Abstract
Immune suppression is one of the 10 hallmarks of cancer. Interleukin-37 (IL-37), a member of the IL-1 family, inhibits both innate and adaptive immunity, and has been shown to modulate immune responses in various disease conditions. Yet, IL-37 has rarely been investigated in cancer patients, and its biological role in cancer remains to be elucidated. In this study, we investigated the gene expression of IL-37 in age- and sex-matched blood samples of healthy individuals and melanoma patients, and demonstrated upregulation of IL-37 mRNA in the blood samples of melanoma patients. By further analyzing immune cell subsets responsible for the upregulated IL-37 expression, we discovered that IL-37 mRNA was highly expressed in T cells and granulocytes, with the highest expression in regulatory T (Treg) cells in healthy individuals, and that IL-37 mRNA was upregulated in lymphocytes (T, B, and NK cells) in melanoma patient blood. Among all cell subsets, Treg cells from melanoma patients exhibited the highest IL-37 gene expression levels. We provided evidence that melanoma-conditioned media induces IL-37 mRNA and protein expression in multiple lymphocyte populations, particularly in Treg cells. We further confirmed that the IL-1-mediated secretome from human melanoma cells, specifically TGF-β, induces IL-37 mRNA expression in human Treg cells. Our results suggest a potential immunosuppressive role for IL-1 and IL-37 in melanoma tumorigenesis. Highly elevated IL-37 in specific lymphocyte populations could serve as a biomarker for tumor-induced immunosuppression.
Keywords: IL-37, Treg cells, blood, melanoma, secretome
1 |. INTRODUCTION
Immune suppression is one of the 10 hallmarks of cancer.1 The immune system interacts with tumor cells throughout the entire process of tumor initiation, development, and progression, where tumor cells induce an immunosuppressive and tumor-promoting environment by educating and recruiting immunosuppressive immune cells and stromal cells.2 Because peripheral whole blood represents many systemic processes including immune responses and intercellular communications, we have examined blood transcriptomes by Affymetrix microarray and identified 78 differentially expressed genes in blood samples from melanoma patients compared to those from healthy control individuals.3 Among these genes was interleukin-37 (IL-37), a unique anti-inflammatory and immunosuppressive gene, which was highly expressed in the blood samples of stage IV melanoma patients compared with those of healthy individuals, as shown in Table S1 of Luo et al. publication.3
IL-37 is a member of the IL-1 family with an increasingly emerging role in both innate and adaptive immune responses.4 Of the eleven IL-1 family members, IL-37 is the only known member that shows a broad anti-inflammatory property. IL-37 was first identified by in silico research in 2000 and designated as IL-1 family member 7 (IL-1F7).5 In 2010, the Dinarello group demonstrated that transgenic mice expressing human IL-37 are protected from non-lethal LPS-induced septic shock, and therefore assigned IL-1F7 the name IL-37 because of its fundamental nature of inhibiting innate immune responses.6 Since then, IL-37 has been extensively investigated for its role in innate immunity.4 Mouse models show that IL-37 protects from septic shock,6 inflammatory bowel disease,7 cardiovascular diseases,8,9 and metabolic syndromes.10 In addition to its inhibitory role in innate immunity, IL-37 has been demonstrated to suppress antigen-specific adaptive immunity by inducing tolerogenic dendritic cells (DCs) and regulatory T (Treg) cells.11 Consistent with these data, many papers have reported upregulation or downregulation of IL-37 in human diseases, including inflammatory diseases and autoimmune diseases.4,12 Although these studies suggest a role for IL-37 in modulating immune responses in various disease conditions, the biological role of IL-37 in cancer remains to be elucidated.
Considering its ability to induce immune tolerance, IL-37 might support tumorigenesis by inducing immunoevasion. Conversely, anti-inflammatory IL-37 might suppress tumorigenesis by inhibiting pro-tumorigenic inflammation. Indeed, the protective role of IL-37 in cancer has been reported when IL-37 was transfected into cancer cells,13–15 or when recombinant IL-37 was administered in animal models of cancers16 (summarized in review papers by Ding et al.17 and Abulkhir et al.18). However, only limited studies have used cancer patient samples to investigate IL-37.16,19,20 Furthermore, given its expression and role in immune cells, the expression of IL-37 in immune cells and its regulatory effects need to be investigated in cancer patients.
In this study, we investigated the expression of IL-37 in age- and sex-matched blood samples of healthy individuals and melanoma patients, and confirmed upregulation of IL-37 mRNA in the blood samples of melanoma patients. We then analyzed immune cell subsets responsible for the upregulated IL-37 expression, and examined the effects of melanoma-conditioned media (MCM) on the overall expression of IL-37 in the immune cell subsets.
