Abstract
Purpose:
The purpose of this study was to determine the effect of a pre-transplant cranial boost (CB) on post-transplant central nervous system (CNS) relapse and survival in acute lymphoblastic leukemia (ALL) patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT) using a total body irradiation (TBI)-containing preparation regimen.
Methods and Materials:
Two hundred thirteen ALL patients were treated consecutively at our institution with allogeneic HSCT. Conditioning included TBI (1320 cGy in 8 fractions given twice daily) and cyclophosphamide (120 mg/kg) with or without fludarabine (75 mg/m2). Patients were divided into four groups based on history of CNS disease and whether a CB was given. Of the 160 patients with no history of CNS disease, none received a cranial boost (CNS−/CB−). Of the 53 patients with prior CNS disease, 41 had not received prior cranial irradiation. Thirty of these 41 received a cranial boost of 900–1000 cGy in five daily fractions (CNS+/CB+), while the other 11 did not receive a boost due to physician preference (CNS+/CB−). The remaining 12 patients with prior CNS involvement had previously received cranial irradiation and thus were not candidates for a boost (CNS+PriorRT). Two-year CNS relapse risk, overall survival (OS), and disease-free survival (DFS) were calculated using Kaplan-Meier analysis.
Results:
Seven patients experienced post-transplant CNS relapse. Four of these were in the CNS−/CB−group, 2 were in the CNS+/CB− group, and 1 was in the CNS+PriorRT group. None of the 30 patients who received a boost relapsed in the CNS. Two-year CNS relapse risk was 0% in the CNS+/CB+ group compared to 21% (95% CI 0–45%) in the CNS+/CB−group (p = 0.03). Two-year OS and DFS did not differ between the groups.
Conclusion:
Among ALL patients with prior CNS leukemia, there was a trend towards a reduced risk of post-transplant CNS relapse in patients who receive a cranial boost. The addition of a cranial boost did not appear to have an impact on OS or DFS.
Keywords: acute lymphocytic leukemia, central nervous system relapse, allogeneic hematopoietic stem cell transplantation, total body irradiation, cranial boost
Introduction
Acute lymphoblastic leukemia (ALL) is the most common pediatric malignancy1,2. Nearly 5,400 new cases of ALL are diagnosed each year in the United States3. The central nervous system (CNS) is the most frequently affected extramedullary site in ALL. Because the CNS is a sanctuary site, prophylactic treatment is warranted to prevent CNS relapse. Due to the concern of neurocognitive toxicity associated with prophylactic cranial irradiation, chemotherapy has emerged as the preferred treatment for CNS prophylaxis4–7. The prognosis of patients who relapse in the CNS despite prophylactic therapy remains poor8.
Allogeneic hematopoietic stem cell transplantation (HSCT) is often used as a consolidation therapy to reduce relapse in high-risk ALL patients. Multiple studies have demonstrated that patients with previous CNS involvement have a higher rate of post-transplant CNS relapse8–12. Several strategies have been employed in the peri-transplant setting to prevent CNS relapse in these patients, including total body irradiation (TBI) conditioning8 and post-transplant prophylactic intrathecal (IT) chemotherapy8,10,12. Previous studies have demonstrated that neither approach successfully decreases CNS recurrence. Therefore, some institutions have integrated a cranial irradiation boost into the TBI conditioning regimen in high-risk patients, although the efficacy of this approach has yet to be validated. The purpose of the current study was to evaluate the impact of a cranial boost on incidence of post-transplant CNS relapse as well as survival in ALL patients treated at a single institution with allogeneic HSCT using TBI as part of the conditioning regimen.
Materials and Methods
Patients
This study was approved by the University of Minnesota Institutional Review Board (IRB study number: 1611E98741). All patients with ALL who received HSCT using a TBI-containing preparative regimen at our institution were identified from the Blood and Marrow Transplantation Database, which prospectively collects baseline patient and transplantation characteristics, post-transplant complications, and outcomes data. A total of 213 such patients who were treated from 2001 to 2016 were included. All patients were in remission at the time of transplantation. These patients were classified into 1 of 4 subgroups based on their history of CNS involvement and whether cranial irradiation was given as a boost (Figure 1). One hundred sixty patients had no history of CNS disease, and of these, none received a cranial boost (CNS−/CB−, n = 160). Fifty-three patients had prior CNS disease, including those with CNS involvement at diagnosis, isolated pre-transplant CNS relapse, or combined CNS and bone marrow relapse. All of these patients had been treated to remission status prior to transplantation. Of the 53 CNS+ patients, 41 had not received prior cranial irradiation and were deemed eligible for a boost. Thirty of these 41 patients received a cranial boost (CNS+/CB+, n = 30), while the other 11 did not receive a boost due to physician preference or patient refusal (CNS+/CB−, n = 11). The remaining 12 CNS+ patients had already received cranial irradiation as part of their initial management and therefore were not candidates for further cranial irradiation at the time of transplantation (CNS+PriorRT, n = 12).
