Abstract
The neuropeptide, corticotropin-releasing factor (CRF), is a key modulator of physiological, endocrine, and behavioral responses during stress. Dysfunction of the CRF system has been observed in stress-related affective disorders including post-traumatic stress disorder, depression, and anxiety. Beyond affective symptoms, these disorders are also characterized by impaired cognition, for which current pharmacological treatments are lacking. Thus, there is a need for pro-cognitive treatments to improve quality of life for individuals suffering from mental illness. In this review, we highlight research demonstrating that CRF elicits potent modulatory effects on higher-order cognition via actions within the prefrontal cortex and subcortical monoaminergic and cholinergic systems. Additionally, we identify questions for future preclinical research on this topic, such as the need to investigate sex differences in the cognitive and microcircuit actions of CRF, and whether CRF may represent a pharmacological target to treat cognitive dysfunction. Addressing these questions will provide new insight into pathophysiology underlying cognitive dysfunction and may lead to improved treatments for neuropsychiatric disorders.
Keywords: Corticotropin-releasing factor (CRF), cognition, cognitive flexibility, working memory, object location memory, sustained attention, cost/benefit decision-making, effort-related choice, motivation, sex differences, norepinephrine (NE), serotonin (5-HT), acetylcholine, dopamine (DA), basal forebrain, medial septum (MS), prefrontal cortex (PFC), dorsal raphe nucleus (DR), locus coeruleus (LC), ventral tegmental area (VTA), nucleus accumbens (NAc)
1. Introduction
Cognitive dysfunction is widely observed in disorders including schizophrenia, post-traumatic stress disorder (PTSD), major depression, and anxiety (Barch and Ceaser, 2012; Millan et al., 2012; Lett et al., 2014). Although available drugs treat affective symptoms in these disorders, there are currently no treatment options for the cognitive deficits (Millan et al., 2012; Al-Sukhni et al., 2015; Buoli and Altamura, 2015). Yet, improvements in cognitive functioning are closely associated with positive disease prognoses and long-term outcomes (Fett et al., 2011). Notably, many psychiatric diseases that are characterized by cognitive impairment are exacerbated by stress (Piazza et al., 1991; Mazure, 1995; Corcoran et al., 2003). Acute and chronic stress impairs higher-order cognitive processes (e.g. working memory, cognitive flexibility) dependent on the prefrontal cortex (PFC), while sparing or improving habitual behaviors and simpler learning (e.g. fear conditioning and memory) dependent on subcortical regions (Wingard and Packard, 2008; Arnsten, 2009; Luethi et al., 2009; Roozendaal et al., 2009; Barsegyan et al., 2010; Shansky and Lipps, 2013). Therefore, a better understanding of the neurobiological mechanisms underlying stress-related cognitive dysfunction may lead to the development of new and improved treatments for many psychiatric disorders.
Emerging evidence demonstrates that the neuropeptide, corticotropin-releasing factor (CRF), modulates the cognition-impairing effects of stress. Although CRF was initially characterized as a hypothalamic neurohormone that initiates pituitary adrenocorticotropic hormone in response to stress, CRF is now widely recognized to orchestrate many aspects of physiological and behavioral effects of stress through networks involving CRF-synthesizing neurons and CRF-expressing receptors in extrahypothalamic brain regions (Swanson et al., 1983; De Souza et al., 1985; Charlton et al., 1987; Lewis et al., 1989). A considerable body of research demonstrates that central CRF administration mimics many behavioral and physiological actions of the stress response, and genetic or pharmacological disruption of CRF function inhibits the physiological and behavioral effects of stress (Dunn and Berridge, 1990; Bale and Vale, 2004; Henckens et al., 2016; Deussing and Chen, 2018). Although the role of CRF in mediating the acute stress response is critical for survival, excessive CRF neurotransmission has been implicated in the pathology of various psychiatric disorders linked to chronic or severe stress, including PTSD, major depression, and anxiety (Nemeroff et al., 1988; Lammers et al., 1995; Bremner et al., 1997; Millan et al., 2012; Bangasser and Kawasumi, 2015).
The past few decades of research have largely focused on studying the role of CRF in affective components relevant to psychiatric disease. However, until recently, efforts to investigate how CRF neurotransmission modulates cognitive function have been sparse. Recent clinical research has observed a link between CRF and cognition, demonstrating that genetic polymorphisms in the CRHR1 gene are associated with impaired cognitive functioning in healthy adults and patients with mental illness (Fuge et al., 2014; Grimm et al., 2015; Davis et al., 2018). Thus, the goal of this review is to highlight preclinical research that reveals a role for CRF in cognitive processes including cognitive flexibility, sustained attention, working memory, spatial memory, and cost/benefit decision-making. This research demonstrates that CRF elicits distinct cognitive and physiological actions via differing receptor mechanisms and intracellular signaling pathways within monoaminergic and cholinergic subcortical nuclei, as well as the medial PFC (mPFC). Where examined, local infusions of CRF do not completely recapitulate the cognitive effects of behavioral stressors or global CRF infusions (Snyder et al., 2012; Bryce and Floresco, 2016; Hupalo and Berridge, 2016), likely due to the fact that stress and global CRF manipulations activate a variety of systems known to modulate cognitive function (Valentino et al., 1983; Dunn and Berridge. 1987; Zahrt et al., 1997; Arnsten et al., 1999; Roozendaal et al., 2004; Lapiz and Morilak, 2006; Barsegyan et al., 2010). Therefore, future studies must utilize a circuit-specific approach that identifies CRF sources, receptor mechanisms, and microcircuitry responsible for the cognitive effects of CRF. Additionally, we describe sex differences in the cognitive actions of CRF signaling and emphasize the need for understanding the impact of sexual differentiation within this system. Finally, we consider whether intervening with CRF signaling may represent a potential treatment for cognitive dysfunction.
2. Regulation of cognitive flexibility by CRF actions on monoamine neurons
CRF axon terminals are widely distributed throughout the brain, providing multiple targets for CRF to regulate cognitive processes (Swanson et al., 1983; Bangasser and Kawasumi, 2015; Henckens et al., 2016). One major mechanism involves CRF action within monoamine nuclei that have widespread cortical projections, including regions critical for executive function. These include the locus coeruleus (LC) and dorsal raphe (DR) nuclei, which represent primary sources of norepinephrine (NE) and serotonin (5-HT) to the cortex, respectively (Amaral and Sinnamon, 1977; Foote et al., 1983; Kosofsky and Molliver, 1987). CRF effects on these nuclei are highly dependent on doses of CRF administered to these regions, suggesting that variations in endogenous CRF levels can have distinct, non-monotonic effects on processes modulated by NE and 5-HT. These differences are in part driven by differential expression of CRF receptors in the rodent LC and DR – whereas the LC solely expresses CRF1 receptors, both CRF1 and CRF2 are found in the DR (Chalmers et al., 1995; Day et al., 2004). Collectively, these studies provide insight into how stress history and coping style regulate and determine varying cognitive strategies.
2.1. CRF, the locus coeruleus-norepinephrine system, and attentional set shifting
The LC-NE system regulates arousal and a diversity of state-dependent behavioral and physiological functions (Foote et al., 1980; Berridge and Foote, 1991; Aston-Jones and Cohen, 2005; Carter et al., 2010). Under conditions associated with moderate arousal, LC neurons display moderate levels of tonic Firing and robust phasic Firing to salient sensory events and sensory-dependent decisions and/or actions (Foote et al., 1980; Aston-Jones and Bloom, 1981). Under higher arousal conditions (e.g. stress), LC neurons display elevated tonic Firing and weakened phasic Firing (Valentino and Foote. 1987). LC axonal projections to the mPFC modulate a diversity of cognitive processes, including working memory, sustained attention, and flexible attention (Arnsten et al., 1999; Birrell and Brown, 2000; McGaughy et al., 2008; Berridge and Spencer, 2016). Under moderate rates of LC activity and NE release, high-affinity postsvnaptic α2 adrenergic receptors in the PFC are preferentially engaged and promote working memory (Franowicz and Arnsten, 1998). Under conditions associated with elevated LC firing and activation of lower affinity α1 adrenergic receptors, working memory is impaired while both flexible and focused attention are improved (Arnsten et al., 1999; Lapiz and Morilak, 2006; Cope et al., 2019; Spencer and Berridge, 2019). Thus, LC projections to the PFC modulate different cognitive processes in a context-sensitive manner.
The LC is densely innervated by CRF axon terminals originating in the central nucleus of the amygdala (CeA), paraventricular nucleus of the hypothalamus (PVN), Barrington’s nucleus, and the nucleus paragigantocellularis (Valentino et al., 1996; Bockstaele et al., 1998; Curtis et al., 2002; Reyes et aL 2005). Local CRF infusions and behavioral stressors shift the mode of LC activity to high tonic discharge rates and blunted sensory-evoked phasic responses (Valentino and Foote, 1987, 1988; Valentino and Wehby, 1988; Curtis et al., 2012). This mode of activity is associated with enhanced scanning attention and behavioral flexibility, which is an adaptive response to a stressor that promotes survival (Aston-Jones and Cohen, 2005). However, different levels of CRF in the LC influence distinct components of behavioral flexibility. For instance, moderate concentrations of intra-LC CRF that increase LC discharge by ~50% enhance cognitive flexibility as measured by extradimensional shifting (EDS) between different rules, strategies, or sets (Curtis et al., 2012; Snyder et al., 2012). This is associated with neuronal activation in the mPFC as measured by Fos immunoreactivity (Snyder et al., 2012). However, higher concentrations of intra-LC CRF, which maximally elevate LC discharge rates, have no effect on EDS performance and do not activate the mPFC (Figure 1A). The dose-dependent effects of CRF on EDS and mPFC activation are consistent with the well documented inverted U-shaped modulatory actions of NE on PFC neuronal activity and function (Arnsten, 2011), associated with preferential engagement of α2 adrenergic receptors at low levels of NE and α1/β receptors at high levels of NE (Devilbiss and Waterhouse, 2000).
