Abstract
Background
Mental health (MH) conditions are common among persons with HIV (PWH). An understanding of factors associated with prescription medication use for these conditions and clinical impact of the prescription medications may improve care of MH disorders in PWH.
Methods
Psychotropic medication use was examined among PWH within the AIDS Clinical Trials Group A5322 (HAILO) study. Multivariable logistic models and Cox regression models estimated the association between psychotropic medications (any/none) with baseline and incident slow gait (>1 sec/m) and neurocognitive impairment (NCI) over 4 years.
Results
Of 1,035 participants, the median age was 51. 81% were men, 30% black, non-Hispanic, and 20% Hispanic. Psychotropic medication use was similar between men (34%) and women (38%; p=0.19). PWH using psychotropic medications had greater odds of baseline slow gait (OR 1.61, [95% CI 1.23–2.10]; p<0.001). Men but not women using psychotropic medications had an increased risk of developing slow gait (hazard ratio 1.85; [1.29–2.65] vs 0.77; [CI 0.35–1.68], p interaction=0.045). The sex-specific ORs for medication use and NCI were qualitatively but not statistically different (men: 1.79; [1.14–2.80]; women: 1.27; [0.56–2.90]; p interaction = 0.47). Psychotropic medication use was associated with an increased risk of incident NCI (HR 2.18; [95% CI 1.23–3.84], p=0.007) in both men and women.
Conclusions
Psychotropic medications are associated with impairment in functional outcomes of aging, with a greater risk of baseline NCI and incident slow gait among men. Further investigation is needed to optimize outcomes in PWH and prescription of psychotropic medications among both men and women.
Keywords: Psychotropic Medications, adverse clinical outcomes
Background
In the United States, one in six people has a mental health disorder, costing nearly $200 billion in 2013.1 Approximately 13% of adults are prescribed psychotropic medications,2 with the use of antidepressants alone increasing almost twofold over the last 20 years.3 Although medication is an essential component of comprehensive mental health care, many psychotropic medications have been associated with increased risk of geriatric syndromes including falls,4,5 fractures,6 slow gait speed,7,8 and impairment in neurocognitive function.9 Whether these outcomes are due to medications themselves, mental health disorders, or a combination is unclear. Furthermore, although some studies suggest a negative impact of psychotropic medications on gait speed7,8 and neurocognitive impairment (NCI),9 other studies suggest a protective effect, with improvements in functional outcomes with greater time on pharmacotherapy.10
Mental health disorders are more prevalent in persons with HIV (PWH)11 than the general population, with some estimates suggesting a three-fold greater risk compared to people without HIV.12,13 In PWH, depression has been linked with less virologic suppression and greater risk of AIDS-related illness and death,14–16 particularly among persons not engaged in mental health care. Evidence suggests that mental health disorders in PWH are often underdiagnosed and undertreated.17,18 Among PWH engaged in mental health care, treatment may involve a high burden of psychotropic prescription medications, with associated costs and adverse side effects. Importantly, many psychotropic medications can interact with antiretroviral therapy, which may increase adverse effects, particularly with the burden of polypharmacy among older PWH.19,20 Although psychotropic medication use has been associated with falls among PWH in cross-sectional studies, the association with other clinical outcomes such as slow gait speed or NCI among PWH is not well described.21
Among PWH, women have high rates of depression (>40% in some studies),22–24 and are more likely than men with HIV to be prescribed psychotropic medications,25 perhaps due to higher mental health treatment-seeking behaviors. Despite treatment, studies of younger women (mostly <40 years of age) living with HIV and depression have shown greater rates of HIV disease progression and increased mortality compared to women without depression.22,23,26 The goals of this analysis were to identify differences in psychotropic medication use and determine the effect on fundamental geriatric outcomes in a cohort of older men and women with HIV. We hypothesized that older PWH who used psychotropic medications would have greater risk for developing slow gait speed or NCI. Furthermore, we speculated that women would have a greater rate of psychotropic medication use and thus poorer clinical outcomes than men.
