To the Editors:
INTRODUCTION
HIV-infected adolescents have poorer outcomes than all other age groups.1,2 In Haiti, 24,000 adolescents and youth are HIV-infected, with HIV prevalence as high as 2.6%.3 Retaining adolescents in HIV care is difficult, and studies in Haiti have demonstrated that only 60% remain in care 1 year after initiating antiretroviral therapy (ART).4
Multiple factors increase adolescents' risk of poor outcomes including physiological and psychological development,5–7 high risk-taking behavior, and poor decision-making.8 In 2012, the World Health Organization (WHO) recommended “youth-friendly services” for HIV-infected adolescents, which have improved linkage to care and ART initiation but have not systematically improved retention to the levels needed to achieve the UNAIDS 90-90-90 targets.6,7 In 2017, the WHO recommended differentiated models of HIV care for adolescents,9,10 defined as patient-centered services tailored to patients' specific needs and designed to reduce health system inefficiencies.9 Despite widespread guidance, description and evaluation of adolescent-specific differentiated models of HIV care are limited.11,12 We report outcomes of a pilot program of a differentiated model of adolescent HIV care called FANMI (“My Family” in Creole) in Haiti.
METHODS
This study took place at GHESKIO (Haitian Group for the Study of Kaposi's Sarcoma and Opportunistic Infections), located in Port-au-Prince, Haiti, which operates the nation's largest adolescent-specific HIV clinic. We conducted a retrospective chart review using routinely collected data from adolescents who participated in the FANMI program from November 2014 to October 2015. Ethics boards at Weill Cornell Medicine and GHESKIO approved this analysis.
We developed the FANMI model of care in response to an analysis of standard HIV care at the GHESKIO adolescent clinic which showed just 66% of patients were in care 12 months after initiating ART.4 We began with focus group discussions with patients and an adolescent community advisory board to identify factors contributing to poor retention in care. Patients reported experiencing extreme rejection by their family and inability to disclose status because of intense stigma, resulting in profound social isolation. Furthermore, patient–provider relationships were limited as visits consisted of short encounters with multiple clinicians. Patients reported peer support groups as the most enjoyable part of care. Our goal was to achieve ≥75% 12-month retention for FANMI to be appropriate for large-scale implementation.
FANMI was designed to address barriers including social isolation, family rejection, stigma, and disjointed care with multiple providers (Fig. 1). It is based on a previous model of group care implemented among pregnant women at GHESKIO13 and was adapted for an adolescent population through quality improvement methods.14 In FANMI, the same cohort of 5–8 adolescents met monthly to facilitate building close relationships with peers, bolster social support, and reduce stigma by making HIV health management a group norm. Cohorts were stratified by age (10–15 years and 16–20 years) to address adolescents' unique developmental needs. Cohort meetings occurred in a community room, separate from the clinic, to decrease the stigma of attending an HIV clinic. Finally, the structure of each group meeting integrated peer counseling, social activities, and medical care by the same providers each time (a nurse and social worker). This both streamlined care and demedicalized the HIV visit by expanding the visit's focus beyond just clinical care. Group counseling topics included stigma, disclosure, family rejection, and developing coping skills as well as ongoing educational counseling on HIV, risk reduction, and adherence. A nurse provided individual clinical check-ups, point-of-care CD4 testing every 6 months after ART initiation, and ART refills. Adolescents could access the HIV clinic whenever desired or referred.
FIGURE 1.
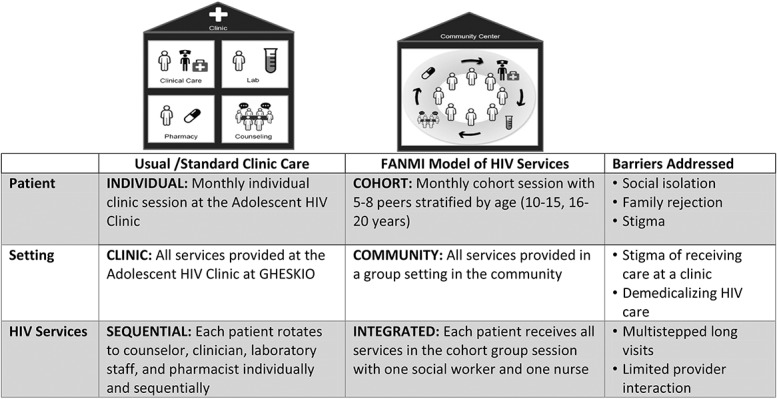
Description of FANMI intervention. HIV services provided were identical in both cohorts and followed the current WHO guidelines for adolescent HIV treatment.