Figure 1. CRF acts on the LC-NE and DR-5-HT systems to modulate cognitive flexibility.
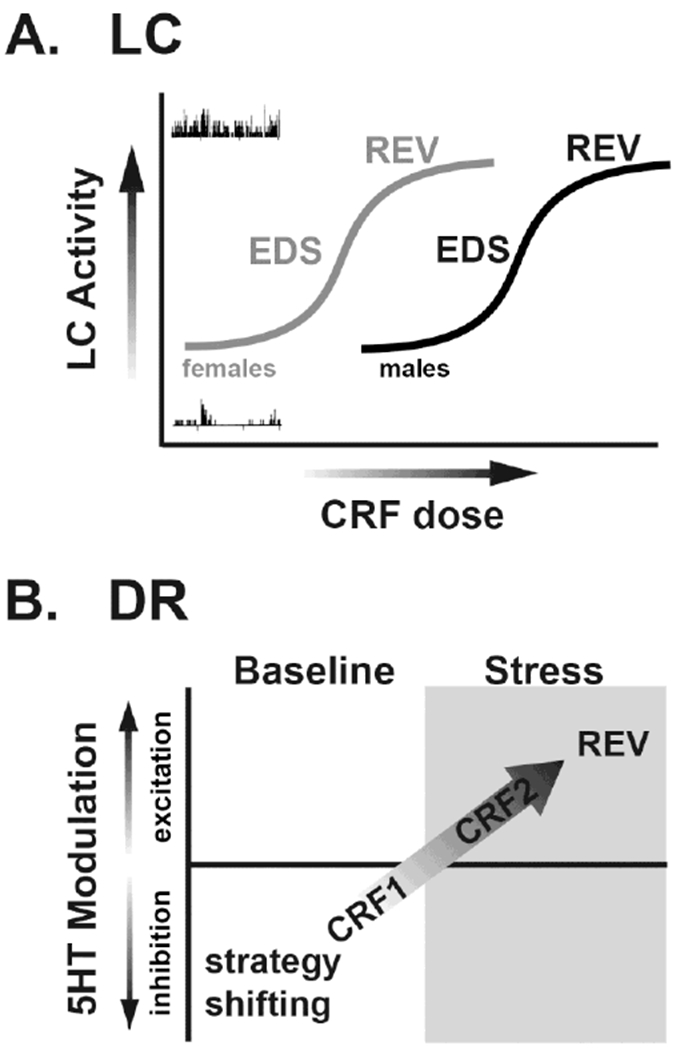
A. In unstressed male rats, low doses of intra-LC CRF that produce moderate increases in LC activity improve EDS performance in an attentional set shifting task. Higher concentrations that produce a maximal increase LC activity have no effect on EDS but improve reversal learning (REV). Although this has not been tested in females, evidence for a shift to the left in the CRF dose-response curve for LC activation in females (gray) compared to males (black) suggests that the dose-response for intra-LC CRF effects on cognitive function would be shifted in a similar manner. B. In unstressed male rats, low doses of CRF infused into the DR inhibit 5-HT neuronal activity via actions at CRF1 receptors while CRF2-selective agonists have no effects. This is associated with improved performance in an operant strategy shifting task. Following stress exposure, CRF2 receptors are recruited to the plasma membrane in the DR and CRF elicits excitatory effects on 5-HT neuronal activity in animals that exhibit a proactive coping strategy. These actions are associated with enhancement of REV.
Interestingly, the higher concentrations of intra-LC CRF that did not alter EDS improved reversal learning, a different form of flexibility entailing shifts between stimulus-reward associations mediated by the orbitofrontal cortex (Figure 1A; (Snyder et al., 2012). Notably, stress and intracerebroventricular (ICV) CRF infusions elicit a similar switch from goal-directed cognitive processes mediated by the mPFC (e.g. EDS) to dorsolateral striatum-dependent reversal learning (Dias-Ferreira et al., 2009; Graybeal et al., 2011; Snyder et al., 2015a). Combined, these observations indicate that under conditions of low stress, moderate levels of CRF in the LC are associated with enhanced EDS and optimal executive functioning. However, severe stress associated with high levels of CRF in the LC contributes to a shift from optimal executive function necessary for goal-directed behavior toward habit-based behavior. This switch may be adaptive in stressful, threatening conditions when goal-oriented behavior may be inefficient or risky and the fallback to well-established habits allows for processing of new information. Notably, LC neuronal recordings during attentional set shifting performance revealed that stress renders LC neurons more sensitive to reward during intradimensional set shifting (IDS) and reversal learning, an action that did not diminish with repeated trials (Chaijale et al., 2015). Together, these results suggest that stress biases circuits that increase reward salience in part through elevated LC-NE signaling.
Nonetheless, it is simplistic to expect that stressors, which engage multiple circuits and evoke release of a diversity of neuromodulators within and outside the LC, would completely recapitulate the effects of local CRF signaling in the LC. For example, social stress promotes IDS performance while having no effects on EDS and reversal learning (Chaijale et al., 2015). Meanwhile, high doses of ICV CRF have no effect on EDS and impair both IDS and reversal learning (Snyder et al., 2012). Together, these results demonstrate that local vs. global CRF manipulations elicit varying effects on cognitive components that are distinct from stress and highlight the importance of studying circuit-specific actions of CRF.
The above-described studies were conducted in male rats. Importantly, recent observations demonstrate that sex differences in CRF regulation of LC function may differentially impact cognition in males vs. females (Bangasser and Wiersielis, 2018). For example, in females, LC tonic firing increases in response to low doses of CRF that have no effect on firing in males, demonstrating that females are more sensitive to LC CRF (Curtis et al., 2006; Bangasser et al., 2010). This sex difference in LC sensitivity to CRF is mediated by sex differences in CRF1 receptor recruitment of intracellular signaling pathways. CRF1 receptors bind Gs proteins to activate the cyclic adenosine monophosphate (cAMP)-protein kinase A (PKA) signaling pathway (Grammatopoulos et al., 2001), which increases neuronal firing in the LC (Jedema and Grace, 2004). In females, increased LC neuronal sensitivity to CRF is mediated by greater activation ofthe cAMP-PKA pathway than in males (Bangasser et al., 2010, 2017). Whether this sex difference in LC sensitivity to CRF results in differential cognitive changes remains unknown. However, if females possess similar inverted-U shaped modulatory effects of LC CRF on cognitive flexibility, lower levels of CRF would be predicted to induce EDS in females relative to males (Figure 1A), and a stressor which enhances behavioral flexibility in males may promote habit-driven behavior in females.
2.3. CRF, the dorsal raphe-serotonin system, and strategy shifting
Beyond the LC-NE system, CRF exerts additional modulatory effects on behavioral flexibility via interactions with the DR-5-HT system. Although the DR contains a small population of local CRF neurons, limited evidence suggests it receives additional sources of CRF from the CeA (Commons et al., 2003; Forster et al., 2008). CRF fibers innervating the DR are topographically organized, providing an opportunity for selective modulation of distinct DR circuits (Valentino et al., 2010). While CRF effects on LC discharge are positively related to dose and mediated by CRF1 receptors, CRF acts via both CRF1 and CRF2 to elicit biphasic effects on DR-5-HT neuronal activity and 5-HT cortical release (Kirby et al., 2000). For instance, low doses of CRF infused into the DR inhibit 5-HT neurons via actions at CRF1 receptors, whereas higher doses are excitatory and CRF2-dependent (Forster et al., 2008; Valentino et al., 2010). This qualitative shift in DR-5-HT neuronal response to CRF is tightly linked to changes in cognitive function. For example, in unstressed male rats, intra-DR CRF doses that inhibit 5-HT neuronal activity and mPFC 5-HT release (via CRF1) facilitate performance in an operant strategy shifting task (Snyder et al., 2015b). However, this effect diminishes with increasing doses of CRF. Meanwhile, intra-DR infusions of a CRF2 selective agonist have no effect on simple discrimination, reversal learning, or strategy shifting (Snyder et al., 2015b).