Methods
Study Population
The HIV Infection, Aging, and Immune Function Long-Term Observational Study (HAILO) study, or AIDS Clinical Trials Group (ACTG) Study A5322, is a prospective observational study of PWH who were ≥40 years of age at the time of enrollment, had received randomized assignment of initial antiretroviral therapy (ART) through an ACTG trial, and were followed in the ACTG A5001 (ALLRT) observational study after their trial participation ended. The HAILO Study enrolled in 2013–2014, and participants are evaluated every six months. This analysis included 1035 individuals with gait speed or neurocognitive function measured at the A5322 entry visit, and for the analyses of incident slow gait and neurocognitive impairment (NCI), those who also had ≥1 additional gait speed or neurocognitive measurement through week 144. 581 participants (495 men and 86 women) were included in the incident gait speed analysis (400 participants with slow gait speed at study entry were excluded). Similarly, 793 participants (650 men and 143 women) were included in the incident NCI analysis (166 participants with NCI at entry were excluded).
Psychotropic Medications
Psychotropic prescription medications were used as a proxy for mental health diagnoses, as only limited primary mental health diagnostic data were collected (ongoing diagnosis of major depression, or ≥ grade 3 major depression symptoms using Division of AIDS criteria27). Psychotropic medications were self-reported or abstracted from chart review as available. Medications were first classified into eight categories to explore sex differences by types of prescribed therapies: first generation antidepressants, second and third generation antidepressants, first generation antipsychotics, addiction treatment medications, benzodiazepines, buspirone (a non-benzodiazepine anxiolytic), mood stabilizers and anticonvulsants, and sleep medications. Because numbers within each class were small, our primary analysis measured the impact of any psychotropic medication (vs none) on outcomes. In exploratory analyses, we further grouped medications into three overarching classes depending on psychotropic properties: sedating medications (e.g., benzodiazepines and sleep aids), stimulating medications (e.g., selective serotonin reuptake inhibitors and serotonin norepinephrine reuptake inhibitors) or neutral medications (e.g., buspirone and mood stabilizers/anticonvulsants). Opioid medication use was analyzed separately due to the known effects of opioid medications on neurocognition.
Outcomes
Gait speed was measured yearly as the average of 2 timed readings on a 4-meter walk, and times were dichotomized as ≤1 meters/second or >1 meters/second, as gait speed ≤1 meters/second in aging persons has been associated with poor prognosis, including increased hospitalizations and death.28 Neurocognitive impairment (NCI) was assessed yearly using the ALLRT Neuroscreen29, with sex, age, race/ethnicity and education-adjusted scores for Trails-Making A, Trails-Making B, and Digit Symbol. NCI was defined as having ≥1 test score ≥2 standard deviations below the mean, or ≥2 separate test scores that were ≥ 1 standard deviation below the mean.
Other Covariates
Insurance was categorized as: no insurance/unknown, public insurance (including Medicaid), Medicare, and private insurance. Education level was grouped as: less than high school completion, high school or GED completion, or greater than high school education (reported at first neurocognitive evaluation during ALLRT follow-up). Self-reported race/ethnicity was categorized as: white non-Hispanic, black non-Hispanic, and Hispanic. Smoking was defined as current, prior, or never smoker. Substance use status was self-reported as use of a non-prescribed psychoactive substance (marijuana, cocaine, heroin, amphetamines, or other non-prescribed drugs) at least once within the last month. Self-reported alcohol use was categorized as abstaining (no drinking); light (men: <7 drinks/week and no binging; women:<3 drinks/week and no binging [binging defined as ≥5 drinks/2 hour period for men, ≥4 drinks for women]); moderate (men: 7–14 drinks/week and no binging; women: 3–7 drinks/week and no binging); and heavy (men: >14 drinks/week, or binging; women: >7 drinks/week, or binging). All covariates were measured at A5322 entry unless otherwise indicated. As efavirenz use has been linked with neurocognitive impairment,30,31 baseline efavirenz use was considered in NCI models only.
