Abstract
Objective
To provide guidance on how systematic review authors, guideline developers, and health technology assessment practitioners should approach the use of the risk of bias in non-randomized studies of interventions (ROBINS-I) tool as part of GRADE’s certainty rating process.
Study Design and Setting
Iterative discussions, testing in systematic reviews, presentation at GRADE working group meetings with feedback from the GRADE Working Group.
Results
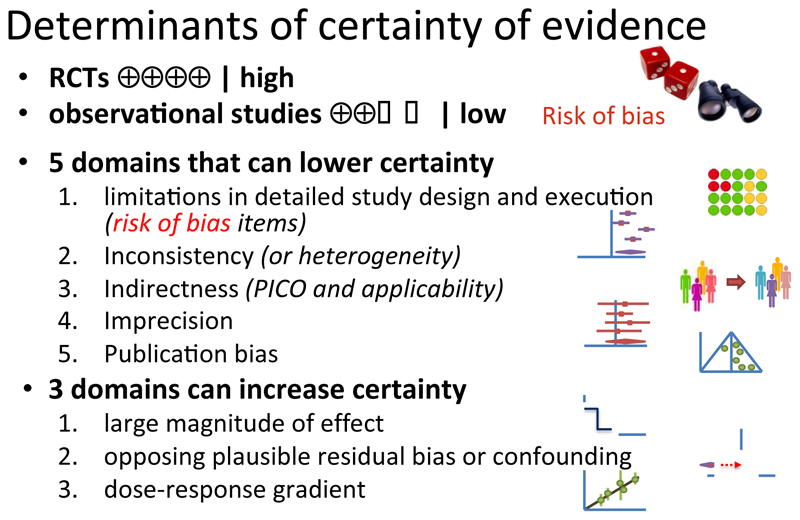
We describe where to start the initial assessment of a body of evidence with the use of ROBINS-I, and where one would anticipate the final rating would end up. GRADE accounted for issues that mitigate concerns about confounding and selection bias by introducing the upgrading domains: large effects, dose-effect relations, and when plausible residual confounders or other biases increase certainty. They will need to be considered in an assessment of a body of evidence when using ROBINS-I.
Conclusions
The use of ROBINS-I in GRADE assessments may allow for a better comparison of evidence from RCTs and NRS because they are placed on a common metric for risk of bias. Challenges remain that include appropriate presentation of evidence from RCTs and NRS for decision-making and how to optimally integrate RCTs and NRS in an evidence assessment.
Keywords: GRADE, quality of evidence, certainty of the evidence, risk of bias, non-randomized studies, ROBINS
1. GRADE’s approach to the certainty of the evidence from observational studies
The GRADE working group has developed a widely accepted approach to rating the certainty of a body of evidence (also known as quality of evidence or confidence in evidence) in the contexts of systematic reviews, developing healthcare recommendations, and supporting decisions. GRADE’s approach to rating the certainty of the evidence is based on a four-level system: high, moderate, low and very low (Table 1). This is the 18th in the ongoing series of articles describing the GRADE approach in the Journal of Clinical Epidemiology and complements articles in other journals. In previous GRADE articles we have described the reasons for decreasing and increasing the certainty of a body of evidence; how an overall rating of the evidence is performed; how evidence is utilized to move to recommendations and decisions; dealt with particular circumstances of diagnostic, prognostic, equity-related, multiple treatment comparison, environmental and public health questions; how GRADE applies to rapid advice; and when there is missing outcome data.(1–17)
Table 1.
Use of GRADE not considering ROBINS-I and similar tools: According to GRADE, certainty, quality, strength of the evidence or the confidence in the estimate of effect, is determined for each outcome based on a systematic review of the evidence for each outcome. For recommendations, the overall certainty is determined across outcomes based on the lowest quality outcome among those critical for decision-making for the specific context.
| 1. Establish initial level of certainty (as implemented in current GRADE) | 2. Consider lowering or raising level of certainty | 3. Final level of certainty rating | ||||
|---|---|---|---|---|---|---|
| Study design | Initial certainty in the evidence |  |
Reasons for considering lowering or raising certainty |  |
Certainty in the evidence across those considerations | |
| ↓ Lower if | ↑ Higher if* | |||||
| Randomized trials → | High certainty | Risk of Bias | Large effect |
High ⊕⊕⊕⊕ |
||
| Inconsistency | Dose response | |||||
| Indirectness |
All plausible confounding and bias
|
Moderate ⊕⊕⊕○ |
||||
| Imprecision | ||||||
| Observational studies → | Low certainty | Publication bias |
Low ⊕⊕○○ |
|||
|
Very low ⊕○○○ |
||||||
Criteria for upgrading the quality are usually only applicable to observational studies without any reason for rating down.
The current GRADE approach for a body of evidence relating to interventions begins by placing studies in one of two categories: randomized controlled trials (RCT) and observational studies (otherwise known as non-randomized studies, or NRS). GRADE considers non-randomized trials, cohort studies, case-control studies, interrupted-time series (if not randomized), cross sectional studies, case series, case reports and other types of non-randomized studies as observational studies.