2 |. MATERIALS AND METHODS
2.1 |. Acquisition of PAX-gene-collected whole blood samples
We determined the whole blood gene expression of IL-37 using mRNAs of PAX-gene-collected blood samples obtained from the Human Research Ethics Committee of Edith Cowan University (No. 2932) and Sir Charles Gardner Hospital (No.2007–123). Briefly, a 2.5 ml sample of whole blood was collected into a PAXgene Blood RNA Tube (PreAnalytiX, Hombrechtikon, Switzerland), containing RNA stabilizers, and total RNA was isolated using the PAXgene Blood RNA Kit (Qiagen, Hilden, Germany) with DNase treatment (DNA-free Kit, Ambion, Carlsbad, CA, U.S.A.).21 All subjects who had their blood drawn provided demographic information. Age- and sex-matched frozen mRNA samples (49 healthy individuals and 49 melanoma patients, stages I-IV) were sent to the University of Colorado. A full description of the cohort, blood collection and RNA extraction was given in the original paper.21
2.2 |. Blood cell fractionation and RNA isolation
Cell fractionation was performed using blood samples of healthy volunteers and melanoma patients recruited from the melanoma clinic in Aurora, Colorado, USA (Cutaneous Oncology Department at the University of Colorado Cancer Center). The use of human blood specimens at the University of Colorado AMC was approved by the Colorado Institutional Review Board (COMIRB#17–0110). Fresh blood samples were collected using Vacutainer® Heparin Tubes (BD, Franklin Lakes, NJ) and kept on ice, followed by labeling with MACS human CD3+, CD14+, CD15+, CD19+, CD45+, and CD56+ microbeads (Miltenyi Biotec, Auburn, CA). Briefly, whole blood (3 ml) was mixed with 150 μl microbeads and incubated for 15 min at 4°C. Labeled cells were washed and resuspended in autoMACS Running Buffer, followed by separation with autoMACS™ separator (Miltenyi Biotec). Programs “posseld2” and “depletes” were used to isolate positive and negative cells, respectively. Further purification of blood cells into T cell subsets was done using flow cytometric sorting of CD4+CD25−CD127hi (conventional T: Tconv cells), CD4+CD25+CD127dim (Treg cells) and CD8+ T cells. RNA was immediately extracted from fractionated cells using a Qiagen RNeasy mini kit (Qiagen, Valencia, CA).
2.3 |. qRT-PCR
Quality of the RNA was verified on an Agilent® 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) and the quantity of RNA was determined using a NanoDrop® ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE). RNA was reverse transcribed into cDNA using MMLV reverse transcriptase (Promega, Madison, WI). PCR amplification and quantitation were performed using Power Up SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) on the AriaMx Real-Time PCR system (Agilent Technologies, Santa Clara, CA). IL-37 mRNA expression was normalized relative to the housekeeping gene GAPDH. IL-37 primers used: forward— 5′-GCA TTC ATG ACC AGG ATC AC-3′; reverse— 5′-CAA AGA AGA TCT CTG GGC GTA-3′. GAPDH primers used: forward— 5′-TGC ACC ACC AAC TGC TTA GC-3′; reverse— 5′-GGC ATG GAC TGT GGT CAT GAG-3′.
2.4 |. Peripheral blood cell preparation and culture with melanoma-conditioned media (MCM)
The blood cell culture study used human peripheral blood obtained from healthy individuals at the Children’s Hospital Blood Donor Center (Aurora, CO). Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll density gradient centrifugation. PBMCs were placed in complete media containing RPMI 1640 with L-glutamine (Gibco, Thermo Fisher) and supplemented with 10% FBS (Gemini BioProducts, Calabasas, CA), 10 nM HEPES (Life Technologies, Thermo Fisher) (San Diego, CA), 1% non-essential amino acids (Life Technologies, Thermo Fisher), and 1 mM sodium pyruvate (Life Technologies, Thermo Fisher) at 1 × 106 / ml.
Purification of PBMCs into CD4+CD25+CD127dim Treg cells was done by using the Human CD4+CD25+CD127dim Regulatory T cell Isolation Kit (Miltenyi Biotech) according to the manufacturer’s instructions. MS column purification steps were performed in duplicate to ensure high purity of CD4+CD25+CD127dim T cells. Flow cytometry analysis of isolated cells using Foxp3, a lineage-specific transcription factor, confirmed the purity of Treg cells (Figure 5A). CD4+CD25- Tconv cells were obtained from the remaining population of CD4+ T cells after CD4+CD25+CD127dim Treg cell purification and used as a comparison to Treg cells (Figure 5A).
MCM was obtained from supernatants of human metastatic melanoma cells, 1205Lu (obtained from Rockland Immunochemicals, Inc., Limerick, PA), after 24 h of cultivation in Opti-MEM I Reduced Serum Media (Life Technologies, Grand Island, NY), and centrifuged at 210 × g for 5 min.22 1205Lu cells were routinely checked for mycoplasma by PCR as previously described,23 and authenticated using STR analysis by Barbara Davis Center Bioresource Core. To treat PBMCs with MCM or IL-1β, cells were cultured for 24 h with complete media containing 10% MCM, 50% MCM or 10 ng/ml IL-1β (Life Technologies, Thermo Fisher). To block IL-1 receptor (IL-1R) signaling in melanoma cells, we pre-treated 1205Lu cells with IL-1 receptor antagonist (IL-1Ra, 10 μg/ml) while generating MCM, and designated MCM as “MCM/IL-1Ra”. To determine the effect of TGF-β, we added an antibody to TGF-β, 1D11 (10 μg/ml), to MCM, and designated MCM as “MCM + 1D11”. To treat Treg cells with MCM, MCM/IL-1Ra or MCM + 1D11, cells were cultured for 24 h with complete media containing 50% MCM, MCM/IL-1Ra or MCM + 1D11. After 24 h, cells were collected and processed for RNA isolation or flow cytometry analysis. Gating strategy for immune cell subset expression IL-37 shown in Figure 4A.