Statistical Analysis
Demographic characteristics were compared by any use versus no use of psychotropic medications, using chi-square and Wilcoxon tests for categorical variables and continuous variables, respectively. We fit separate logistic regression models to evaluate the associations between each socioeconomic variable (race/ethnicity, education and medical insurance) and any psychotropic medication use (overall and then stratified by sex). When the effect estimates for the sex-stratified models suggested qualitative sex differences (effect modification), we included an interaction term in the logistic regression models. Multivariable models were fit to adjust for age and/or race/ethnicity; additional covariates were entered into the model one at a time. If the covariate changed the odds ratio (OR) for medication use and the outcome by ≥10% it was retained in the final, multivariable model.
Logistic regression modeling was used to examine the association between medication use and prevalent outcomes of slow gait speed and NCI. Age was forced into all models and education was forced into the models for NCI. Multivariable models were fit to adjust for confounding and assessed for effect modification, as described above. Additional logistic regression models were fit to explore the association between medication class (stimulating, combination of sedating/stimulating, neutral) and prevalent slow gait speed and NCI.
Cox proportional hazards models were used to examine the association between psychotropic medication use and the outcomes of incident slow gait and NCI. These analyses were restricted to those without the outcome at entry and at least one additional visit through week 144. Participants without the outcome were censored on the date of their last evaluation. Date of outcome occurrence was defined as the midpoint from the previous evaluation to the date of the evaluation where the outcome was recorded. The same covariates described above were evaluated as potential confounding variables, and effect modification was evaluated as described above. Since the assumption of proportional hazards was violated for several covariates in their original form, in the Cox proportional hazards models for incident NCI covariate categories were reduced for education (≤high school [HS] vs > HS), alcohol consumption (abstainer vs any alcohol consumption), and smoking status (non-smoker vs any smoking history).
Results
Of 1035 participants, the majority (81%) were men, white non-Hispanic (48%), privately insured (42%), with post-high school education (61%) and a median age of 51 years. Median CD4 count was 624 cells/μL, and 94% of participants were virally suppressed (HIV RNA <200 copies/mL) (Table 1). Marijuana was the most common substance used (17%), followed by non-prescribed medications (4%), and then cocaine (2%). At study entry, 705 participants (68%) were prescribed no psychotropic prescription medications, 193 (19%) were prescribed one, 75 participants (7%) were prescribed two, and 62 participants (6%) were prescribed three or more. The use of any psychotropic medication differed significantly by race/ethnicity, medical insurance, substance use, and smoking status, but not by sex (Table 1). The number of medications did not significantly differ between sexes. The most commonly-prescribed classes included second and third generation antidepressants (12% of participants), followed by mood stabilizers/anticonvulsants (11%). The type of medication class also did not differ by sex (i.e., 12% of men versus 13% of women were on second/third generation antidepressants, p=0.61; Table 2).
Table 1.
