Abstract
Objective:
The presentation of cognitive impairments in HIV-infected individuals has transformed since the introduction of antiretroviral therapies (ART). Although the overall prevalence of cognitive impairments has not changed considerably, frank dementia is now infrequent, and milder forms of cognitive impairments predominate. Mechanistic insights to the underlying causes of these residual cognitive impairments have been elusive, in part due to the heterogenous etiology of cognitive dysfunction in this population. Here we sought to categorize longitudinal change in HIV-infected patients based on the performance in specific cognitive domains.
Design:
This study consisted of 193 participants from the CHARTER cohort with detailed demographic, clinical and neuropsychological testing data obtained from two study visits interspersed by ~6 months. Cognitive testing assessed executive function, learning and delayed recall, working memory, verbal fluency, speed of information processing, and motor skills. Change scores were calculated for each domain between the two study visits. Dimension reduction and clustering was accomplished by principal component analysis of change scores and k-means clustering to identify cognitive domains that group together, as well as groups of subjects with similar patterns of change.
Results:
We identified four distinct cognitive change phenotypes that included declines in: 1) verbal fluency, 2) executive function 3) learning and recall, and 4) motor function, with approximately equal numbers of participants in each phenotype.
Conclusion:
Each of the four cognitive change phenotypes identify deficits that imply perturbations in specific neural networks. Future studies will need to validate if cognitive change phenotypes are associated with alterations in associated neural pathways.
INTRODUCTION
The HIV epidemic is well into its third decade, and since the advent of antiretroviral therapy (ART), patients are living much longer lives. This longer lifespan is accompanied by a greater frequency of comorbid conditions compared with uninfected individuals including cardiac disease, hypertension, hyperlipidemia, renal disease, endocrine disease, decreases in bone density, and cognitive changes (among others) (reviewed in 1–3). Early in the epidemic, cognitive impairments were frequent, progressive, and severe. Frank dementia (HIV-Associated Dementia; HAD) became infrequent with widespread ART, and the nosology of cognitive impairments in HIV-infected patients was redefined in 20074, to better reflect the current symptomology. HIV-Associated Neurocognitive Disorders (HAND) were grouped as Asymptomatic Neurocognitive Impairment (ANI), Mild Neurocognitive Disorder (MND), and HAD, with the largest proportion of current patients on ART falling into the ANI and MND categories. Most investigators would agree that HAND is a neurological disease of heterogeneous etiology with some combination of viral infection, long-term ART, genetics, and legacy effects contributing to the development and trajectory of HAND. Staging strategies that combine the results from neuropsychological testing of multiple cognitive domains may not readily identify the neurological representation of different disease phenotypes.
The presence and severity of HIV-Associated Neurocognitive Disorders (HAND) has been defined by a Global Deficit Score (GDS; among other measures) that considers the number and severity of impairments in neuropsychological testing across 7 cognitive domains (executive function, learning, delayed recall, working memory, verbal fluency, speed of information processing, and motor skills) to produce a summary score. Longitudinal changes in cognition have been defined using a summary regression change score (sRCS) that considers change in performance for all cognitive domains to also produce a summary score. Although these approaches have been extremely useful to identify HIV-infected subjects with cognitive impairments, to stratify severity of cognitive impairments, and to track the trajectory of cognitive impairments, these summary scores do not take into account possible heterogeneity in the direction and trajectory of change in different cognitive domains. In this work we used a progressive modelling approach to identify 4 cognitive change phenotypes based on longitudinal declines in specific or related cognitive domains.
METHODS
Participants and cognitive testing:
This study consisted of 193 participants from the CHARTER cohort5. Subjects were predominantly male (84.5% n=163). All subjects were HIV+ and on ART for at-least three months prior to the baseline visit (V1), with a second study visit (V2) approximately six months later (median 6.5 months). Visit 1 occurred between the years of 1999 and 20011, with 75% of participants falling between 2004 and 2009. At each study visit participants underwent a comprehensive neuropsychological (NP) test battery evaluating seven cognitive domains: executive function 6,7, learning and delayed recall 8–10, working memory 11,12, verbal fluency 6, speed of information processing 7,11,12, and motor skills 13. Raw test scores were converted to standardized T-Scores for each cognitive domain that were corrected for age, education, sex, and ethnicity with the best available normative standards 5 to have a mean of 50 and a standard deviation of 10. Baseline impairment in each cognitive domain at V1 was defined as a T-Score of 45 or less. Change scores for each cognitive domain were calculated by subtracting the T-Scores at V1 from V2.
Statistical Analysis:
Subject demographics and disease characteristics at V1 were summarized descriptively. Normally distributed data was compared using a one-way analysis of variance followed by Tukey’s multiple comparison. Non-normally distributed data was analyzed using a Kruskal-Wallis test, and categorical data was compared using chi-square. Correlations between cognitive domains were calculated using Pearson’s correlation and visualized using the ‘corrplot’ package in R 14. Dimension reduction and clustering was accomplished by conducting a principal components analysis (PCA) of change scores for each domain to produce a new smaller set of uncorrelated variables (the principal components; PCs)15. The minimum number of PCs that explained at-least 85% of the total variance in the data were retained for further analyses. Coefficients (loadings) obtained from the PCA show the relative contribution of each domain change score to a PC. We further examined the loadings using k-means clustering16 with the Hartigan-Wong algorithm17 to identify cognitive domains that grouped together using the ‘stats’ package in R18. This partitions the cognitive domains into k-groups that best fit the contributions of the domain change scores to the PCs, creating clusters of related cognitive trajectories. The optimal number of clusters were determined by the “elbow” in the total within group sum of squares (WSS) plot for n=1 to 6 clusters19. The goodness of fit for clusters was evaluated using the ratio of the between groups sum of squares to the total sum of squares (BSS:TSS)20, followed by k-means clustering of the PCs to identify groups of subjects with similar cognitive trajectories. Silhouette plots of these groupings were used to confirm the clustering21. Within each group, an effect size (Cohen’s D) was calculated to determine meaningful change using a standard deviation of 0.5SD22.