According to existing GRADE guidance for interventions, the process of rating a body of evidence (typically several or many studies) begins by classifying the design the relevant studies have used. If the relevant studies are randomized trials, the body of evidence begins as high certainty. If the relevant studies are observational, the body of evidence begins as low certainty. This initial rating is followed by consideration of eight domains, five of which may result in rating down certainty, and three in rating up.(8)
The separation of randomized and observational studies was primarily a result of recognition that randomization is the only way to fully protect against confounding (i.e. imbalance in prognostic factors between intervention and control groups), and that confounding is always a concern in even the most rigorously conducted observational studies. The imbalance in unknown prognostic factors that exists after statistical adjustment or stratified analysis to account for known variables which are not balanced in the exposed and the control groups is known as residual confounding.
The choice of starting observational studies at low rather than moderate or very low certainty followed intense discussion in the GRADE working group’s early days, and was based on the group’s assessment of the magnitude of the potential for residual confounding, and the limited protection against bias provided by adjusted analysis in observational studies. An alternative way of understanding GRADE is that randomization is one of the reasons for rating certainty up as a measure to protect against confounding and selection bias.
2. Rating risk of bias in individual observational studies
Consider now the assessment of risk of bias in individual observational studies, which in the GRADE approach might lead to further rating down quality from low to very low. Investigators have developed many assessment tools for rating risk of bias in observational studies. Most of the instruments address a specific type of observational or non-randomized design (e.g. cohort or case-control) (18), and seek to determine how well, relative to a perfect observational study of that particular design, the individual study at hand was conducted. An alternative approach is to determine risk of bias of observational studies in relation to the effect that would be seen in a high quality randomized trial. Such a trial avoids both confounding (through random allocation to interventions) as well as other sources of bias such as selection or information biases.
The risk of bias in non-randomized studies of interventions (ROBINS-I) tool, rather than using the ideal observational study as a standard, addresses risk of bias using an absolute scale approach.(19) ROBINS-I evaluates risk of bias in estimates of the effects (harm or benefit) of one or more interventions from studies that did not use randomization to allocate units (individuals or clusters of individuals) to comparison groups (in GRADE terminology observational studies).
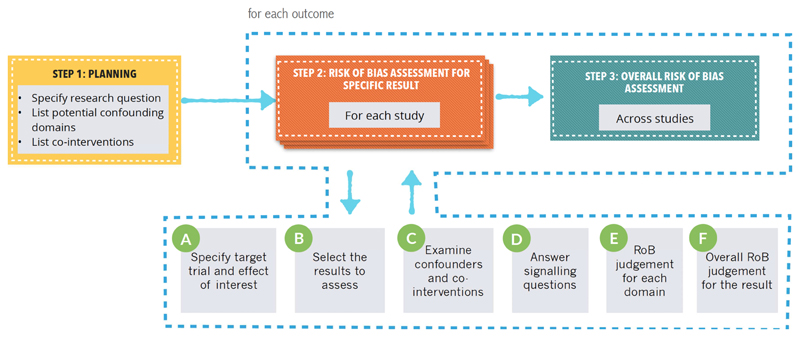
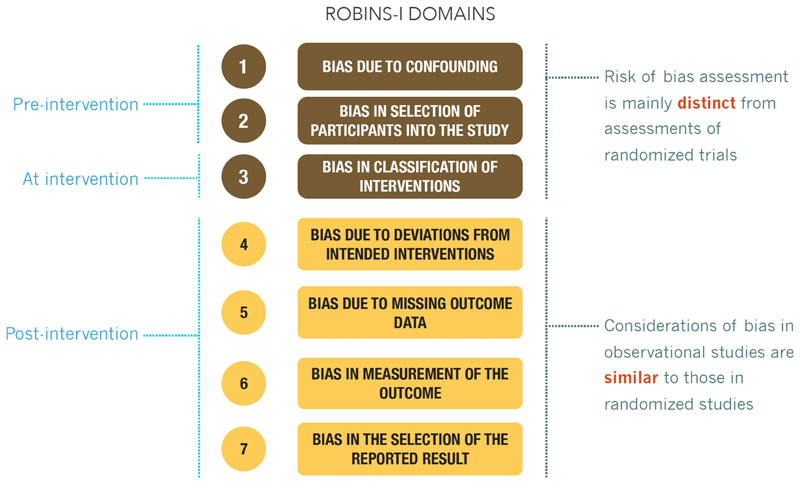
ROBINS-I’s fundamental underlying principles are that (1) the study’s risk of bias is compared against a target RCT, even if this RCT may not be feasible or ethical; (2) the assessment of confounding and selection bias are integral parts of the tool; and (3) for a given result for a specific outcome, evidence from an NRS is assessed, addressing a number of domains and then giving an overall rating per outcome for each study. Figure 1 describes the application of ROBINS-I. Signaling questions in the ROBINS-I instrument ask respondents to rate RoB in domains of 1) Bias due to confounding, 2) Bias in selection of participants into the study, 3) Bias in classification of interventions, 4) Bias due to departures from intended interventions, 5) Bias due to missing data, 6) Bias in measurement of outcomes, and 7) Bias in selection of reported results (Figure 2). In addition, ROBINS-I includes an optional judgment about the direction of the bias for each domain. ROBINS-I has undergone careful development by a large group of experienced investigators. It has been tested and scientists have begun to validate it, and experience will continue to accumulate.