Adolescents aged 10–20 years who tested HIV-positive at the GHESKIO adolescent HIV clinic or in a community-based HIV testing campaign3 between November 2014 and October 2015 participated in FANMI. All participants received HIV services according to national and WHO guidelines.15,16 Verbal parental consent and adolescent assent were provided for all HIV testing and treatment. Upon testing HIV-positive, all adolescents received counseling and initiated ART by a physician, with a first-line regimen of tenofovir, lamivudine, and efavirenz.
Age, sex, and CD4 count were measured at time of HIV testing. Enrollment in HIV care was defined as the date of an HIV-positive test. ART initiation was defined as documentation of ART start in the clinic electronic medical record (EMR). Retention at 12 months was defined as having a clinic visit between 11 and 13 months from HIV testing. Participants completed a standard survey at enrollment and a follow-up survey at 12 months to assess mental health, social support, and sexual risk behaviors. Viral load was recommended at 12 months after ART initiation.
Kaplan–Meier methods estimated the incidence of retention at 12 months with patients censored at time of transfer or attrition from care. Statistical comparisons between survey responses at enrollment and 12 months were made using a 2 proportion z-test.
RESULTS
Fifty adolescents participated in the FANMI program. Median age was 17.5 years [interquartile range (IQR) 15–19], and 76% (n = 38) were female. Median CD4 cell count was 537 cells/mm3 (IQR 339–805) at HIV testing. All adolescents were assessed for ART eligibility and initiated ART the same day as HIV testing (100%; 50/50). At 12 months after ART initiation, 86% (43/50; 95% confidence interval: 74 to 92) of adolescents were retained in care. No deaths were reported, and there was no significant association between age or sex and being retained in care.
Surveys administered at enrollment (n = 50) and 12 months (n = 45) assessed depression, social support, and sexual risk behaviors. The proportion of adolescents who reported feeling hopeless in the past 30 days decreased from 38% at enrollment to 20% at 12 months (P = 0.05), and the proportion who reported depression decreased from 34% at enrollment to 17% at 12 months (P = 0.07). The proportion of adolescents reporting a desire for more emotional support from family and friends decreased from 94% at enrollment to 50% at 12 months (P < 0.01), with 52% reporting they had 1 or less close friends with whom they felt at ease to talk about private matters or call on for help at enrollment and only 40% reporting this at 12 months (P = 0.11). The proportion of participants reporting they used a condom every time they had sex increased from 8% at enrollment to 26% at 12 months (P = 0.02). Among 40 adolescents with available viral load test results 12 months from enrollment, 33% had a viral load <1000 copies/μL. This proportion increased to 55% with further follow-up (IQR 1.6–2.6 years).
Additional outcomes that underscore the vulnerability of these patients include 10% of adolescents who reported they had exchanged sex for food, rent, or other necessities; 12% reported having been raped; and 90% reported consistent food insecurity. No adolescent was referred to the adolescent HIV clinic for medical issues, and none reported any social harms including increased stigma or unintended disclosure.
DISCUSSION
This study describes a promising model of adolescent-specific HIV care that achieved high retention in care, reduced risk behaviors, and improved mental health. The success of FANMI likely reflects the bundling of multiple structural and behavioral interventions into a combined strategy which collectively intensified social support, reduced stigma, demedicalized HIV care, and integrated clinical care and counseling in a single visit with the same provider to facilitate long-term patient–provider relationships and streamlined services.
FANMI builds on several other adolescent models of HIV care. For example, “Saturday Teen Clubs” in Malawi expanded clinic hours to Saturdays, when adolescents received individualized care and participated in crafts and games with peers.17 Those who attended a club were less likely to be lost from care (odds ratio 0.27; 95% confidence interval: 0.16 to 0.45). Second, in the Zvandiri program in Zimbabwe, HIV-infected adolescents were trained as community adolescent treatment supporters and managed a caseload of patients in their district, providing support through home visits and mobile phone communication.12,18 Data from 161 participating sites report 12-month retention was 98% among adolescents on ART. FANMI is unique in that it includes adolescents newly diagnosed, not yet defined as stable, and combines multiple strategies into a combination approach.
Although FANMI improved behavioral outcomes, rates of depression and desire for more social support at 12 months remain markedly high, reflecting the persistent hardships and complexities of adolescence. The hardships may influence medication adherence as our rates of viral suppression remain far from optimal. Additional behavioral and biomedical interventions coupled with the FANMI model of care are needed to improve adherence. Examples include strengthened adherence counseling, mhealth reminders or virtual group platforms,19 family interventions, and more potent and/or better tolerated ART regimens such as integrase inhibitors or long-action regimens.20
In summary, FANMI is a promising, novel model of care for HIV-infected adolescents to improve retention in care as well as risk behaviors and mental health for this extremely vulnerable population. Although this analysis reports data from a small pilot, FANMI is now being evaluated in a large randomized control trial (NCT03286504) in Haiti. If found effective, this model could be scaled up in other resource-limited settings and among other vulnerable populations.