RESULTS
Baseline T-Scores for all cognitive domains were normally distributed with means ranging from 44.84 to 48.24 (Fig.1A). At baseline, all cognitive domain T-Scores were positively correlated, with the highest correlations between the learning and recall (R = 0.89, p p<0.0001), followed by speed of information processing and working memory (R = 0.58, p<0.0001), speed of information processing and executive function (R = 0.58, p<0.0001). The weakest (but still significant) correlation was between verbal fluency and motor function (R = 0.19, p<0.01) (Fig.1B). We calculated baseline impairment in each cognitive domain and found that 16.6% (n=42) of subjects did not exhibit impairment in any domain, 18.1% (n=35) showed impairment in one domain, 15.5% (n=30) showed impairment in two domains, 7.7% (n=15) showed impairment in three domains, 12.4% (n=24) showed impairment in 4 domains, and 29.5% (n=57) showed impairment in 5 or more domains. At baseline, 32.1%(n=62) of subjects were impaired in Verbal Fluency, 43.0%(n = 83) were impaired in Executive Function, 36.8%(n=71) were impaired in Speed of Information Processing, 45.6%(n=88) were impaired in Learning, 43.05(n=83) were impaired in Recall, and 48.7%(n=94) were impaired in Motor Function.
Figure1:
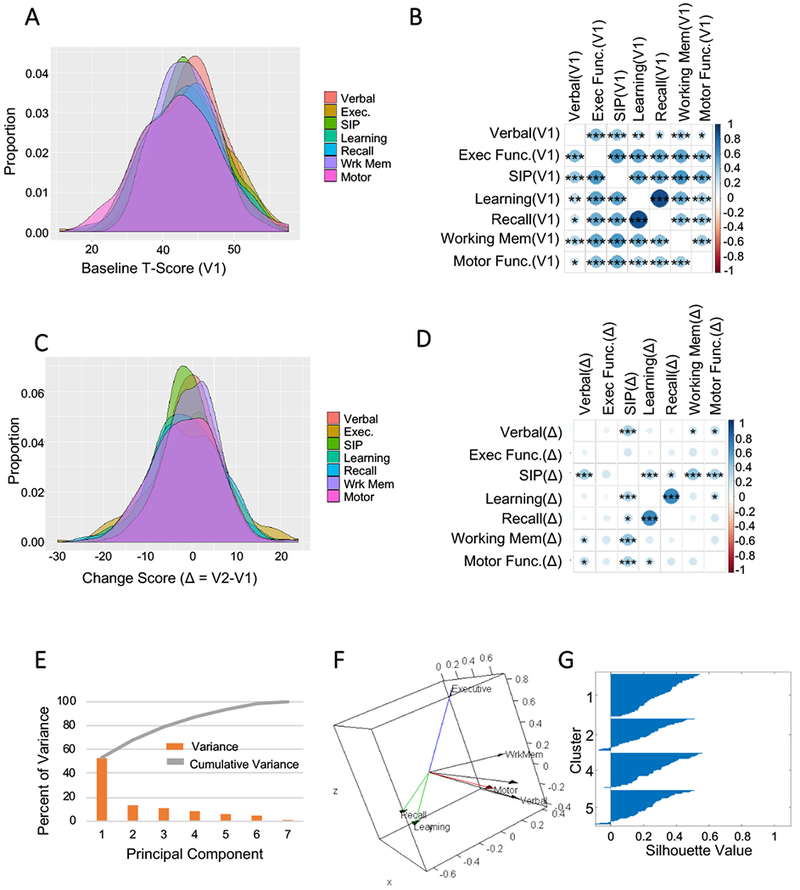
Identification of cognitive domain phenotypes. A) Distribution of baseline T-Scores in all domains. T-Scores are normalized so that the average in healthy controls is 50 with a standard deviation of 10. B) Correlation plot of the baseline (V1) T-Scores in each cognitive domain. C) Distribution of change scores (V2-V1) for each cognitive domain. D) Correlation plot of the change scores for each of the indicated cognitive domains. The size and color of the circles corresponds to the strength of Pearson’s correlation coefficient. Larger and darker circles are more strongly correlated. E) Individual and cumulative percent of the variance explained by each principal component of the cognitive change scores. F) Loading plot of the domain variables for the first three principal components. The vectors represent the coefficient of the variables on the components. Cognitive domain change scores that cluster together are represented by color. Executive function (green), Working memory, speed of information processing, and verbal fluency (red), motor function (black), and learning with recall (blue). G) Silhouette plot showing subject clustering based on their principal component values. n=193 subjects. * = p <0.01, ** = p < 0.001, *** = p < 0.0001. Pearson’s Product Moment Correlation.
Cognitive change scores in each domain were normally distributed with means ranging from −1.68 to −0.08 (Fig.1C). Pearson’s correlation showed that not all change scores in all domains were highly correlated (Fig.1D). However, the change scores in the cognitive domains of learning and recall were highly correlated (R=0.65, p<0.0001), as were the domains of working memory and speed of information processing (R=0.38, p<0.0001), motor function and memory (R= 0.23, p<0.05). Change scores in executive function were not highly correlated with any other cognitive domain (Fig.1D). All significant correlations were positive.
We next conducted a PCA of change scores for all seven domains to produce a smaller set of uncorrelated components. PC1 accounted for 33.2% of the total variance, with the first four components cumulatively accounting for 87.1% of the total variance (Fig.1E). Visual inspection of the loading plot showed apparent groupings between some, but not all domains (Fig.1D). K-means clustering was performed to identify cognitive domains that grouped together. Calculating the WSS for n=1 to 6 clusters demonstrated that 4 clusters optimally grouped the 7 cognitive domains (data not shown) with the following groupings: 1) executive function, 2) recall & learning, 3) motor, 4) working memory, verbal fluency & speed of information processing (Fig.1F). The BSS:TSS ratio was 0.909, indicating that 90.9% of total variance in the variable loadings was explained by these 4 clusters. Next, we identified cognitive trajectory phenotypes by conducting k-means clustering on the using k=4 components. This produced a model where cluster 1 included 23.7%(n=45), cluster 2 included 16.3%(n=31), cluster 3 included 28.4%(n=54), and cluster 4 included 31.6%(n=60) of the subjects. The silhouette plot confirmed an excellent separation of subjects using these 4 clusters (Fig.1G). The distribution of domain-specific change scores in each cluster is summarized in Fig.2A. Phenotype 1 consisted of an isolated decline in verbal fluency, phenotype 2 consisted of an isolated decline in executive function, phenotype 3 showed declines in learning and recall, while phenotype 4 showed a selective decline in motor function (Fig 2A–B). We used Cohen’s D to calculate the effect size for each cognitive change phenotype and found medium effect sizes associated with change in verbal fluency (phenotype 1, d=−.86). Large effects sizes were associated with change in executive function (phenotype 2, d=−2.18), changes in learning and recall (phenotype 3, d=−1.7, −1.7), and change in motor function (phenotype 4, d=−1.9)(Fig 2C).