Figure 1. The process for using ROBINS-I.
Figure 2. ROBINS-I risk of bias domains.
In GRADE risk of bias is a domain and ROBINS-I domains are called items)
3. ROBINS-I and GRADE
The arrival of ROBINS-I presents a number of opportunities for the GRADE approach. First, it offers an alternative terminology: establishing NRS rather than observational studies. Although not different in intended meaning in the GRADE approach, substituting NRS for observational studies will lead to a more transparent separation of studies based on their design. For instance, some have struggled with the classification of certain types of studies, such as non-randomized before-after studies as observational; in the alternative nomenclature, such studies are clearly non-randomized. How to classify studies that allocate by essentially random processes such as date of birth or hospital ID number, in which the concern is lack of concealment rather than confounding bias per se, may remain a matter of debate that we will not address here. We will use the term NRS from here on in this article.
Second, the use of ROBINS-I in GRADE assessments may allow for a better comparison of evidence from RCTs and NRS because they are placed on a common metric for risk of bias. This article provides guidance regarding how systematic review authors, guideline developers, and health technology assessment practitioners using GRADE might approach the use of ROBINS-I as part of the certainty rating process. The article focuses on where to start the initial assessment of a body of evidence with the use of ROBINS-I, and where one would anticipate the final rating would end up. Implications and requirement for further work are dealt with in the final sections of this article. This article will not resolve all relevant issues, and we plan subsequent articles describing the work of the GRADE RoB in NRS and environmental health project groups (www.gradeworkinggroup.org).
4. Concerns about GRADE’s approach to start NRS at low certainty
Despite GRADE’s broad acceptance in the evidence synthesis community, GRADE’s initial certainty rating of outcome data from NRS as low has led to challenges for some GRADE users. First, users of GRADE may inappropriately double count the risk of confounding and selection bias, initially by starting a body of evidence from NRS as low certainty of the evidence followed by again rating down for unknown confounders (although rating down additionally for failure to accurately measure known confounders and to adjust for these confounders in the analysis would be appropriate (Figure 3)). Second, those working in fields in which RCTs are sparse or not feasible have expressed concerns that NRS in their fields will seldom be rated as high or perhaps even moderate certainty. GRADE has accepted that criticism, highlighted how one may rate up certainty for large effects, a dose-response gradient, and if all plausible biases will strengthen rather than undermine inferences from study results. In this article, we note the merits of a rating system that follows the underlying logic of ROBINS-I and thus may better integrate RCTs and NRS and allow for more detailed assessment of different types of NRS.
Figure 3. The current GRADE approach for certainty of evidence: initial certainty and rating domains.
While best evidence must be used for decision-making, relying on the best available or achievable rather than least biased evidence as a reference standard would lead to differing certainty in decisions based on the questions asked (20). Picture the following: in one scenario for a health care decision RCTs are neither ethical nor feasible and we, therefore, accept that possible confounded NRS are the reference standard for highest feasible certainty. If these studies are available we would express that we have high certainty in the decision despite the fact that confounding may bias the results. In the second scenario, RCTs are feasible and ethical and they become our reference standard for highest feasible certainty. If for this situation, only NRS are available, we would label our health care decision as based on low certainty. Should the certainty of the decision that is based on the respective evidence differ because of what evidence is available or should the certainty depend on what would be the highest possible certainty? It would be illogical to express different certainty for the same degree of bias because of feasibility and ethical reasons. A comparison on an absolute rather than relative (to the feasibility and ethics of an RCT) provides greater transparency. A decision can still be made for both scenarios and for both we should acknowledge the (same) degree of uncertainty. In fact, in most, if not all, areas of health care some interventions are supported by evidence from RCTs and others are not, requiring a common reference standard in order to ensure appropriate communication with target populations.
Third, by beginning the rating of evidence from a body of NRS studies as low certainty, the current GRADE approach fails to consider that a body of evidence from particular NRS designs may more appropriately be rated higher than conventional NRS designs. For instance, interrupted time series with multiple periods and measurements during each period and no other limitations may constitute moderate quality evidence without meeting any of the criteria for rating up (though our efforts to identify examples for such a body of evidence have not yet proved successful) (21).