Stress exposure can profoundly change CRF receptor distribution in the DR. Following swim stress in male rats, CRF receptors within the DR redistribute such that CRF1 becomes internalized while CRF2 is recruited to the plasma membrane, resulting in a qualitative switch from CRF-induced neuronal inhibition to an excitation that is sensitive to CRF2 antagonists (Waselus et al., 2009). A similar pattern of effects occurs after exposure to the resident-intruder model of psychosocial stress, but only for rats that exhibit a proactive coping strategy to the stressor (Wood et al., 2013). Repeated resident-intruder stress results in the emergence of two phenotypes defined by animals’ propensity to become subordinate (Wood et al., 2010). The population is bimodally distributed into rats having either short or long latencies (SL or LL) to assume the subordinate defeat posture. It is only in the LL rats that the CRF response switches from CRF1-mediated inhibition to CRF2-mediated excitation due to receptor redistribution in the DR (Wood et al., 2010). Following repeated resident-intruder psychosocial stress exposure, the effects of intra-DR CRF change from facilitation of strategy shifting to an enhancement of reversal learning in LL rats. This is consistent with observations demonstrating that CRF induces a shift in DR-5-HT neuronal inhibition to excitation only in LL rats (Figure 1B; (Snyder et al., 2015b). Given the modulatory role of 5-HT in the orbitofrontal cortex in reversal learning (Clarke et al., 2005), the qualitative switch toward CRF2-mediated excitation of 5-HT neuronal activity and 5-HT release may account for the shift toward facilitation of reversal learning.
Although these studies utilized male subjects, reported sex differences in CRF1 localization and physiology suggest that CRF signaling in the DR could have different effects on strategy shifting in females compared to males. In the female DR, CRF1 receptors are less frequently expressed on GABAergic neurons relative to males (Howerton et al., 2014). Moreover, in vitro studies demonstrate that CRF prolongs inhibitory currents in the male DR but has the opposite effect in females (Howerton et al., 2014). A diminished inhibitory effect of CRF in the female DR may result in impaired strategy shifting. However, to date, the contribution of DR CRF1 and CRF2 receptors to strategy shifting in females is unknown and remains to be investigated.
Collectively, these studies demonstrate that CRF influences different forms of cognitive flexibility by regulating monoamine neuronal activity in the LC and DR. The cognitive and cellular actions of CRF signaling in these regions vary as a function of sex, stress history, and coping style. The observation that varying levels of CRF elicit distinct neurophysiological and cognitive actions suggests that fluctuations in stress and arousal produce diverse behavioral outcomes to optimize adaptive coping strategies and ensure survival.
3. CRF regulation of cholinergic basal forebrain-dependent processes
The basal forebrain cholinergic system modulates processes ranging from memory to attention (Sarter et al., 2003). Cholinergic neurons in the nucleus basalis of Meynert (NBM) are critical for sustained attention, the ability to monitor a situation for intermittent and unpredictable events (Sarter et al., 2001). Although it has long been known that CRF1 receptors are present on cholinergic neurons in this region, the sources of CRF to the NBM have not been definitively identified. One potential region that contains CRF cell bodies and projects to the NBM is the CeA (Krettek and Price, 1978; Sauvage and Steckler, 2001). These anatomical findings have led to the hypothesis that CRF regulates cognitive processes dependent on the cholinergic basal forebrain. As such, ICV CRF dose-dependently disrupts aspects of attention mediated by NBM cholinergic neurons in both male and female rats (Van’t Veer et al., 2012; Cole et al., 2016). In females, ovarian hormones regulate these actions, such that CRF impairs attention in the estrous stages with low ovarian hormones, but not in phases with high ovarian hormones (Cole et al., 2016). Thus, ovarian hormones can protect females from the negative effects of CRF signaling on sustained attention. However, the degree to which CRF activity locally within the NBM modulates components of sustained attention is still unknown.
Female resistance to the cognition-impairing effects of CRF is also observed in the medial septum (MS), which is a major source of cholinergic projections to the hippocampus critical for spatial learning and memory (Amaral and Kurz, 1985; Cai et al., 2012). Similar to the NBM, the MS contains the CRF1 subtype (Van Pett et al., 2000). Although the sources of CRF to the MS have not been systematically investigated, limited evidence indicates CRF cell bodies are sparsely located in the MS (Merchenthaler et al., 1984; Hupalo et al., 2019). CRF administered into the MS impairs hippocampal-dependent object location memory in both sexes, while having no effect on hippocampal-independent object recognition memory (Wiersielis et al., 2019). Males are more sensitive to this effect, as a low dose of CRF impairs performance in this task in male, but not female rats (Figure 2). There exist no sex differences in CRF1 expression in the MS, suggesting receptor number cannot explain the male vulnerability. Instead, the decreased sensitivity to low CRF doses in the MS observed in females is linked to higher levels of CRF binding protein, which reduces CRF bioavailability (Wiersielis et al., 2019). Combined, this suggests that females are less sensitive to CRF-induced impairments in basal forebrain-dependent cognitive processes but are highly vulnerable to CRF’s effect on the LC-NE system.
Figure 2. CRF1 receptor activity in the MS impairs spatial memory.

Local infusions of CRF into the MS impair hippocampal-dependent object location memory. Male rats are more sensitive to these actions, such that a low CRF dose that is impairing in males has no effect in females. Compared to males, female rats have greater MS expression levels of CRF-binding protein (CRF-bp), which sequesters CRF and limits its bioavailability. Increased levels of CRF-bp in females could reduce the behavioral and physiological effects of CRF and may account for their decreased sensitivity to the behavioral actions of CRF transmission in the MS. However, causal manipulations demonstrating that the sex difference in CRF-bp fully accounts for the sex difference in behavior still need to be completed.
4. Working memory actions of CRF signaling in the prefrontal cortex
The PFC supports higher cognitive processes critical for goal-directed behavior (Fuster, 2015). Stress-related impairment in PFC-dependent cognition is associated with excessive catecholamine signaling and altered PFC neurophysiology (Arnsten, 2009). In addition to catecholamines, CRF is another stress-related neuromodulator that is prominent in the PFC. In rodents, CRF-containing cell bodies and CRF1-expressing neurons are present in all layers of the mPFC, and represent two distinct neuronal populations (Swanson et al., 1983; De Souza et al., 1985; Charlton et al., 1987; Sakanaka et al., 1987; Potter et al., 1994; Uribe-Mariño et al., 2016). Besides local CRF-synthesizing neurons, it is possible that additional sources arise from CRF neurons in regions known to project to the PFC, including the MS (Hupalo et al., 2019) and bed nucleus of the stria terminalis (BNST; (Swanson et al., 1983; Hoover and Vertes, 2007). Although very low levels of CRF2 mRNA have been identified in the cortex (Kostich et al., 1998; Van Pett et al., 2000), CRF binds CRF1 receptors with a tenfold greater affinity compared to CRF2, and CRF1 is presumed to be the primary functional receptor subtype in the PFC (Perrin et al., 1995).
Recent evidence demonstrates that CRF is a potent modulator of PFC-dependent cognition. For instance, ICV and intra-PFC infusions of CRF impair performance in a delayed alternation test of spatial working memory in male rats (Hupalo and Berridge, 2016). Notably, ICV CRF elicits a more robust cognitive impairment than intra-PFC CRF, likely due to activation of additional systems that influence PFC function (Valentino et al., 1983; Dunn and Berridge, 1987; Arnsten et al., 1999; Lapiz and Morilak, 2006; Barsegyan et al., 2010). The working memory actions of CRF signaling in the PFC are topographically organized, with impairment observed only following CRF infusions into the caudal dorsomedial PFC (dmPFC; (Hupalo and Berridge 2016). Moreover, intra-PFC and systemic administration of CRF1 antagonists improves working memory, demonstrating that endogenous CRF signaling modulates PFC-dependent cognition under basal, non-stressful conditions (Figure 3). Interestingly, the working memory-enhancing actions of CRF antagonists mimic the pro-cognitive effects of all FDA-approved drugs for attention-deficit/hyperactivity disorder (ADHD; (Berridge and Arnsten, 2015). Together, these findings suggest that targeting PFC CRF neurotransmission may represent a novel therapeutic approach for the treatment of PFC-dependent cognitive dysfunction.
Figure 3. CRF1 signaling in the caudal dmPFC impairs working memory.
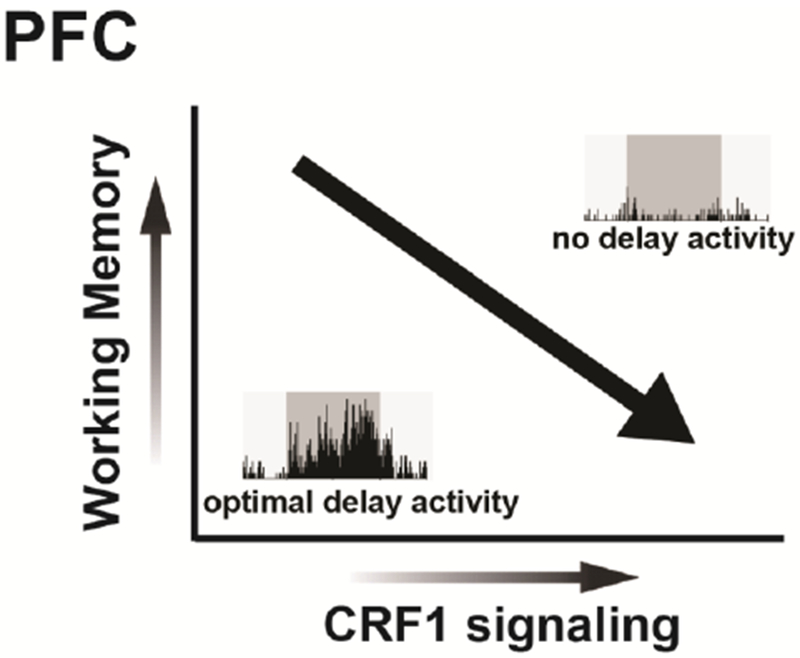
In male rats, local PFC CRF release and subsequent activation of CRF1 receptors coupled to PKA impairs performance in a delayed alternation test of spatial working memory. These actions are associated with a robust degradation in delay-related activity of PFC pyramidal neurons in animals undergoing working memory testing.