2.5 |. Intracellular and extracellular flow cytometry staining
Flow cytometry was performed using the following antibodies (ThermoFisher) directed against human antigens: CD45RB (MEM-55), CD25 (BC96), CD3 (OKT3), CD4 (RPA-T4), CD8 (SK1), CD16 (eBiocb16), CD19 (SJ25C1), CD56 (CMSSB), IL-37 (37D12), and Foxp3 (236A/E7). PBMCs treated with or without MCM were washed with FACS buffer (5% BSA (Sigma-Aldrich) in 1x PBS), and then further prepared for surface or intracellular staining, as described previously.24 For surface staining, 1 μg of fluorescently conjugated antibody specific for the surface marker to be visualized was added to 1 × 106 cells resuspended in 100 μl of FACS buffer for 30 min at 4°C, then washed twice with FACS buffer prior to be either analyzed or further processed. For intercellular IL-37 cytokine staining and Foxp3 staining, the Foxp3 Cytoperm/Cytofix staining kit (BD Pharmingen, San Diego, CA) was used.
2.6 |. Human Cytokine Array
The Human Cytokine Antibody Array C5 (RayBiotech Life, Norcross, GA) was used to detect 80 human cytokines according to the manufacturer’s instructions. In brief, MCM were collected from 1205Lu cells either untreated or treated with IL-1Ra for 24 h. After incubation in blocking buffer for 30 min at room temperature, the array membranes containing 80 human cytokine specific antibodies were incubated with MCM or MCM/IL-1Ra overnight at 4°C. The membranes were then washed and incubated with the biotinylated antibody cocktail for 2 h at room temperature. Membranes were washed again and then probed with HRP-streptavidin for 2 h at room temperature. The signals were developed using Detection Buffer mix and imaged using the Odyssey Fc system (Li-cor). Relative cytokine intensities were normalized in comparison to control spots on the same membrane. Relative density (AU, arbitrary units) contained in each region of interest for 80 antibodies was measured using FIJI (National Institutes of Health: http://rsb.info.nih.gov/ij/), and background intensity was subtracted from each measurement using negative controls present on each array membrane prior to determining the final area. This protocol can be found on the Gel Analysis tab on http://rsb.info.nih.gov/ij/.
2.7 |. TGF-β1 ELISA.
TGF-β1 secretion into MCM was analyzed from 1205Lu cells either untreated or treated with IL-1Ra. MCM and MCM/IL1Ra were then collected and analyzed using DuoSet® human TGF-β1 ELISA kits (R&D Systems) to measure TGF-β1 protein abundance, according to the manufacturer’s instructions.
2.8 |. Statistical analysis
All of the experiments were replicated at least twice. Patient data in tables 1–3 were processed by the biostatistics and informatics group of the Colorado School of Public Health (D. Gao). Data were expressed throughout as mean ± standard error of the mean (SEM). To assess if there is an association between IL-37 expression and disease status (case or control), linear regression model including disease status and clusters (case and control 1:1 matched on sex and age and form 49 clusters) as covariates was performed with log transformed IL-37 measurement as outcome to approximate normal distribution. The estimated mean expression level in melanoma patients and healthy controls on the original scale of IL-37 was then calculated based on the coefficients from the model and log normal distribution for IL-37. Data sets were compared using the two-tailed unpaired Student’s t-test if the test was not specified. Statistical analysis and graphing were performed using Prism 8 (GraphPad, Lo Jolla, CA) and SAS software 9.4 (SAS Institute Inc. NC, Cary, USA). Differences were considered statistically significant when p < 0.05.
3 |. RESULTS
3.1 |. IL-37 mRNA expression is elevated in the blood samples of melanoma patients
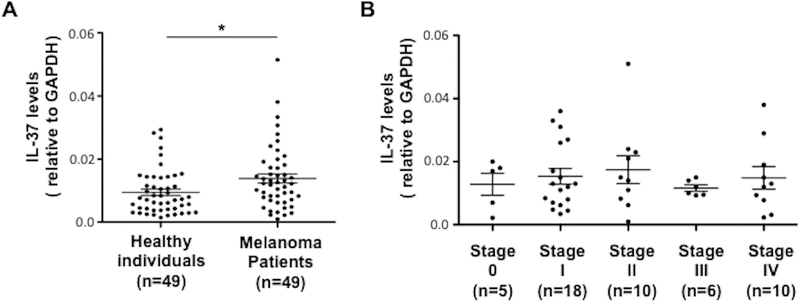
Age and sex-matched blood samples of 49 healthy individuals and 49 melanoma patients were investigated for the expression of IL-37 mRNA. The sample parameters are shown in Tables 1 and 2. Regression analysis results indicated that melanoma patients had a statistically significant higher IL-37 mRNA expression (0.383 on log scale of IL-37 measurement, Table 3) in their blood compared to health control individuals (p = 0.025), which was 1.47 times higher than the control group on the original (anti-log) scale (see the Statistical Analysis for the method of calculation). In addition, a two-group t-test was used to verify the result, because t-test is relatively robust to distribution violation. The two-group t-test showed that IL-37 was upregulated 1.53-fold in whole blood samples from 49 melanoma patients compared to healthy individuals (Figure 1A). However, no statistical difference was observed among IL-37 expressions from different stages (Figure 1B). These data confirmed our previous microarray data,3 which revealed upregulated IL-37 gene expression in the blood of melanoma patients compared to healthy individuals.