5. Certainty of evidence for a body of evidence from NRS when using ROBINS-I for assessing risk of bias in individual NRS
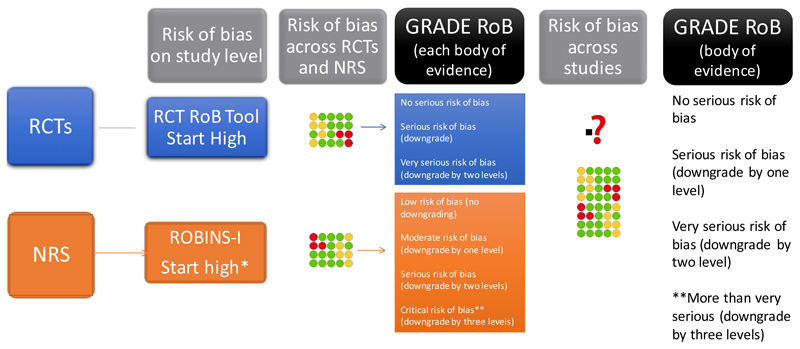
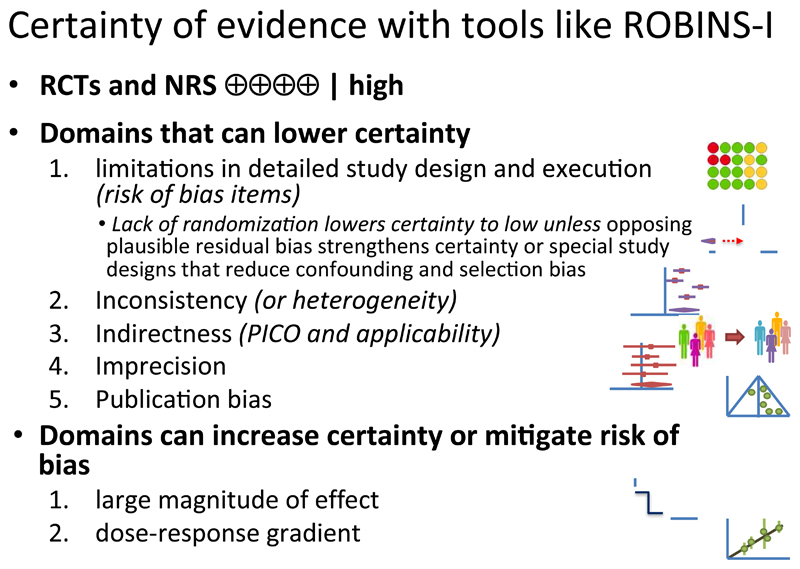
Here, we provide general guidance for the use of GRADE in the context of ROBINS-I. ROBINS-I compares an assessment of an individual NRS against a target RCT. The initial description of the underlying study design, such as cohort, case-control, case series or cross sectional study, is not considered as a risk of bias feature in ROBINS-I. Thus, when using ROBINS-I for assessing risk of bias in NRS, given that assessment of selection bias and confounding is an integral part of the ROBINS-I tool, the initial GRADE certainty in the evidence from a body of studies using an NRS design would be high (Figures 4 and 5). This does not mean that GRADE has changed the view that randomization is the only secure way to guard against confounding bias; that view remains the same. Thus, we would anticipate that whether one begins with a body of evidence from NRS studies as low certainty and looks for reasons to rate up or down, or starts with that evidence as high quality and looks for reasons to rate down, the final certainty rating should be the same.
Figure 4. Assessing randomized trials and non-randomized studies with GRADE.
Rating of risk of bias for randomized and non-randomized studies. Different tools are used to address for the two types of bodies of evidence (randomized and non-randomized). The risk of bias is then assessed across studies and integrated if possible. When ROBINS-I is used rating down by more than two levels is required for a body of evidence that is rated as critical on that instrument.
*In practice this will generally lead to rating down by at least two levels to low or very low certainty for NRS. However, for results with large effects, or dose response, or results in which inference is strengthened by the plausible biases that exist, the extent of rating down may be lowered. We have not identified bodies of evidence in which a ROBINS-I assessment alone leads to no rating down, or rating down by only one level. How to integrate RCTs and NRS will be further discussed in upcoming GRADE guidance articles.
** For GRADE the corresponding terminology is not serious, serious, very serious and a fourth level of risk of bias. GRADE is currently exploring the appropriate term for the fourth level
Figure 5. GRADE approach for certainty of evidence with tools like ROBINS-I.
This approach implies that ROBINS-I users rating conventional NRS of any design (e.g. cohort, case-control) following their assessment of confounding and selection bias, will often arrive at a rating of high risk of bias. Using ROBINS-I it nevertheless remains possible that a body of evidence from NRS studies will receive a final rating of high or moderate certainty of evidence. This could result from rating up for large effect, dose-response, or the direction of plausible confounding. Or it could result from use of NRS designs and analyses with greater protection against risk of bias – for instance, interrupted time series – that would lead to rating down by only 1 level or not at all. As we have already noted, however, while we have many examples of rating up certainty, efforts to identify a body of evidence from innovative designs meriting, simply for design considerations, moderate quality evidence, have thus far proved unsuccessful (Figure 4). Methodological developments in this area that describe how NRS may have greater protection against risk of bias than those typically available should help making such judgments but GRADE requires careful examination of these examples.(22)
6. What makes us confident in results of NRS and does GRADE already account for this?
At the end of the previous section we have noted how, within current GRADE thinking, a body of evidence from NRS studies may emerge from the rating exercise as moderate or high quality evidence. We will now expand on these issues.