Figure 2:
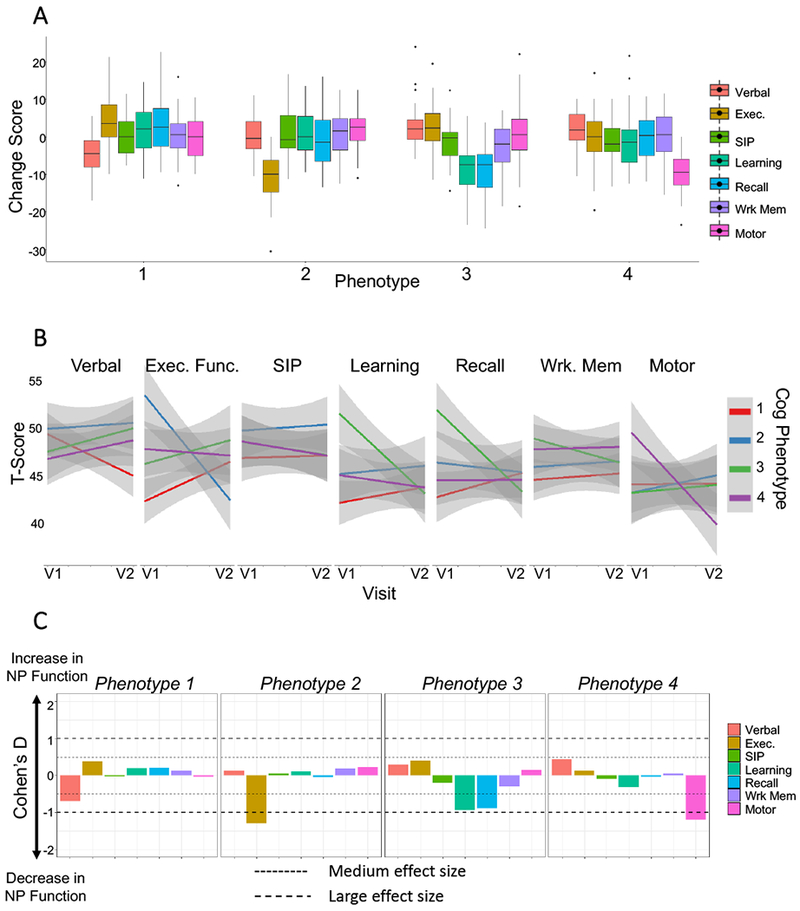
Cognitive trajectory phenotypes. A) Boxplots representing the pattern of cognitive domain change scores for each of the indicated phenotypes. B) Line plots showing the average change in T-scores from visit 1 to visit 2 for each of the 4 cognitive phenotypes. The 95% confidence intervals are indicated by shaded areas. C) Effect size calculated for each cognitive domain stratified by cognitive phenotype.
When we stratified subjects at baseline based on cognitive change phenotype, the T-scores for executive function were higher in phenotype 2 (p<0.0001), learning and recall were higher in phenotype 3 (0<0.001), baseline working memory was highest in phenotype 3 (p<0.05), and baseline motor function was highest in phenotype 4 (p<0.01). Patients in phenotype 1 (Verbal) had significantly lower cholesterol levels (p<0.01). A greater number of patients in phenotype 3 (learning and recall; p<0.01) had Emtricitabine included in their ARV regimen, and a greater number of patients in phenotype 4 (motor; p<0.05) had Nevirapine included in their ARV regimen. Phenotype 1 (Verbal), had the lowest number of patients with lifetime depressive symptoms (p<0.05) . There were no significant differences in baseline age, ethnicity, CDC stage, current or nadir CD4, plasma viral load, HIV duration, HDL or triglycerides, HCV infection, duration of HIV infection, plasma glucose, CPE score, or any drugs of abuse between the 4 cognitive phenotypes (Table 1).
Table 1. Demographic and clinical information.
Data are represented as mean ± SD and n (%), or median (IQR). Student’s t-test, Chi-square test, Kruskal Wallis Test.
| Variable | Phenotype 1 (n = 57) |
Phenotype 2 (n=43) |
Phenotype 3 (n = 47) |
Phenotype 4 (n=46) |
p-val |
|---|---|---|---|---|---|
| Age, years | 44.35(8.03) | 45.86(8.96) | 46.11(7.95) | 45.3(8.7) | 0.7172 |
| Education, years | 12.91(3.07) | 12.44(2.68 | 13.11(2.24) | 13.5(2.07) | 0.2764 |
| Race/ethnicity, n (%) | 0.0598 | ||||
| White | 20(35.09) | 28(65.12) | 29(61.7) | 26(56.52) | |
| Black | 18(31.58) | 9(20.93) | 13(27.66) | 12(26.09) | |
| Hispanic | 16(28.07) | 5(11.63) | 4(8.51) | 5(10.87) | |
| Other | 3(5.26) | 1(2.33) | 1(2.13) | 3(6.52) | |
| Male, n (%) | 46(80.7) | 39(90.7) | 40(85.11) | 38(82.61) | 0.5677 |
| AIDS, n (%) | 42(73.68) | 31(72.09) | 36(76.6) | 32(69.57) | 0.9950 |
| Current CD4, median [IQR] | 391(423.25) | 487(390.75) | 360(355.75) | 517(349) | 0.0995 |
| Nadir CD4, median [IQR] | 126(182) | 127.5(206) | 72(181.5) | 97.5(261) | 0.4049 |
| Viral Load, median [IQR] | 115(13715) | 374(7247.25) | 409(7877.5) | 189(21547.75) | 0.8056 |
| Undetectable Viral Load, n (%) | 14(24.56) | 8(18.6) | 10(21.28) | 4(8.7) | 0.2096 |
| HCV, n (%) | 10(17.54) | 2(4.65) | 6(12.77) | 4(8.7) | 0.1668 |
| HIV Duration (years) | 12.41(6.7) | 13.51(7.9) | 11.51(6.09) | 12.78(6.49) | 0.5806 |
| Glucose, median [IQR] | 92.5(30) | 96(24.75) | 91(25.75) | 93(24.25) | 0.6979 |
| Cholesterol | 169.9(33.69)* | 192.44(47.52) | 196.14(42.47) | 176.95(37.15) | 0.0083 |
| CPE | 7.86(2.06) | 7.86(1.75) | 7.67(1.79) | 8.27(2.45) | 0.7448 |
| Baseline GDS-defined impaired, n(%) | 32(56.14) | 17(39.53) | 12(25.53) | 15(32.61) | 0.0121 |
| Follow-up GDS-defined impaired, n(%) | 26(45.61) | 19(44.19) | 17(36.17) | 19(41.3) | 0.7694 |
| Change Score: Verbal Fluency | −4.32(4.91)* | 0.62(5.36) | 2.43(5.32) | 1.94(5.22) | <0.0001 |
| Change Score: Executive Function | 4.13(6.99) | −10.93(6.3)* | 2.48(6.18) | −0.68(6.98) | <0.0001 |
| Change Score: Speed of Information Processing | 0.26(5.08) | 0.63(6.07) | −1.43(5.33) | −1.49(5.73) | 0.1336 |
| Change Score: Learning | 1.63(6.37) | 0.87(5.37) | −8.32(6.54)* | −1.3(6.94) | <0.0001 |
| Change Score: Recall | 2.54(6.76) | −1(7.6) | −8.54(6.82)* | 0.06(6) | <0.0001 |
| Change Score: Working Memory | 0.66(5.09) | 0.59(5.73) | −2.5(5.74)* | 0.26(6.91) | 0.0253 |
| Change Score: Motor | 0.04(5.28) | 1.81(5.54) | 0.83(7.25) | −9.62(4.97)* | <0.0001 |
| Lifetime MDD, n (%) | 20(35.09)* | 27(62.79) | 21(44.68) | 25(54.35) | 0.0286 |
| Lifetime Cannabis Abuse, n (%) | 7(12.28) | 6(13.95) | 13(27.66) | 7(15.22) | 0.1152 |
| Lifetime Cocaine Abuse, n (%) | 6(10.53) | 1(2.33) | 4(8.51) | 5(10.87) | 0.3623 |
| Lifetime Methamphetamine Abuse, n (%) | 1(1.75) | 2(4.65) | 1(2.13) | 2(4.35) | 0.7828 |