Table 1 –
Analysis variable: Age
| Group | N Obs | Mean | Std Dev | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Healthy | 49 | 55.63 | 12.33 | 54.00 | 28.00 | 78.00 |
| Melanoma | 49 | 55.61 | 12.39 | 56.00 | 28.00 | 78.00 |
| Stage 0 | 5 | 62.80 | 9.834 | 66.00 | 52.00 | 74.00 |
| Stage I | 18 | 57.67 | 11.17 | 58.00 | 43.00 | 78.00 |
| Stage II | 10 | 54.10 | 11.15 | 57.00 | 36.00 | 68.00 |
| Stage III | 6 | 45.50 | 17.40 | 42.50 | 28.00 | 67.00 |
| Stage IV | 10 | 55.90 | 11.81 | 56.50 | 38.00 | 73.00 |
Table 2 –
Analysis variable: Sex
| Group | N Obs | Female | Male |
|---|---|---|---|
| Healthy | 49 | 25 (51.02%) | 24 (48.98%) |
| Melanoma | 49 | 25 (51.02%) | 24 (48.98%) |
| Stage 0 | 5 | 4 (80.00%) | 1 (20.00%) |
| Stage I | 18 | 11 (61.11%) | 7 (38.89%) |
| Stage II | 10 | 5 (50.00%) | 5 (50.00%) |
| Stage III | 6 | 3 (50.00%) | 3 (50.00%) |
| Stage IV | 10 | 2 (20.00%) | 8 (80.00%) |
Table 3 –
Results of linear regression for log (IL-37-GAPDH)
| Effect* | Estimate | Standard Error | p-value | 95% CI Lower | 95%CI Upper |
|---|---|---|---|---|---|
| Disease status | 0.3826 | 0.1651 | 0.0248 | 0.05070 | 0.7145 |
The model adjusted for the clustering effect. The estimated expression level of IL-37 in melanoma is on average 1.47 times higher than those in healthy control.
Figure 1. IL37 gene expression of whole blood cells from healthy individuals and melanoma patients.
(A) qRT-PCR was conducted using mRNAs extracted from PAX-gene-collected whole blood samples from 49 healthy individuals and 49 melanoma patients. (B) qRT-PCR of IL-37 from PAX-gene-collected whole blood samples from 49 melanoma patients in different stages of melanoma: stage 0 (n = 5), stage I (n = 18), stage II (n = 10), stage III (n = 6), and stage IV (n = 10). IL-37 gene expression was determined based on the relative levels to GAPDH mRNA. Each symbol represents an individual sample; horizontal lines indicate mean ± SEM. * p < 0.05 (Student’s t-test).
3.2 |. IL-37 mRNA is highly expressed in Treg cells and its expression is much higher in melanoma patients
Blood contains various cellular subsets with distinct functions: in addition to leukocytes, erythrocytes and platelets/thrombocytes, many cells (such as endothelial cells, fibroblasts, bone-marrow-derived cells and tumor cells) circulate and interact with each other in the blood of cancer patients. To define the physiological relevance and biological mechanisms of IL-37 upregulation in blood samples, we used magnetic cell separation kits from Miltenyi Biotec to fractionate blood samples into CD45 cells (leukocytes) and the subsets of CD45 cells, including CD3 (T cells), CD14 (monocytes/macrophages), CD15 (granulocytes: basophils, eosinophils and neutrophils), CD19 (B cells), and CD56 (NK cells). IL-37 was almost exclusively expressed in CD45+ cells but rarely detected in CD45− cells (Figure 2A). Among CD45+ cells, IL-37 was highly expressed in T cells and in granulocytes from healthy individuals (Figure 2B). The comparison between healthy individuals and melanoma patients revealed significantly higher levels of IL-37 gene expression in T cells, B cells and NK cells in the melanoma patient blood. On the contrary, monocytes and granulocytes did not show significant differences in IL-37 gene expression between healthy individual and melanoma patient blood. Further purification of blood cells into T cell subsets revealed that IL-37 was highly expressed in Treg cells of healthy individuals, with the expression levels 9.5-fold higher than CD45+ cells and 3.1-fold higher than T cells (Figure 2C). This elevated IL-37 gene expression in Treg cells was further upregulated in Treg cells from melanoma patients and found to be 1.7-fold higher than those from healthy individuals. Among all cell subsets, Treg cells from melanoma patients exhibited the highest levels of IL-37 gene expression: 3.5-fold and 2.7-fold higher than CD45+ cells and T cells from the same patient group, respectively; and 17.5-fold and 5.6-fold higher than CD45+ cells and T cells from healthy controls, respectively. Conversely, IL-37 expression remained low in CD8+ T cells, and did not show differences between healthy individuals and melanoma patients. These data demonstrate increased IL-37 gene expression in subsets of lymphocytes from melanoma patients compared to those from healthy individuals, indicating stimulatory effects from melanoma cells.
