Abstract
Background:
Postoperative delirium and postoperative cognitive dysfunction share risk factors and may co-occur, but their relationship is not well-established. The primary goals of this study were to describe the prevalence of postoperative cognitive dysfunction and to investigate its association with in-hospital delirium. We hypothesized that delirium would be a significant risk factor for postoperative cognitive dysfunction during follow-up.
Materials and Methods:
This study utilized data from an observational study of cognitive outcomes after major noncardiac surgery, the Successful Aging after Elective Surgery (SAGES) study. Postoperative delirium was evaluated each hospital day with Confusion Assessment Method-based interviews supplemented by chart reviews. Postoperative cognitive dysfunction was determined using methods adapted from the International Study of Postoperative Cognitive Dysfunction (ISPOCD). Associations between delirium and postoperative cognitive dysfunction were examined at 1, 2, and 6 months.
Results:
134 of 560 participants (24%) developed delirium during hospitalization. Slightly less than half (47%, 256/548) met the ISPOCD-defined threshold for postoperative cognitive dysfunction at 1 month, but this proportion decreased at 2 months (23%, 123/536) and 6 months (16%, 85/528). At each follow-up, the level of agreement between delirium and postoperative cognitive dysfunction was poor (kappa < .08) and correlations were small (r < .16). The relative risk (RR) of postoperative cognitive dysfunction was significantly elevated for patients with a history of postoperative delirium at 1 month (RR = 1.34, 95% CI 1.07–1.67), but not 2 months (RR = 1.08, 95% CI 0.72–1.64), or 6 months (RR = 1.21, 95% CI 0.71–2.09).
Conclusions:
Delirium significantly increased the risk of POCD in the first postoperative month; this relationship did not hold in longer-term follow-up. At each evaluation, postoperative cognitive dysfunction was more common among patients without delirium. Postoperative delirium and postoperative cognitive dysfunction may be distinct manifestations of perioperative neurocognitive deficits.
1. INTRODUCTION
Older adults represent a large and increasing proportion of surgical patients in the United States; although adults 65 years and older comprised only 14% of the general population in 2014, they underwent more than one-third of all inpatient surgical procedures.1,2 Advances in surgical and anesthesia techniques, coupled with better preoperative risk assessment have resulted in safer operations and lower rates of some serious complications (e.g. infections);3 however, much less is known about effectively safeguarding the aging brain from perioperative stress.
Perioperative disturbances of cognition may occur acutely, in the form of postoperative delirium (POD)4 or after hospital discharge, as postoperative cognitive dysfunction (POCD).5 The incidence of POD is 20–45% among older adult surgery patients;4,6 POCD is experienced by 20–50% of older patients three months after cardiac surgery7,8 and in 5–55% of those undergoing other major surgeries.9,10 In general, higher rates have been reported in studies that defined POCD using less stringent statistical thresholds, and conversely, studies using more stringent statistical methods have found lower rates of POCD. This point is nontrivial because unlike delirium, POCD is not a clinical diagnosis, but a variably operationalized concept defined by decline in postoperative cognitive performance as measured by a neuropsychological tests.5,11,12
At present, little is known about how to effectively prevent POCD, or how to successfully treat either POD or POCD. Neither condition is benign. Delirium is linked with persistent impairments in brain function, including cognitive decline13–15, and increased risk of dementia,13,16 as well as numerous negative outcomes, including longer hospitalizations, decline in physical functioning,17 increased risk of institutionalization, and death.21,22 POCD has been associated with delay in returning to work and premature retirement, as well as increased mortality.9,23,24
That delirium has been linked to cognitive decline following delirium13,15,18 raises the question of whether POD and POCD are distinct disorders or overlapping conditions on a continuum of neurocognitive deficits.19,20 Prior observations that POD and POCD sometimes occur in the same individuals with overlapping risk factors have resulted in the suggestion of a common underlying neuropathogenesis.21,22
Considering this, we hypothesized that delirium is an independent risk factor for POCD. Thus, the goals of this study were to: 1) investigate the incidence of POCD up to 6 months following surgery, and 2) evaluate relationships between POD and subsequent development of POCD during follow-up among older adults undergoing major noncardiac surgery in the Successful Aging after Elective Surgery (SAGES) cohort.
2. MATERIALS AND METHODS
2.1. Data Source and Participants
Data for this retrospective cohort study were obtained from the SAGES study, an ongoing observational study of older adults undergoing major elective surgery.22,23 The study design and methods have been detailed previously.22,23 Eligible participants were age 70 years and older, English-speaking and able to communicate verbally, scheduled to undergo elective surgery at two Harvard-affiliated academic medical centers with an anticipated length of stay of at least 3 days, and available for in-person follow-up interviews. Qualifying surgical procedures were: total hip or knee replacement, lumbar, cervical, or sacral laminectomy, lower extremity arterial bypass surgery, open abdominal aortic aneurysm repair, and open or laparoscopic colectomy. Exclusion criteria were delirium, prior hospitalization within 3 months, legal blindness, severe deafness, terminal condition, history of schizophrenia or psychosis, history of alcohol abuse or withdrawal, and evidence of dementia at the pre-surgery assessment. Dementia diagnosis was determined through a rigorous process fulfilling the National Institute on Aging-Alzheimer’s Association (NIA-AA) criteria that included case review by an expert consensus panel, but did not include biomarker data.24,25
In addition to the surgical patients, 118 patients without dementia were recruited in primary care clinics at the Beth Israel Deaconess Medical Center (BIDMC) to serve as a non-surgical control (NSC) for measurement of practice effects associated with serial cognitive testing.26 The NSC group met the same inclusion and exclusion criteria as the SAGES sample, other than undergoing major surgery, and were assessed with the same neuropsychological test battery, administered at identical intervals as the surgical sample. Written informed consent was obtained according to procedures approved by the institutional review boards of BIDMC and Brigham and Women’s Hospital, the two study hospitals, and Hebrew SeniorLife, the study coordinating center, all located in Boston, Massachusetts.
2.2. Data Collection
Baseline interviews were performed on average within 2 weeks (mean 13 ± 15 days) before surgery and assessed demographics, cognition (Modified Mini-Mental State (3MS)),25 comorbidities, and daily living activities. Participants were evaluated for POD daily (described below). The SAGES neuropsychological test battery was administered to participants upon study entry, at one and two months after hospital discharge, and every 6 months thereafter, up to 36 months. Separate teams conducted inpatient and outpatient assessments so that the research staff assessing postoperative cognitive decline were blinded to the participants’ delirium status.
2.3. Assessment of Delirium
Delirium assessment was conducted at the pre-surgical baseline visit (as an exclusionary factor), then daily during hospitalization until discharge. The initial delirium assessment was conducted on postoperative Day 1 following transfer to the surgical floor; emergence delirium was not evaluated. Assessments included a brief cognitive screen (orientation, short-term recall, attention testing with Digit Span), the Delirium Symptom Interview,27 and interviews with family members and hospital staff.28 This information was used to score the Confusion Assessment Method (CAM), which has been demonstrated to be highly sensitive (94%; 95% confidence interval [CI] 91 – 97), and specific (89%; 95% CI 85 – 94) for detection of delirium compared with reference standard ratings.29 Participants were determined to have incident POD by a positive CAM rating or by validated chart review evidence of delirium recorded at any time prior to hospital discharge.28,30
2.4. Assessment of Cognitive Function
The SAGES neuropsychological test battery consists of the Hopkins Verbal Learning Test (HVLT),31 Digit Span Forwards and Backwards,32,33 Phonemic (F-A-S) and Categorical (Supermarket) Fluency Tasks,34 Boston Naming Test,35 Visual Search and Attention Test (VSAT),36 Trail Making Test (A and B),37 and the Digit Symbol Test.38 Overall performance was summarized with the General Cognitive Performance (GCP), a weighted composite measure derived following standard procedures, and calibrated to a nationally representative sample of older adults to yield a mean score of 50 and standard deviation of 10.39,40
2.5. Postoperative Cognitive Dysfunction
There is considerable variability in the measurement and definition of POCD.11 To operationalize POCD, we adapted methods utilized in the International Study of Postoperative Cognitive Dysfunction (ISPOCD), a landmark study of postoperative cognitive change.5 The ISPOCD battery consisted of a total of seven test variables; the Rey Visual Verbal Learning Test41 (number of words, delayed recall), the Concept Shifting Test42 (time, number of errors), the Stroop Color-Word Test43 (time, number of errors), and Letter-Digit Substitution44 (number of correct responses). POCD was defined based on change from baseline; a composite z-score of ≥ 1.96 across all tests, or z-scores for two or more tests scores ≥ 1.96.
The SAGES and ISPOCD neuropsychological test batteries are not identical in the number and type of cognitive test variables. Thus, we developed an approach to defining POCD in the SAGES dataset that was consistent with the ISPOCD methods and accommodated differences in cognitive assessments between the two studies. First, we matched similar tests in the ISPOCD and SAGES batteries. Three of the eight SAGES neuropsychological tests (HVLT, Trail-Making, RBANS); directly matched ISPOCD tests by both content and cognitive domain to yield a core set of five component test variables (Table 1).