6.1. All plausible residual confounders or other biases increase our certainty in the estimated effect
GRADE allows higher certainty ratings for bodies of evidence when all plausible residual confounders or other biases increase our confidence in the range of an estimated effect, that is the effect is either larger or smaller than that observed (23, 24). GRADE suggests that judgments about the direction of the possible bias are important to assess certainty of the evidence from NRS. One example from the public health field comes from a systematic review of NRS including a total of 38 million patients that demonstrated a very small relative increase (relative risk 1.020, 95% confidence interval 1.003-1.038) in death rates in private for-profit compared with private not-for-profit hospitals (23, 25). The evaluation of risk of bias across studies revealed that all residual plausible confounding – the major issues being that for-profit hospitals have on average higher income patients and greater resources - would have decreased the observed effect (further towards a RR of 1.0). Despite the biases in favour of for-profit hospitals, those hospitals demonstrated higher mortality – therefore the true effect, if it differs from the estimate, is almost certain to be greater.
Currently, ROBINS-I allows for an optional judgment regarding the direction of confounding and selection bias (“Risk of bias judgment. Optional: What is the predicted direction of the of bias due to confounding/selection …”). If this optional judgment is indeed used in ROBINS-I, in scenarios such as the hospital profit status example, not rating an individual NRS as high risk of bias (and thus not rating the body of evidence from a number of such studies as low certainty) is justified. This may happen, albeit rarely, even in the context of small effects such as the one observed for the mortality risk in for-profit private hospitals.
While GRADE has accounted for this situation in its approach, when users of GRADE apply ROBINS-I to assess risk of bias, the direction and degree of residual plausible confounding requires considering during the risk of bias assessment. Rather than rating up NRS from low to moderate at the study and body of evidence level, raters using ROBINS-I may not rate risk of bias as very serious, but only rate it as moderate. Whether or not one starts at low certainty in the traditional GRADE approach and rates up or does not rate down to low when using ROBINS-I, the end result is identical and depends on the risk of bias judgment (Figure 5).
6.2. Large effects and dose responses
GRADE suggests that large effects and dose-response relations mitigate concerns regarding residual confounding. In one of our prior articles we described that a systematic review of NRS investigating the effect of cyclooxygenase-2 inhibitors on cardiovascular events found that the summary estimate of RR with rofecoxib of 1.33 (95% CI: 1.00 to 1.79) with doses less than 25mg/d and 2.19 (95% CI: 1.64 to 2.91) with doses more than 25 mg/d. Can we infer that rofecoxib will increase the risk for cardiovascular events? Although only NRS are available to address the question, we can have moderate, or perhaps even high, certainty of the causal connection. The reasons are that, although residual confounding is likely to exist in the NRS that address this issue, the existence of a dose-response gradient and the large apparent effect of higher doses of rofecoxib markedly increase our strength of inference that the association cannot be explained by residual confounding, and is therefore likely to be both causal and, at high levels of exposure, substantial.1 Given the large effect and the observed dose-response relation this could lead to a high certainty rating, for the outcome of increasing cardiovascular events.
The previous paragraph dealt with evaluation of the entire body of evidence, which begs the question of how the rating of individual NRS of rofecoxib using ROBINS-I would impact on a GRADE assessment of the body of evidence. The rater, in dealing with the confounding and selection bias domains would rate individual study as high risk of bias because of the possibility that residual confounding or selection bias may be influencing the estimates of association. How would one then deal with the dose-response and large effect size considerations when dealing with the body of evidence?
In one way of looking at the situation, the subsequent rating up for large effects for the higher doses of rofecoxib would make, in retrospect, some of the items on the ROBINS-I tool potentially irrelevant. The possible solutions are to a) rate the confounding in ROBINS-I as moderate or low risk of bias because large effects are observed and the larger the effect the stronger the confounding would have to be to explain the effect which makes an explanation by confounding unlikely; or b) leave the initial grading as low following the guidance above, and then rate up for large effects when one considers the entire body of evidence. The same options exist with respect to dose-response relationships. GRADE has thus accounted for issues that mitigate concerns about confounding and selection bias by introducing the upgrading domains. They will need to be considered in an assessment of a body of evidence when using ROBINS-I.