Figure 2. IL37 gene expression in fractionated blood cell subpopulations from healthy individuals and melanoma patients.
(A) qRT-PCR of IL-37 in CD45+ and CD45− cells fractionated using autoMACS from healthy individuals. (B) qRT-PCR of IL-37 in autoMACS-fractionated leukocytes (CD45), T cells (CD3), monocytes/macrophages (CD14), granulocytes (CD15), B cells (CD19) and NK cells (CD56), from healthy individuals and melanoma patients. (C) qRT-PCR of IL-37 in FACS-sorted Tconv cells, Treg cells and CD8+ cells, from healthy individuals and melanoma patients. Data indicate mean ± SEM, n = 7–21 (A, B), or 5–6 (C) per group. NS, not significant (p > 0.05); *, p < 0.05, **, p < 0.01, *** p < 0.001 (Student’s t-test). Data are representative of two (C) or three (A, B) independent experiments.
3.3 |. Melanoma conditioned media (MCM) induces IL-37 mRNA and protein expression in multiple lymphocyte populations, including Treg cells
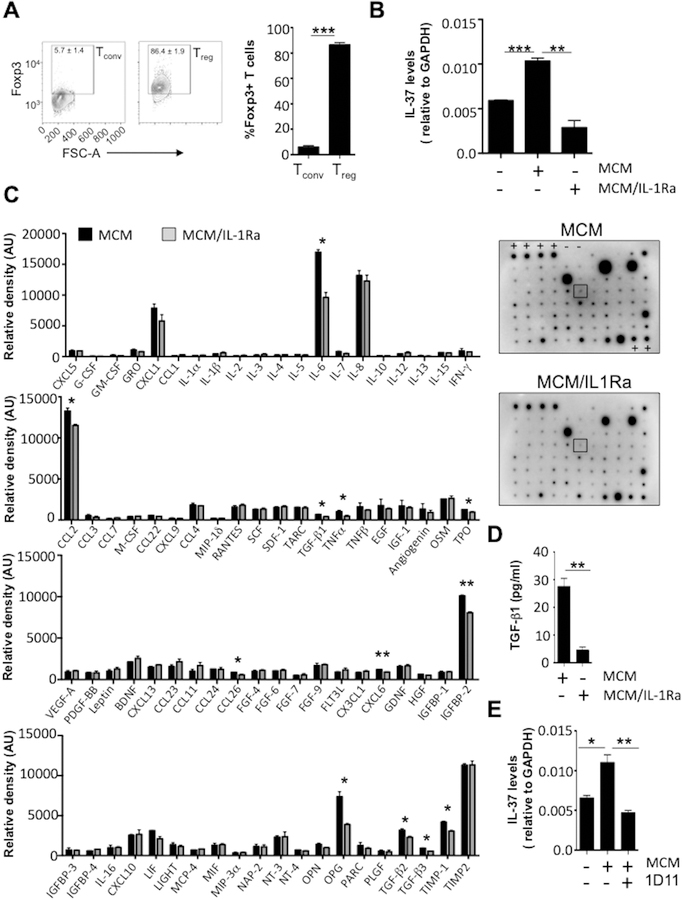
The levels of IL-37 mRNA and protein are usually low due to the instability elements within the coding region in exon 5, which limit the half-life of IL-37 mRNA.25 However, stimulation with TLR ligands and pro-inflammatory cytokines such as IL-1β, TNF-α, IL-18, IFN-γ or TGF-β1 induces IL-37.5,26–28 Because these cytokines are secreted in the tumor microenvironment, we speculated that melanoma cells could induce IL-37 expression in lymphocyte populations, specifically Treg cells. To explore this hypothesis, we first cultured human PBMCs from healthy individuals with 10% or 50% vol/vol MCM derived from the human metastatic melanoma cell line 1205Lu for 18 h. IL-1β was used as a positive control to induce IL-37 expression in PBMCs. Culturing PBMCs with MCM upregulated IL-37 gene expression in a dose-dependent fashion, reaching 3.4-fold (in #1 individual) to 2.6-fold (in #2 individual) increase of IL-37, comparable to those observed with IL-1β (Figure 3). Therefore, we next cultured healthy individual-derived PBMCs with 1205Lu-derived MCM for 24 h, and examined IL-37 protein expression in cell subsets. Figure 4A illustrates the gating strategy of those immune subsets. Consistent with our hypothesis, MCM induced IL-37 protein expression in multiple lymphocyte populations including Tconv cells, Treg cells, CD8+ T cells, B cells and NK cells (Figure 4B). Among them, Treg cells contained the highest proportion of IL-37 protein-expressing cells compared to other immune subsets, and the frequency of IL-37 protein-expressing Treg cells was further increased from 69% to 84% after cultivation with MCM (Figure 4B). The mean fluorescence intensity of IL-37 staining in Treg cells was also increased 1.6-fold (Figure 4C).
Figure 3. qRT-PCR of IL-37 in PBMCs cultured with MCM.