Table 1.
Crosswalk of neuropsychological tests utilized in the SAGES and ISPOCD studies
| Cognitive Domain | SAGES Tests | ISPOCD Tests |
|---|---|---|
| Verbal episodic memory | Hopkins Verbal Learning, Revised (HVLT-R), sum of trials | Visual Verbal Learning, cumulative number of words |
| HVLT-R, delayed recall | Visual Verbal Learning, delayed recall | |
| Executive, Visuospatial | DKEFS Trail Making, Part B time | Concept Shifting test, part C, time |
| DKEFS Trail Making, Part B, number of errors | Concept Shifting Test, part C, number of errors | |
| RBANS Digit Symbol, Errors, time | Letter Digit Coding, Errors, time | |
| Visual Search and Attention Test |  |
|
| Confrontation naming, Language | Boston Naming Test | |
| Attention | WAIS Digit Span Forward and Backward | |
| Executive, Semantic memory, Language | Category Fluency Phonemic Fluency |
|
| Executive, Selective attention | NA | Stroop, errors |
| Stroop, time |
Two tests from this group of five were selected at random to construct all 10 possible 7-test battery combinations—see text for details.
Next, we repeatedly selected two of the remaining five unmatched tests from the SAGES battery (Digit Span Forwards and Backwards, Category Fluency, Phonemic Fluency, Boston Naming Test, VSAT) and sequentially added these pairs to the set of five ISPOCD-matched tests. This process yielded a total of ten unique SAGES POCD test batteries, each with seven component variables that provided the same number of tests used in ISPOCD.
We then applied the approach utilized in ISPOCD to identify the prevalence of POCD in the surgical cohort at each follow-up. Neuropsychological test results from the NSC group were used in the calculation of z-scores to account for practice (and retest) effects across repeated cognitive testing sessions. Individual z-scores were computed for each test, by subtracting the mean change score in the NSC group from the change score between baseline and follow-up for that test in the surgical patients. This result was then divided by the standard deviation of the mean change score in the NSC group to obtain the individual cognitive test z-scores. The z-score for each test at baseline was then subtracted from the z-score at each follow-up visit, and the individual test z-scores were summed across all tests to create a composite z-score. POCD was defined as 1) z-scores for two or more individual tests ≤ −1.96; or, 2) the sum of the seven z-scores (composite z-score) ≤ −1.96.45 Cohort members meeting neither criterion were considered not to have experienced POCD.
2.6. Statistical analysis
For the primary analysis, we evaluated the prevalence of POD and POCD for all participants with available data at 1, 2, and 6 months and assessed the correlation between POD and POCD at each timepoint. No power calculation was performed for this retrospective study as the sample size was pre-determined by the number of patients with available data at each follow-up.
For each patient, ten possible definitions of POCD were generated from the SAGES cognitive tests, as described in Section 2.5. The different POCD definitions were combined into a single analysis treating each definition as one of ten multiply-imputed outcomes. Multiple imputation is a statistical technique for analyzing incomplete data in which missing information is replaced with plausible values to create multiple complete datasets.46 The imputed datasets are analyzed individually, and then the results are combined. We adapted this method by treating each of the ten unique SAGES POCD test batteries (each with 7 component variables) as a multiply imputed dataset which was then combined into a single multiply imputed definition of POCD. This approach gives equal weight to each of the ten possible combinations and limits the number of multiple comparisons.
We calculated the prevalence of POCD, correlations (tetrachoric) and kappa coefficients for agreement between POD and POCD, and relative risk (RR) estimates with 95% confidence intervals (CI) to compare the risk of subsequent POCD when POD was present during hospitalization, with the risk of POCD when POD was not present. These calculations were performed for each of the 10 possible definitions of POCD, as well as the overall (multiply-imputed) definition of POCD, which was used for our primary level of inference.
In post hoc secondary analyses, we investigated the concordance (persistence) of POCD across time points. Generalized linear models for binomial outcomes were used to estimate associations between in-hospital delirium and persistent POCD at 2 and 6 months.
Sensitivity analyses
The ISPOCD-defined threshold has been criticized as an overly conservative cut-off that may miss subtle, but clinically relevant, cognitive decline. Similarly, the 2018 Recommendations for the Nomenclature of Cognitive Change Associated with Anesthesia and Surgery criteria for postoperative mild neurocognitive disorder designate a threshold of 1–2 standard deviations below population norms or controls.47 Therefore, we re-defined POCD as 1) z-scores for two or more individual tests ≤ −1.0; or 2) the sum of the seven z-scores ≤ −1.0.
As described previously,23 cognitive test and other data were subject to rigorous quality procedures to minimize missing data and evaluation of outliers. No observations were omitted on the basis of their relative distribution. All tests were two-tailed and statistical significance was defined at the p < .05 level. Statistical analyses were performed using Stata version 14.1 (Stata Corp, College Station, Texas).
3. RESULTS
Study Population
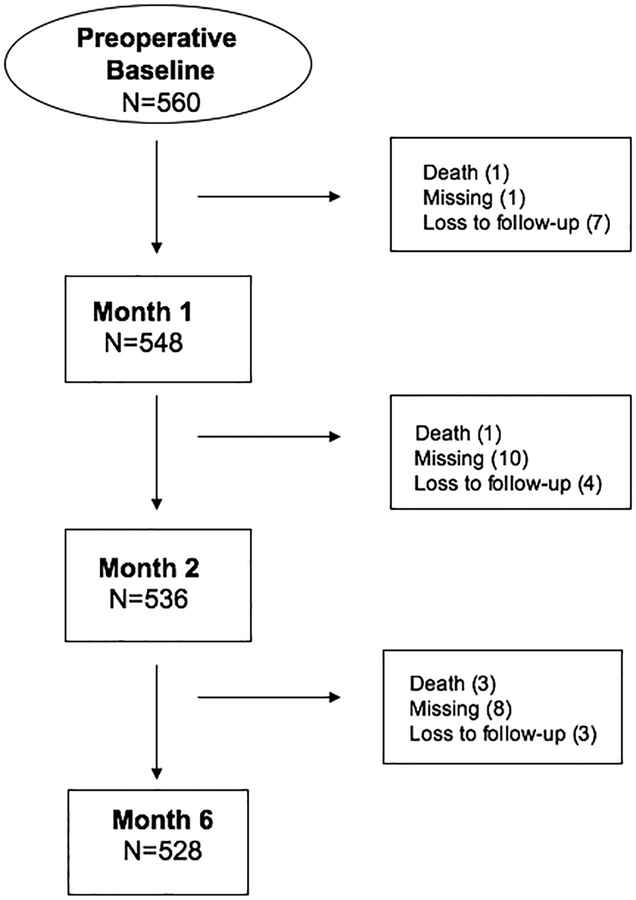
Data were available for 98% (551/560) of SAGES participants at baseline. Figure A1 shows a participant flow diagram. For this study, we compared baseline cognitive performance to the postoperative test results at 1 month (median, 40 days, interquartile range [IQR] 34–45), 2 months (86 days, IQR 74–103), and 6 months (210 days, IQR 192–245).
The surgical cohort was predominantly female (58%, 320/551), with a mean age (standard deviation, SD) of 77 (SD 5) years (Table 2). Characteristics of the NSC group, including their relatively stable cognitive trajectories over 6 months, have been described.15,26 In summary, both groups were comparable at baseline in age and measures of cognitive performance; however, the proportion of men was greater in the control group (NSC [56%, 67/119] vs. surgical [42%, 236/551])15 (Table A1).
Table 2.