Figure 1: Patient groups.
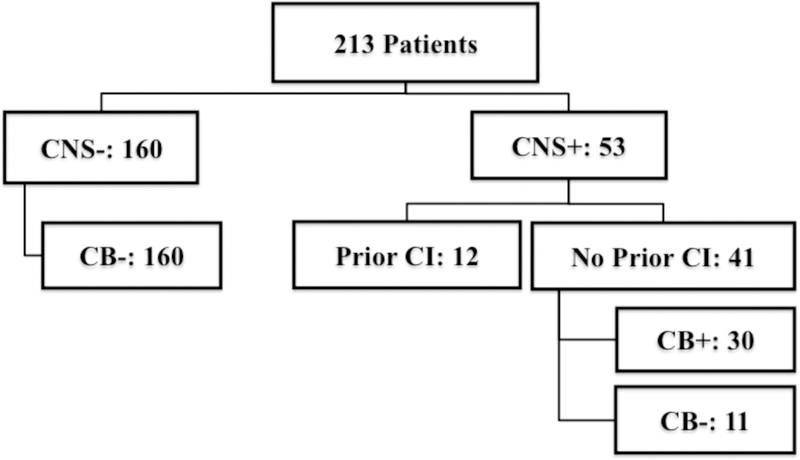
CNS+ indicates patients with a history of central nervous system disease; CB+, patients who received a cranial boost; CI, cranial irradiation.]
Transplantation procedure and conditioning regimens
Graft sources were umbilical cord blood (n = 137, 64.3%), related donor bone marrow (n = 74, 34.7%), and unrelated donor bone marrow (n = 2, 0.9%). All patients received pre-transplant conditioning with TBI at 165 cGy per fraction given twice daily over four days to a total dose of 1320 cGy at a dose rate of 10–19 cGy per minute. TBI was delivered via an opposed lateral technique with arms placed at the sides. Majority of the patients were placed in a semi-recumbent position while infants and young children requiring general anesthesia were treated in supine position. Custom aluminum compensators for head/neck and legs to account for the transverse tissue deficit were used to provide a uniform dose homogeneity within 10% of the prescribed dose. An anterior chest film was used to determine lung thickness, taking density into account. In cases where arm thickness is not sufficient to reduce lung dose, additional tissue compensator was placed13. All males received a testicular boost of 400 cGy using en face electrons.
All patients received cyclophosphamide 60 mg/kg/day for two days (120 mg/kg total). One hundred thirty-one patients also received fludarabine 25 mg/m2/day for three days (75 mg/m2 total). Graft-versus-host disease prophylaxis included either cyclosporine and mycophenolate mofetil or cyclosporine and methotrexate in the majority of patients.
Cranial boost and prior cranial irradiation
Patients with a history of CNS disease not previously treated with cranial irradiation were candidates for a cranial boost in addition to TBI prior to transplantation. Patients with prior cranial irradiation whose total cranial dose, including TBI, would exceed 2400 cGy, were not candidates14. Because all patients with prior irradiation received a dose of at least 1200 cGy, none of them were offered a boost. Some providers and/or patients declined a boost due to concern of neurotoxicity. For these patients, a cranial boost was not administered.
Thirty patients received a cranial boost while 11 did not. Cranial boost was completed within two weeks before initiating TBI. Twenty-nine of 30 patients received 900 cGy given in five daily fractions of 180 cGy each. One patient received 1000 cGy in five daily fractions of 200 cGy each. Thus, the total dose to the brain was 2220–2320 cGy after accounting for the TBI dose. None of the boosted patients received additional spinal irradiation. Of the 12 patients who were precluded from receiving a cranial boost due to prior cranial irradiation, previous doses to the brain were 1200 cGy in 5 patients, 1800 cGy in 5, 2400 cGy in 1, and unknown in 1. Among these patients, one also received 75 cGy to the spine.
Post-transplant prophylactic intrathecal chemotherapy
A total of 13 patients received post-transplant prophylactic IT chemotherapy, 12 of whom were in the CNS−/CB−group and 1 of whom was in the CNS+PriorRT group. Three of the 13 (23.1%) had T-cell disease, and 4 (30.8%) were t(9;22) positive. Intrathecal chemotherapeutic regimens consisted of methotrexate, cytarabine, and/or hydrocortisone.