Measurement of IL-37 mRNA in PBMCs cultured without (−) or with either 10% MCM, 50% MCM or IL-1β (10 ng/ml) for 18 h. MCM was obtained from supernatants of 1205Lu cells after 24 h of cultivation. #1 and #2 are biological replicates. Data indicate mean ± SEM, n = 3. *, p < 0.05, **, p < 0.01, *** p < 0.001 compared to the expression level without MCM or IL-1β. Data are representative of two independent experiments.
Figure 4. Flow cytometric analysis of IL-37 protein expression in immune cell subsets cultured with MCM.
(A) Gating strategy of human PBMCs for IL-37-expressing immune cell subsets. Following live cell gating, cell subsets were determined by antibodies against CD3, CD4, CD8, CD14, CD19, Foxp3, and CD56. Dot plots are representative of one of five donor PBMCs. (B) PBMCs were cultured in the absence (−) or presence (+) of 1205Lu-derived MCM (50%) for 24 h and subjected for antibody staining. Contour plots (left) and frequency of IL-37-expressing cells (right) in Tconv cells (gated CD4+Foxp3−), Treg cells (gated CD4+Foxp3+), CD8+ T cells, B cells (gated CD19+) and NK cells (gated CD56+), measured by flow cytometry. (C) Histogram (left) and quantification by mean fluorescent intensity (MFI) (right) of IL-37 expression in T cell subsets (Tconv cells in red, Treg cells in black, and CD8+T cells in blue) in the absence (−) or presence (+) of MCM. IgG control staining shown in gray. Data indicate mean ± SEM, n = 5. NS, not significant (p > 0.05); *, p < 0.05, **, p < 0.01, *** p < 0.001. Data are representative of two independent experiments.
IL-37 is induced by many pro-inflammatory cytokines.5,26–28 We have previously shown that human melanoma cell-derived IL-1β is biologically active and can induce production and secretion of other cytokines and chemokines from melanoma cells.22 Therefore, we speculated that melanoma-derived IL-1β and its downstream mediators (IL-1-mediated secretome from melanoma cells) induce IL-37 expression in lymphocyte populations, particularly in Treg cells. To define the biological roles of IL-1-mediated secretome from melanoma cells in Treg cells, we treated 1205Lu cells with an IL-1R signaling blockade, IL-1Ra, while generating MCM (MCM/IL-1Ra), and incubated human Treg cells (Treg cell purity was confirmed in Figure 5A) in the absence or presence of MCM or MCM/IL-1Ra. While MCM significantly increased IL-37 expression in Treg cells, MCM/IL-1Ra abolished the IL-37-inducing effect of MCM (Figure 5B). To identify secretome proteins mediated by IL-1R signaling in 1205Lu cells, we used a cytokine array. Consistent with our previous report,22 IL-1R signaling blockade reduced the secretion of many cytokines from 1205Lu melanoma cells, including IL-6 (−43.4%), CCL2 (−13.2%), TGF-β1 (−49.4%), TGF-β2 (−30.7%), TGF-β3 (−39.1%), TNF-α (−58.2%), TPO (−24.5%), CCL26 (−33.0%), CXCL6 (−24.6%), IGFBP-2 (−20.0%), OPG (−47.4%), and TIMP-1 (−27.0%) (Figure 5C). Importantly, we observed the largest reduction in the secretion of TGF-β1, a key cytokine involved in Treg cell induction and a known inducer of IL-37.6 This result was confirmed by ELISA for TGF-β1, which showed decreased secretion of TGF-β1 (−83.7%) from 1205Lu cells when IL-1R signaling was blocked (Figure 5D). Therefore, we examined the effect of TGF-β on inducing IL-37 in Treg cells by neutralizing TGF-β. While MCM significantly increased IL-37 expression in Treg cells, adding 1D11, an anti-TGF-β antibody, to MCM (MCM + 1D11) abolished the IL-37-inducing effect of MCM in Treg cells (Figure 5E). These results confirm that the secretome from melanoma cells including TGF-β induces the expression of IL-37 in Treg cells.
Figure 5. qRT-PCR of IL-37 in Treg cells and cytokine analysis of MCM.
(A) Flow cytometry analysis of Foxp3 in CD4+CD25- Tconv cells and CD4+CD25+CD127dim Treg cells prior to treatment with MCM. Left, contour plots of Foxp3+ cell gating in human Tconv cells and Treg cells. Right, quantification of Foxp3+ Tconv and Treg cells. (B) Measurement of IL-37 mRNA in Treg cells cultured without (−) or with (+) 50% MCM or 50% MCM/IL-1Ra for 18 h. (C) Cytokine protein antibody array. MCM or MCM/IL1Ra was immunoblotted using the array membranes, and the relative density (AU, arbitrary units) of each dot (representing 80 different cytokines) was measured. Left, quantification of 80 cytokines. Right, representative array membrane images. The order of cytokines in bar graphs matches the order of dots in the blots, from left to right, top to bottom. Positive (+) and negative (−) controls are labeled on the blots. Dots highlighted in black box represent TGF-β1. (D) ELISA of TGF-β1 from MCM or MCM/IL1Ra. (E) Measurement of IL-37 mRNA in Treg cells cultured without (−) or with (+) 50% MCM or 50% MCM + 1D11 (10 μg/ml of 1D11 added to MCM) for 18 h. MCM or MCM/IL1Ra was obtained from supernatants of 1205Lu cells after 24 h of cultivation without or with IL-1Ra (10 μg/ml), respectively. Data indicate mean ± SEM, n = 2 (Figure 5C) or 3 (Figures 5A, 5B, 5D, 5E). *, p < 0.05, **, p < 0.01, *** p < 0.001. Data are representative of two independent experiments.