Sociodemographic and Clinical Characteristics
| Stratified by POD and POCD at 2 Months (N=536) | |||||
|---|---|---|---|---|---|
| Characteristics | Total Study Sample* | POD− POCD− |
POD+ POCD− |
POD− POCD+ |
POD+ POCD+ |
| Number of observations [N (%)] | 551 | 319 | 95 | 91 | 31 |
| Age (years) [mean (SD)] | 77 (5) | 76 (5) | 77 (5) | 77 (5) | 78 (5) |
| Sex [n (%)] | |||||
| Men | 231 (42) | 129 (40) | 37 (39) | 47 (52) | 13 (42) |
| Women | 320 (58) | 190 (60) | 58 (61) | 44 (48) | 18 (58) |
| Race [n (%)] | |||||
| White | 510 (93) | 298 (93) | 84 (88) | 85 (93) | 29 (94) |
| All other race and ethnicity groups | 41 (7) | 21 (7) | 12 (13) | 6 (6) | 1 (4) |
| Education (years) [mean (SD)] | 15 (3) | 15 (3) | 15 (3) | 14 (3) | 14 (2) |
| 3MS Score [mean (SD)] | 93.6 (5.3) | 94.5 (4.8) | 91.6 (5.7) | 92.7 (5.8) | 92.5 (5.9) |
| General cognitive performance, (GCP) [mean (SD)] | 57.7 (7.2) | 58.9 (7.2) | 54.7 (6.3) | 57.5 (7.4) | 55.6 (6.7) |
| Proxy IQCODE [mean (SD)] | 3.1 (0.2) | 3.1 (0.2) | 3.2 (0.3) | 3.1 (0.2) | 3.3 (0.3) |
| Charlson Comorbidity Index, [mean (SD)] | 1.02 (1.3) | 0.90 (1.2) | 1.29 (1.3) | 1.14 (1.3) | 1.10 (1.4) |
| Surgery type [n (%)] | |||||
| Orthopedic | 446 (81) | 265 (83) | 74 (78) | 72 (79) | 24 (77) |
| Vascular | 34 (6) | 13 (4) | 8.5 (9) | 8.7 (10) | 1.5 (5) |
| Gastrointestinal | 71 (13) | 41 (13) | 13 (14) | 10 (11) | 5.2 (17) |
| Anesthesia type [n (%)] | |||||
| General | 455 (85) | 265 (84) | 83 (87) | 78 (86) | 29 (97) |
| Spinal | 77 (14) | 52 (16) | 12 (13) | 12 (13) | 1 (3) |
| General & Spinal | 4 (1) | 2 (1) | 0 (0) | 1 (1) | 0 (0) |
| Duration of surgery (minutes) [mean (SD)] | 144 (77) | 133 (67) | 166 (88) | 153 (87) | 157 (79) |
| Duration of anesthesia (minutes) [mean (SD)] | 193 (81) | 182 (73) | 219 (96) | 202 (85) | 204 (77) |
N=551, baseline characteristics, excludes those who had died or were lost to follow-up at 2 months.
POCD = postoperative cognitive dysfunction, proportions estimated from multiply-imputed data; POD = postoperative delirium; 3MS = Modified Mini-Mental State.
The majority of surgical patients entered the study without cognitive impairment (e.g. 3MS mean 93.6, SD 5.3) and none met criteria for dementia. Among the surgical procedures, orthopedic surgeries were most frequent (81%, 444/551), followed by gastrointestinal (13%, 71/551), and vascular surgeries (6%, 34/551). The majority of surgeries (85%, 455/551) were performed under general anesthesia.
Postoperative Neurocognitive Change
Delirium occurred in nearly a quarter (24%, 134/560; 95% CI 21 – 28) of the surgical patients while hospitalized. On average, POCD was observed more frequently than POD; slightly less than half of all patients (47%, 256/548; 95% CI 43 – 51) met the ISPOCD-defined threshold for POCD at 1 month, but this proportion decreased at 2 months (23%, 123/536; 95% CI 19–26) and 6 months (16%, 85/528; 95% CI 13 – 19) (Table 3). Similarly, the proportion of those with POD, who subsequently met criteria for POCD during follow-up also declined: 1 month (14%, 75/548; 95% CI 11 – 17), 2 months (6%, 31/536; 95% CI 4 – 8), and 6 months (4%, 23/528; 95% CI 3 – 6) (Table 3, Figure 1).
Table 3.
Proportions of in-hospital POD and POCD during follow-up*
| In-hospital Delirium and 1-month POCD (N=548) | |||
|---|---|---|---|
| POCD – 1 Month | Total | ||
| In-hospital Delirium (n (%)) | No | Yes | |
| No | 238 (43.4) | 181 (33) | 419 (76.4) |
| Yes | 54 (9.9) | 75 (13.7) | 129 (23.5) |
| Total | 292 (53.3) | 256 (46.7) | 548 (100) |
| In-hospital Delirium and 2-month POCD (N=536) | |||
| POCD – 2 Months | Total | ||
| In-hospital Delirium | No | Yes | |
| No | 318 (59.3) | 92 (17.2) | 410 (76.5) |
| Yes | 95 (17.8) | 31 (5.7) | 126 (23.5) |
| Total | 413 (77.1) | 123 (22.9) | 536 (100) |
| In-hospital Delirium and 6-month POCD (N=528) | |||
| POCD – 6 Months | Total | ||
| In-hospital Delirium | No | Yes | |
| No | 342 (64.8) | 62 (11.7) | 404 (76.5) |
| Yes | 101 (19.1) | 23 (4.4) | 124 (23.5) |
| Total | 443 (83.9) | 85 (16.1) | 528 (100) |
Postoperative cognitive dysfunction (POCD); Postoperative delirium (POD)
Proportion with POCD was estimated from multiply-imputed data for all participants with complete neuropsychiatric test data at each time point
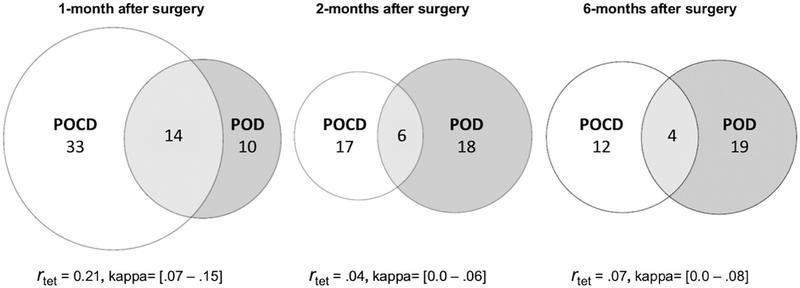
Figure 1.
Incidence of in-hospital POD and POCD during follow-up. Venn diagrams for overlap of POD and POCD at postoperative months 1 (left), 2 (center), and 6 (right). The three circles in each diagram illustrate the relative proportions of patients who a) met criteria for POCD (left) at 1, 2, or 6 months after surgery; b) developed POD while hospitalized (right); and c) developed in-hospital POD and also met criteria for POCD (center) at each follow-up.
POCD = postoperative cognitive dysfunction, proportions estimated from multiply-imputed data; POD = postoperative delirium. Tetrachoric correlations (rtet) and kappa coefficients are displayed for each month.
At each follow-up, the level of agreement between POD and POCD was poor (kappa coefficient, κ =.02 – .11)48 and the tetrachoric correlations (r) were small (r =.04 – .21), with the strongest associations at 1 month. The risk for POCD was significantly elevated for patients with a history of POD at 1 month (RR =1.34, 95% CI 1.07 – 1.67, p =.010) but not at 2 months (RR = 1.08, 95% CI 0.720 – 1.63, p = .699) or 6 months (RR =1.21, 95% CI 0.70 – 2.09, p =.489) (Table 4).
Table 4.