Footnotes
Supported by NIH/NIAID 5K24AI098627, MACAIDS Foundation, Flora Family Foundation, Camela Basin Family Foundation, NIH/FIC 5D43TW009606, and NIH/FIC 5D43TW010062.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Idele P, Gillespie A, Porth T, et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr. 2014;66(suppl 2):S144–S153. [DOI] [PubMed] [Google Scholar]
- 2.Sohn AH, Hazra R. The changing epidemiology of the global paediatric HIV epidemic: keeping track of perinatally HIV-infected adolescents. J Int AIDS Soc. 2013;16:18555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reif LK, Rivera V, Louis B, et al. Community-based HIV and health testing for high-risk adolescents and youth. AIDS Patient Care STDS. 2016;30:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reif LK, Bertrand R, Benedict C, et al. Impact of a youth-friendly HIV clinic: 10 years of adolescent outcomes in Port-au-Prince, Haiti. J Int AIDS Soc. 2016;19:20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adejumo OA, Malee KM, Ryscavage P, et al. Contemporary issues on the epidemiology and antiretroviral adherence of HIV-infected adolescents in sub-Saharan Africa: a narrative review. J Int AIDS Soc. 2015;18:20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abubakar A, Van de Vijver FJ, Fischer R, et al. “Everyone has a secret they keep close to their hearts”: challenges faced by adolescents living with HIV infection at the Kenyan coast. BMC Public Health. 2016;16:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choudhury S, Blakemore SJ, Charman T. Social cognitive development during adolescence. Soc Cogn Affect Neurosci. 2006;1:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willoughby T, Good M, Adachi PJC, et al. Examining the link between adolescent brain development and risk taking from a social-developmental perspective. Brain Cogn. 2013;83:315–323. [DOI] [PubMed] [Google Scholar]
- 9.Differentiated Care. Differentiated care for HIV: a decision framework for differentiated antiretroviral therapy delivery for children, adolescents, and pregnant and breastfeeding women. Available at: http://www.differentiatedcare.org/Portals/0/adam/Content/9ErIJtsSfUmj_Ska6BoN0Q/File/Decision%20Framework%20for%20children%20adolescents%20and%20pregnant%20and%20breastfeeding%20women.pdf. Accessed April 8, 2019.
- 10.The Global Fund. A Toolkit for Health Facilities: Differentiated Care for HIV and Tuberculosis. Geneva, Switzerland; 2015. Available at: https://www.theglobalfund.org/media/2569/core_differentiatedcare_toolkit_en.pdf. Accessed April 8, 2019. [Google Scholar]
- 11.Reif LK, McNairy ML, Lamb MR, et al. Youth-friendly services and differentiated models of care are needed to improve outcomes for young people living with HIV. Curr Opin HIV AIDS. 2018;13:249–256. [DOI] [PubMed] [Google Scholar]
- 12.Willis N, Napei T, Armstrong A, et al. Zvandiri-bringing a differentiated service delivery program to scale for children, adolescents, and young people in Zimbabwe. J Acquir Immune Defic Syndr. 2018;78(suppl 2):S115–S123. [DOI] [PubMed] [Google Scholar]
- 13.Heidkamp RA, Stoltzfus RJ, Fitzgerald DW, et al. Growth in late infancy among HIV-exposed children in urban Haiti is associated with participation in a clinic-based infant feeding support intervention. J Nutr. 2012;142:774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langley G, Moen R, Nolan K, et al. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. San Francisco, CA: Jossey-Bass; 2009. [Google Scholar]
- 15.Guideline on When to Start Antiretroviral Therapy and on Pre-exposure Prophylaxis for HIV. Geneva, Switzerland: World Health Organization; 2015. Available at: http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/. Accessed April 8, 2019. [PubMed] [Google Scholar]
- 16.World Health Organization. Rapid Advice: Antriretroviral Therapy for HIV Infection in Adults and Adolescents. Geneve, Switzerland: WHO; 2009. [Google Scholar]
- 17.MacKenzie RK, van Lettow M, Gondwe C, et al. Greater retention in care among adolescents on antiretroviral treatment accessing “Teen Club” an adolescent-centred differentiated care model compared with standard of care: a nested case-control study at a tertiary referral hospital in Malawi. J Int AIDS Soc. 2017;20:e25028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Africaid. Zimbabwe Africaid-Zvandiri Community Adolescent Treatment Supporters (CATS). CHAI. Available at: https://docs.wixstatic.com/ugd/a5b780_fcfd9f3221474eba8d3f971ab780c793.pdf. Accessed April 8, 2019.
- 19.Dillingham R, Ingersoll K, Flickinger TE, et al. PositiveLinks: a mobile health intervention for retention in HIV care and clinical outcomes with 12-month follow-up. AIDS Patient Care STDS. 2018;32:241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017;390:1499–1510. [DOI] [PubMed] [Google Scholar]