7. Advantages and disadvantages of, in the context of GRADE, assessing risk of bias for individual studies using the ROBINS-I approach of specifying target trials
7.1. Advantages
Among other features, ROBINS-I allows review authors to assess how failure to use randomization in individual studies has impacted on risk of bias. For example, ROBINS-I allows a categorization of the magnitude of bias from lack of randomization through the selection and confounding bias domains, allows application of this assessment across risk of bias domains, and evaluation of how this differs across individual studies that address different health care questions. Furthermore, ROBINS-I will facilitate assessment of a study that has been described as randomized but when assessed in detail is found to be not appropriately randomized. In those cases, users of GRADE have struggled with whether to start the certainty of evidence as high and then rate it down, or ignore descriptions of the study authors and treat the studies as NRS by starting the certainty of evidence as low. All these features of the assessment of individual studies can then be taken into account when evaluating a group of individual studies that constitute a body of evidence.
Another potential advantage of using an approach such as ROBINS-I is that it may harmonize GRADE approaches across different study types for different types of questions such as prognosis or test accuracy. In the current GRADE approach, NRS studies for these types of questions begin with high certainty ratings. In particular, with prognostic studies, in which the issue is association and not causation, prognostic NRS begin as high certainty evidence. If GRADE assessments for all types of studies were to start at high certainty, questions of intervention, prognosis, values and preferences, and test accuracy, would not require different initial certainty ratings. What will be required, however, are different versions of ROBINS, such as ROBINS tool for prognosis.
Finally, those applying GRADE in fields where RCTs are sparse such as environmental and certain areas of public health, reframing the certainty assessment with a focus on the actual items that randomization addresses, i.e. confounding and selection bias, rather than labeling a study design feature, i.e. randomization, will find GRADE more acceptable.
6.2. Disadvantages
The disadvantages of offering an alternative to the existing GRADE system include mistakes if users of GRADE do not follow the approach appropriately. First, ROBINS-I is currently the only available tool that explicitly includes a comparison against RCTs (and this situation is unlikely to change) and thus this guidance only applies to the situations when ROBINS-I is used. Second, because of the advantage of assessing risk of bias on an absolute scale, use of ROBINS-I may facilitate combining results of RCTs and NRS. However, under what conditions one should combine results from randomized and non-randomized studies remains uncertain (this uncertainty also applies whether one uses ROBINS-I or other instruments for assessing risk of bias in NRS) (Figure 4).
Third, there is a possibility of misuse by those wanting to assign a higher certainty of the evidence to a body of evidence from NRS than is appropriate. Evaluators of evidence may rate the risk of bias from a group of NRS as moderate risk of bias if they are not appropriately cautious about the impact of confounding and selection bias or if reporting is poor. Users of GRADE may then take the results of such studies and classify them as moderate certainty (if no problems in other GRADE domains exist) when, following current GRADE guidance, they should be classified as low certainty. This may result from a higher threshold and requirement for documenting upgrading rather than a potentially higher threshold for rating down NRS when using ROBINS-I. Fourth, further, detailed guidance is required for appropriate application of ROBINS-I with more examples as concerns about the amount of time required and the lack of detailed reporting of risk of bias related items in current NRS. Fifth, until now there is no practical example on which to base a rating of initial high or even moderate certainty in the evidence that comes from a body of evidence from NRS where no traditional GRADE upgrading domain applies.
8. Unresolved Issues
GRADE recognizes that there are a number of unresolved issues related to the arrival of ROBINS-I. The GRADE working group is addressing those in the near future. The unresolved issues are as follows:
If systematic review authors use ROBINS-I, should the results from NRSs and RCTs be considered together, including potentially in a meta-analysis (Figure 4). If RCTs and NRS are indeed considered together, when should they be combined? Should non-randomized studies be utilized to provide more precise estimates in summary effects when in fact NRS may dominate such estimates? Should they be used to alleviate concerns about indirectness because they are often including broader populations and more practice oriented interventions? Should we continue to follow GRADE guidance to generally separate randomized and non-randomized study results in GRADE summary tables such as evidence profiles and Summary of Findings tables or should the guidance be modified? Until clear advice on when to combine data form randomized and non-randomized studies is available, we suggest following current GRADE guidance: if certainty of evidence differs in a body of randomized trials and a body of NRS, one need only present in summary of findings (SoF) tables, the higher certainty evidence (almost invariably that from RCTs). If certainty ratings are the same (typically low certainty) one presents results from the two bodies of evidence separately. If the results are consistent, then the overall certainty assessment is that of the two bodies of evidence (typically low certainty). If the results are inconsistent, and one believes both bodies of evidence should be taken into consideration, then one will rate down further for this inconsistency, and the final rating will be one category lower (typically very low certainty).
How should we deal with publication bias in the context of including NRS, clearly posing more challenges than evaluating publication bias in RCTs (available evidence suggests publication bias is a greater problem in NRS than in RCTs)?
Under what circumstances should evidence syntheses broaden their scope of search and consider NRS routinely?
GRADE needs to develop more detailed guidance than currently exists regarding the presence of large effects and dose-effect relations. With regards to large effects, if a body of evidence from NRS is indeed rated as high-certainty in the evidence prior to the consideration of size of effect and very large effects exist, no further rating up is possible or required. This is also the situation regarding how GRADE currently deals with large effects observed in a body of evidence from RCTs. For instance, the large relative risk reduction observed with oral anticoagulation for the treatment of DVT for prevention of stroke in patients with atrial fibrillation does not lead to an ultimate rating of the certainty of the evidence beyond high.