Survival, recurrence, and follow-up
Patients’ overall (OS) and disease free (DFS) survival were calculated from the date of transplantation. CNS relapse was defined as occurrence of any of the following: leukemic blasts in cerebrospinal fluid, contrast-enhancing brain or spinal lesion(s) on imaging attributed to leukemic involvement, or clinical symptoms consistent with CNS relapse. For the purposes of this study, CNS recurrence was restricted to patients in whom the CNS was the first site of post-transplant relapse. The rationale for this definition was to exclude cases in which the CNS was a secondary relapse site following bone marrow failure, a scenario that pre-transplant cranial irradiation could not have prevented. For patients who experienced relapse or death, the follow-up duration was defined from the date of HSCT to first relapse or death. Patients who did not experience post-transplant relapse were censored at the time of last clinical follow-up visit.
Statistical analysis
Demographic data and transplantation characteristics were summarized by standard descriptive statistical methods. Comparisons between groups were conducted using chi-square or Fisher’s exact test for categorical data and Wilcoxon’s rank sum test for continuous data. The cut-off significance level for p-values was 0.05.
The Kaplan-Meier method15 was used to estimate the probabilities of DFS and OS through 2 years after HSCT. A Cox proportional hazard regression model was used to estimate the adjusted survival curves16.
A cumulative incidence estimator was used to calculate the probability of CNS relapse, reflecting the nonevent deaths as a competing risk17. Fine and Gray regression analysis was used to compare the differences between cumulative incidence curves for relapse rate18. The log-rank test was used for univariate comparisons. The following risk factors were included on univariate analysis: patient group, presence of Philadelphia chromosome, leukemic cell subtype, age at transplantation, remission status, stem cell source, and post-transplant IT chemotherapy. Two-year relapse rate was compared between the four groups as well as between the CNS+/CB+ and CNS+/CB− groups. A p-value cutoff of 0.025 was used to correct for multiple comparisons.
All statistical analyses were implemented using Statistical Analysis System statistical software version 9.3 (SAS Institute Inc., Cary, NC).
Results
Patient, disease and transplant characteristics
Median duration of follow-up from the time of HSCT for the entire cohort was 839 days (range: 16–4390 days). One hundred twenty-eight (60.1%) patients were male. Median age at transplantation was 17.9 years (range: 1.6–56.0). Leukemic cell subtypes were as follows: 137 (64.3%) pre-B-cell, 26 (12.2%) T-cell, 16 (7.5%) mature-B-cell, and 34 (16.0%) unknown. At diagnosis, 49 (23.0%) were t(9;22) positive. One hundred and twenty-one (56.8%) patients were transplanted while in first remission, 72 (33.8%) in second remission, and 20 (9.4%) in third remission or beyond.
When comparing the 4 groups based on their history of CNS involvement and use of a cranial boost, patients in the CNS+PriorRT group were the youngest (p = <0.01), whereas the CNS−/CB−group had the highest proportion (62.5%) of patients in CR1 (p < 0.01). There were otherwise no significant differences among the 4 groups in the patient and treatment characteristics studied, including gender, leukemic cell subtype, presence of t(9:22), year of transplantation, stem cell source, HLA matches, total nucleated cells transplanted, and the use of post-transplant IT chemotherapy (Table 1). Since we were particularly interested in the role of a cranial boost among CNS+ patients, we also compared the demographic and transplant-related characteristics between those who received cranial boost (CNS+/CB+) and those for whom it was deferred (CNS+/CB−). No significant differences were detected between the two groups (Table 1).
Table 1:
Patient, disease, and transplantation characteristics.