| Lifetime Hallucinogen Abuse, n (%) | 4(7.02) | 3(6.98) | 2(4.26) | 4(8.7) | 0.8394 |
| Lifetime Inhalant Abuse, n (%) | 1(1.75) | 1(2.33) | 1(2.13) | 2(4.35) | 0.8162 |
| Lifetime Opioid Abuse, n (%) | 1(1.75) | 0(0) | 1(2.13) | 0(0) | 0.6170 |
| Lifetime PCP Abuse, n (%) | 1(1.75) | 0(0) | 1(2.13) | 1(2.17) | 0.8014 |
| Lifetime Sedative Abuse, n (%) | 0(0) | 3(6.98) | 2(4.26) | 1(2.17) | 0.2573 |
| Baseline BDI, median[IQR] | 7[13] | 10[16] | 6[8.25] | 8.5[15] | 0.6772 |
| Follow-up BDI, median[IQR] | 6.5[2.5] | 10[12] | 9[9.5] | 9[15] | 0.4755 |
| PAOFI, median[IQR] | 1[5] | 3[10] | 1[5] | 3[7] | 0.4609 |
| Efavirenz, n (%) | 15(26.32) | 11(25.58) | 12(25.53) | 9(19.57) | 0.8585 |
| Atazanavir, n (%) | 15(26.32) | 14(32.56) | 14(29.79) | 9(19.57) | 0.5388 |
| Emtricitabine, n (%) | 21(36.84) | 18(41.86) | 25(53.19)* | 11(23.91) | 0.0343 |
| Ritonavir, n (%) | 21(36.84) | 18(41.86) | 24(51.06) | 15(32.61) | 0.2937 |
| Tenofovir, n (%) | 25(43.86) | 26(60.47) | 31(65.96) | 21(45.65) | 0.0708 |
| Abacavir, n (%) | 12(21.05) | 13(30.23) | 6(12.77) | 11(23.91) | 0.2419 |
| Zidovudine, n (%) | 13(22.81) | 4(9.3) | 7(14.89) | 9(19.57) | 0.3191 |
| Lopinavir, n (%) | 5(8.77) | 2(4.65) | 7(14.89) | 5(10.87) | 0.4260 |
| Lamivudine, n (%) | 28(49.12) | 15(34.88) | 13(27.66) | 20(43.48) | 0.1294 |
| Stavudine, n (%) | 5(8.77) | 1(2.33) | 3(6.38) | 2(4.35) | 0.5501 |
| Nevirapine, n (%) | 5(8.77) | 4(9.3) | 2(4.26) | 10(21.74)* | 0.0441 |
DISCUSSION
Using longitudinal neuropsychological testing data obtained from HIV-infected subjects enrolled in the CHARTER study, we analyzed cognitive trajectory as a function of change in individual or groups of cognitive domains. We identified 4 distinct cognitive change phenotypes with approximately equal numbers of subjects falling into each phenotype. Each of the phenotypes are discussed below with reference to known biological, demographic, and behavioral covariates that have been associated with performance in the respective domains. Although we discuss these relationships separately for each phenotype, it is important to note that it is uncommon for a biological, demographic or behavioral covariate to associate with performance in a single cognitive domain; in the vast majority of reports, covariates associate with performance across multiple cognitive domains. In our longitudinal analysis of change in performance we did not find any baseline clinical, demographic or behavioral (i.e drug use) covariates that associated with any of the identified phenotypes.
In a 2005 study, Lojek and Bernstein identified 4 individual neurocognitive patterns in HIV-infected patients that they termed “clusters”. These cross-sectional clusters were incredibly similar to those we identified using a separate cohort with a distinct modeling scheme. The clusters they identified included psychomotor speed (Cluster 1), deficits in learning and memory (Cluster 2), deficits in multiple domains (Cluster 3), and normal neurocognitive performance (Cluster 4). These patterns of impairment were relatively stable over time24. A more recent cross-sectional study used a cluster variable selection algorithm to identify 3 cognitive phenotypes in patients from the Multicenter AIDS Cohort Study23. Patients that grouped into Profile 1 showed below average performance in all cognitive domains. Patients in Profile 2 exhibited a mixed phenotype with average performance in executive functioning, motor, and speed of information processing, but exhibited below average performance in learning and memory. Patients in Profile 3 performed above average in all cognitive domains. There were several demographic, cognitive, and social factors that were associated with profile membership23. In the current study, we used a cognitive change approach to identify longitudinal patterns of change in individual cognitive domains, and identified 4 cognitive change phenotypes which included a decline in verbal fluency (Phenotype 1), a decline in executive function (Phenotype 2), declines in learning and recall (Phenotype 3), and declines in motor function (Phenotype 4). As the clinical presentation of cognitive decline is likely related to the brain regions involved, each of the 4 cognitive phenotypes is described below with reference to the underlying neural circuitry, as informed by data from multiple types of imaging modalities.
Phenotype 1: Decline in verbal fluency.