Currently GRADE has only three labels for risk of bias: not serious, serious or very serious on the risk of bias domain levels. For RCTs, this corresponds to a rating of the body of evidence as high, moderate or low certainty of evidence after considering risk of bias; for NRS this means that when GRADE currently uses serious risk of bias for NRS they are rated down from low initial certainty to very low. When raters use ROBINS-I with NRS beginning at high certainty, three levels for rating down for risk of bias are required so that NRS can arrive at a rating of very low certainty after considering risk of bias. GRADE is now exploring the best labelling options which include the use of not serious, serious, very serious and very, very serious leading to certainty ratings of high, moderate, low and very low after risk of bias assessment.
9. Summary and next steps
Risk of bias can be best mitigated by a well conducted RCT that balances known and unknown confounders, and using the Cochrane RoB 2.0 tool or similar assessment tools for RCTs to assess risk of bias. For situations in which NRS are used instead or in addition to RCTs, the arrival of ROBINS-I poses a number of opportunities and challenges to summarizing RoB in GRADE, and raises a need for clarification about how ROBINS-I and GRADE are used together. Given the inherent limitations of studies that do not use randomization, a body of evidence from NRS studies should generally not lead to moderate or high certainty in the evidence in relation to risk of bias. Raters using GRADE should always consider confounding and selection bias as reasons for rating down a body of evidence, and this is achieved in the current GRADE by assigning an initial rating of low certainty.
For studies of interventions that are assessed with ROBINS-I in the context of GRADE, we suggest that an initial rating of high is used, with appropriate consideration of the impact of lack of randomization leading to rating down for risk of bias according to the ROBINS-I tool. In practice this will generally lead to rating down by at least two levels to low or very low certainty for NRS. However, for results with large effects, or dose response, or results in which inference is strengthened by the plausible biases that exist, the extent of rating down may be lowered. We have not identified bodies of evidence in which a ROBINS-I assessment alone leads to no rating down, or rating down by only one level. We therefore invite users of ROBINS-I and those who produce summary of findings tables or evidence profiles to submit to the GRADE working group any examples of when they believe that NRS studies without reasons for rating up warrant moderate or high certainty evidence.
Box 1. Clarification of terminology.
GRADE uses the term “criteria” for all criteria in the evidence to decision frameworks of GRADE. Within these criteria the “certainty in the evidence” (or quality or strength of evidence) is one criterion. Certainty of the evidence is assessed based on “certainty domains” with individual items within each domain. RoB is one domain, therefore we will, in the context of GRADE, use the term RoB items to describe the 7 areas of judgment that ROBINS-I calls domains.
Highlights.
Key findings
The risk of bias in non-randomized studies of interventions (ROBINS-I) tool addresses risk of bias in relation to a randomized trial presenting a number of opportunities for the GRADE approach. The GRADE Working Group addresses here how tools like ROBINS-I to assess risk of bias in observational or non-randomized studies should be used. The GRADE approach already accounted for issues that mitigate concerns about confounding and selection bias by introducing the upgrading domains: large effects, dose-effect relations, and when plausible residual confounders or other biases increase certainty.
What this adds to what is known?
In GRADE, the separation of randomized and observational studies was primarily a result of recognition that randomization is the only way to fully protect against confounding, and that confounding is always a concern in even the most rigorously conducted observational studies. Using the term non-randomized studies clarifies that all other designs but randomized trials are meant when evaluating observational studies in GRADE.
What are the implications, what should change now?
The use of ROBINS-I in GRADE assessments may allow for a better comparison of evidence from RCTs and non-randomized studies because they are placed on a common metric for risk of bias. The article describes the initial assessment of a body of evidence with the use of ROBINS-I, and where one would anticipate the final rating would end up.
Acknowledgment
The members of the GRADE Working Group who contributed to writing this article are: Holger J. Schünemann, Carlos Cuello, Elie A. Akl, Reem Mustafa, Kris Thayer, Rebecca Morgan, Joerg Meerpohl, Julian Higgins, Gordon Guyatt. We would like to acknowledge from the GRADE Working Group for input on the work. Article history: Slides presented at GRADE meetings in Barcelona (2014), Amsterdam (2015), Philadelphia (2016) and Seoul (2016); Approved at GRADE meeting May 2017
Footnotes
GRADE guidance suggests the possibility of rating up one level for a large effect if the relative effect is greater than 2.0. Here, the fact that the point estimate of the relative effect is greater than 2.0, but the confidence interval is appreciably below 2.0 might make some hesitate in the decision to rate up for a large effect.
Conflict of interest
HJS has no direct financial conflict of interest and other authors have not declared financial conflicts of interest. Part of the work has been presented scientific conferences and at GRADE Working Group meetings. This article has been officially endorsed by the GRADE Working Group.