To investigate whether local CRF-synthesizing neurons are a source of CRF to the caudal dmPFC, recent studies utilized a dual virus approach to chemogenetically activate or inhibit PFC CRF neurons in male wild-type rats. Chemogenetic activation of CRF neurons in the caudal dmPFC impaired spatial working memory, and this effect was reversed with local infusions of a CRF antagonist or a PKA inhibitor (Hupalo et al., 2019). Together, these studies demonstrate that CRF neurons in the caudal dmPFC impair working memory via local release and subsequent activation CRF receptors coupled to intracellular PKA signaling. Moreover, chemogenetic inhibition of caudal dmPFC CRF neurons improved working memory performance, similar to the actions of CRF receptor blockade. These latter findings suggest that endogenous PFC CRF neurotransmission impairs cognitive processes necessary for successful working memory performance. The cognition-impairing actions of PFC CRF neurotransmission contrast with evidence demonstrating that CRF signaling in subcortical circuits promotes fear-related learning and memory (Lee et al., 1992; Roozendaal et al., 2002, 2008; Sanford et al., 2017). Thus, CRF may enhance subcortical memory processes at the expense of higher-order cognition.
The working memory-impairing actions of PFC CRF are associated with a degradation in PFC neuronal coding critical for goal-directed behaviors. Specifically, chemogenetic activation of CRF neurons in the caudal dmPFC suppresses delay- and reward-related signaling of putative pyramidal neurons in the dmPFC of working memory-tested rats (Hupalo et al., 2019). This is accompanied by a dramatic decrease in the population size of strongly tuned delay neurons in the dmPFC (Figure 3). Importantly, PFC CRF neuronal activation has no effects on the broader population of dmPFC neurons not displaying task-related activity, indicating that PFC CRF signaling does not broadly increase inhibitory tone within the dmPFC. Nonetheless, prior studies demonstrate that at least some PFC CRF neurons are GABAergic interneurons (Mohila and Onn, 2005; Helmeke et al., 2008). Taken together, this suggests PFC CRF neurons are neurochemically heterogenous and exert complex neuromodulatory actions on PFC neurophysiology, warranting further investigation into the contribution of distinct PFC CRF neuronal subpopulations to working memory. Nevertheless, these observations demonstrate that PFC CRF neuronal activity weakens coding of information required for successful goal attainment in a working memory task.
Given these studies were limited to male subjects, it remains to be seen whether there exist sex differences in the cognitive actions of PFC CRF. This is a pressing question given that cortical CRF1 receptors have been shown to recruit different intracellular signaling pathways in males vs. females (Valentino et al., 2013). In females, cortical CRF1 receptors bind more Gs protein, while in males cortical CRF1 receptors bind more β-arrestin. which activates signaling cascades (e.g. Rho GTPases) distinct from those activated by G-proteins (Lefkowitz and Shenoy, 2005; Bangasser et al., 2010). Consistent with this, in a model of CRF hypersecretion, the CRF overexpressing mouse (CRF-OE), phosphorylation of cortical proteins within the cAMP-PKA signaling pathway is greater in females compared to males (Bangasser et al., 2017). In contrast, male CRF-OE mice exhibit enriched cortical phosphopeptides related to the Rho signaling pathway activated by β-arrestin. Together, these studies identify how sex differences in CRF signaling could lead to sex differences in PFC-dependent processes, and demonstrate the importance of understanding the broad cognitive actions of PFC CRF neurotransmission in both sexes.
Ample evidence indicates PFC CRF neurotransmission contributes to stress-related cognitive impairment. For example, acute stress impairs multiple forms of PFC-dependent cognition and increases CRF and CRF1 mRNA levels within the PFC (Meng et al., 2011; Uribe-Mariño et al., 2016). In addition, stress is associated with excessive PKA signaling, which is recruited by CRF1 receptor activity in the PFC (Arnsten, 2009; Miguel et al., 2014; Uribe-Mariño et al., 2016; Hupalo et al., 2019). Similar to the effects of increased PFC CRF neuronal activity, stress suppresses task-related activity of mPFC neurons in animals engaged in a working memory task (Devilbiss et al., 2017). Importantly, viral knockdown of mPFC CRF1 receptors reverses the cognition-impairing effects of stress (Uribe-Mariño et al., 2016).
Although PFC CRF neurotransmission contributes to the behavioral and physiological effects of stress, the cognition-enhancing actions of CRF antagonists and CRF neuronal suppression may not solely reflect an anti-stress effect. Specifically, in studies where CRF antagonists were observed to improve working memory (Hupalo and Berridge, 2016), animals were well-habituated to the testing procedures, motivated to engage in the task, and displayed relatively high baseline performance accuracy. Moreover, PFC CRF release has been associated with non-stressful events, including anticipatory responses to appetitive cues (Merali et al., 2004). Combined, these observations suggest that PFC CRF neurons are active across a range of conditions associated with higher arousal and motivation states, both stressful and non-stressful.
5. CRF modulation of motivational and decision-making processes
In addition to regulating cognitive processes such as attention, working memory, and cognitive flexibility, CRF modulates complex evaluative processes involving assessments of costs and benefits to optimize rewards of greater subjective value. Perturbations in cost/benefit decision-making are observed in stress-related psychiatric illnesses associated with CRF hypersecretion (Nemeroff et al., 1984; Treadway et al., 2012). Among the various types of costs (e.g. delays, uncertainty) that a decision-maker may consider in pursuit of different rewards, decisions involving choices between easily obtained, less valuable rewards vs. larger, more valuable ones that require greater effort to obtain appear to be particularly sensitive to increases in CRF neurotransmission. For example, central infusions of exogenous CRF markedly shift choice biases away from larger rewards obtainable after multiple lever presses in favor of smaller ones delivered after a single press in an operant, effort-discounting task in male rats (Bryce and Floresco, 2016). Notably, acute restraint stress induces a similar effect on effort-related choice, yet this is not mediated by glucocorticoids, as corticosterone treatment does not recapitulate the effects of stress on this form of decision-making (Shafiei et al., 2012). Instead, CRF antagonism blocks the effects of restraint stress on effort-related choice, demonstrating that stress-related increases in CRF diminish preference for larger rewards associated with a greater effort cost (Bryce and Floresco, 2016). Importantly, increased CRF activity has no effect on choice for larger vs. smaller rewards of equal costs. Thus, rather than reducing the incentive value of larger rewards, increased CRF transmission may amplify the perceived effort costs required to obtain them. Consistent with this hypothesis, central infusions of CRF reduce responding for rewards delivered on a progressive ratio schedule of reinforcement (Wanat et ah, 2013; Bryce and Floresco, 2016).
The ability to overcome effort costs in the pursuit of larger or “better” rewards is critically dependent on mesolimbic dopamine (DA) signaling, particularly in the nucleus accumbens (NAc). DA depletion or DA receptor blockade in this nucleus diminishes choice of larger, more costly rewards in a manner similar to increased CRF neurotransmission (Salamone et al., 1991, 1994; Cousins et al., 1993; Cousins and Salamone, 1994; Nowend et al., 2001; Floresco et al., 2008). As such, it is plausible that CRF reduces motivation to pursue more costly rewards via actions on DA transmission. This idea is supported by anatomical observations demonstrating strong interactions between the DA and CRF systems (Kelly and Fudge, 2018). In rodents, the ventral tegmental area (VTA) receives CRF innervation from the lateral BNST, CeA, and the PVN (Rodaros et al., 2007; Dabrowska et al., 2016), and both CRF1 and CRF2 receptors are localized within the DA cell body region of this structure (Van Pett et al., 2000; Tan et al., 2017). Moreover, the NAc contains local CRF cell bodies (Swanson et al., 1983; Merchenthaler et al., 1984) and additional fibers that may originate from the thalamic paraventricular nucleus, BNST, basolateral amygdala, and mPFC (Itoga et al., 2019). Tyrosine-hydroxylase-immunoreactivity co-localizes with CRF fibers and both CRF1 and CRF2 receptor subtypes in the NAc (Lemos et al., 2012), suggesting that CRF is well poised to modulate DA terminal release.
Intra-VTA infusion of CRF reduces instrumental responding on a progressive ratio and shifts choice away from larger rewards in an effort discounting task in male rats (Wanat et al., 2013; Bryce and Floresco, 2016). Notably, intra-VTA infusions of CRF do not completely recapitulate the effects of ICV infusions or acute stress, in that the latter also increases choice latencies whereas the former does not. Furthermore, CRF infusions in the NAc core do not cause an overall reduction in choice of larger, high cost rewards. Instead, these treatments make rats less sensitive to changes in effort costs and induced a more static pattern of choice, with rats preferring the larger reward less when costs were low and more when effort costs were high (Bryce and Floresco, 2019). These latter observations further highlight that CRF modulates DA-dependent motivational and decision-making functions in a circuit-specific manner and that increasing CRF activity in certain nodes of these circuits may induce differential effects on these behaviors compared to acute stress or global increases in CRF.