4 |. DISCUSSION
Accumulating evidence demonstrates that IL-37 has anti-inflammatory and immunosuppressive roles.4 In the present study, we not only observed the increased expression of IL-37 in the blood of melanoma patients but also discovered that the increased IL-37 occurred in lymphocytes (T, B, and NK cells), with the highest expression in Treg cells. Further, we provide evidence that the melanoma secretome induces the expression of IL-37 mRNA and protein in the lymphocyte populations, particularly in Treg cells.
Suppression of the adaptive T cell immune response plays a significant role in tumorigenesis.1 Studies have shown that Treg frequencies are increased within tumors and circulation, and correlate with tumor burden and disease outcome in cancer patients including melanoma.29 Our discovery of the melanoma-induced upregulation of IL-37 in adaptive immune cells, including Treg cells, suggests that IL-37 could be a mechanism for immune suppression and immune evasion by melanoma cells. We have previously shown that IL-37 participates in peripheral tolerance through the generation of semi-mature tolerogenic DCs, thereby impairing activation of effector T-cell responses and inducing Treg cells in vitro and in vivo.11 Xu et al. reported IL-37 expression in Treg cells and its contribution to the increased levels of Foxp3 and CTLA-4 as well as the enhanced suppressive activity of Treg cells.30 Therefore, IL-37 would reduce an anti-tumor response and enhance tumorigenesis. Similar to our data, Huo et al. reported that serum IL-37 was elevated in patients with epithelial ovarian cancer, and that the elevated IL-37 was correlated with poor overall survival and shorter progression-free survival of patients.19 On the other hand, several studies show a protective role of IL-37 in cancer by inhibiting multiple signaling pathways and enhancing anti-tumor effects.13–16 The difference between our data and those from several other studies is that the former examined clinical samples whereas the latter used transfected cancer cells or animal models. Another important difference is that our study analyzed the expression of IL-37 in immune cells from blood samples but not in tumor cells. Therefore, the effect of IL-37 on tumor cells could be different because of the indirect effect through immune cells or the direct effect in tumor cells themselves.
Although we measured IL-37 expression (mRNA and protein) in immune cells from melanoma patients, we have not measured secreted IL-37. Unlike most cytokines, IL-37 functions both intracellularly and extracellulary.4 Intracellularly, IL-37 is processed and translocates into the nucleus to induce negative signals.4,6 Additionally, IL-37 is released or secreted from the cells, and the extracellular form of IL-37 binds the IL-18 receptor, IL-18Rα, and recruits the inhibitory receptor IL-1R8 (formerly TIR8: Toll/IL-1 receptor 8, SIGIRR: single Ig IL-1-related receptor), thereby delivering an inhibitory signal and suppressing NF-κB pathway. Therefore, expressed IL-37 and secreted IL-37 may possess different biological functions in cancer.
We have yet to study the mechanism by which tumor cells induce IL-37 expression in lymphocytes. IL-37 can be induced by many cytokines such as IL-1β, IFN-γ, TNF-α, IL-18, and TGF-β, and melanoma cells are reported to produce IL-1β, TNF-α, and TGF-β.22,30 We have shown in the current study that the secretion of TNF-α, TGF-β and other cytokines from melanoma cells is mediated by IL-1R signaling (Figures 5C, 5D) and that IL-1-mediated secretome, specifically TGF-β, is responsible for the increase in IL-37 expression in Treg cells (Figure 5E). The mechanistic role of tumor cell-derived cytokines in the induction of IL-37 from blood samples and/or MCM remains to be further investigated.
In conclusion, we show increased expression of IL-37 in the blood of melanoma patients. IL-37 is highly expressed in lymphocyte populations, particularly in Treg cells, and its expression is even higher in melanoma patients, which can be explained by our findings that the IL-1-mediated melanoma secretome including TGF-β induces the expression of IL-37 in Treg cells. Considering the association between IL-37 expression and the enhanced suppressive ability in Treg cells, our data suggest a potential immunosuppressive role for IL-1 and IL-37 in melanoma tumorigenesis. Highly elevated IL-37 in specific lymphocyte populations could serve as a biomarker for tumor-induced immunosuppression.
ACKNOWLEDGEMENTS
We thank the University of Colorado Denver (UCD) melanoma tissue bank for providing human melanoma samples and information. We also thank the University of Colorado Cancer Center (UCCC) Support Grant (P30CA046934), the Skin Diseases Research Cores (SDRC) Grant (P30AR057212) and the Flow Cytometry Core (Alistaire S. Acosta and Karen Helm) for helping with FACS sorting.
