NA, Daselaar S, Cabeza R, 2007. Effects of aging on transient and sustained successful memory encoding activity. Neurobiol. Aging 28, 1749–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R, 2008. Effects of aging on the neural correlates of successful item and source memory encoding. J. Exp. Psychol. Learn Mem. Cogn 34, 791–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J, Frith C, 2000. Shifting baselines in attention research. Nat. Rev. Neurosci 1, 147. [DOI] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Graham KS, 2008. The effects of aging on the neural correlates of subjective and objective recollection. Cereb. Cortex 18, 2169–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Trujillo C, Knight RT, 2006. Intact recollection memory in high-performing older adults: ERP and behavioral evidence. J. Cogn. Neurosci 18, 33–47. [DOI] [PubMed] [Google Scholar]
- Dulas MR, Duarte A, 2012. The effects of aging on material-independent and material-dependent neural correlates of source memory retrieval. Cereb. Cortex 22, 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzel E, Penny WD, Burgess N, 2010. Brain oscillations and memory. Curr. Opin. Neurobiol 20, 143–149. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C, 2007. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci 30, 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P, 2010. Beta-band oscillationsdsignalling the status quo? Curr. Opin. Neurobiol 20, 156–165. [DOI] [PubMed] [Google Scholar]
- Fell J, Ludowig E, Staresina BP, Wagner T, Kranz T, Elger CE, Axmacher N, 2011. Medial temporal theta/alpha power enhancement precedes successful memory encoding: evidence based on intracranial EEG. J. Neurosci 31, 5392–5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner MC, Bauml KH, Hanslmayr S, 2013. Brain oscillatory subsequent memory effects differ in power and long-range synchronization between semantic and survival processing. Neuroimage 79, 361–370. [DOI] [PubMed] [Google Scholar]
- Ferrand CT, 2002. Harmonics-to-noise ratio: an index of vocal aging. J. voice 16, 480–487. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Snyder AC, 2011. The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front. Psychol 2, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, 2000. Event-related brain potential investigations of memory and aging. Biol. Psychol 54, 175–206. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Korthauer LE, McDonough IM, Teshale S, Johnson EL, 2011. Age-related positivity effects and autobiographical memory detail: evidence from a past/future source memory task. Memory 19, 641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky EL, Rubin SR, Davidson PS, 2001. Source memory in older adults: an encoding or retrieval problem? J. Exp. Psychol. Learn. Mem. Cogn 27, 1131. [DOI] [PubMed] [Google Scholar]
- Golby AJ, Poldrack RA, Brewer JB, Spencer D, Desmond JE, Aron AP, Gabrieli JD, 2001. Material-specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain 124, 1841–1854. [DOI] [PubMed] [Google Scholar]
- Gruber MJ, Otten LJ, 2010. Voluntary control over prestimulus activity related to encoding. J. Neurosci 30, 9793–9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber MJ, Watrous AJ, Ekstrom AD, Ranganath C, Otten LJ, 2013. Expected reward modulates encoding-related theta activity before an event. Neuroimage 64, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber T, Tsivilis D, Giabbiconi CM, Muller MM, 2008. Induced electroencephalogram oscillations during source memory: familiarity is reflected in the gamma band, recollection in the theta band. J. Cogn. Neurosci 20, 1043–1053. [DOI] [PubMed] [Google Scholar]
- Guderian S, Schott BH, Richardson-Klavehn A, Duzel E, 2009. Medial temporal theta state before an event predicts episodic encoding success in humans. Proc. Natl. Acad. Sci. U S A 106, 5365–5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Matuschek J, Fellner M-C, 2014. Entrainment of prefrontal beta oscillations induces an endogenous echo and impairs memory formation. Curr. Biol 24, 904–909. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Spitzer B, Bauml KH, 2009. Brain oscillations dissociate between semantic and nonsemantic encoding of episodic memories. Cereb. Cortex 19, 1631–1640. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Staresina BP, Bowman H, 2016. Oscillations and episodic memory: addressing the synchronization/desynchronization conundrum. Trends Neurosci 39, 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T, 2014. How brain oscillations form memories–a processing based perspective on oscillatory subsequent memory effects. Neuroimage 85 (Pt 2), 648–655. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T, Fellner MC, 2012. Oscillatory power decreases and long-term memory: the information via desynchronization hypothesis. Front. Hum. Neurosci 6, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Volberg G, Wimber M, Raabe M, Greenlee MW, Bauml KH, 2011. The relationship between brain oscillations and BOLD signal during memory formation: a combined EEG-fMRI study. J. Neurosci 31, 15674–15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashtroudi S, Johnson MK, Chrosniak LD, 1990. Aging and qualitative characteristics of memories for perceived and imagined complex events. Psychol. Aging 5, 119. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Eichenbaum H, 2005. Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Netw 18, 1172–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S, Falkenstein M, 2008. The correction of eye blink artefacts in the EEG: a comparison of two prominent methods. PLoS One 3, e3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh L-T, Ranganath C, 2014. Frontal midline theta oscillations during working memory maintenance and episodic encoding and retrieval. Neuroimage 85, 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, 1996. Feature memory and binding in young and older adults. Mem. Cognit 24, 403–416. [DOI] [PubMed] [Google Scholar]