| Characteristics | All patients (n = 213) |
CNS−/CB− (n = 160) |
CNS+/CB+ (n = 30) |
CNS+/CB− (n = 11) |
CNS+PriorRT (n = 12) |
4-group p | 2-group p* |
|---|---|---|---|---|---|---|---|
| Gender: Male | 128 (60.1)** | 93 (58.1) | 21 (70.0) | 8 (72.7) | 6 (50.0) | 0.44 | 0.86 |
| Median age: years (range) | 17.9 (1.6–56.0) | 20.5 (1.6–56.0) | 16.2 (3.245.9) | 20.5 (7.8–52.9) | 10.2 (5.3–36.5) | <0.01 | 0.13 |
| Age ≥ 18 years at transplant | 106 (49.8) | 87 (54.4) | 12 (40.0) | 6 (54.5) | 1 (8.3) | <0.01 | 0.41 |
|
Cell subtype Pre-B T-cell Mature-B Unknown |
137 (64.3) 26 (12.2) 16 (7.5) 34 (16.0) |
103 (64.4) 18 (11.2) 12 (7.5) 27 (16.9) |
21 (70.0) 2 (6.7) 2 (6.7) 5 (16.7) |
5 (45.5) 3 (27.3) 2 (18.2) 1 (9.1) |
8 (66.7) 3 (25.0) 0 1 (8.3) |
0.39 | 0.12 |
| t(9;22): present | 49 (23.0) | 42 (26.3) | 4 (13.3) | 2 (18.2) | 1 (8.3) | 0.25 | 0.70 |
|
Remission status CR1 CR2 CR3+ |
121 (56.8) 72 (33.8) 20 (9.4) |
101 (63.1) 48 (30.0) 11 (6.9) |
11 (36.7) 15 (50.0) 4 (13.3) |
7 (63.6) 4 (36.4) 0 |
2 (16.7) 5 (41.7) 5 (41.7) |
<0.01 | 0.46 |
|
Transplant year 2001 – 2007 2008 – 2016 |
90 (42.3) 123 (57.7) |
67 (40.4) 93 (58.1) |
15 (50.0) 15 (50.0) |
3 (27.3) 8 (72.7) |
5 (41.7) 7 (58.3) |
0.62 | 0.19 |
|
Stem cell source UCB MRD URD |
137 (64.3) 74 (34.7) 2 (0.9) |
100 (62.5) 58 (36.3) 2 (1.3) |
19 (63.3) 11 (36.7) 0 |
7 (63.6) 4 (36.4) 0 |
11 (91.7) 1 (8.3) 0 |
0.58 | 0.99 |
|
HLA
match 4/6 5/6 6/6 7/8 8/8 Unknown |
52 (24.4) 64 (30.0) 48 (22.5) 2 (0.9) 31 (14.6) 16 (7.5) |
40 (25.0) 47 (29.4) 35 (21.9) 2 (1.3) 22 (13.8) 14 (8.8) |
7 (23.3) 9 (30.0) 8 (26.7) 0 4 (13.3) 2 (6.7) |
1 (9.1) 5 (45.5) 1 (9.1) 0 4 (36.4) 0 |
4 (33.3) 3(25.0) 4 (33.3) 0 1 (8.3) 0 |
0.77 | 0.21 |
| Median TNC transplanted (# cells × 108/kg, range) | 0.6 (0.2–15.7) | 0.7 (0.2–15.7) | 0.5 (0.2–11.0) | 0.6 (0.2–10.9) | 0.5 (0.2–3.0) | 0.44 | 0.85 |
| Post-transplant prophylactic IT chemotherapy | 13 (6.1) | 12 (7.5) | 0 | 0 | 1 (8.3) | 0.44 | NA |
CNS indicates central nervous system; CB, cranial boost; PriorRT, prior cranial radiation therapy; CR, complete remission; UCB, umbilical cord blood; MRD, matched related donor; URD, unrelated donor; HLA, human leukocyte antigen; TNC, total nucleated cells; IT, intrathecal.
Two-group p-values compare CNS+/CB+ vs. CNS+/CB−.
Values are number (percentage) unless otherwise
Survival
A total of 111 (52.1%) patients were alive at last follow-up. Two-year OS was 60% (95% CI 53–67%) and DFS was 54% (95% CI 47–61%) for all patients. There were no significant differences in survival among the 4 groups (p = 0.59 for OS and p = 0.81 for DFS). When only the CNS+/CB+ patients and CNS+/CB− patients were compared, significant differences were still not observed. Two-year OS for the CNS+/CB+ group was 63% (95% CI 44–78%) compared to 80% (95% CI 39–95%) for the CNS+/CB−group (p = 0.25). The 2-year DFS in these two groups were 60% (95% CI 40–75%) and 61% (95% CI 25–83%), respectively (p = 0.63). Survival curves are illustrated in Figure 2.
Figure 2: Two-year post-transplant (A) overall and (B) disease free survival.

CNS indicates central nervous system; CB, cranial boost; PriorRT, prior cranial radiation therapy. Parentheses indicate 95% confidence intervals. 2-group p-values compare CNS+/CB+ vs. CNS+/CB−.
Relapse
Fifty-three of the 213 patients experienced relapse following HSCT. As expected, the most common site of initial relapse was hematologic only, occurring in 34 patients. Seven patients relapsed initially in the CNS; three of these patients experienced isolated CNS recurrence while 4 had concurrent hematologic involvement. Twelve patients relapsed at other extramedullary sites with or without concurrent hematologic recurrence. There were 43 relapses in the CNS−/CB−group (43/160; 26.9%), 2 in the CNS+/CB+ group (2/30; 6.7%), 5 in the CNS+/CB−group (5/11; 45.5%), and 3 in the CNS+PriorRT group (3/12; 25.0%). Incidence of post-transplant relapse did not differ significantly among the four groups (p = 0.40).
Seven patients relapsed in the CNS initially, ranging from 59 to 708 days following HSCT. Of these, 4 had no prior history of CNS disease and 2 had a history of CNS disease but did not receive a cranial boost. An additional patient who received previous cranial irradiation and hence was not eligible for a boost also experienced post-transplant CNS relapse. Characteristics and clinical outcomes of the patients who relapsed in the CNS are presented in Table 2.