POCD definitions and summary statistics for associations with in-hospital POD during 6 months of postoperative follow up
| MONTH 1 | |||||
|---|---|---|---|---|---|
| Component neuropsychological tests* | P(POCD) | r | κ | RR (95% CI) | p |
| BNT, Digit Span | 0.43 | 0.13 | 0.07 | 1.22 (0.99, 1.50) | .058 |
| Digit Span, Phonemic Fluency | 0.49 | 0.20 | 0.10 | 1.30 (1.09, 1.55) | .003 |
| Digit Span, Category Fluency | 0.47 | 0.18 | 0.09 | 1.27 (1.06, 1.53) | .010 |
| VSAT, Digit Span | 0.46 | 0.20 | 0.10 | 1.32 (1.09, 1.59) | .004 |
| BNT, Phonemic Fluency | 0.48 | 0.20 | 0.10 | 1.30 (1.09, 1.56) | .004 |
| Category Fluency, Phonemic Fluency | 0.49 | 0.22 | 0.11 | 1.34 (1.13, 1.60) | .001 |
| BNT, Category Fluency | 0.43 | 0.23 | 0.12 | 1.39 (1.14, 1.69) | .001 |
| VSAT, BNT | 0.45 | 0.26 | 0.15 | 1.44 (1.20, 1.74) | <.001 |
| VSAT, Category Fluency | 0.46 | 0.29 | 0.15 | 1.47 (1.23, 1.76) | <.001 |
| VSAT, Phonemic Fluency | 0.51 | 0.24 | 0.11 | 1.34 (1.13, 1.58) | .001 |
| Overall (Month 1)† | 0.47 | 0.21 | 0.11 | 1.34 (1.08, 1.66) | .008 |
| MONTH 2 | |||||
| Component neuropsychological tests* | P(POCD) | r | κ | RR (95% CI) | p |
| BNT, Digit Span | 0.22 | −0.02 | −0.01 | 0.94 (0.65, 1.38) | .769 |
| Digit Span, Phonemic Fluency | 0.25 | 0.05 | 0.03 | 1.11 (0.79, 1.55) | .553 |
| Digit Span, Category Fluency | 0.24 | 0.04 | 0.02 | 1.08 (0.77, 1.53) | .646 |
| VSAT, Digit Span | 0.22 | 0.02 | 0.02 | 1.11 (0.77, 1.60) | .576 |
| BNT, Phonemic Fluency | 0.24 | 0.03 | 0.02 | 1.07 (0.76, 1.52) | .688 |
| Category Fluency, Phonemic Fluency | 0.24 | −0.02 | −0.01 | 0.97 (0.68, 1.38) | .851 |
| BNT, Category Fluency | 0.23 | 0.00 | 0.00 | 1.00 (0.70, 1.45) | .983 |
| VSAT, BNT | 0.20 | 0.04 | 0.02 | 1.10 (0.74, 1.62) | .636 |
| VSAT, Category Fluency | 0.22 | 0.09 | 0.05 | 1.21 (0.85, 1.72) | .289 |
| VSAT, Phonemic Fluency | 0.22 | 0.11 | 0.06 | 1.29 (0.91, 1.81) | .151 |
| Overall (Month 2)† | 0.23 | 0.04 | 0.02 | 1.08 (0.72, 1.63) | .698 |
| MONTH 6 | |||||
| Component neuropsychological tests* | P(POCD) | r | κ | RR (95% CI) | p |
| BNT, Digit Span | 0.15 | 0.08 | 0.04 | 1.21 (0.78, 1.90) | .393 |
| Digit Span, Phonemic Fluency | 0.16 | 0.02 | 0.08 | 1.49 (0.98, 2.26) | .063 |
| Digit Span, Category Fluency | 0.18 | 0.04 | 0.02 | 1.10 (0.73, 1.67) | .650 |
| VSAT, Digit Span | 0.17 | 0.12 | 0.06 | 1.35 (0.91, 2.02) | .140 |
| BNT, Phonemic Fluency | 0.13 | 0.02 | 0.01 | 1.05 (0.63, 1.74) | .865 |
| Category Fluency, Phonemic Fluency | 0.16 | 0.15 | 0.07 | 1.46 (0.96, 2.22) | .075 |
| BNT, Category Fluency | 0.15 | 0.03 | 0.01 | 1.07 (0.67, 1.70) | .780 |
| VSAT, BNT | 0.15 | −0.02 | −0.01 | 0.95 (0.58, 1.54) | .822 |
| VSAT, Category Fluency | 0.18 | 0.07 | 0.04 | 1.20 (0.79, 1.81) | .391 |
| VSAT, Phonemic Fluency | 0.18 | 0.13 | 0.07 | 1.36 (0.92, 2.02) | .124 |
| Overall (Month 6)† | 0.16 | 0.07 | 0.04 | 1.21 (0.71, 2.08) | .486 |
Prevalence of POCD [P(POCD)], tetrachoric correlations [r], level of agreement, kappa [κ], relative risk [RR] for the association of POD as a risk factor for POCD, and significance level [p] for each of the 10 unique subsets of seven component neuropsychological test variables used to define POCD at Months 1, 2, and 6.
Neuropsychological tests: Visual Search and Attention Test (VSAT); Boston Naming Test (BNT); Digit Symbol, RBANS Digit Symbol; Digit Span, WAIS Digit Span Forward and Backward; Category Fluency; Phonemic Fluency.
Each of the POCD definitions includes the following five test variables: Hopkins Verbal Learning Test, revised (HVLT-R) Sum of learning trials, HVLT-R Delayed Recall, Delis-Kaplan Executive Function System (D-KEFS) Trail Making Test (part B time), D-KEFS Trail Making Test (part B errors).
Overall refers to the definition of POCD at each time point estimated from multiply-imputed data.
Agreement, correlations, and relative risks varied by the subset of tests used to operationalize POCD, but the interpretation (e.g. direction of effect and statistical significance) was generally consistent with the overall (multiply imputed) definition (Table 3). For instance, the strength of the association of POD as a risk factor for POCD at 1 month (RR) ranged from 1.22 to 1.47, and all but one subset was significant (p<.05).
Persistence of POCD across time points and its association with POD was investigated in secondary analyses. Of the estimated 240 patients who were classified POCD+ at 1 month, 76 (32%) were POCD+ at month 2 and 30 (40%) were also POCD+ at month 6 (Table A2). The estimated percent of patients who had persistent POCD through month 2 (e.g. POCD+ at months 1 and 2) was 14% (76/535), and the estimated percent with persistent POCD through month 6 (e.g. POCD+ at months 1, 2, and 6) was 6% (29/522). In-hospital delirium was weakly associated with increased risk of persistent POCD through month 2 (odds ratio (OR) = 1.30, 95% CI 0.99–1.70, p=.062), but was not associated with having persistent POCD through month 6 (OR = 1.07, 95% CI 0.70–1.66, p=.744) (Table A3).
Sensitivity Analyses
Using the less stringent cut-off (z-score 1.0) for POCD, 60% (324/540) of patients met criteria at 1 month, of which 27% (89/324) had a history of delirium. Consistent with the main results, the proportion of patients with POCD declined at each follow up: 2 months (36%, 192/535) and 6 months (27%, 141/522). Among patients with a history of POD, the proportion with POCD declined to 8% (16/192) at 2 months, and then remained relatively unchanged (7%, 10/140) at 6 months (Table A4).
As before, the level of agreement between POD and POCD was poor and correlations remained weak. The risk for POCD, when POD was present, reached statistical significance only at 1 month (RR =1.24, 95% CI 1.06 – 1.45, p =.007). Results are reported in Table A5.
4. DISCUSSION
POD and POCD are neurocognitive complications with adverse consequences that may extend far beyond surgical recovery.4,13 We found that POD significantly increased the risk of POCD, but only in the first month; this relationship did not hold in longer-term follow-up. At each evaluation, POCD was more common among patients without delirium. These findings may suggest that POD and POCD (variously defined) could be distinct manifestations of neurocognitive deficits, triggered by interactions between surgery, anesthesia, and one or more preoperative vulnerabilities (e.g. inflammation, preclinical Alzheimer neuropathology, blood-brain barrier dysfunction).
The nature of the relationship between POD and POCD is not well-characterized.49,50 Delirium is associated with long-term cognitive decline,50 an observation that has been taken to suggest a putative role for POD in the pathogenesis of POCD. Furthermore, delirium has been associated with POCD in a number of prior studies of cognitive outcomes following noncardiac surgeries;9,51–59 however, not all utilized rigorous delirium assessment, sensitive cognitive tests, and consideration of practice effects. A strength of this study is the SAGES data,22 which is remarkable for its longitudinal follow-up of more than 500 surgical patients using well-validated delirium measures and a neuropsychological test battery sensitive to global and domain-specific cognitive change.60,61
Others have also reported associations between POD and POCD at hospital discharge or 1 month, but not at later follow-up.9,57,58 Franck et al only found an association between POD and POCD in a subgroup of participants, and concluded there was no clear evidence that delirium is independently associated with POCD beyond 1 week after surgery.54 Our findings support those of others that suggest a link between POD and POCD in early recovery. However, it is important to recognize that cognitive outcomes in the first postoperative month may be influenced by multiple factors, including waning effects of anesthesia, pain, sleep disturbance, and burden of sedative and analgesic medications.
Absence of associations between POD and POCD beyond the first postoperative month does not contradict the growing appreciation of delirium as a neurotoxic event that precipitates a trajectory of cognitive deterioration. In fact, a number of studies have shown that POD is a predictor of progression to dementia and cognitive decline years after surgery.15,62–65 Moreover, it remains possible that the effects of POD and POCD may converge after 6-months, whereby patients who developed both POD and POCD may experience the greatest long-term cognitive decline.
Another consideration is a potential statistical issue that may have lessened the correlation between POD and POCD. The finding that POCD and POD would show a very low correlation could have been expected considering prior research and the definition of POCD. Specifically, we have shown that preoperative cognitive functioning is a moderately strong predictor of postoperative delirium and dominates all other risk factors for POD with a strong negative polyserial correlation (r = −.33).66 Thus, higher baseline cognitive performance scores are associated with lower risk of POD. In contrast, the opposite association might be expected for POCD using the current definition (based on cognitive change scores) and the phenomena of regression to the mean,67 which refers to the observation that higher baseline scores are frequently associated with larger (negative) change scores. Regression to the mean would imply, therefore, that high baseline cognitive scores should be associated with greater rates of POCD (i.e. larger negative change scores) and low baseline cognitive scores should be associated with lower rates of POCD (i.e. less negative change scores). Taking these observations together, a major risk factor for delirium (baseline cognitive ability) would potentially be inherently associated with lower rates of POCD (as defined by change scores). As a result, we might expect low or moderately low correlations between these two constructs, all other factors held constant. Our findings of low correlations between POD and POCD may, therefore, reflect a statistical phenomenon rather than absence of an underlying biological association. Thus, for future work and to advance the field, it may be important to consider alternative methods to defining POCD that do not induce a correlation between baseline cognitive performance and POCD, (e.g. residualizing follow-up cognitive performance for baseline cognitive performance, rather than computing difference or change scores). This or other more sophisticated methods for modeling change68 might help to clarify relationships among variables rather than revealing potentially spurious correlations (or their lack thereof) induced by methodology.