- Kardos Z, Toth B, Boha R, File B, Molnar M, 2014. Age-related changes of frontal-midline theta is predictive of efficient memory maintenance. Neuroscience 273, 152–162. [DOI] [PubMed] [Google Scholar]
- Karrasch M, Laine M, Rapinoja P, Krause CM, 2004. Effects of normal aging on event-related desynchronization/synchronization during a memory task in humans. Neurosci. Lett 366, 18–23. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Allard ER, Krendl AC, 2014. The effects of age on memory for socioemotional material: an affective neuroscience perspective. In: The Oxford Handbook of Emotion, Social Cognition, and Problem Solving in Adulthood Oxford University Press, New York, NY, pp. 26–46. [Google Scholar]
- Khader PH, Rösler F, 2011. EEG power changes reflect distinct mechanisms during long-term memory retrieval. Psychophysiology 48, 362–369. [DOI] [PubMed] [Google Scholar]
- Klimesch W, 1999. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Brain Res. Rev 29, 169–195. [DOI] [PubMed] [Google Scholar]
- Klimesch W, 2012. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci 16, 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Schimke H, Doppelmayr M, Ripper B, Schwaiger J, Pfurtscheller G, 1996. Event-related desynchronization (ERD) and the Dm effect: does alpha desynchronization during encoding predict later recall performance? Int. J. Psychophysiol 24, 47–60. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schwaiger J, Auinger P, Winkler T, 1999. Paradoxical’ alpha synchronization in a memory task. Brain Res. Cogn. Brain Res 7, 493–501. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S, 2007. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res. Rev 53, 63–88. [DOI] [PubMed] [Google Scholar]
- Koen JD, Horne ED, Hauck N, Rugg MD, 2018. Age-related differences in prestimulus subsequent memory effects assessed with event-related potentials. J. Cogn. Neurosci 30, 829–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kučera H, Francis WN, 1967. Computational Analysis of Present-Day American English Dartmouth Publishing Group, Providence, R.I. [Google Scholar]
- Leshikar ED, Dulas MR, Duarte A, 2015. Self-referencing enhances recollection in both young and older adults. Aging Neuropsychol. Cogn 22, 388–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Calderon J, Luck SJ, 2014. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front Hum. Neurosci 8, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R, 1997. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J. Neurophysiol 77, 24–42. [DOI] [PubMed] [Google Scholar]
- Mackiewicz KL, Sarinopoulos I, Cleven KL, Nitschke JB, 2006. The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proc. Natl. Acad. Sci. U S A 103, 14200–14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R, 2007. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190. [DOI] [PubMed] [Google Scholar]
- Mark RE, Rugg MD, 1998. Age effects on brain activity associated with episodic memory retrieval. An electrophysiological study. Brain 121 (Pt 5), 861–873. [DOI] [PubMed] [Google Scholar]
- Mazaheri A, van Schouwenburg MR, Dimitrijevic A, Denys D, Cools R, Jensen O, 2014. Region-specific modulations in oscillatory alpha activity serve to facilitate processing in the visual and auditory modalities. Neuroimage 87, 356–362. [DOI] [PubMed] [Google Scholar]
- Merkow MB, Burke JF, Stein JM, Kahana MJ, 2014. Prestimulus theta in the human hippocampus predicts subsequent recognition but not recall. Hippocampus 24, 1562–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minarik T, Berger B, Sauseng P, 2018. The involvement of alpha oscillations in voluntary attention directed towards encoding episodic memories. Neuroimage 166, 307–316. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD, 2007. Age effects on the neural correlates of episodic retrieval: increased cortical recruitment with matched performance. Cereb. Cortex 17, 2491–2506. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Chertkow H, 2005. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc 53, 695–699. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Brav TK, Levy O, 2007. The associative memory deficit of older adults: the role of strategy utilization. Psychol. Aging 22, 202–208. [DOI] [PubMed] [Google Scholar]
- Nuwer MR, Comi G, Emerson R, Fuglsang-Frederiksen A, Guerit JM, Hinrichs H, Rappelsberger P, 1999. IFCN standards for digital recording of clinical EEG. The international federation of clinical neurophysiology. Electroencephalogr. Clin. Neurophysiol 52, 11–14. [PubMed] [Google Scholar]