Superficially, the manner in which CRF reduces effort-related choice and motivated responding suggests these effects are driven by reductions in DA transmission. However, a survey of the literature on CRF-DA interactions reveals more complex interplay between these systems. For example, in vitro studies demonstrate that CRF can excite VTA DA neurons (Korotkova et al., 2006), although as has been observed with other monoamine systems, these effects show bimodal, dose-dependent actions with higher concentrations reducing excitatory currents on DA cells (Williams et al., 2014). Conversely, CRF can increase inhibitory currents driven by D2 and GABAB receptors on DA neurons (Beckstead et al., 2009) and increase firing of VTA GABA neurons (Korotkova et al., 2006), making it unclear if the net effect of VTA CRF receptor activation is an increase or decrease in DA neural firing and terminal release. Indeed, other in vivo studies suggest that CRF may exert an inhibitory tone on VTA DA neurons. This is supported by observations that central administration of a CRF1 receptor antagonist increases the number of spontaneously active DA neurons (Lodge and Grace, 2005), which drives slower, extrasynaptic or “tonic” DA levels within the NAc (Floresco et al., 2003).
With respect to CRF effects on different modes of DA release, microdialysis studies demonstrate that centrally administered CRF increases tonic DA release in the NAc (Dunn and Berridge, 1987; Matsuzaki et al., 1989; Kalivas and Duffy, 1995), and blockade of VTA CRF2 receptors attenuates increases in mesoaccumbens DA release induced by social defeat stress (Holly et al., 2015). Moreover, administration of CRF on NAc slices in vitro potentiates evoked DA release, suggesting that it may facilitate DA transmissions via local mechanisms within this nucleus (Lemos et al., 2012). On the other hand, intra-VTA CRF reduces phasic accumbal DA release evoked by stimulation of the pedunculopontine tegmentum inputs to the VTA and attenuates reward-associated phasic DA responses in rats working for food on a progressive ratio (Wanat et al., 2013). These studies illuminate the complexity of CRF actions on DA neuron physiology and release, which may arise from different experimental conditions or suggests that CRF differentially shapes tonic vs. phasic DA activity.
Given the complex and somewhat opposing manners by which CRF influences DA activity, how CRF modulates DA transmission to reduce effort-related choice remains an open question. As discussed above, the effects of CRF on effort-related decision-making appear to mimic a reduction in DA tone (Salamone et al., 1991; Floresco et al., 2008; Bryce and Floresco, 2016). However, excessive increases in DA activity can also reduce preference for larger rewards associated with a greater effort cost. For example, systemic administration of higher doses of amphetamine, which increases DA tone, also shifts preference away from larger rewards during effort discounting (Floresco et al., 2008). Likewise, overexpression of striatal D2 receptors reduces effort-related choice (Filla et al., 2018). Consistent with this, pharmacological activation of NAc D2 receptors reduces choice of larger, more costly rewards in a manner similar to acute stress, central, and intra-VTA CRF infusion (Bryce and Floresco, 2019). Taken together, it is tempting to speculate that reductions in motivated responding induced by excessive CRF activity are driven by increased mesoaccumbens DA levels and recruitment of D2 receptors, combined with an attenuation of reward-related phasic DA responses (Wanat et al., 2013). This hypothesis remains to be tested in future studies.
Moving forward, a key question pertains to the receptor mechanisms involved in the modulatory actions of CRF on effort-related choice. In addition, it would be of interest to investigate how CRF modulates decision-making involving other types of costs, such as delays or risk. For example, it is unclear how CRF may modulate choice for larger, delayed rewards vs. smaller, immediate ones, although it is interesting to note that acute restraint stress does not appear to alter this form of cost/benefit decision-making (Shafiei et al., 2012). On the other hand, there is a dearth of preclinical research investigating how stress or CRF affect decision-making under uncertainty. In human studies, acute stress influences risk/reward decision-making in a sex dependent manner, increasing risky choices in males but reducing risky choices in females (Putman et al., 2009; van den Bos et al., 2009; Mather and Lighthall, 2012). Given that most studies in this field have exclusively used male rodents, this represents a significant gap in our understanding of the modulatory role CRF plays in decision-making. Therefore, it is critical that future studies include both sexes to identify potential sex differences in CRF modulation of effort-related choice.
6. Conclusions and future directions
Recent evidence demonstrates that CRF is a potent modulator of cognition and decisionmaking (Figure 4). This research has identified interesting new questions regarding the neurocircuitry and mechanisms underlying the cognitive actions of CRF. As noted, there remain significant gaps in our understanding of sex differences in the behavioral and circuit actions of CRF in brain regions that contribute to different forms of cognition. Given rates of certain stress-related affective disorders (e.g. depression, PTSD) vary between men and women (Kessler et al., 2012), a better understanding of sex differences in CRF function across multiple brain systems is critical for improving treatments for psychiatric disorders. Moreover, future studies investigating how CRF1 and CRF2 signaling in the VTA and NAc impacts cost/benefit decision-making may provide mechanistic insight about CRF-DA interactions responsible for disrupted motivation and decision-making in various psychiatric illnesses.
Figure 4. Schematic of rodent brain depicting distribution of CRF cell bodies and receptor subtypes known to modulate cognition.
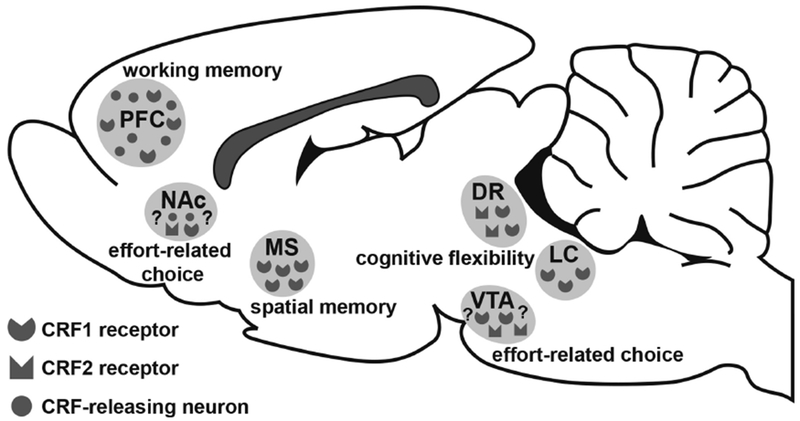
Areas discussed in this review include the prefrontal cortex (PFC), medial septum (MS), dorsal raphe (DR) nucleus, ventral tegmental area (VTA), and locus coeruleus (LC). It is unknown whether CRF acts via CRF1, CRF2, or both receptor subtypes in the VTA to modulate effort-related choice. Moreover, although evidence suggests CRF may also act within the nucleus accumbens (NAc) to modulate effort-related choice, this hypothesis remains to be explicitly tested.
It is promising that systemic CRF1 antagonists improve PFC-dependent cognition as measured in a spatial working memory task, an action that is identical to methylphenidate (Ritalin) and other ADHD-related drugs (Berridge and Arnsten, 2015; Hupalo and Berridge, 2016). Future studies are necessary to determine the broader cognitive actions of CRF antagonists in both male and female subjects. Given previous wide scale efforts to develop CRF antagonists to treat depression and anxiety, many CRF antagonist compounds have already passed safety tests in human subjects (Kehne and Cain, 2010; Koob and Zorrilla, 2012). Additionally, long-term CRF antagonist treatment appears to have limited effects on hypothalamic pituitary adrenal axis function. For example, 2-week treatment with CRF1 antagonists had no effects on diurnal or evoked adrenocorticotropic hormone and cortisol levels in healthy human subjects (Ising et al., 2007). However, subsequent studies in subjects with major depression reported moderate, yet significant decreases in cortisol levels following 6 weeks of antagonist treatment (Binneman et al., 2008).
Admittedly, most clinical trials concluded that CRF antagonists are not effective at treating affective symptoms in depression or anxiety disorders (Binneman et al., 2008; Coric et al., 2010). Limited evidence suggests sex differences may play a role in determining therapeutic responses to CRF antagonists (Howerton et al., 2014). Nevertheless, the cognitive actions of CRF antagonists have not been explicitly assessed in humans. Given CRF antagonists have been observed to improve PFC-dependent cognition similar to all approved treatments for ADHD, this may represent a relatively low-cost path toward a novel therapeutic treatment for cognitive dysfunction.
Highlights.