We chose to adapt the statistical approach developed by the ISPOCD consortium to define POCD, as these methods (and their variations) have been extensively studied. The use of multiple imputation is an innovative modification that addressed differences in the number and types of cognitive tests utilized in the ISPOCD and SAGES studies. The number of cognitive test variables is an important determinant of POCD incidence (as well as the risk of Type 1 error), since the likelihood of detecting POCD increases with the number of individual tests. The demonstration of consistent associations between POD and POCD at each postoperative timepoint indicates that our construct of POCD was relatively robust to different combinations of neuropsychological tests. Furthermore, results of the sensitivity analyses (which employed a more permissive POCD threshold) confirm that the generally weak associations between delirium and POCD beyond the first month were not a result of “setting the bar too high”.
Variability in the timing of cognitive assessments across studies is another frequently cited methodologic challenge in POCD research; notably, most contemporary POCD studies have assessed cognition at 3- and 12-months following surgery. Using available data for the SAGES cohort, our findings (POCD prevalence; 47% at 1 month, 23% at 2 months, 15% at 6 months) are not inconsistent with those of the few prior studies that reported the incidence of POCD at one or more of these intervals, e.g. 41% at 2 months, 36% at 3 months, and 24% at 6 months69 and more recently, 72% at 6 days and 30% at 6 months.70
It is important to recognize the recent efforts by the International Perioperative Cognition Nomenclature Working Group to address these and other methodologic challenges, which resulted in a proposal to harmonize criteria for POCD and establish new nomenclature for conditions related to delayed postsurgical cognitive recovery (e.g. POD and POCD), now termed “postoperative neurocognitive disorders”.47 In many aspects, this study provides support for a number of recommendations in the report.
Several limitations deserve mention. First, we were unable to ascertain delirium that might have occurred, and subsequently resolved, during the interval between hospital discharge and one month. Second, this study was not designed to investigate associations between POD, our construct of POCD, and long-term cognitive recovery. However, Inouye et al., recently described the long-term consequences of POD in the SAGES cohort and found that delirium was associated with a 2.8-fold increase in the rate of cognitive decline over three years.17 Similarly, we were unable to investigate associations between potential risk factors (e.g. age, education, surgery type, complications, delirium severity or subtype) and POCD incidence or severity; clarifying these complex relationships is an important area of future research. Third, SAGES participants were nearly a decade older (mean age, 77 years) than those enrolled in ISPOCD1 (mean age, 68 years),5 and subsequent POCD studies.71 The cohort was also relatively cognitively and physically healthy, primarily white, and well-educated; thus, it is possible that our findings may not generalize to other populations. Fourth, it is possible that our negative findings were due in part to insufficient sample sizes, which may have limited our ability to detect small effects. However, post-hoc simulation reveals that if a population correlation between POCD and POD of r = .50 (25% shared variance in the tendency to satisfy POCD and POD criteria) exists, the probability of observing a correlation of less than 0.1 in our sample is less than 1 in 10000. If the association was more modest (r = .20, about 4% shared variance in the tendency to satisfy POCD and POD criteria), there is about a 1 in 12 probability of observing a correlation less than r = .1, given our sample size. Finally, our results may differ from other POCD studies because we did not log-transform performance data from timed tests.
Delayed or incomplete cognitive recovery complicates recuperation from surgery for many older adults.72,73 Our results provide evidence for considering POD and POCD as distinct constructs. The finding that POD is a significant risk factor for POCD at one month may have clinical implications. At least 40% of delirium cases may be avoidable;6 consequently, interventions aimed at delirium prevention might also serve to decrease the risk of POCD. Because POD and POCD may be more strongly related in subpopulations, such as those with multiple comorbidities or preoperative cognitive impairment, studies evaluating factors associated with vulnerabilities for POCD in the absence of POD are needed. Finally, future research should investigate the effects of POD, POCD, and their co-occurrence on functional decline, dementia, and mortality to better understand long-term prognosis for patients with either or both conditions.
Acknowledgements:
The authors gratefully acknowledge the contributions of the patients, family members, nurses, physicians, staff members, and members of the Executive Committee who participated in the Successful Aging after Elective Surgery (SAGES) Study. This work is dedicated to the memory of Joshua Bryan Inouye Helfand and Jane Ann McDowell.
Funding Statement: This work was supported by the National Institutes of Health [P01AG031720 (SKI), K07AG041835 (SKI), R24AG054259 (SKI), R01AG044518 (SKI/RNJ), R01AG030618 (ERM), K24AG035075 (ERM), and T32AG023480(AMR)]. Dr. Inouye holds the Milton and Shirley F. Levy Family Chair. The funding sources had no role in the design, conduct, or reporting of this study.
APPENDIX
The information below is provided for transparency about study participants both in the primary cohort study, Successful Aging after Elective Surgery (SAGES; Figure A1, Table A1) and a non-surgical comparison (NSC) cohort that was recruited for assessment of retest and practice effects (Table A1). Table A2 describes results from the secondary analysis of POCD concordance across timepoints. Association of in-hospital POD with persistent POCD is shown in Table A3. Detailed results of sensitivity analyses in which the threshold for POCD was re-defined as 1) z-scores for two or more individual tests ≤ -1.0; or 2) the sum of the seven z-scores ≤ -1.0 are presented in Tables A3 and A4.
Figure A1.
SAGES Participant Flow (Primary Analyses)
Table A1.
Selected baseline characteristics of the SAGES surgical patients and non-surgical control group*
| Characteristic | Full SAGES (Surgical) Sample N= 560 |
Non-surgical Control Group N=118 |
|---|---|---|
| Age- mean years (SD) | 77 (5) | 77 (5) |
| Female- n (%) | 326 (58) | 52 (44) |
| Nonwhite- n (%) | 42 (8) | 16 (13) |
| Education- mean years (SD) | 15 (3) | 16 (3) |
| GCP score- mean (SD) | 57.6 (7.3) | 58.0 (9.8) |
| 3MS score- mean (SD) | 93.5 (5.4) | 93.8 (5.4) |
GCP= General Cognitive Performance; 3MS = Modified Mini-Mental State Exam, range (0–100), lower scores indicate greater impairment; SD= standard deviation
Table A2.
Concordance of POCD* across time points
| POCD at 2 months (N=535) | Total | ||
|---|---|---|---|
| POCD at 1 month, n (%) | No POCD | POCD | |
| No POCD | 265 (49.5) | 30 (5.6) | 295 (55.1) |
| POCD | 164 (30.7) | 76 (14.2) | 240 (44.9) |
| Total | 429 (80.2) | 106 (19.8) | 535 (100) |
| POCD at 6 months (N = 525) | Total | ||
|---|---|---|---|
| POCD at 1 month | No POCD | POCD | |
| No POCD | 268 (51.1) | 23 (4.3) | 291 (55.4) |
| POCD | 177 (33.7) | 57 (10.9) | 234 (44.6) |
| Total | 445 (84.8) | 80 (15.2) | 525 (100) |
| POCD at 6 months (N = 523) | Total | ||
|---|---|---|---|
| POCD at 2 months | No POCD | POCD | |
| No POCD | 381 (72.9) | 42 (8.0) | 423 (80.9) |
| POCD | 62 (11.8) | 38 (7.3) | 100 (19.1) |
| Total | 443 (84.7) | 80 (15.3) | 523 (100) |
| POCD at 6 months (N = 522) | Total | ||
|---|---|---|---|
| POCD at 1 & 2 months | No POCD | POCD | |
| No POCD | 398 (76.3) | 51 (9.7) | 449 (86.0) |
| POCD | 44 (8.4) | 29 (5.6) | 73 (14.0) |
| Total | 442 (84.7) | 80 (15.3) | 522 (100) |
Table A3.
Association of in-hospital POD with persistent POCD*
| POCD at 1 & 2 months (N = 535) | Total | ||
|---|---|---|---|
| POD, n (%) | No POCD | POCD | |
| No POD | 308 (57.5) | 48 (9.0) | 356 (66.5) |
| POD | 151 (28.3) | 28 (5.2) | 179 (33.5) |
| Total | 459 (85.8) | 76 (14.2) | 535 (100) |
| OR = 1.30, 95% CI 0.99, 1.70, p=.062 | |||
| POCD at 1, 2 & 6 months (N = 522) | Total | ||
|---|---|---|---|
| POD | No POCD | POCD | |
| No POD | 327 (62.6) | 19 (3.7) | 346 (66.3) |
| POD | 166 (31.8) | 10 (1.9) | 176 (33.7) |
| Total | 493 (94.4) | 29 (5.6) | 522 (100) |
| OR = 1.08, 95% CI, 0.70, 1.66, p=.744 | |||
POCD, Postoperative Cognitive Dysfunction; POD, Postoperative Delirium; Odds Ratio, (OR)
Proportion with POCD was estimated from multiply-imputed data for all participants with complete neuropsychological test data at each time point
Table A4.