The first phenotype we identified showed a selective decline in verbal fluency. This cognitive domain is considered a measure of both verbal ability and executive control. Verbal ability is a measure of a subjects’ ability to retrieve grammatical representations and sound forms of words from their mental lexicon25. It most frequently used as a screening instrument for general verbal functioning. Executive control regulates goal directed behaviors through a series of complex cognitive abilities that include attention, working memory, cognitive flexibility, and impulse inhibition. Impairments of verbal fluency appear early in HIV infection26, and can continue, or reemerge HIV-infected patients despite ART with well-controlled plasma viral loads27. HIV-infection appears to accelerate age-related declines in verbal fluency, with impairments in the executive components of semantic fluency, and relative sparing of semantic memory stores28. Childhood trauma29, poor sleep quality30,31, use of efavirenz32, alcohol and substance abuse26, low CD4 nadir and current CD4 are associated with poor verbal fluency performance. While most of these studies are cross sectional, longitudinal approaches have found that impairments in verbal fluency predict frailty, and incident cognitive impairment at follow up33,34.
Verbal fluency tasks are thought to be a reflection of frontostriatal network integrity. In the early stages of neural degeneration, when performance on verbal fluency tasks is similar in HIV+ compared with HIV− subjects, BOLD fMRI studies show evidence for hyperactivity of basal ganglia in HIV+ patients during a phonemic fluency task, with no activation differences apparent during a semantic fluency task35. This hyper-recruitment of basal ganglia structures suggests that HIV+ patients may recruit basal ganglia to compensate for deficits in verbal learning networks. In HIV-infected patients with symptomatic cognitive impairment, or frank dementia, brain regional volumes in subcortical frontal and caudate regions correlate with verbal fluency performance36. BOLD fMRI imaging studies have shown that performance on word generation tasks which use auditory presentation of a letter (i.e. F, A, S) to cue subjects to generate as many different words as possible beginning with that letter correlate with activity in the caudate, putamen, and inferior frontal gyrus. The number of phonemic switches correlates with the supplementary motor area, caudate, putamen, and inferior frontal gyrus, and the number of semantic switches correlates with activity of the supplementary motor area, and inferior frontal gyrus. In this phonemic fluency task, HIV+ individuals demonstrate significantly greater activation in BG structures (caudate, putamen) than HIV− individuals. There were no significant differences in frontal brain activation between HIV status groups during the phonemic fluency task, nor were there significant brain activation differences during the semantic fluency task35. These data suggest that phenotype 1 may reflect frontostriatal network degeneration.
Phenotype 2: Decline in executive function.
The second cognitive phenotype we identified showed a selective deterioration in executive function. Although impairments in executive function are common in HIV-infected patients, most often executive dysfunction has been reported simultaneously with impairments in other cognitive domains. Worse EF has been associated with monocyte activation (increased sCD14), inflammation (increased sCD163 levels)37, age (older), sex (female), education (lower), current and nadir CD4 (lower)38, frailty39, substance and alcohol use disorders38,40, race (Latinos)41. Impairments in executive function often persist despite ART with effective viral suppression42. Moreover, some ARVs such as efavirenz may worsen executive function32,43. Deficits in executive function are apparent in acute and early HIV infection44, and executive function deficits are associated with substance abuse40,45, and risky sexual behavior46. This complex relationship of EF function to risky behavior makes it difficult to determine if individuals with lower executive function are more likely to become infected by HIV, or if HIV-infection (and/or ART) itself impairs executive function networks. Regardless, our longitudinal findings suggest that there may be a population of HIV-infected individuals who are vulnerable to a selective worsening of executive function.
Executive functions are largely (but not exclusively) regulated through prefrontal regions including the dorsolateral prefrontal cortex, anterior cingulate cortex, and the orbitofrontal cortex. As these frontal regions have multiple connections to cortical, subcortical, and brain stem regions, executive functions are often referred to as top-down processing. Functional imaging studies in HIV-infected patients have identified reduced connectivity in the executive function networks that connect the dorso-lateral prefrontal cortex to the striatum and posterior inferior parietal areas47. HIV-infected patients also adapt less quickly to changing task demands and this behavior is correlated with reduced brain activations in the dorsal anterior cingulate cortex48. Neuroimaging findings suggest that in HIV-infected patients, EF dysfunction is associated with abnormalities in subcortical structures that include smaller volumes in the right and left caudate, accumbens, right putamen, and globus pallidum49, reduced white matter volume, white matter hyperintensities50.
Phenotype 3: Declines in Learning and Recall.
The third phenotype identified showed selective declines in learning and recall. It is worth noting that in this neuropsychiatric test battery, the learning and recall domain scores were calculated based on aspects of the same cognitive tests (the Hopkins Verbal Learning Test-Revised and the Brief Visuospatial Memory Test – Revised), which may account for the high correlation between learning and subsequent recall.
Verbal learning and recall tests evaluate a wide diversity of functions including short-term auditory-verbal memory, rate of learning, learning strategies, retroactive, and proactive interference, retention of information, and differences between learning and retrieval. Impairments of learning and recall in HIV-infected patients can occur early following infection51, and are observed in patients with asymptomatic neurocognitive impairment52 suggesting that learning and recall may be impaired in some individuals at very early stages of HIV infection. HIV patients with a Impairments in verbal learning are have greater odds of being-non adherent with ARVs53, and verbal learning impairments are associated with monocyte activation (increased sCD14), inflammation (increased sCD163 levels)37, negatively correlate with viral loads51, are worse in HIV-infected patients who are diabetic54, heavy current alcohol users55, heavy marijuana users56, cocaine (crack) users57, and decline faster with age compared to non-infected age-matched subjects58. HIV-infected patients with a HAND defined cognitive impairment show the highest rates of retrieval vs. encoding deficits compared to other neurodegenerative diseases, consistent with a subcortical retrieval deficit in HIV59. High stress is associated with worse verbal memory performance60, as are smaller volumes in the parahippocampal gyrus, superior frontal gyrus, middle frontal gyrus, and inferior frontal gyrus61. Low-dose hydrocortisone improves verbal memory in HIV-infected patients for a short time following drug administration in accordance with the time-frame for elevation of plasma cortisol levels62, suggesting that chronic inflammation and/or immune activation may contribute to impairments in verbal learning that are associated with abnormalities in hippocampal and parahippocampal structures. This conclusion is supported by magnetic resonance spectroscopy findings that showed negative correlations between Cho and Cho/Cr in hippocampus and parahippocampal gyrus, and positive correlations between N-acetylaspartate/creatine ratios of the left hippocampus and verbal learning59.
Phenotype 4. Declines in Motor Function.