Differing levels of CRF modulate distinct forms of cognitive flexibility
CRF acts in the medial septum to regulate spatial memory
Females are less sensitive to CRF in basal forebrain-dependent processes but are highly vulnerable to CRF actions in the locus coeruleus
Local CRF signaling in the medial PFC impairs working memory
CRF diminishes preference for larger rewards associated with a greater effort cost
Acknowledgements
Some of the work reviewed in this manuscript was supported by National Institutes of Health grants MH102211, MH116526 (CWB), and MH107140 (SH); MH040008 and MH058350 (RJV); NSF Career Grant IOS-1552416 (DAB); and grants from the Canadian Institutes of Health Research (MOP 133579) to SBF. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Sukhni M, Maruschak NA, McIntyre RS (2015) Vortioxetine: a review of efficacy, safety and tolerability with a focus on cognitive symptoms in major depressive disorder. Expert Opin Drug Saf 14:1291–1304. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Kurz J (1985) An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J Comp Neurol 240:37–59. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Sinnamon HM (1977) The locus coeruleus: neurobiology of a central noradrenergic nucleus. Prog Neurobiol 9:147–196. [DOI] [PubMed] [Google Scholar]
- Arnsten AF., Mathew R, Ubriani R, Taylor JR, Li B-M (1999) α-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol Psychiatry 45:26–31. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT (2009) Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 10:410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT (2011) Catecholamine Influences on Dorsolateral Prefrontal Cortical Networks. Biol Psychiatry 69:e89–e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE (1981) Nonrepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci 1:887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD (2005) An integrative theory of locus coemleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28:403–450. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW (2004) CRF and CRF Receptros: Role in Stress Responsivity and Other Behaviors. Annu Rev Pharmacol Toxicol 44:525–557. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BAS, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ (2010) Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 15:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Dong H, Carroll J, Plona Z, Ding H, Rodriguez L, McKennan C, Csemansky JG, Seeholzer SH, Valentino RJ (2017) Corticotropin-releasing factor overexpression gives rise to sex differences in Alzheimer’s disease-related signaling. Mol Psychiatry 22:1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Kawasumi Y (2015) Cognitive dismptions in stress-related psychiatric disorders: A role for corticotropin releasing factor (CRF). Horm Behav 76:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Wiersielis KR (2018) Sex differences in stress responses: a critical role for corticotropin-releasing factor. Hormones 17:5–13. [DOI] [PubMed] [Google Scholar]
- Barch DM, Ceaser A (2012) Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci 16:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsegyan A, Mackenzie SM, Kurose BD, McGaugh JL, Roozendaal B (2010) Glucocorticoids in the prefrontal cortex enhance memory consolidation and impair working memory by a common neural mechanism. ProcNatl Acad Sci 107:16655–16660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Gantz SC, Ford CP, Stenzel-Poore MP, Phillips PE, Mark GP, Williams JT (2009) CRF Enhancement of GIRK Channel-Mediated Transmission in Dopamine Neurons. Neuropsychopharmacology 34:1926–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Arnsten AF (2015) Catecholamine mechanisms in the prefrontal cortex: proven strategies for enhancing higher cognitive function. Curr Opin Behav Sci 4:33–40. [Google Scholar]
- Berridge CW, Foote SL (1991) Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. J Neurosci 11:3135–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Spencer RC (2016) Differential cognitive actions of norepinephrine a2 and a1 receptor signaling in the prefrontal cortex. Brain Res 1641:189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binneman B, Feltner D, Kolluri S, Shi Y, Qiu R, Stiger T (2008) A 6-Week Randomized, Placebo-Controlled Trial of CP-316,311 (a Selective CRH 1 Antagonist) in the Treatment of Major Depression. Am J Psychiatry 165:617–620. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ (2000) Medial Frontal Cortex Mediates Perceptual Attentional Set Shifting in the Rat. J Neurosci 20:4320–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockstaele EJV, Colago EEO, Valentino RJ (1998) Amygdaloid Corticotropin-Releasing Factor Targets Locus Coeruleus Dendrites: Substrate for the Co-ordination of Emotional and Cognitive Limbs of the Stress Response. J Neuroendocrinol 10:743–758. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Chamey DS (1997) Elevated CSF Corticotropin-Releasing Factor Concentrations in Posttraumatic Stress Disorder. Am J Psychiatry 154:624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce CA, Floresco SB (2016) Perturbations in Effort-Related Decision-Making Driven by Acute Stress and Corticotropin-Releasing Factor. Neuropsychopharmacology 41:2147–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce CA, Floresco SB (2019) Alterations in effort-related decision making induced by stimulation of dopamine D1, D2, D3 and corticotropin-releasing factor receptors in nucleus accumbens subregions. Psychopharmacology (Berl). [DOI] [PubMed] [Google Scholar]
- Buoli M, Altamura AC (2015) May Non-antipsychotic Drugs Improve Cognition of Schizophrenia Patients? Pharmacopsychiatry 48:41–50. [DOI] [PubMed] [Google Scholar]
- Cai L, Gibbs RB, Johnson DA (2012) Recognition of novel objects and their location in rats with selective cholinergic lesion of the medial septum. Neurosci Lett 506:261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L (2010) Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci 13:1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaijale NN, Snyder K, Amer J, Curtis AL, Valentino RJ (2015) Repeated Social Stress Increases Reward Salience and Impairs Encoding of Prediction by Rat Locus Coeruleus Neurons. Neuropsychopharmacology 40:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, Souza ED (1995) Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci 15:6340–6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton BG, Ferrier IN, Perry RH (1987) Distribution of corticotropin-releasing factor-like immunoreactivity in human brain. Neuropeptides 10:329–334. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dailey JW, Robbins TW, Roberts AC (2005) Prefrontal Serotonin Depletion Affects Reversal Learning But Not Attentional Set Shifting. J Neurosci 25:532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RD, Kawasumi Y, Parikh V, Bangasser DA (2016) Corticotropin releasing factor impairs sustained attention in male and female rats. Behav Brain Res 296:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ (2003) A Neurochemically Distinct Dorsal Raphe-Limbic Circuit with a Potential Role in Affective Disorders. Neuropsychopharmacology 28:206–215. [DOI] [PubMed] [Google Scholar]
- Cope ZA, Vazey EM, Floresco SB, Aston Jones GS (2019) DREADD-Mediated Modulation of Locus Coeruleus Inputs to mPFC Improves Strategy Set-Shifting. Neurobiol Learn Mem [DOI] [PubMed] [Google Scholar]
- Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L, Malaspina D (2003) The stress cascade and schizophrenia: etiology and onset. Schizophr Bull 29:671–692. [DOI] [PubMed] [Google Scholar]
- Coric V, Feldman HH, Oren DA, Shekhar A, Pultz J, Dockens RC, Wu X, Gentile KA, Huang S-P, Emison E, Delmonte T, D’Souza BB, Zimbroff DL, Grebb JA, Goddard AW, Stock EG (2010) Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress Anxiety 27:417–425. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Salamone JD (1994) Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacol Biochem Behav 49:85–91. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Sokolowski JD, Salamone JD (1993) Different effects of nucleus accumbens and ventrolateral striatal dopamine depletions on instrumental response selection in the rat. Pharmacol Biochem Behav 46:943–951. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bello NT, Connolly KR, Valentino RJ (2002) Corticotropin-Releasing Factor Neurones of the Central Nucleus of the Amygdala Mediate Locus Coeruleus Activation by Cardiovascular Stress. J Neuroendocrinol 14:667–682. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ (2006) Sexually Dimorphic Responses of the Brain Norepinephrine System to Stress and Corticotropin-Releasing Factor. Neuropsychopharmacology 31:544–554. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Leiser SC, Snyder K, Valentino RJ (2012) Predator stress engages corticotropin-releasing factor and opioid systems to alter the operating mode of locus coeruleus norepinephrine neurons. Neuropharmacology 62:1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Martinon D, Moaddab M, Rainnie DG (2016) Targeting corticotropin-releasing factor (CRF) projections from the oval nucleus of the BNST using cell-type specific neuronal tracing studies in mouse and rat brain. J Neuroendocrinol 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EG, Keller J, Hallmayer J, Pankow HR, Murphy GM, Gotlib IH, Schatzberg AF (2018) Corticotropin-releasing factor 1 receptor haplotype and cognitive features of major depression. Transl Psychiatry 8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HEW, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S (2004) Differential expression of 5HT-1A, α1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, γ-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol 474:364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ (1985) Corticotropin-releasing factor receptors are widely distributed within the rat central nervous system: an autoradiographic study. J Neurosci Off J Soc Neurosci 5:3189–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deussing JM, Chen A (2018) The Corticotropin-Releasing Factor Family: Physiology of the Stress Response. Physiol Rev 98:2225–2286. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Spencer RC, Berridge CW (2017) Stress Degrades Prefrontal Cortex Neuronal Coding of Goal-Directed Behavior. Cereb Cortex 27:2970–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD (2000) Norepinephrine exhibits two distinct profdes of action on sensory cortical neuron responses to excitatory synaptic stimuli. Synapse 37:273–282. [DOI] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N (2009) Chronic Stress Causes Frontostriatal Reorganization and Affects Decision-Making. Science 325:621–625. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW (1987) Corticotropin-releasing factor administration elicits a stress-like activation of cerebral catecholaminergic systems. Pharmacol Biochem Behav 27:685–691. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW (1990) Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Rev 15:71–100. [DOI] [PubMed] [Google Scholar]