Proportions of in-hospital POD and POCD during follow up* using threshold of 1 SD decline to define POCD
| In-hospital Delirium and 1-month POCD (N=548) | |||
|---|---|---|---|
| POCD – 1 Month | Total | ||
| In-hospital Delirium, n (%) | No | Yes | |
| No | 181 (33.1) | 238 (43.5) | 419 (76.6) |
| Yes | 38 (6.9) | 90 (16.5) | 128 (23.4) |
| Total | 219 (40.0) | 328 (60.0) | 547 (100) |
| In-hospital Delirium and 2-month POCD (N=536) | |||
| POCD – 2 Months | Total | ||
| In-hospital Delirium | No | Yes | |
| No | 262 (48.8) | 149 (27.7) | 411 (76.5) |
| Yes | 82 (15.3) | 43 (8.2) | 125 (23.5) |
| Total | 344 (64.1) | 192 (35.9) | 536 (100) |
| In-hospital Delirium and 6-month POCD (N=528) | |||
| POCD – 6 Months | Total | ||
| In-hospital Delirium | No | Yes | |
| No | 302 (57.1) | 103 (19.6) | 405 (76.7) |
| Yes | 84 (16) | 39 (7.3) | 123 (23.3) |
| Total | 386 (73.1) | 142 (26.9) | 528 (100) |
Postoperative cognitive dysfunction (POCD); Postoperative delirium (POD)
Proportion with POCD was estimated from multiply-imputed data for all participants with complete neuropsychiatric test data at each time point
Table A5.
POCD definitions and summary statistics for associations with in-hospital POD during 6 months of postoperative follow up, using threshold of 1 SD decline to define POCD
| MONTH 1 | |||||
|---|---|---|---|---|---|
| Component neuropsychological tests* | P(POCD) | r | κ | RR (95% CI) | p |
| BNT, Digit Span | 0.58 | 0.16 | 0.07 | 1.19 (1.02 – 1.39) | 0.025 |
| Digit Span, Phonemic Fluency | 0.62 | 0.20 | 0.08 | 1.21 (1.06 – 1.38) | 0.006 |
| Digit Span, Category Fluency | 0.59 | 0.21 | 0.09 | 1.24 (1.07 – 1.43) | 0.003 |
| VSAT, Digit Span | 0.61 | 0.19 | 0.08 | 1.22 (1.06 –1.40) | 0.006 |
| BNT, Phonemic Fluency | 0.60 | 0.23 | 0.10 | 1.27 (1.10 – 1.46) | 0.001 |
| Category Fluency, Phonemic Fluency | 0.63 | 0.22 | 0.09 | 1.24 (1.08 – 1.41) | 0.002 |
| BNT, Category Fluency | 0.56 | 0.17 | 0.07 | 1.21 (1.03 – 1.41) | 0.018 |
| VSAT, BNT | 0.58 | 0.26 | 0.11 | 1.32 (1.14 – 1.51) | 0.000 |
| VSAT, Category Fluency | 0.59 | 0.27 | 0.11 | 1.32 (1.15 – 1.52) | 0.000 |
| VSAT, Phonemic Fluency | 0.65 | 0.22 | 0.08 | 1.22 (1.08 – 1.39) | 0.002 |
| Overall (Month 1) † | 0.60 | 0.21 | 0.09 | 1.24 (1.06 – 1.45) | 0.007 |
| MONTH 2 | |||||
| Component neuropsychological tests* | P(POCD) | r | κ | RR (95% CI) | p |
| BNT, Digit Span | 0.35 | -0.06 | -0.03 | 0.90 (0.68 – 1.19) | 0.443 |
| Digit Span, Phonemic Fluency | 0.38 | -0.06 | -0.03 | 0.91 (0.68 – 1.18) | 0.471 |
| Digit Span, Category Fluency | 0.38 | -0.07 | -0.04 | 0.89 (0.67 – 1.16) | 0.381 |
| VSAT, Digit Span | 0.34 | -0.07 | -0.04 | 0.88 (0.65 – 1.17) | 0.371 |
| BNT, Phonemic Fluency | 0.36 | -0.01 | -0.01 | 0.97 (0.74 – 1.28) | 0.849 |
| Category Fluency, Phonemic Fluency | 0.39 | 0.00 | 0.00 | 1.00 (0.78 – 1.29) | 0.983 |
| BNT, Category Fluency | 0.36 | -0.04 | -0.02 | 0.93 (0.70 – 1.22) | 0.586 |
| VSAT, BNT | 0.33 | 0.00 | 0.00 | 1.00 (0.75 – 1.33) | 0.976 |
| VSAT, Category Fluency | 0.36 | 0.05 | 0.03 | 1.09 (0.84 – 1.42) | 0.505 |
| VSAT, Phonemic Fluency | 0.35 | 0.07 | 0.04 | 1.14 (0.88 – 1.47) | 0.321 |
| Overall (Month 2) † | 0.36 | -0.02 | -0.01 | 0.97 (0.69 – 1.34) | 0.835 |
| MONTH 6 | |||||
| Component neuropsychological tests* | P(POCD) | r | κ | RR (95% CI) | p |
| BNT, Digit Span | 0.26 | 0.11 | 0.06 | 1.25 (0.91 – 1.71) | 0.167 |
| Digit Span, Phonemic Fluency | 0.28 | 0.08 | 0.04 | 1.17 (0.86 – 1.59) | 0.331 |
| Digit Span, Category Fluency | 0.28 | 0.03 | 0.01 | 1.06 (0.77 – 1.45) | 0.737 |
| VSAT, Digit Span | 0.27 | 0.08 | 0.04 | 1.17 (0.85 – 1.60) | 0.335 |
| BNT, Phonemic Fluency | 0.25 | 0.14 | 0.08 | 1.34 (0.98 – 1.85) | 0.071 |
| Category Fluency, Phonemic Fluency | 0.28 | 0.05 | 0.03 | 1.11 (0.81 – 1.53) | 0.497 |
| BNT, Category Fluency | 0.27 | 0.13 | 0.07 | 1.31 (0.97 – 1.77) | 0.081 |
| VSAT, BNT | 0.26 | 0.18 | 0.10 | 1.43 (1.05 – 1.95) | 0.022 |
| VSAT, Category Fluency | 0.27 | 0.10 | 0.05 | 1.22 (0.90 – 1.66) | 0.205 |
| VSAT, Phonemic Fluency | 0.28 | 0.15 | 0.08 | 1.34 (1.00 – 1.80) | 0.054 |
| Overall (Month 6) † | 0.27 | 0.11 | 0.05 | 1.23 (0.86 – 1.78) | 0.259 |
Prevalence of POCD [P(POCD)], tetrachoric correlations [r], level of agreement, kappa [κ], relative risk [RR] for the association of POD as a risk factor for POCD, and significance level [p] for each of the unique subsets of seven component neuropsychological test variables used to define POCD in the SAGES cohort.
Neuropsychological tests: Visual Search and Attention Test (VSAT); Boston Naming Test (BNT); Digit Symbol, RBANS Digit Symbol; Digit Span, WAIS Digit Span Forward and Backward; Category Fluency; Phonemic Fluency.
Each of the ten POCD definitions includes the following five test variables: Hopkins Verbal Learning Test, revised (HVLT-R) Sum of learning trials, HVLT-R Delayed Recall, Delis-Kaplan Executive Function System (D-KEFS) Trail Making Test (part B time), D-KEFS Trail Making Test (part B errors).
Overall refers to the definition of POCD at each time point estimated from multiply-imputed data.