The final phenotype identified showed a selective decline in motor function. Similar to the other three phenotypes identified, deficits in motor functions are known to associate with multiple disease markers including monocyte activation (increased sCD14), inflammation (increased sCD163 levels)37, and viral loads63. Motor impairments are apparent in roughly one third of patients early following HIV-infection, and largely remit following initiation of ART64. However, in HIV-infected individuals on stable ART there are significant interactions between age and motor speed65,66, with gait and balance impairments that are more pronounced during challenging conditions67. These results are supported by the findings of an separate study that showed a multitasking paradigm unmasked “latent” impairments in the motor component of the multitask while sparing cognitive performance68. HIV-infected women show greater impairments in fine motor functions compared with men, while no sex differences are apparent in uninfected women compared with men69, and these motor impairments appear equally in virally suppressed and non-virally suppressed women42. Heavy alcohol use55, opiate dependence70, and psychostimulant use, that was associated with decreased white matter integrity in all white matter tracts71. Impairments in fine motor skills are accompanied by decreased cortical thickness that is pronounced in the bilateral primary sensimotor areas, extending to the prefrontal cortices with left retrosplenial cortical thinning72. Reduced grey matter volume has likewise been reported in bilateral posterior insula cortex, premotor cortex and supramarginal gyrus73. Although we found that approximately half of patients taking and ARV regimen containing nevirapine were in phenotype 4 (motor; 10 of 20 patients taking nevirapine), the implications of this association are not straight forward, as this drug has not be linked to neuropathy or motor impairment.
Some caveats of this study include a relatively small sample size, and skewed sex (patients were largely male). We also cannot determine from this study if these specific cognitive change phenotypes will be widely applicable to HIV-infected patients regardless of their current cognitive status. For example the cognitive change phenotypes we identified in this study may be more applicable to patients with mild forms of cognitive dysfunction where there is impairment in single cognitive domain. However, 65% of our patients were impaired in 2 or more domains at baseline, but the majority of these individuals showed progressive worsening in a single cognitive domain, or domains related by phenotypical functions, suggesting that this approach could be more widely used. Likewise, we cannot fully determine if functional changes in specific cognitive domains are due to legacy damage to brain parenchyma, or are the result of cumulative damage resulting from low-level inflammation, chronic low-level ciral production or long-term ARV therapy, or how other comorbid conditions would contribute to these phenotypes. Future studies using larger patient numbers are necessary to confirm the frequency of the cognitive change phenotypes identified, and to determine if additional cognitive change phenotypes can be identified. Approaches that identify cognitive change phenotypes may be useful to differentiate groups of HIV-infected patients with active degeneration in specific brain regions and/or neural circuits that could be selectively targeted using personalized therapeutic approaches.
Table 2. Domain-specific classification of cognitive changes.
Meaningful change was defined as a T-Score difference of at least 5 (half a standard deviation) in any direction between the two visits. Subjects classified as “Improved” had a change score ≥ 5, subjects classified as “Declined” had a change score ≤ −5, and all others were classified as “Stable”.
| Verbal n(%) |
Executive n(%) |
SIP n(%) |
Learning n(%) |
Recall n(%) |
WrkMem n(%) |
Motor n(%) |
||
|---|---|---|---|---|---|---|---|---|
|
Overall n = 193 |
Improved | 38 (19.7%) | 44(22.8%) | 34(17.6%) | 40(20.1%) | 41(21.2%) | 40(20.7%) | 33(17.1%) |
| Declined | 40(20.7%) | 54(28.0%) | 40(20.7%) | 67(34.7%) | 72(37.3%) | 43(22.3%) | 67(34.7%) | |
| Stable | 115(59.6%) | 95(49.2%) | 119(61.7%) | 86(44.6%) | 80(41.5%) | 110(57.0%) | 93(48.2%) | |
|
Phenotype1 n = 57 |
Improved | 2(3.5%) | 22(38.6%) | 11(19.3%) | 18(31.6%) | 22(38.6%) | 11(19.3%) | 11(19.3%) |
| Declined | 27(47.4%) | 4(7.0%) | 9(15.8%) | 9(15.8%) | 7(12.3%) | 8(14.0%) | 15(26.3%) | |
| Stable | 28(49.1%) | 31(54.4%) | 37(64.9%) | 30(52.6%) | 28(49.1%) | 38(66.7%) | 31(54.4%) | |
|
Phenotype 2 n = 43 |
Improved | 9(21.0%) | 0(0%) | 13(30.2%) | 14(32.6%) | 10(23.3%) | 11(25.6%) | 11(25.6%) |
| Declined | 7(16.3%) | 35(81.4%) | 7(16.3%) | 6(14.0%) | 17(39.5%) | 9(20.9%) | 5(11.6%) | |
| Stable | 27(62.8%) | 8(18.6%) | 23(53.5%) | 23(53.5%) | 16(37.2%) | 23(53.5%) | 27(62.8%) | |
|
Phenotype 3 n = 47 |
Improved | 11(23.4%) | 15(31.9%) | 5(10.6%) | 1(2.1%) | 0(0%) | 5(10.6%) | 11(23.4%) |
| Declined | 1(2.1%) | 5(10.6%) | 13(27.7%) | 36(76.6%) | 35(74.5%) | 15(31.9%) | 8(17.0%) | |
| Stable | 35(74.5%) | 27(57.4%) | 29(61.7%) | 10(21.2%) | 12(25.5%) | 27(57.4%) | 28(59.6%) | |
|
Phenotype 4 n = 46 |
Improved | 16(34.8%) | 7(15.2%) | 5(10.9%) | 7(15.2%) | 9(19.6%) | 13(28.3%) | 0(o%) |
| Declined | 5(10.9%) | 10(21.7%) | 11(23.9%) | 16(34.8%) | 13(28.3%) | 11(23.9%) | 39(84.8%) | |
| Stable | 25(53.3%) | 29(63.0%) | 30(65.2%) | 23(50%) | 24(52.2%) | 22(47.8%) | 7(15.2%) |
Acknowledgments
Study Funding: This work was supported by the National Institutes of Health awards AA0017408, MH077542, MH075673, AG034849 (NJH and JCM), MH071150 (NS), the CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) was supported by awards N01 MH22005, HHSN271201000036C and HHSN271201000030C (IG, TM, SL).