- Fett A-KJ, Viechtbauer W, Dominguez M-G, Penn DF, van Os J, Krabbendam F (2011) The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A metaanalysis. Neurosci Biobehav Rev 35:573–588. [DOI] [PubMed] [Google Scholar]
- Filla I, Bailey MR, Schipani E, Winiger V, Mezias C, Balsam PD, Simpson EH (2018) Striatal dopamine D2 receptors regulate effort but not value-based decision making and alter the dopaminergic encoding of cost. Neuropsychopharmacology 43:2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Tse MTF, Ghods-Sharifi S (2008) Dopaminergic and Glutamatergic Regulation of Effort- and Delay-Based Decision Making. Neuropsychopharmacology 33:1966–1979. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA (2003) Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6:968–973. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE (1980) Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci 77:3033–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G (1983) Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev 63:844–914. [DOI] [PubMed] [Google Scholar]
- Forster GL, Pringle RB, Mouw NJ, Vuong SM, Watt MJ, Burke AR, Lowry CA, Summers CH, Renner KJ (2008) Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. Eur J Neurosci 28:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franowicz JS, Arnsten AFT (1998) The α-2a noradrenergic agonist, guanfacine, improves delayed response performance in young adult rhesus monkeys. Psychopharmacology (Berl) 136:8–14. [DOI] [PubMed] [Google Scholar]
- Fuge P, Aust S, Fan Y, Weigand A, Gärtner M, Feeser M, Bajbouj M, Grimm S (2014) Interaction of Early Life Stress and Corticotropin-Releasing Hormone Receptor Gene: Effects on Working Memory. Biol Psychiatry 76:888–894. [DOI] [PubMed] [Google Scholar]
- Fuster J (2015) The Prefrontal Cortex. Academic Press. [Google Scholar]
- Grammatopoulos DK, Randeva HS, Levine MA, Kanellopoulou KA, Hillhouse EW (2001) Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple G-proteins. J Neurochem 76:509–519. [DOI] [PubMed] [Google Scholar]
- Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, Holmes A (2011) Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nat Neurosci 14:1507–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Matti G, Phillipp F, Yan F, Anne W, Melanie F, Sabine A, Hauke H, Arthur J, Isabella H, Malek B (2015) Variation in the corticotropin-releasing hormone receptor 1 (CRHR1) gene modulates age effects on working memory. J Psychiatr Res 61:57–63. [DOI] [PubMed] [Google Scholar]
- Helmeke C, Ovtscharoff W, Poeggel G, Braun K (2008) Imbalance of immunohistochemically characterized intemeuron populations in the adolescent and adult rodent medial prefrontal cortex after repeated exposure to neonatal separation stress. Neuroscience 152:18–28. [DOI] [PubMed] [Google Scholar]
- Henckens MJAG Deussing JM, Chen A (2016) Region-specific roles of the corticotropin-releasing factor–urocortin system in stress. Nat Rev Neurosci 17:636–651. [DOI] [PubMed] [Google Scholar]
- Holly EN, DeBold JF, Miczek KA (2015) Increased mesocorticolimbic dopamine during acute and repeated social defeat stress: modulation by corticotropin releasing factor receptors in the ventral tegmental area. Psychopharmacology (Berl) 232:4469–4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP (2007) Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212:149–179. [DOI] [PubMed] [Google Scholar]
- Howerton AR, Roland AV, Fluharty JM, Marshall A, Chen A, Daniels D, Beck SG, Bale TL (2014) Sex Differences in Corticotropin-Releasing Factor Receptor-1 Action Within the Dorsal Raphe Nucleus in Stress Responsivity. Biol Psychiatry 75:873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupalo S, Berridge CW (2016) Working Memory Impairing Actions of Corticotropin-Releasing Factor (CRF) Neurotransmission in the Prefrontal Cortex. Neuropsychopharmacology 41:2733–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupalo S, Martin AJ, Green RK, Devilbiss DM, Berridge CW (2019) Prefrontal Corticotropin-Releasing Factor (CRF) Neurons Act Locally to Modulate Frontostriatal Cognition and Circuit Function. J Neurosci:2701–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising M, Zimmermann US, Künzel HE, Uhr M, Foster AC, Leamed-Coughlin SM, Holsboer F, Grigoriadis DE (2007) High-Affinity CRF1 Receptor Antagonist NBI-34041: Preclinical and Clinical Data Suggest Safety and Efficacy in Attenuating Elevated Stress Response. Neuropsychopharmacology 32:1941–1949. [DOI] [PubMed] [Google Scholar]
- Itoga CA, Chen Y, Fateri C, Echeverry PA, Lai JM, Delgado J, Badhon S, Short A, Baram TZ, Xu X (2019) New viral-genetic mapping uncovers an enrichment of corticotropin-releasing hormone-expressing neuronal inputs to the nucleus accumbens from stress-related brain regions. J Comp Neurol 0 Available at: https://onlinelibrary.wiley.com/doi/abs/10.1002/cne.24676 [Accessed March 22, 2019]. [DOI] [PMC free article] [PubMed]
- Jedema HP, Grace AA (2004) Corticotropin-Releasing Hormone Directly Activates Noradrenergic Neurons of the Locus Ceruleus Recorded In Vitro. J Neurosci 24:9703–9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P (1995) Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res 675:325–328. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Cain CK (2010) Therapeutic utility of non-peptidic CRF1 receptor antagonists in anxiety, depression, and stress-related disorders: evidence from animal models. Pharmacol Ther 128:460–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly EA, Fudge JL (2018) The neuroanatomic complexity of the CRF and DA systems and their interface: What we still don’t know. Neurosci Biobehav Rev 90:247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen H-U (2012) Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res 21:169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ (2000) Effects of Corticotropin-Releasing Factor on Neuronal Activity in the Serotonergic Dorsal Raphe Nucleus. Neuropsychopharmacology 22:148–162. [DOI] [PubMed] [Google Scholar]
- Koob GF, Zorrilla EP (2012) Update on Corticotropin-Releasing Factor Pharmacotherapy for Psychiatric Disorders: A Revisionist View. Neuropsychopharmacology 37:308–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL (2006) Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci 23:2677–2685. [DOI] [PubMed] [Google Scholar]
- Kosofsky BE, Molliver ME (1987) The serotoninergic innervation of cerebral cortex: Different classes of axon terminals arise from dorsal and median raphe nuclei. Synapse 1:153–168. [DOI] [PubMed] [Google Scholar]
- Kostich WA, Chen A, Sperle K, Largent BL (1998) Molecular Identification and Analysis of a Novel Human Corticotropin-Releasing Factor (CRF) Receptor: The CRF2γ Receptor. Mol Endocrinol 12:1077–1085. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL (1978) Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol 178:225–254. [DOI] [PubMed] [Google Scholar]
- Lammers C-H, Garcia-Borreguero D, Schmider J, Gotthardt U, Dettling M, Holsboer F, Heuser IJE (1995) Combined dexamethasone/corticotropin-releasing hormone test in patients with schizophrenia and in normal controls: II. Biol Psychiatry 38:803–807. [DOI] [PubMed] [Google Scholar]
- Lapiz MDS, Morilak DA (2006) Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience 137:1039–1049. [DOI] [PubMed] [Google Scholar]
- Lee EHY, Hung HC, Lu KT, Chen WH, Chen HY (1992) Protein synthesis in the hippocampus associated with memory facilitation by corticotropin-releasing factor in rats. Peptides 13:927–937. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK (2005) Transduction of Receptor Signals by β-Arrcstins. Science 308:512–517. [DOI] [PubMed] [Google Scholar]
- Lemos JC, Wanat MJ, Smith JS, Reyes BAS, Hollon NG, Van Bockstaele EJ, Chavkin C, Phillips PEM (2012) Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature 490:402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett TA, Voineskos AN, Kennedy JL, Levine B, Daskalakis ZJ (2014) Treating Working Memory Deficits in Schizophrenia: A Review of the Neurobiology. Biol Psychiatry 75:361–370. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Foote SL, Cha CI (1989) Corticotropin-releasing factor immunoreactivity in monkey neocortex: An immunohistochemical analysis. J Comp Neurol 290:599–613. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA (2005) Acute and Chronic Corticotropin-Releasing Factor 1 Receptor Blockade Inhibits Cocaine-Induced Dopamine Release: Correlation with Dopamine Neuron Activity. J Pharmacol Exp Ther 314:201–206. [DOI] [PubMed] [Google Scholar]
- Luethi M, Meier B, Sandi C (2009) Stress Effects on Working Memory, Explicit Memory, and Implicit Memory for Neutral and Emotional Stimuli in Healthy Men. Front Behav Neurosci 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Lighthall NR (2012) Risk and Reward Are Processed Differently in Decisions Made Under Stress. Curr Dir Psychol Sci 21:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki I, Takamatsu Y, Moroji T (1989) The effects of intracerebroventricularly injected corticotropinreleasing factor (CRF) on the central nervous system: Behavioural and biochemical studies. Neuropeptides 13:147–155. [DOI] [PubMed] [Google Scholar]
- Mazure CM (1995) Does Stress Cause Psychiatric Illness? American Psychiatric Pub. [Google Scholar]
- McGaughy J, Ross RS, Eichenbaum H (2008) Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience 153:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q-Y, Chen X-N, Tong D-L, Zhou J-N (2011) Stress and glucocorticoids regulated corticotropin releasing factor in rat prefrontal cortex. Mol Cell Endocrinol 342:54–63. [DOI] [PubMed] [Google Scholar]
- Merali Z, McIntosh J, Anisman H (2004) Anticipatory Cues Differentially Provoke In Vivo Peptidergic and Monoaminergic Release at the Medial Prefrontal Cortex. Neuropsychopharmacology 29:1409–1418. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Vigh S, Schally AV, Stumpf WE, Arimura A (1984) Immunocytochemical localization of corticotropin releasing factor (CRF)-like immunoreactivity in the thalamus of the rat. Brain Res 323:119–122. [DOI] [PubMed] [Google Scholar]