*Sages Study Group
Overall Principal Investigator: Sharon K. Inouye, MD, MPH
Project and Core Leaders: David Alsop, PhD; Richard Jones; Thomas Travison, PhD; Edward R. Marcantonio, MD, SM
Executive Committee: Steven Arnold, MD; Zara Cooper, MD, MSc; Bradford Dickerson, MD; Tamara Fong, MD, PhD; Eran Metzger, MD; Alvaro Pascual-Leone, MD; Eva M. Schmitt, PhD; Mouhsin Shafi, MD
Other Co-investigators: Michele Cavallari, MD, PhD; Weiying Dai, PhD; Simon T. Dillon, PhD; Janet McElhaney, MD; Charles Guttmann, MD; Tammy Hshieh, MD; George Kuchel, MD, FRCP; Towia Libermann, PhD; Long Ngo, PhD; Daniel Press, MD; Jane Saczynski, PhD; Sarinnapha Vasunilashorn, PhD
Clinical Consensus Panel: Margaret O’Connor, PhD; Eyal Kimchi, MD, Jason Strauss, MD; Bonnie Wong, PhD
Surgical Leaders: Michael Belkin, MD; Douglas Ayres, MD; Mark Callery, MD; Frank Pomposelli, MD; John Wright, MD; Marc Schermerhorn, MD
Epidemiology Core: Tatiana Abrantes; Asha Albuquerque; Sylvie Bertrand; Amanda Brown M.Ed; Amy Callahan; Madeline D’Aquila, BS; Sarah Dowal, MSW, LCSW, MPH; Meaghan Fox, BS; Jacqueline Gallagher, MS; Rebecca Anna Gersten MD; Ariel Hodara RN; Ben Helfand, MPH; Jennifer Inloes, BS; Jennifer Kettell; Aleksandra Kuczmarska, AB; Jacqueline Nee, BA; Emese Nemeth, BS; Lisa Ochsner; Kerry Palihnich, BA; Katelyn Parisi, BA; Margaret Puelle, MD; Sarah Rastegar, MA; Margaret Vella, BS, Guoquan Xu, MD, PhD
Data Management and Statistical Analysis Core: Margaret Bryan, BA; Jamey Guess, MS; Dee Enghorn; Alden Gross, PhD, MHS; Yun Gou, MA; Daniel Habtemariam, BA; Ilean Isaza, PhD; Cyrus Kosar, MA; Christopher Rockett, PhD; Douglas Tommet, MPH
Fiscal Management Committee: Ted Gruen, BA, MBA; Meg Ross; Katherine Tasker, BA
Scientific Advisory Board: James Gee, PhD; Ann Kolanowski, PhD, RN, FAAN;
Margaret Pisani, MD, MPH; Sophia de Rooij, MD, PhD; Selwyn Rogers, MD, MPH; Stephanie Studenski, MD; Yaakov Stern, PhD; Anthony Whittemore, MD
Internal Advisory Board: Gary Gottlieb, MD, MBA; John Orav, PhD; Reisa Sperling, MD, MMSc
Footnotes
Prior Presentations: This work was presented, in part, at the Alzheimer’s Association International Conference, London, UK (July 2017).
Conflicts of Interest: The authors declare no competing interests.
REFERENCES
- 1.U.S. Census Bureau PD. Annual Estimates of the Resident Population for Selected Age Groups by Sex for the United States, States, Counties, and Puerto Rico Commonwealth and Municipios: April 1, 2010. to July 1, 2014.
- 2.McDermott KFW, Elixhauser A Overview of Operating Room Procedures During Inpatient Stays in US Hospitals, 2014: Statistical Brief# 233. In. Rockville, MD: Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- 3.Kim S, Brooks AK, Groban L. Preoperative assessment of the older surgical patient: honing in on geriatric syndromes. Clin Interv Aging. 2015;10:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudolph JL, Marcantonio ER. Review articles: postoperative delirium: acute change with long-term implications. Anesth Analg. 2011;112(5):1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351(9106):857–861. [DOI] [PubMed] [Google Scholar]
- 6.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evered L, Scott DA, Silbert B, Maruff P. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112(5):1179–1185. [DOI] [PubMed] [Google Scholar]
- 8.Meybohm P, Renner J, Broch O, Caliebe D, Albrecht M, Cremer J, Haake N, Scholz J, Zacharowski K, Bein B. Postoperative neurocognitive dysfunction in patients undergoing cardiac surgery after remote ischemic preconditioning: a double-blind randomized controlled pilot study. PLoS One. 2013;8(5):e64743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18–30. [DOI] [PubMed] [Google Scholar]
- 10.McDonagh DL, Mathew JP, White WD, Phillips-Bute B, Laskowitz DT, Podgoreanu MV, Newman MF, Neurologic Outcome Research G. Cognitive function after major noncardiac surgery, apolipoprotein E4 genotype, and biomarkers of brain injury. Anesthesiology. 2010;112(4):852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudolph JL, Schreiber KA, Culley DJ, McGlinchey RE, Crosby G, Levitsky S, Marcantonio ER. Measurement of post-operative cognitive dysfunction after cardiac surgery: a systematic review. Acta Anaesthesiol Scand. 2010;54(6):663–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funder KS, Steinmetz J, Rasmussen LS. Cognitive dysfunction after cardiovascular surgery. Minerva Anestesiol. 2009;75(5):329–332. [PubMed] [Google Scholar]
- 13.Gross AL, Jones RN, Habtemariam DA, Fong TG, Tommet D, Quach L, Schmitt E, Yap L, Inouye SK. Delirium and Long-term Cognitive Trajectory Among Persons With Dementia. Arch Intern Med. 2012;172(17):1324–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, Jones RN. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inouye SK, Marcantonio ER, Kosar CM, Tommet D, Schmitt EM, Travison TG, Saczynski JS, Ngo LH, Alsop DC, Jones RN. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12(7):766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olofsson B, Persson M, Bellelli G, Morandi A, Gustafson Y, Stenvall M. Development of dementia in patients with femoral neck fracture who experience postoperative delirium-A three-year follow-up study. Int J Geriatr Psychiatry. 2018;33(4):623–632. [DOI] [PubMed] [Google Scholar]
- 17.Kiely DK, Jones RN, Bergmann MA, Murphy KM, Orav EJ, Marcantonio ER. Association between delirium resolution and functional recovery among newly admitted postacute facility patients. J Gerontol A Biol Sci Med Sci. 2006;61(2):204–208. [DOI] [PubMed] [Google Scholar]
- 18.Fong TG, Jones RN, Marcantonio ER, Tommet D, Gross AL, Habtemariam D, Schmitt E, Yap L, Inouye SK. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Intern Med. 2012;156(12):848–856, W296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devinney MJ, Mathew JP, Berger M. Postoperative Delirium and Postoperative Cognitive Dysfunction: Two Sides of the Same Coin? Anesthesiology. 2018;129(3):389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger M, Terrando N, Smith SK, Browndyke JN, Newman MF, Mathew JP. Neurocognitive Function after Cardiac Surgery: From Phenotypes to Mechanisms. Anesthesiology. 2018;129(4):829–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fong TG, Jones RN, Shi P, Marcantonio ER, Yap L, Rudolph JL, Yang FM, Kiely DK, Inouye SK. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72(18):1570–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt EM, Marcantonio ER, Alsop DC, Jones RN, Rogers SO Jr., Fong TG, Metzger E, Inouye SK, Group SS. Novel risk markers and long-term outcomes of delirium: the successful aging after elective surgery (SAGES) study design and methods. J Am Med Dir Assoc. 2012;13(9):818 e811–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt EM, Saczynski JS, Kosar CM, Jones RN, Alsop DC, Fong TG, Metzger E, Cooper Z, Marcantonio ER, Travison T, Inouye SK, Group SS. The Successful Aging after Elective Surgery (SAGES) Study: Cohort Description and Data Quality Procedures. J Am Geriatr Soc. 2015;63(12):2463–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimchi EY, Hshieh TT, Guo R, Wong B, O’Connor M, Marcantonio ER, Metzger ED, Strauss J, Arnold SE, Inouye SK, Fong TG. Consensus Approaches to Identify Incident Dementia in Cohort Studies: Systematic Review and Approach in the Successful Aging after Elective Surgery Study. J Am Med Dir Assoc. 2017;18(12):1010–1018 e1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 26.Racine AM, Gou Y, Fong TG, Marcantonio ER, Schmitt EM, Travison TG, Inouye SK, Jones RN. Correction for retest effects across repeated measures of cognitive functioning: a longitudinal cohort study of postoperative delirium. BMC Med Res Methodol. 2018;18(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albert MS, Levkoff SE, Reilly C, Liptzin B, Pilgrim D, Cleary PD, Evans D, Rowe JW. The delirium symptom interview: an interview for the detection of delirium symptoms in hospitalized patients. J Geriatr Psychiatry Neurol. 1992;5(1):14–21. [DOI] [PubMed] [Google Scholar]
- 28.Saczynski JS, Kosar CM, Xu G, Puelle MR, Schmitt E, Jones RN, Marcantonio ER, Wong B, Isaza I, Inouye SK. A tale of two methods: chart and interview methods for identifying delirium. J Am Geriatr Soc. 2014;62(3):518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. [DOI] [PubMed] [Google Scholar]
- 30.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST Jr., Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53(2):312–318. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol. 1999;13(3):348–358. [DOI] [PubMed] [Google Scholar]
- 32.Wechsler DS. Manual for the Wechsler Adult Intelligence Scale—Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 33.Benton AH, de SK. Multilingual Aphasia Examination: Manual Instructions. Iowa City: University of Iowa; 1989. [Google Scholar]