Author Contributions
HJS conceived and designed the article and wrote the first draft of this manuscript. All other authors contributed to the writing. All authors have read and commented on the manuscript, and have given written agreement of their authorship.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Carlos Cuello, Email: cuelloca@mcmaster.ca.
Elie A. Akl, Email: ea32@aub.edu.lb.
Reem A. Mustafa, Email: ramustafa@gmail.com.
Jörg J. Meerpohl, Email: meerpohl.jj@gmail.com.
Kris Thayer, Email: thayer.kris@epa.gov.
Rebecca L. Morgan, Email: morganrl@mcmaster.ca.
Gerald Gartlehner, Email: Gerald.Gartlehner@donau-uni.ac.at.
Regina Kunz, Email: Regina.Kunz@usb.ch.
S Vittal Katikireddi, Email: vittal.katikireddi@glasgow.ac.uk.
Jonathan Sterne, Email: Jonathan.Sterne@bristol.ac.uk.
Julian PT Higgins, Email: julian.higgins@bristol.ac.uk.
Gordon Guyatt, Email: guyatt@mcmaster.ca.
References
- 1.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 2.Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knotterus A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2010 doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 4.Schunemann HJ, Best D, Vist G, Oxman AD, Group GW Letters, numbers, symbols and words: how to communicate grades of evidence and recommendations. CMAJ. 2003;169(7):677–80. [PMC free article] [PubMed] [Google Scholar]
- 5.Schunemann HJ, Oxman AD, Brozek J, Glasziou P, Bossuyt P, Chang S, et al. GRADE: assessing the quality of evidence for diagnostic recommendations. ACP J Club. 2008;149(6):2. [PubMed] [Google Scholar]
- 6.Spencer FA, Iorio A, You J, Murad MH, Schunemann HJ, Vandvik PO, et al. Uncertainties in baseline risk estimates and confidence in treatment effects. BMJ. 2012;345:e7401. doi: 10.1136/bmj.e7401. [DOI] [PubMed] [Google Scholar]
- 7.Schunemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336(7653):1106–10. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol. 2013;66(2):151–7. doi: 10.1016/j.jclinepi.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016;353:i2089. doi: 10.1136/bmj.i2089. [DOI] [PubMed] [Google Scholar]
- 10.Alonso-Coello P, Schunemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016. doi: 10.1136/bmj.i2016. [DOI] [PubMed] [Google Scholar]
- 11.Schunemann HJ, Mustafa R, Brozek J, Santesso N, Alonso-Coello P, Guyatt G, et al. GRADE Guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. J Clin Epidemiol. 2016;76:89–98. doi: 10.1016/j.jclinepi.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 12.Burford BJ, Rehfuess E, Schunemann HJ, Akl EA, Waters E, Armstrong R, et al. Assessing evidence in public health: the added value of GRADE. J Public Health (Oxf) 2012;34(4):631–5. doi: 10.1093/pubmed/fds092. [DOI] [PubMed] [Google Scholar]
- 13.Thayer KA, Schunemann HJ. Using GRADE to respond to health questions with different levels of urgency. Environ Int. 2016;92–93:585–9. doi: 10.1016/j.envint.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Schunemann HJ, Hill SR, Kakad M, Vist GE, Bellamy R, Stockman L, et al. Transparent development of the WHO rapid advice guidelines. PLoS Med. 2007;4(5):e119. doi: 10.1371/journal.pmed.0040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. doi: 10.1136/bmj.h870. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt GH, Ebrahim S, Alonso-Coello P, Johnston BC, Mathioudakis AG, Briel M, et al. GRADE guidelines 17: assessing the risk of bias associated with missing participant outcome data in a body of evidence. J Clin Epidemiol. 2017 doi: 10.1016/j.jclinepi.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Morgan RL, Thayer KA, Bero L, Bruce N, Falck-Ytter Y, Ghersi D, et al. GRADE: Assessing the quality of evidence in environmental and occupational health. Environ Int. 2016 doi: 10.1016/j.envint.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson S, T I, Higgins JPT. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36(3):666–76. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- 19.Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katikireddi SV, Egan M, Petticrew M. How do systematic reviews incorporate risk of bias assessments into the synthesis of evidence? A methodological study. J Epidemiol Community Health. 2015;69(2):189–95. doi: 10.1136/jech-2014-204711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craig P, Katikireddi SV, Leyland A, Popham F. Natural Experiments: An Overview of Methods, Approaches, and Contributions to Public Health Intervention Research. Annu Rev Public Health. 2017;38:39–56. doi: 10.1146/annurev-publhealth-031816-044327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64(12):1311–6. doi: 10.1016/j.jclinepi.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 doi: 10.1016/j.jclinepi.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devereaux PJ, Choi PT, Lacchetti C, Weaver B, Schunemann HJ, Haines T, et al. A systematic review and meta-analysis of studies comparing mortality rates of private for-profit and private not-for-profit hospitals. CMAJ. 2002;166(11):1399–406. [PMC free article] [PubMed] [Google Scholar]