REFERENCES
- 1.Sillman B, Woldstad C, McMillan J, Gendelman HE. Neuropathogenesis of human immunodeficiency virus infection. Handbook of clinical neurology. 2018;152:21–40. [DOI] [PubMed] [Google Scholar]
- 2.Stoff DM, Goodkin K, Jeste D, Marquine M. Redefining Aging in HIV Infection Using Phenotypes. Current HIV/AIDS reports. 2017;14(5):184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vance DE, Fazeli PL, Dodson JE, Ackerman M, Talley M, Appel SJ. The synergistic effects of HIV, diabetes, and aging on cognition: implications for practice and research. J Neurosci Nurs. 2014;46(5):292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heaton RK, Clifford DB, Franklin DR Jr., et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5(2):135–140. [Google Scholar]
- 7.Reitan RM, Davison LA. Clinical neuropsychology: Current status and applications. VH Winston & Sons; 1974. [Google Scholar]
- 8.Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test–Revised: normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12(1):43–55. [Google Scholar]
- 9.Heaton RK, Grant I, Matthews CG. Comprehensive norms for an expanded Halstead-Reitan Battery: Demographic corrections, research findings, and clinical applications. Psychological Assessment Resources; Odessa, FL; 1991. [Google Scholar]
- 10.Heaton R, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- 11.Wechsler D WAIS-III: Wechsler adult intelligence scale. Psychological Corporation; San Antonio; 1997. [Google Scholar]
- 12.Diehr MC, Heaton RK, Miller W, Grant I. The Paced Auditory Serial Addition Task (PASAT): norms for age, education, and ethnicity. Assessment. 1998;5(4):375–387. [DOI] [PubMed] [Google Scholar]
- 13.Kløve H Grooved pegboard. Indiana: Lafayette Instruments; 1963. [Google Scholar]
- 14.Wei T, Wei MT. Package ‘corrplot’. Statistician. 2016;56:316–324. [Google Scholar]
- 15.Fo Husson, Lê Sb, Pagès Jrm Exploratory multivariate analysis by example using R. Boca Raton: CRC Press; 2011. [Google Scholar]
- 16.MacQueen J Some methods for classification and analysis of multivariate observations. Paper presented at: Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, Volume 1: Statistics; 1967, 1967; Berkeley, Calif. [Google Scholar]
- 17.Hartigan JA, Wong MA. Algorithm AS 136: A k-means clustering algorithm. Journal of the Royal Statistical Society Series C (Applied Statistics). 1979;28(1):100–108. [Google Scholar]
- 18.R Core Team. R: A Language and Environment for Statistical Computing. 2017. [Google Scholar]
- 19.Ketchen DJ, Shook CL. The application of cluster analysis in strategic management research: An analysis and critique. Strategic Manage J. 1996;17(6):441–458. [Google Scholar]
- 20.Hastie T, Tibshirani R, Friedman J. Unsupervised learning The elements of statistical learning: Springer; 2009:485–585. [Google Scholar]
- 21.Rousseeuw PJ. Silhouettes - a Graphical Aid to the Interpretation and Validation of Cluster-Analysis. J Comput Appl Math. 1987;20:53–65. [Google Scholar]
- 22.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. [DOI] [PubMed] [Google Scholar]
- 23.Molsberry SA, Cheng Y, Kingsley L, et al. Neuropsychological phenotypes among men with and without HIV disease in the multicenter AIDS cohort study. AIDS. 2018;32(12):1679–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lojek E, Bornstein RA. The stability of neurocognitive patterns in HIV infected men: classification considerations. J Clin Exp Neuropsychol. 2005;27(6):665–682. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt BM, Meyer AS, Levelt WJ. Lexical access in the production of pronouns. Cognition. 1999;69(3):313–335. [DOI] [PubMed] [Google Scholar]
- 26.Weber E, Morgan EE, Iudicello JE, et al. Substance use is a risk factor for neurocognitive deficits and neuropsychiatric distress in acute and early HIV infection. Journal of neurovirology. 2013;19(1):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vance DE, Rubin LH, Valcour V, Waldrop-Valverde D, Maki PM. Aging and Neurocognitive Functioning in HIV-Infected Women: a Review of the Literature Involving the Women’s Interagency HIV Study. Current HIV/AIDS reports. 2016;13(6):399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iudicello JE, Woods SP, Deutsch R, Grant I, Group HIVNRPH. Combined effects of aging and HIV infection on semantic verbal fluency: a view of the cortical hypothesis through the lens of clustering and switching. Journal of clinical and experimental neuropsychology. 2012;34(5):476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malan-Muller S, Hemmings SM, Spies G, Kidd M, Fennema-Notestine C, Seedat S. Shorter telomere length - A potential susceptibility factor for HIV-associated neurocognitive impairments in South African women [corrected]. PloS one. 2013;8(3):e58351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gamaldo CE, Gamaldo A, Creighton J, et al. Evaluating sleep and cognition in HIV. Journal of acquired immune deficiency syndromes. 2013;63(5):609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gamaldo CE, Spira AP, Hock RS, et al. Sleep, function and HIV: a multi-method assessment. AIDS and behavior. 2013;17(8):2808–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Q, Vaida F, Wong J, et al. Long-term efavirenz use is associated with worse neurocognitive functioning in HIV-infected patients. Journal of neurovirology. 2016;22(2):170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oppenheim H, Paolillo EW, Moore RC, et al. Neurocognitive functioning predicts frailty index in HIV. Neurology. 2018;91(2):e162–e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheppard DP, Woods SP, Bondi MW, et al. Does Older Age Confer an Increased Risk of Incident Neurocognitive Disorders Among Persons Living with HIV Disease? Clin Neuropsychol. 2015;29(5):656–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thames AD, Sayegh P, Terashima K, et al. Increased subcortical neural activity among HIV+ individuals during a lexical retrieval task. Neurobiology of disease. 2016;92(Pt B):175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hestad K, McArthur JH, Dal Pan GJ, et al. Regional brain atrophy in HIV-1 infection: association with specific neuropsychological test performance. Acta neurologica Scandinavica. 1993;88(2):112–118. [DOI] [PubMed] [Google Scholar]
- 37.Imp BM, Rubin LH, Tien PC, et al. Monocyte Activation Is Associated With Worse Cognitive Performance in HIV-Infected Women With Virologic Suppression. The Journal of infectious diseases. 2017;215(1):114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker KA, Brown GG. HIV-associated executive dysfunction in the era of modern antiretroviral therapy: A systematic review and meta-analysis. Journal of clinical and experimental neuropsychology. 2018;40(4):357–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paul RH, Cooley SA, Garcia-Egan PM, Ances BM. Cognitive Performance and Frailty in Older HIV-Positive Adults. Journal of acquired immune deficiency syndromes. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fama R, Sullivan EV, Sassoon SA, Pfefferbaum A, Zahr NM. Impairments in Component Processes of Executive Function and Episodic Memory in Alcoholism, HIV Infection, and HIV Infection with Alcoholism Comorbidity. Alcoholism, clinical and experimental research. 