- 34.Benton AL. Differential Behavioural Effects in Frontal Lobe Disease Neuropsychologica. 1968;6:53–60. [Google Scholar]
- 35.Goodglass HK. The Assessment of Aphasia and Related Disorders 2nd ed. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 36.Trenerry MR C B D J, & Leber WR Visual Search and Attention Test. Odessa, FL: Psychological Assessment Resources; 1990. [Google Scholar]
- 37.Delis D K, E, Kramer J Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 38.Randolph CTM, Mohr E, Chase TN The Repeatable Battery for the Assessment of Neuropsychological Status (Rbans): Preliminary Clinical Validity. Journal of Clinical and Experimental Neuropsychology 1998;20(3):310–319. [DOI] [PubMed] [Google Scholar]
- 39.Jones RN, Rudolph JL, Inouye SK, Yang FM, Fong TG, Milberg WP, Tommet D, Metzger ED, Cupples LA, Marcantonio ER. Development of a unidimensional composite measure of neuropsychological functioning in older cardiac surgery patients with good measurement precision. J Clin Exp Neuropsychol. 2010;32(10):1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gross AL, Jones RN, Fong TG, Tommet D, Inouye SK. Calibration and validation of an innovative approach for estimating general cognitive performance. Neuroepidemiology. 2014;42(3):144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rey A. L’examen Psychologique Dans Les Cas D’encéphalopathie Traumatique. Archives de Psychologie. 1941. 28: 215–285. [Google Scholar]
- 42.Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19(5):393–394. [DOI] [PubMed] [Google Scholar]
- 43.Bohnen N, Twijnstra A, Jolles J. Performance in the Stroop color word test in relationship to the persistence of symptoms following mild head injury. Acta Neurol Scand. 1992;85(2):116–121. [DOI] [PubMed] [Google Scholar]
- 44.Lezak M Neuropsychological Assessment. 3 ed. New York: Oxford University Press; 1995. [Google Scholar]
- 45.Krenk L, Rasmussen LS, Siersma VD, Kehlet H. Short-term practice effects and variability in cognitive testing in a healthy elderly population. Exp Gerontol. 2012;47(6):432–436. [DOI] [PubMed] [Google Scholar]
- 46.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, Oh ES, Crosby G, Berger M, Eckenhoff RG, Nomenclature Consensus Working G. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery. Br J Anaesth. 2018;121(5):1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fleiss J Statistical Methods for Rates and Proportions. New York: John Wiley & Sons; 2000. [Google Scholar]
- 49.Silverstein JH, Deiner SG. Perioperative delirium and its relationship to dementia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacLullich AM, Beaglehole A, Hall RJ, Meagher DJ. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21(1):30–42. [DOI] [PubMed] [Google Scholar]
- 51.Beishuizen SJE, van Munster BC, de Jonghe A, Abu-Hanna A, Buurman BM, de Rooij SE. Distinct Cognitive Trajectories in the First Year After Hip Fracture. J Am Geriatr Soc. 2017;65(5):1034–1042. [DOI] [PubMed] [Google Scholar]
- 52.Bryson GL, Wyand A, Wozny D, Rees L, Taljaard M, Nathan H. A prospective cohort study evaluating associations among delirium, postoperative cognitive dysfunction, and apolipoprotein E genotype following open aortic repair. Can J Anaesth. 2011;58(3):246–255. [DOI] [PubMed] [Google Scholar]
- 53.Benoit AG, Campbell BI, Tanner JR, Staley JD, Wallbridge HR, Biehl DR, Bradley BD, Louridas G, Guzman RP, Fromm RA. Risk factors and prevalence of perioperative cognitive dysfunction in abdominal aneurysm patients. J Vasc Surg. 2005;42(5):884–890. [DOI] [PubMed] [Google Scholar]
- 54.Franck M, Nerlich K, Neuner B, Schlattmann P, Brockhaus WR, Spies CD, Radtke FM. No convincing association between post-operative delirium and post-operative cognitive dysfunction: a secondary analysis. Acta Anaesthesiol Scand. 2016;60(10):1404–1414. [DOI] [PubMed] [Google Scholar]
- 55.Neufeld KJ, Leoutsakos JM, Sieber FE, Wanamaker BL, Gibson Chambers JJ, Rao V, Schretlen DJ, Needham DM. Outcomes of early delirium diagnosis after general anesthesia in the elderly. Anesth Analg. 2013;117(2):471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radtke FM, Franck M, Lendner J, Kruger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110 Suppl 1:i98–105. [DOI] [PubMed] [Google Scholar]
- 57.Rudolph JL, Marcantonio ER, Culley DJ, Silverstein JH, Rasmussen LS, Crosby GJ, Inouye SK. Delirium is associated with early postoperative cognitive dysfunction. Anaesthesia. 2008;63(9):941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sauer AC, Veldhuijzen DS, Ottens TH, Slooter AJC, Kalkman CJ, van Dijk D. Association between delirium and cognitive change after cardiac surgery. Br J Anaesth. 2017;119(2):308–315. [DOI] [PubMed] [Google Scholar]
- 59.Krogseth M, Watne LO, Juliebo V, Skovlund E, Engedal K, Frihagen F, Wyller TB. Delirium is a risk factor for further cognitive decline in cognitively impaired hip fracture patients. Arch Gerontol Geriatr. 2016;64:38–44. [DOI] [PubMed] [Google Scholar]
- 60.Price CC, Garvan CW, Monk TG. Type and severity of cognitive decline in older adults after noncardiac surgery. Anesthesiology. 2008;108(1):8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Price CC, Tanner JJ, Schmalfuss I, Garvan CW, Gearen P, Dickey D, Heilman K, McDonagh DL, Libon DJ, Leonard C, Bowers D, Monk TG. A pilot study evaluating presurgery neuroanatomical biomarkers for postoperative cognitive decline after total knee arthroplasty in older adults. Anesthesiology. 2014;120(3):601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK. The interface between delirium and dementia in elderly adults. Lancet Neurol. 2015;14(8):823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis DH, Muniz-Terrera G, Keage HA, Stephan BC, Fleming J, Ince PG, Matthews FE, Cunningham C, Ely EW, MacLullich AM, Brayne C, Epidemiological Clinicopathological Studies in Europe (EClipSE) Collaborative Members. Association of Delirium With Cognitive Decline in Late Life: A Neuropathologic Study of 3 Population-Based Cohort Studies. JAMA Psychiatry. 2017;74(3):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Devore EE, Fong TG, Marcantonio ER, Schmitt EM, Travison TG, Jones RN, Inouye SK. Prediction of Long-term Cognitive Decline Following Postoperative Delirium in Older Adults. J Gerontol A Biol Sci Med Sci. 2017;72(12):1697–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kat MG, Vreeswijk R, de Jonghe JF, van der Ploeg T, van Gool WA, Eikelenboom P, Kalisvaart KJ. Long-term cognitive outcome of delirium in elderly hip surgery patients. A prospective matched controlled study over two and a half years. Dement Geriatr Cogn Disord. 2008;26(1):1–8. [DOI] [PubMed] [Google Scholar]
- 66.Jones RN, Marcantonio ER, Saczynski JS, Tommet D, Gross AL, Travison TG, Alsop DC, Schmitt EM, Fong TG, Cizginer S, Shafi MM, Pascual-Leone A, Inouye SK. Preoperative Cognitive Performance Dominates Risk for Delirium Among Older Adults. J Geriatr Psychiatry Neurol. 2016;29(6):320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rogosa D. Myths About Longitudinal Research In: Schaie KC, Meredith W, Rawlings S, ed. Methodological Issues in Aging Research. New York: Springer Publishing Company; 1988:177–209. [Google Scholar]
- 68.Castro-Schilo L, G KJ. Using Residualized Change Versus Difference Scores for Longitudinal Research. Journal of Social and Personal Relationships 2018;35(1):32–58. [Google Scholar]
- 69.Abildstrom H, Christiansen M, Siersma VD, Rasmussen LS, Investigators I. Apolipoprotein E genotype and cognitive dysfunction after noncardiac surgery. Anesthesiology. 2004;101(4):855–861. [DOI] [PubMed] [Google Scholar]
- 70.Deo H, West G, Butcher C, Lewis P. The prevalence of cognitive dysfunction after conventional and computer-assisted total knee replacement. Knee. 2011;18(2):117–120. [DOI] [PubMed] [Google Scholar]
- 71.Paredes S, Cortinez L, Contreras V, Silbert B. Post-operative cognitive dysfunction at 3 months in adults after non-cardiac surgery: a qualitative systematic review. Acta Anaesthesiol Scand. 2016;60(8):1043–1058. [DOI] [PubMed] [Google Scholar]
- 72.Gleason LJ, Schmitt EM, Kosar CM, Tabloski P, Saczynski JS, Robinson T, Cooper Z, Rogers SO Jr., Jones RN, Marcantonio ER, Inouye SK. Effect of Delirium and Other Major Complications on Outcomes After Elective Surgery in Older Adults. JAMA Surg. 2015;150(12):1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berger M, Nadler JW, Browndyke J, Terrando N, Ponnusamy V, Cohen HJ, Whitson HE, Mathew JP. Postoperative Cognitive Dysfunction: Minding the Gaps in Our Knowledge of a Common Postoperative Complication in the Elderly. Anesthesiol Clin. 2015;33(3):517–550. [DOI] [PMC free article] [PubMed] [Google Scholar]