Table 2:
Characteristics of patients who developed initial relapse in the CNS following transplantation.
| Patient No. | Group | Gender | Age at transplantation (years) | Leukemic cell subtype | t(9;22) | Remission status | Year of HSCT | GVHD prophylaxis | Stem cell source | HLA match | Median TNC transplanted (# cells × 108/kg) | Post-transplant prophylactic IT chemotherapy | Days from HSCT to CNS relapse | Additional relapse site | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CNS−/CB− | F | 31 | Pre-B | + | CR1 | ≤2007 | None | MRD | 6/6 | 10.0 | No | 708 | None | Dead |
| 2 | CNS−/CB− | M | 44 | Unknown | + | CR1 | ≥ 2008 | C/MMF | UCB | 4/6 | 0.3 | No | 73 | Hematologic | Dead |
| 3 | CNS−/CB− | M | 39 | Pre-B | + | CR1 | ≥ 2008 | C/MTX | MRD | 8/8 | 6.5 | No | 273 | Hematologic | Alive |
| 4 | CNS−/CB− | M | 20 | Pre-B | − | CR2 | ≥ 2008 | C/MTX | MRD | 8/8 | 2.6 | Yes | 365 | Hematologic | Dead |
| 5 | CNS+/CB− | F | 11 | T | − | CR2 | ≥ 2008 | C/MTX | MRD | 8/8 | 4.2 | No | 504 | None | Alive |
| 6 | CNS+/CB− | M | 14 | Pre-B | − | CR2 | ≥ 2008 | C/MMF | UCB | 6/6 | 0.2 | No | 99 | None | Alive |
| 7 | CNS+PriorRT | F | 12 | Unknown | − | CR3 | ≥ 2008 | C/MMF | UCB | 5/6 | 1.5 | Yes | 59 | Hematologic | Dead |
CNS indicates central nervous system; CB, cranial boost; PriorRT, prior cranial radiation therapy; CR, complete remission; UCB, umbilical cord blood; MRD, matched related donor; HLA, human leukocyte antigen; TNC, total nucleated cells; IT, intrathecal, GVHD, graft versus host disease; C, cyclosporine; MTX, methotrexate; MMF, mycophenolate mofetil.
Of the 41 patients who had a history of CNS disease and were eligible for a cranial boost, none of the 30 patients who received a boost experienced relapse in the CNS, whereas 2 of the 11 patients who omitted a boost relapsed in the CNS, corresponding to 2-year CNS relapse rates of 0% (CNS+/CB+) and 21% (CNS+/CB−, 95% CI 0–45%) respectively (p = 0.03).
Univariate analysis was performed to analyze factors predictive of CNS relapse (Table 3). Using a p-value cutoff of 0.025 to correct for multiple comparisons, treatment group was found to approach significance for 2-year CNS relapse rate (p = 0.04). A subgroup comparison between the CNS+/CB+ and CNS+/CB−groups also yielded a difference that approached significance (p = 0.03). Patients who received post-transplant IT chemotherapy had a higher CNS relapse rate of 15% (95% CI 0–34%) compared to 3% (95% CI 0–5%) in patients not receiving it (p = 0.01). No other factors included on univariate analysis, including age at transplantation, leukemic cell subtype, presence of Philadelphia chromosome, remission status, and stem cell source affected 2-year CNS relapse rate.
Table 3:
Univariate analysis of 2-year CNS relapse.
| N | Events | Risk (95% CI) | P | |
|---|---|---|---|---|
| Total | 213 | 7 | 3% (1–6%) | |
| Group | 0.04* | |||
| CNS−/CB− | 160 | 4 | 3% (0–5%) | 0.03** |
| CNS+/CB+ | 30 | 0 | 0% | |
| CNS+/CB− | 11 | 2 | 21% (0–45%) | |
| CNS+PriorRT | 12 | 1 | 8% (0–23%) | |
| Age at transplantation | 0.51 | |||
| < 18 years | 107 | 3 | 3% (0–6%) | |
| ≥ 18 years | 106 | 4 | 4% (0–8%) | |
| Cell subtype | 0.72 | |||
| Pre-B | 137 | 4 | 3% (0–6%) | |
| T-cell | 26 | 1 | 5% (0–13%) | |
| Mature-B | 16 | 0 | 0% | |
| Unknown | 34 | 2 | 6% (0–14%) | |
| t(9;22) | 0.19 | |||
| Yes | 49 | 3 | 6% (0–13%) | |
| No | 164 | 4 | 3% (0–5%) | |
| Remission status | 0.81 | |||
| CR1 | 121 | 3 | 3% (0–6%) | |
| CR2 | 72 | 3 | 4% (0–9%) | |
| CR3+ | 20 | 1 | 5% (0–14%) | |
| Source of stem cells | 0.50 | |||
| UCB | 137 | 3 | 2% (0–5%) | |
| MRD | 74 | 4 | 6% (0–13%) | |
| URD | 2 | 0 | 0% | |
| Post-transplant prophylactic IT chemotherapy | ||||
| Yes | 13 | 2 | 15% (0–34%) | 0.01 |
| No | 200 | 5 | 3% (0–5%) | |
CNS indicates central nervous system; CB, cranial boost; PriorRT, prior cranial radiation therapy; CR, complete remission; UCB, umbilical cord blood; MRD, matched related donor; URD, unrelated donor; IT, intrathecal. A p-value cutoff of 0.025 was used to correct for multiple subgroup comparisons.