GRANT SUPPORT
This work was supported by NIH/NCI R01CA197919 (to M. Fujita), Veterans Affairs Merit Review Award 5I01BX001228 (to M. Fujita), Cancer League of Colorado (to M. Fujita), 1R03CA125833 (to M. Fujita), Interleukin Foundation (to M. Fujita), NHMRC application number 1013349 (to M. Ziman), Cancer and Palliative Care Research and Evaluation Unit WAPCN Small Grants 2010/11 (to M. Ziman), and Cancer Council of WA Research Grants (to M. Ziman).
ABBREVIATION
- IL-37
interleukin-37
- Treg
regulatory T cell
- Tconv
conventional T cell
- NK
natural killer cell
- MCM
melanoma-conditioned media
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
DATA AVAILIBILITY STATEMENT
Raw data were generated at the University of Colorado Anschutz campus in the department of Dermatology. Derived data supporting the findings of this study are available from the corresponding author MF on request.
REFERENCES
- 1.Hanahan D & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Wellenstein MD & de Visser KE Cancer-Cell-Intrinsic Mechanisms Shaping the Tumor Immune Landscape. Immunity 48, 399–416 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Luo Y, et al. Transcriptome profiling of whole blood cells identifies PLEK2 and C1QB in human melanoma. PLoS One 6, e20971 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinarello CA, et al. Suppression of innate inflammation and immunity by interleukin-37. Eur J Immunol 46, 1067–1081 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith DE, et al. Four new members expand the interleukin-1 superfamily. J Biol Chem 275, 1169–1175 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Nold MF, et al. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol 11, 1014–1022 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNamee EN, et al. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci U S A 108, 16711–16716 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji Q, et al. Exogenous interleukin 37 ameliorates atherosclerosis via inducing the Treg response in ApoE-deficient mice. Sci Rep 7, 3310 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng Q, et al. Interleukin-37 suppresses the osteogenic responses of human aortic valve interstitial cells in vitro and alleviates valve lesions in mice. Proc Natl Acad Sci U S A 114, 1631–1636 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavalli G, et al. Treating experimental arthritis with the innate immune inhibitor interleukin-37 reduces joint and systemic inflammation. Rheumatology (Oxford) 55, 2220–2229 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo Y, et al. Suppression of antigen-specific adaptive immunity by IL-37 via induction of tolerogenic dendritic cells. Proc Natl Acad Sci U S A 111, 15178–15183 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen HM & Fujita M IL-37: a new player in immune tolerance. Cytokine 72, 113–114 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Gao W, et al. Innate immunity mediated by the cytokine IL-1 homologue 4 (IL-1H4/IL-1F7) induces IL-12-dependent adaptive and profound antitumor immunity. J Immunol 170, 107–113 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Zhao JJ, et al. Interleukin-37 mediates the antitumor activity in hepatocellular carcinoma: role for CD57+ NK cells. Sci Rep 4, 5177 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge G, et al. Interleukin-37 suppresses tumor growth through inhibition of angiogenesis in non-small cell lung cancer. J Exp Clin Cancer Res 35, 13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Y, et al. IL-37 mediates the antitumor activity in renal cell carcinoma. Med Oncol 32, 250 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Ding VA, et al. The role of IL-37 in cancer. Med Oncol 33, 68 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Abulkhir A, et al. A protective role of IL-37 in cancer: a new hope for cancer patients. J Leukoc Biol 101, 395–406 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Huo J, Hu J, Liu G, Cui Y & Ju Y Elevated serum interleukin-37 level is a predictive biomarker of poor prognosis in epithelial ovarian cancer patients. Arch Gynecol Obstet 295, 459–465 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Jiang M, et al. IL-37 inhibits invasion and metastasis in non-small cell lung cancer by suppressing the IL-6/STAT3 signaling pathway. Thorac Cancer 9, 621–629 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reid AL, et al. Markers of circulating tumour cells in the peripheral blood of patients with melanoma correlate with disease recurrence and progression. Br J Dermatol 168, 85–92 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okamoto M, et al. Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of interleukin-1beta. J Biol Chem 285, 6477–6488 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravindran Menon D, et al. CDK1 interacts with Sox2 and promotes tumor initiation in human melanoma. Cancer Res (2018). [DOI] [PMC free article] [PubMed]
- 24.Osborne DG & Wetzel SA Trogocytosis results in sustained intracellular signaling in CD4(+) T cells. J Immunol 189, 4728–4739 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boraschi D, et al. IL-37: a new anti-inflammatory cytokine of the IL-1 family. Eur Cytokine Netw 22, 127–147 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Pan G, et al. IL-1H, an interleukin 1-related protein that binds IL-18 receptor/IL-1Rrp. Cytokine 13, 1–7 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Bufler P, et al. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc Natl Acad Sci U S A 99, 13723–13728 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bufler P, Gamboni-Robertson F, Azam T, Kim SH & Dinarello CA Interleukin-1 homologues IL-1F7b and IL-18 contain functional mRNA instability elements within the coding region responsive to lipopolysaccharide. Biochem J 381, 503–510 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shuai X, et al. Expression of IL-37 contributes to the immunosuppressive property of human CD4+CD25+ regulatory T cells. Sci Rep 5, 14478 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elias EG, Hasskamp JH & Sharma BK Cytokines and growth factors expressed by human cutaneous melanoma. Cancers (Basel) 2, 794–808 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]