- Nyhus E, Curran T, 2010. Functional role of gamma and theta oscillations in episodic memory. Neurosci. Biobehav Rev 34, 1023–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old SR, Naveh-Benjamin M, 2008. Differential effects of age on item and associative measures of memory: a meta-analysis. Psychol. Aging 23, 104. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen J-M, 2011. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci 2011, 156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ, Quayle AH, Akram S, Ditewig TA, Rugg MD, 2006. Brain activity before an event predicts later recollection. Nat. Neurosci 9, 489–491. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Quayle AH, Puvaneswaran B, 2010. Prestimulus subsequent memory effects for auditory and visual events. J. Cogn. Neurosci 22, 1212–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD, 2001. Electrophysiological correlates of memory encoding are task-dependent. Brain Res. Cogn. Brain Res 12, 11–18. [DOI] [PubMed] [Google Scholar]
- Padovani T, Koenig T, Brandeis D, Perrig WJ, 2011. Different brain activities predict retrieval success during emotional and semantic encoding. J. Cogn. Neurosci 23, 4008–4021. [DOI] [PubMed] [Google Scholar]
- Paller KA, Kutas M, Mayes AR, 1987. Neural correlates of encoding in an incidental learning paradigm. Electroencephalogr Clin. Neurophysiol 67, 360–371. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD, 2002. Observing the transformation of experience into memory. Trends Cogn. Sci 6, 93–102. [DOI] [PubMed] [Google Scholar]
- Park H, Rugg MD, 2010. Prestimulus hippocampal activity predicts later recollection. Hippocampus 20, 24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastotter B, Schicker S, Niedernhuber J, Bauml KHT, 2011. Retrieval during learning facilitates subsequent memory encoding. J. Exp. Psychol. Learn. Mem. Cogn 37, 287–297. [DOI] [PubMed] [Google Scholar]
- Percival DB, Walden AT, 1993. Spectral Analysis for Physical Applications: Multitaper and Conventional Univariate Techniques Cambridge University Press, Cambridge, New York, NY. [Google Scholar]
- Rondina R II, Olsen RK, McQuiggan DA, Fatima Z, Li L, Oziel E, Meltzer JA, Ryan JD, 2016. Age-related changes to oscillatory dynamics in hippocampal and neocortical networks. Neurobiol. Learn. Mem 134, 15–30. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Johnson JD, Park H, Uncapher MR, 2008. Encoding-retrieval overlap in human episodic memory: a functional neuroimaging perspective. Prog. Brain Res 169, 339–352. [DOI] [PubMed] [Google Scholar]
- Salari N, Rose M, 2016. Dissociation of the functional relevance of different prestimulus oscillatory activity for memory formation. Neuroimage 125 (Suppl C), 1013–1021. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gruber WR, Hanslmayr S, Freunberger R, Doppelmayr M, 2007. Are event-related potential components generated by phase resetting of brain oscillations? A critical discussion. Neuroscience 146, 1435–1444. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR, 2003. Theta and gamma oscillations during encoding predict subsequent recall. J. Neurosci 23, 10809–10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J, 1988. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J. Exp. Psychol. Gen 117, 34–50. [DOI] [PubMed] [Google Scholar]
- Speer NK, Jacoby LL, Braver TS, 2003. Strategy-dependent changes in memory: effects on behavior and brain activity. Cogn. Affect Behav. Neurosci 3, 155–167. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N, 1995. Differential effects of aging on memory for content and context: a meta-analysis. Psychol. Aging 10, 527–539. [DOI] [PubMed] [Google Scholar]
- Staudigl T, Hanslmayr S, 2013. Theta oscillations at encoding mediate the context-dependent nature of human episodic memory. Curr. Biol 23, 1101–1106. [DOI] [PubMed] [Google Scholar]
- Staudigl T, Hanslmayr S, Bauml KH, 2010. Theta oscillations reflect the dynamics of interference in episodic memory retrieval. J. Neurosci 30, 11356–11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Stein A, Sarnthein J, 2000. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int. J. Psychophysiol 38, 301–313. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Poldrack RA, Eldridge LL, Desmond JE, Glover GH, Gabrieli JD, 1998. Material-specific lateralization of prefrontal activation during episodic encoding and retrieval. Neuroreport 9, 3711–3717. [DOI] [PubMed] [Google Scholar]
- Waldhauser GT, Braun V, Hanslmayr S, 2016. Episodic memory retrieval functionally relies on very rapid reactivation of sensory information. J. Neurosci 36, 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhauser GT, Johansson M, Hanslmayr S, 2012. alpha/beta oscillations indicate inhibition of interfering visual memories. J. Neurosci 32, 1953–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox RR, Keselman H, 2003. Modern robust data analysis methods: measures of central tendency. Psychol. Methods 8, 254. [DOI] [PubMed] [Google Scholar]
- Wilson M, 1988. Mrc psycholinguistic database - machine-usable dictionary, version 2.00. Behav. Res. Methods Instrum. Comput 20, 6–10. [Google Scholar]
- Xia J, Galli G, Otten LJ, 2018. Brain state before a memory probe and associative retrieval in older adults. Neurobiol. Aging 68, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, 1994. Receiver-operating characteristics in recognition memory: evidence for a dual-process model. J. Exp. Psychol. Learn. Mem. Cogn 20, 1341. [DOI] [PubMed] [Google Scholar]