Four-group p-value.
Two-group p-values compare CNS+/CB+ vs. CNS+/CB−.
Given the small number of events and the fact that the only factor reaching significance was post-transplant IT chemotherapy, multivariate analysis was not performed.
Discussion
Despite advances in the treatment of ALL, rates of post-transplant relapse remain high, especially in high-risk patients. Hematologic relapse is the most frequent obstacle to achieving long-term survival. Relapse in the CNS, while not as prevalent, remains a significant barrier to cure and poses a therapeutic challenge, as the CNS is a sanctuary site. This study demonstrates that, in ALL patients with previous CNS involvement, there is a trend towards reduced rate of post-transplant CNS relapse in those who receive a cranial boost in addition to TBI conditioning. None of the 30 CNS+ patients treated with a cranial boost experienced initial CNS recurrence, whereas 2 of the 11 CNS+ patients for whom a boost was deferred recurred in the CNS. Other prognostic factors including age at transplantation, leukemic cell subtype, presence of Philadelphia chromosome, remission status, and stem cell source did not influence CNS recurrence risk.
In our cohort, isolated CNS relapse was observed between 59 days and 708 days after transplant. Although late CNS relapses may have been missed with our median follow-up of 839 days, previous retrospective studies have demonstrated that the median time to CNS relapse is less than 1 year following HSCT, and that the risk appears to level off beyond 2 years9,12. Because of the small number of events, we were only able to detect a difference that approached significance. Further studies are warranted to validate this finding. Despite an improvement in CNS disease control, there was no difference in survival between the CNS+/CB+ and CNS+/CB− groups, likely due to the potential competing risk of death and/or the use of salvage therapy.
Previous evidence on the benefit of a cranial boost has been limited. Grow et al. demonstrated in a retrospective analysis of 26 patients that a cranial or craniospinal boost protects against CNS recurrence19. Another retrospective study performed by Su et al. concluded that a 600 cGy boost, in addition to 1200 cGy TBI, is well-tolerated and may reduce the likelihood of CNS recurrence20. The minimal cranial dose needed to protect against CNS relapse is unclear. In our single institution cohort, patients received a cumulative dose of 2220 to 2320 cGy to the brain. Prior reported total cranial doses range from 180018 to 3200 cGy21.
Although we found a possible role of a cranial boost in protecting against CNS relapse, this benefit must be balanced against added CNS toxicity. The long-term adverse effects of cranial irradiation on neurocognition have been well documented22–24. For this reason, the use of prophylactic cranial irradiation in the treatment of standard risk ALL has gradually been eliminated in favor of systemic and IT therapy25–28. Formal neuropsychological testing unfortunately was not routinely performed among the patients who received a CB. In our study, only 9 such patients (median age: 11.4 years) received formal neuropsychological testing, all of whom were referred due to subjective cognitive decline. Seven of the 9 patients were found to have cognitive abilities within or above the average range, while 2 demonstrated slightly below average cognitive abilities. Because baseline neurocognitive testing prior to transplantation was not performed, it is difficult to assess the detriment directly attributable to the use of a cranial boost. It is somewhat reassuring, however, that the majority of patients who were formally tested were found to have cognitive function in the average or above average range. There is at least one other study which showed no adverse effect on overall intellectual function when a cranial boost was given prior to HSCT29. Additional work is needed to determine a dose that is both effective at reducing CNS relapse and has minimal neurotoxicity.