2016;40(12):2656–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marquine MJ, Heaton A, Johnson N, et al. Differences in Neurocognitive Impairment Among HIV-Infected Latinos in the United States. Journal of the International Neuropsychological Society : JINS. 2018;24(2):163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubin LH, Maki PM, Springer G, et al. Cognitive trajectories over 4 years among HIV-infected women with optimal viral suppression. Neurology. 2017;89(15):1594–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tovar-y-Romo LB, Bumpus NN, Pomerantz D, et al. Dendritic spine injury induced by the 8-hydroxy metabolite of efavirenz. The Journal of pharmacology and experimental therapeutics. 2012;343(3):696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamat R, Doyle KL, Iudicello JE, et al. Neurobehavioral Disturbances During Acute and Early HIV Infection. Cogn Behav Neurol. 2016;29(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marquine MJ, Iudicello JE, Morgan EE, et al. “Frontal systems” behaviors in comorbid human immunodeficiency virus infection and methamphetamine dependency. Psychiatry research. 2014;215(1):208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenberg M, Pettifor A, Duta M, et al. Executive function associated with sexual risk in young South African women: Findings from the HPTN 068 cohort. PloS one. 2018;13(4):e0195217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaganti JR, Heinecke A, Gates TM, Moffat KJ, Brew BJ. Functional Connectivity in Virally Suppressed Patients with HIV-Associated Neurocognitive Disorder: A Resting-State Analysis. AJNR Am J Neuroradiol. 2017;38(8):1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang X, Barasky R, Olsen H, Riesenhuber M, Magnus M. Behavioral and neuroimaging evidence for impaired executive function in “cognitively normal” older HIV-infected adults. AIDS care. 2016;28(4):436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Correa DG, Zimmermann N, Netto TM, et al. Regional Cerebral Gray Matter Volume in HIV-Positive Patients with Executive Function Deficits. J Neuroimaging. 2016;26(4):450–457. [DOI] [PubMed] [Google Scholar]
- 50.Watson C, Busovaca E, Foley JM, et al. White matter hyperintensities correlate to cognition and fiber tract integrity in older adults with HIV. Journal of neurovirology. 2017;23(3):422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akolo C, Royal W 3rd, Cherner M, et al. Neurocognitive impairment associated with predominantly early stage HIV infection in Abuja, Nigeria. Journal of neurovirology. 2014;20(4):380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinez-Banfi M, Velez JI, Perea MV, et al. Neuropsychological performance in patients with asymptomatic HIV-1 infection. AIDS care. 2018;30(5):623–633. [DOI] [PubMed] [Google Scholar]
- 53.Cattie J, Marquine MJ, Bolden KA, et al. Predictors of Attrition in a Cohort Study of HIV Infection and Methamphetamine Dependence. J Subst Use. 2015;20(6):407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang J, Jacobson LP, Becker JT, et al. Impact of glycemic status on longitudinal cognitive performance in men with and without HIV infection. Aids. 2018;32(13):1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woods AJ, Porges EC, Bryant VE, et al. Current Heavy Alcohol Consumption is Associated with Greater Cognitive Impairment in Older Adults. Alcoholism, clinical and experimental research. 2016;40(11):2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thames AD, Mahmood Z, Burggren AC, Karimian A, Kuhn TP. Combined effects of HIV and marijuana use on neurocognitive functioning and immune status. AIDS care. 2016;28(5):628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyer VJ, Little DM, Fitzgerald DA, et al. Crack cocaine use impairs anterior cingulate and prefrontal cortex function in women with HIV infection. Journal of neurovirology. 2014;20(4):352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seider TR, Luo X, Gongvatana A, et al. Verbal memory declines more rapidly with age in HIV infected versus uninfected adults. Journal of clinical and experimental neuropsychology. 2014;36(4):356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doyle KL, Woods SP, McDonald CR, et al. Verbal episodic memory profiles in HIV-Associated Neurocognitive Disorders (HAND): A comparison with Huntington’s disease and mesial temporal lobe epilepsy. Appl Neuropsychol Adult. 2017:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rubin LH, Cook JA, Weber KM, et al. The association of perceived stress and verbal memory is greater in HIV-infected versus HIV-uninfected women. Journal of neurovirology. 2015;21(4):422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rubin LH, Meyer VJ, R JC, et al. Prefrontal cortical volume loss is associated with stress-related deficits in verbal learning and memory in HIV-infected women. Neurobiology of disease. 2016;92(Pt B):166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mordecai KL, Rubin LH, Eatough E, et al. Cortisol reactivity and emotional memory after psychosocial stress in oral contraceptive users. Journal of neuroscience research. 2017;95(1–2):126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lyons JL, Uno H, Ancuta P, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr. 2011;57(5):371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hellmuth J, Fletcher JL, Valcour V, et al. Neurologic signs and symptoms frequently manifest in acute HIV infection. Neurology. 2016;87(2):148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ding Y, Lin H, Shen W, Wu Q, Gao M, He N. Interaction Effects between HIV and Aging on Selective Neurocognitive Impairment. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2017;12(4):661–669. [DOI] [PubMed] [Google Scholar]
- 66.Goodkin K, Miller EN, Cox C, et al. Effect of ageing on neurocognitive function by stage of HIV infection: evidence from the Multicenter AIDS Cohort Study. Lancet HIV. 2017;4(9):e411–e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berner K, Morris L, Baumeister J, Louw Q. Objective impairments of gait and balance in adults living with HIV-1 infection: a systematic review and meta-analysis of observational studies. BMC musculoskeletal disorders. 2017;18(1):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kronemer SI, Mandel JA, Sacktor NC, Marvel CL. Impairments of Motor Function While Multitasking in HIV. Front Hum Neurosci. 2017;11:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maki PM, Rubin LH, Springer G, et al. Differences in Cognitive Function Between Women and Men With HIV. Journal of acquired immune deficiency syndromes. 2018;79(1):101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Applebaum AJ, Otto MW, Richardson MA, Safren SA. Contributors to neuropsychological impairment in HIV-infected and HIV-uninfected opiate-dependent patients. Journal of clinical and experimental neuropsychology. 2010;32(6):579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang VM, Lang DJ, Giesbrecht CJ, et al. White matter deficits assessed by diffusion tensor imaging and cognitive dysfunction in psychostimulant users with comorbid human immunodeficiency virus infection. BMC Res Notes. 2015;8:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shin NY, Hong J, Choi JY, Lee SK, Lim SM, Yoon U. Retrosplenial cortical thinning as a possible major contributor for cognitive impairment in HIV patients. Eur Radiol. 2017;27(11):4721–4729. [DOI] [PubMed] [Google Scholar]
- 73.Zhou Y, Li R, Wang X, et al. Motor-related brain abnormalities in HIV-infected patients: a multimodal MRI study. Neuroradiology. 2017;59(11):1133–1142. [DOI] [PubMed] [Google Scholar]