- Miguel TT, Gomes KS, Nunes-de-Souza RL (2014) Tonic modulation of anxiety-like behavior by corticotropin-releasing factor (CRF) type 1 receptor (CRF1) within the medial prefrontal cortex (mPFC) in male mice: Role of protein kinase A (PKA). Horm Behav 66:247–256. [DOI] [PubMed] [Google Scholar]
- Millan MJ et al. (2012) Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov 11:141–168. [DOI] [PubMed] [Google Scholar]
- Mohila CA, Onn S-P (2005) Increases in the density of parvalbumin-immunoreactive neurons in anterior cingulate cortex of amphetamine-withdrawn rats: evidence for corticotropin-releasing factor in sustained elevation. Cereb Cortex N Y N 1991 15:262–274. [DOI] [PubMed] [Google Scholar]
- Nemeroff C, Owens MJ, Bissette G, Andom AC, Stanley M (1988) Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry 45:577–579. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlöv E, Bissette G, Walléus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W (1984) Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 226:1342–1344. [DOI] [PubMed] [Google Scholar]
- Nowend KL, Arizzi M, Carlson BB, Salamone JD (2001) D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav 69:373–382. [DOI] [PubMed] [Google Scholar]
- Perrin M, Donaldson C, Chen R, Blount A, Berggren T, Bilezikjian L, Sawchenko P, Vale W (1995) Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci 92:2969–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminière JM, Le Moal M, Mormede P, Simon H (1991) Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci U S A 88:2088–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W (1994) Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci 91:8777–8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman P, Antypa N, Crysovergi P, van der Does WAJ (2009) Exogenous cortisol acutely influences motivated decision making in healthy young men. Psychopharmacology (Berl) 208:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BAS, Valentino RJ, Xu G, Bockstaele EJV (2005) Hypothalamic projections to locus coeruleus neurons in rat brain. Eur J Neurosci 22:93–106. [DOI] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J (2007) Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience 150:8–13. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ (2002) Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci 99:13908–13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattaqi S (2009) Stress, memory and the amygdala. Nat Rev Neurosci 10:423–433. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, McGaugh JL (2004) The Basolateral Amygdala Interacts with the Medial Prefrontal Cortex in Regulating Glucocorticoid Effects on Working Memory Impairment. J Neurosci 24:1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Schelling G, McGaugh JL (2008) Corticotropin-Releasing Factor in the Basolateral Amygdala Enhances Memory Consolidation via an Interaction with the -Adrenoceptor-cAMP Pathway: Dependence on Glucocorticoid Receptor Activation. J Neurosci 28:6642–6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K (1987) Corticotropin releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidase-diaminobenzidine method. J Comp Neurol 260:256–298. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S (1994) Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res 65:221–229. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K (1991) Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology (Berl) 104:515–521. [DOI] [PubMed] [Google Scholar]
- Sanford CA, Soden ME, Baird MA, Miller SM, Schulkin J, Palmiter RD, Clark M, Zweifel LS (2017) A Central Amygdala CRF Circuit Facilitates Learning about Weak Threats. Neuron 93:164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Givens B (2003) Attentional functions of cortical cholinergic inputs: What does it mean for learning and memory? Neurobiol Leam Mem 80:245–256. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP (2001) The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Rev 35:146–160. [DOI] [PubMed] [Google Scholar]
- Sauvage M, Steckler T (2001) Detection of corticotropin-releasing hormone receptor 1 immunoreactivity in cholinergic, dopaminergic and noradrenergic neurons of the murine basal forebrain and brainstem nuclei – potential implication for arousal and attention. Neuroscience 104:643–652. [DOI] [PubMed] [Google Scholar]
- Shafiei N, Gray M, Viau V, Floresco SB (2012) Acute stress induces selective alterations in cost/benefit decision-making. Neuropsychopharmacology 37:2194–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Lipps J (2013) Stress-induced cognitive dysfunction: hormone-neurotransmitter interactions in the prefrontal cortex. Front Hum Neurosci 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder K, Barry M, Plona Z, Ho A, Zhang X-Y, Valentino RJ (2015a) The impact of social stress during adolescence or adulthood and coping strategy on cognitive function of female rats. Behav Brain Res 286:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder K, Wang W-W, Han R, McFadden K, Valentino RJ (2012) Corticotropin-Releasing Factor in the Norepinephrine Nucleus, Locus Coeruleus, Facilitates Behavioral Flexibility. Neuropsychopharmacology 3 7:520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder KP, Hill-Smith TE, Lucki I, Valentino RJ (2015b) Corticotropin-releasing Factor in the Rat Dorsal Raphe Nucleus Promotes Different Forms of Behavioral Flexibility Depending on Social Stress History. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RC, Berridge CW (2019) Receptor and circuit mechanisms underlying differential procognitive actions of psychostimulants. Neuropsychopharmacology: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson FW, Sawchenko PE, Rivier J, Vale WW (1983) Organization of Ovine Corticotropin-Releasing Factor Immunoreactive Cells and Fibers in the Rat Brain: An Immunohistochemical Study. Neuroendocrinology 36:165–186. [DOI] [PubMed] [Google Scholar]
- Tan FA, Vaughan JM, Perrin MH, Rivier JE, Sawchenko PE (2017) Distribution of corticotropin-releasing factor (CRF) receptor binding in the mouse brain using a new, high-affinity radioligand, [125 I]-PD-Sauvagine. J Comp Neurol 525:3840–3864. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH (2012) Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol 121:553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe-Mariño A, Gassen NC, Wiesbeck MF, Balsevich G, Santarelli S, Solfrank B, Doumes C, Fries GR, Masana M, Fabermeier C, Wang X-D, Hairier K, Schmid B, Rein T, Chen A, Deussing JM, Schmidt MV (2016) Prefrontal Cortex Corticotropin-Releasing Factor Receptor 1 Conveys Acute Stress-Induced Executive Dysfunction. Biol Psychiatry 80:743–753. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SF (1987) Corticotropin-Releasing Factor Disrupts Sensory Responses of Brain Noradrenergic Neurons. Neuroendocrinology 45:28–36. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SF (1988) Corticotropin-releasing hormone increases tonic but not sensory-evoked activity of noradrenergic locus coeruleus neurons in unanesthetized rats. J Neurosci 8:1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Foote SF, Aston-Jones G (1983) Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res 270:363–367. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Fucki I, Van Bockstaele E (2010) Corticotropin-releasing factor in the dorsal raphe nucleus: Finking stress coping and addiction. Brain Res 1314:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Sheng C, Yan Z, Aston-Jones G (1996) Evidence for divergent projections to the brain noradrenergic system and the spinal parasympathetic system from Barrington’s nucleus. Brain Res 732:1–15. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E, Bangasser D (2013) Sex-specific cell signaling: the corticotropinreleasing factor receptor model. Trends Pharmacol Sci 34:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Wehby RG (1988) Corticotropin-Releasing Factor: Evidence for a Neurotransmitter Role in the Focus ceruleus during Hemodynamic Stress. Neuroendocrinology 48:674–677. [DOI] [PubMed] [Google Scholar]
- van den Bos R, Harteveld M, Stoop H (2009) Stress and decision-making in humans: Performance is related to cortisol reactivity, albeit differently in men and women. Psychoneuroendocrinology 34:1449–1458. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RKW, Li H-Y, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE (2000) Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428:191–212. [DOI] [PubMed] [Google Scholar]
- Van’t Veer A, Yano JM, Carroll FI, Cohen BM, Carlezon WA (2012) Corticotropin-Releasing Factor (CRF)-Induced Disruption of Attention in Rats Is Blocked by the κ-Opioid Receptor Antagonist JDTic. Neuropsychopharmacology 37:2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Bonci A, Phillips PEM (2013) CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat Neurosci 16:383–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ (2009) Stress-Induced Redistribution of Corticotropin-Releasing Factor Receptor Subtypes in the Dorsal Raphe Nucleus. Biol Psychiatry 66:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersielis KR, Ceretti A, Hall A, Famularo ST, Salvatore M, Ellis AS, Jang H, Wimmer ME, Bangasser DA (2019) Sex differences in corticotropin releasing factor regulation of medial septum-mediated memory formation. Neurobiol Stress 10:100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Buchta WC, Riegel AC (2014) CRF-R2 and the Heterosynaptic Regulation of VTA Glutamate during Reinstatement of Cocaine Seeking. J Neurosci 34:10402–10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingard JC, Packard MG (2008) The amygdala and emotional modulation of competition between cognitive and habit memory. Behav Brain Res 193:126–131. [DOI] [PubMed] [Google Scholar]
- Wood SK, Walker HE, Valentino RJ, Bhatnagar S (2010) Individual Differences in Reactivity to Social Stress Predict Susceptibility and Resilience to a Depressive Phenotype: Role of Corticotropin-Releasing Factor. Endocrinology 151:1795–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Zhang X-Y, Reyes BAS, Lee CS, Van Bockstaele EJ, Valentino RJ (2013) Cellular Adaptations of Dorsal Raphe Serotonin Neurons Associated with the Development of Active Coping in Response to Social Stress. Biol Psychiatry 73:1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF (1997) Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci Off J Soc Neurosci 17:8528–8535. [DOI] [PMC free article] [PubMed] [Google Scholar]