An alternative strategy to cranial irradiation for CNS prophylaxis is the use of IT chemotherapy. In our study, 13 patients received IT chemotherapy in the post-transplant setting. Despite receiving IT therapy, 2 of the 13 relapsed in CNS. Patients who received IT chemotherapy had a higher percentage of T-cell disease and t(9;22) positivity compared to the overall cohort, both of which are predictors for CNS relapse30, although this difference was not statistically significant. Prophylactic IT therapy in the immediate post-transplant period may somehow be detrimental. Alexander et al. reported in their study of 67 patients that all 3 CNS failures occurred in the 10 patients who received post-transplant IT chemotherapy21. Oshima et al. also demonstrated an increased risk of CNS relapse in similarly treated patients, while two other studies showed no impact of post-transplant prophylactic IT chemotherapy on CNS relapse risk8,12. Aldoss et al. posited that chemotherapy may destroy donor-derived T lymphocytes in recipient cerebrospinal fluid which normally exert a graft-versus-leukemia effect that protects against CNS disease in allogeneic HSCT recipients8.
We acknowledge the limitations of our study. Our event number was small, with only 7 patients experiencing post-transplant CNS relapse. This is partially because we included only those CNS relapses that occurred as the initial event following transplantation. This definition allowed us to directly assess the effect of a cranial boost in preventing CNS relapse, as it would not prevent secondary CNS relapse following hematologic failure. As with any retrospective analysis, selection bias likely influenced our results. Among the 41 CNS+ patients who were eligible to receive a cranial boost, our practice was not uniform, with only 30 receiving the treatment. The reason for omitting a boost in the remaining 11 patients was due to physician bias or patient refusal. Lastly, no formal pre-transplant neurocognitive assessment was performed on patients who were boosted.
In conclusion, in this cohort of ALL patients receiving allogeneic HSCT, augmenting TBI with a cranial boost may protect against post-transplant CNS relapse among patients with a history of CNS leukemia, although the improvement seen in CNS control does not translate into an OS or DFS benefit. Our results require validation from a larger cohort or multi-institutional database.
Footnotes
Conflicts of Interest: none
References
- 1.Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: Challenges for the twenty-first century. J Clin Oncol. 2010;28(15):2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992–2004). Cancer. 2008;112(2):416–432. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 3.Hourigan MJ, Goldstone AH. Acute Lymphoblastic Leukemia: Epidemiology In: Advani AS, Lazarus HM, eds. Adult Acute Lymphocytic Leukemia: Biology and Treatment. Totowa, NJ: Humana Press; 2011:77–87. doi: 10.1007/978-1-60761-707-5_6. [DOI] [Google Scholar]
- 4.Pui C-H, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamps WA, Bökkerink JPM, Hakvoort-Cammel FGAJ, et al. BFM-oriented treatment for children with acute lymphoblastic leukemia without cranial irradiation and treatment reduction for standard risk patients: Results of DCLSG protocol ALL-8 (1991–1996). Leukemia. 2002;16(6):1099–1111. doi: 10.1038/sj/leu/2402489. [DOI] [PubMed] [Google Scholar]
- 6.Annino L, Vegna ML, Camera A, et al. Treatment of adult acute lymphoblastic leukemia (ALL): long-term follow-up of the GIMEMA ALL 0288 randomized study. Blood. 2002;99(3):863–871. doi: 10.1182/blood.V99.3.863. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian H, Thomas D, O’Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101(12):2788–2801. doi: 10.1002/cncr.20668. [DOI] [PubMed] [Google Scholar]
- 8.Aldoss I, Al Malki MM, Stiller T, et al. Implications and Management of Central Nervous System Involvement before Allogeneic Hematopoietic Cell Transplantation in Acute Lymphoblastic Leukemia. Biol Blood Marrow Transplant. 2016;22(3):575–578. doi: 10.1016/j.bbmt.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Ganem G, Kuentz M, Bernaudin F, et al. Central nervous system relapses after bone marrow transplantation for acute lymphoblastic leukemia in remission. Cancer. http://www.ncbi.nlm.nih.gov/pubmed/2676139. Published 1989. [DOI] [PubMed] [Google Scholar]
- 10.Oshima K, Kanda Y, Yamashita T, et al. Central Nervous System Relapse of Leukemia after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2008;14(10): 1100–1107. doi: 10.1016/j.bbmt.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Thompson CB, Sanders JE, Flournoy N, Buckner CD, Thomas ED. The risks of central nervous system relapse and leukoencephalopathy in patients receiving marrow transplants for acute leukemia. Blood. http://www.ncbi.nlm.nih.gov/pubmed/3510066. Published 1986. [PubMed] [Google Scholar]
- 12.Hamdi A, Mawad R, Bassett R, et al. Central nervous system relapse in adults with acute lymphoblastic leukemia after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2014;20(11):1767–1771. doi: 10.1016/j.bbmt.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levitt SH, Khan FM, Potish RA. Levitt and Tapley ‘s Technological Basis of Radiation Therapy: Practical Clinical Application. 2nd ed Lea & Febiger; 1992. [Google Scholar]
- 14.Halberg FE, Kramer JH, Moore IM, Wara WM, Matthay KK, Ablin AR. Prophylactic cranial irradiation dose effects on late cognitive function in children treated for acute lymphoblastic leukemia. Int JRadiat Oncol Biol Phys. 1992;22(1): 13–16. http://www.ncbi.nlm.nih.gov/pubmed/1727109. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 16.Cox DR, Society S, Methodological SB. Regression Models and Life-Tables. JR StatSoc Ser B. 1972;34(2):187–220. doi: 10.2307/2985181. [DOI] [Google Scholar]
- 17.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16(8):901–910. doi:. [DOI] [PubMed] [Google Scholar]
- 18.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 19.Grow JL, Stea B, Katsanis E. Treatment of Pediatric CNS Leukemia With Cranial or Craniospinal Boost in Conjunction With Total Body Irradiation as Part of the Conditioning Regimen for Bone Marrow Transplantation. Int J Radiat Oncol Biol Phys. 2016;96:S166. [Google Scholar]
- 20.Su W, Thompson M, Sheu R-D, et al. Low-dose cranial boost in high-risk adult acute lymphoblastic leukemia patients undergoing bone marrow transplant. PractRadiat Oncol. 2017;7(2): 103–108. doi: 10.1016/j.prro.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexander BM, Wechsler D, Braun TM, et al. Utility of cranial boost in addition to total body irradiation in the treatment of high risk acute lymphoblastic leukemia. Int J Radiat Oncol Biol Phys. 2005;63(4): 1191–1196. doi: 10.1016/j.ijrobp.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Clayton PE, Shalet SM, Morris-Jones PH, Price DA. Growth in children treated for acute lymphoblastic leukaemia. Lancet (London, England). 1988;1(8583):460–462. [DOI] [PubMed] [Google Scholar]
- 23.Cousens P, Waters B, Said J, Stevens M. COGNITIVE EFFECTS OF CRANIAL IRRADIATION IN LEUKAEMIA: A SURVEY AND META-ANALYSIS. J Child Psychol Psychiatry. 1988;29(6):839–852. doi: 10.1111/j.1469-7610.1988.tb00757.x. [DOI] [PubMed] [Google Scholar]
- 24.Löning L, Zimmermann M, Reiter a, et al. Secondary neoplasms subsequent to Berlin-Frankfurt-Münster therapy of acute lymphoblastic leukemia in childhood: significantly lower risk without cranial radiotherapy. Blood. 2000;95(9):2770–2775. [PubMed] [Google Scholar]
- 25.Eden OB, Harrison G, Richards S, et al. Long-term follow-up of the United Kingdom Medical Research Council protocols for childhood acute lymphoblastic leukaemia, 1980–1997. Medical Research Council Childhood Leukaemia Working Party. Leuk Off JLeuk Soc Am LeukRes Fund, UK. 2000;14(12):2307–2320. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=11187922&retmode=ref&cmd=prlinks%Cnpapers3://publication/uuid/32CDDD5D-AF13-4817-8AD0-FEC4A99ACCEE. [DOI] [PubMed] [Google Scholar]
- 26.Hill F, Richards S, Gibson S, et al. Successful treatment without cranial radiotherapy of children receiving intensified chemotherapy for acute lymphoblastic leukaemia: Results of the risk-stratified randomized central nervous system trial MRC UKALL XI (ISRC TN 16757172). Br JHaematol. 2004;124:33–46. [DOI] [PubMed] [Google Scholar]
- 27.Gaynon PS, Trigg ME, Heerema NA, et al. Children’s Cancer Group trials in childhood acute lymphoblastic leukemia: 1983–1995. Leuk Off J Leuk Soc Am Leuk Res Fund, UK. 2000;14(12):2223–2233. doi: 10.1038/sj.leu.2401939. [DOI] [PubMed] [Google Scholar]
- 28.Pui CH, Boyett JM, Rivera GK, et al. Long-term results of Total Therapy studies 11, 12 and 13A for childhood acute lymphoblastic leukemia at St Jude Children’s Research Hospital. Leuk Off J Leuk Soc Am Leuk Res Fund, UK. 2000;14(12):2286–2294. doi: 10.1038/sj.leu.2401938. [DOI] [PubMed] [Google Scholar]
- 29.Hiniker SM, Agarwal R, Modlin LA, et al. Survival and neurocognitive outcomes after cranial or craniospinal irradiation plus total-body irradiation before stem cell transplantation in pediatric leukemia patients with central nervous system involvement. Int J Radiat Oncol Biol Phys. 2014;89(1):67–74. doi: 10.1016/j.ijrobp.2014.01.056. [DOI] [PubMed] [Google Scholar]
- 30.Pui CH, Evans WE. Acute lymphoblastic leukemia. N Engl J Med. 1998;339(9):605–615. doi: 10.1056/NEJM199808273390907. [DOI] [PubMed] [Google Scholar]