Demographic Characteristics at Study Entry by Psychotropic Medication Use
| Medication Use at Study Entry | |||||
|---|---|---|---|---|---|
| Characteristic | No psychotropic medication (N=705) | ≥1 psychotropic medication (N=330) | Total (N=1035) | P-Value | |
| Sex | Male | 570 (81%) | 264 (80%) | 834 (81%) | 0.75 |
| Female | 135 (19%) | 66 (20%) | 201 (19%) | ||
| Age, years | Median (Q1-Q3) | 50 (46–56) | 51 (46–56) | 51 (46–56) | 0.39 |
| Race/Ethnicity | White Non-Hispanic | 307 (44%) | 186 (56%) | 493 (48%) | <.001 |
| Black Non-Hispanic | 214 (30%) | 91 (28%) | 305 (29%) | ||
| Hispanic | 160 (23%) | 47 (14%) | 207 (20%) | ||
| Other/Missing | 24 (3%) | 6 (2%) | 30 (3%) | ||
| Medical insurance | None/unknown | 163 (23%) | 52 (16%) | 215 (21%) | <.001 |
| Public | 164 (23%) | 102 (31%) | 266 (26%) | ||
| Private | 315 (45%) | 118 (36%) | 433 (42%) | ||
| Medicare | 63 (9%) | 58 (18%) | 121 (12%) | ||
| Education | <High School | 109 (15%) | 44 (13%) | 153 (15%) | 0.50 |
| High School or GED | 146 (21%) | 78 (24%) | 224 (22%) | ||
| >High school | 428 (61%) | 201 (61%) | 629 (61%) | ||
| Alcohol Consumption | Abstainer | 277 (39%) | 136 (41%) | 413 (40%) | 0.74 |
| Light Drinker | 244 (35%) | 104 (32%) | 348 (34%) | ||
| Moderate Drinker | 33 (5%) | 18 (5%) | 51 (5%) | ||
| Heavy Drinker | 133 (19%) | 66 (20%) | 199 (19%) | ||
| Substance use in past month | No | 531 (75%) | 236 (71%) | 767 (74%) | 0.008 |
| Yes | 125 (18%) | 85 (26%) | 210 (20%) | ||
| Smoking Status | Never | 318 (45%) | 108 (33%) | 426 (41%) | <.001 |
| Prior Smoker | 235 (33%) | 112 (34%) | 347 (34%) | ||
| Current Smoker | 152 (22%) | 110 (33%) | 262 (25%) | ||
| Absolute CD4 count (cells/μL) | Median (Q1-Q3) | 624(455–833) | 620(450–809) | 624(451–828) | 0.78 |
| Virally Suppressed (<200 HIV-1 RNA copies/mL) | No | 36 (5%) | 21 (6%) | 57 (6%) | 0.41 |
| Yes | 669 (95%) | 209 (94%) | 978 (94%) | ||
Table 2.
Psychotropic Medication Prescription by Class
| Percentage of Participants Prescribed Each Drug Class, by Sex | |||
|---|---|---|---|
| Drug Class | Men (%) | Women (%) | P value |
| 1st Generation Antidepressants | 22 (3) | 8 (4) | 0.31 |
| 2nd and 3rd Generation Antidepressants | 101 (12) | 27 (13) | 0.61 |
| 1st Generation Antipsychotics | 40 (5) | 12 (6) | 0.49 |
| Addiction Medications | 3 (<1) | 1 (<1) | 0.78 |
| Benzodiazepines | 61 (7) | 12 (6) | 0.50 |
| Buspirone | 2 (<1) | 0 | 0.49 |
| Mood Stabilizers/Anticonvulsants | 89 (11) | 28 (14) | 0.19 |
| Sleep Medications | 78 (9) | 18 (9) | 0.86 |
| Opioids | 44 (5) | 26 (12) | <0.001 |
Demographic Characteristics Associated with Psychotropic Medication Use
In univariable analyses, Hispanic participants (OR=0.48 [95% confidence interval (CI) 0.33, 0.70], p<0.001) and Black non-Hispanic participants (OR=0.70 [0.52, 0.95], p = 0.02) had lower odds of psychotropic medication use compared to white, non-Hispanic participants. When stratified by sex, the odds for the association of Hispanic ethnicity with psychotropic medication use was substantially lower for women (OR=0.16 [0.06, 0.41]) than for men (OR=0.59 [0.39, 0.89], p interaction=0.013). This sex difference was not present for black (vs white) participants. Compared to those without insurance, the odds of taking psychotropic medications was greater for publicly-insured participants (OR=1.95 [1.31, 2.90], p=0.001) and participants on Medicare (OR=2.89 [1.80, 4.63], p<0.001) but similar to privately insured participants (OR=1.17 [0.81, 1.71], p=0.40). No associations were seen between education level and psychotropic medication use. The impact of insurance status and education level on psychotropic medication use did not vary by sex (Table 3).
Table 3.
Associations between Demographic Information and Psychotropic Medication Use, Adjusted for Demographic Characteristics
| Variables | Unadjusted Odds Ratio | Adjusted Odds Ratio (95% CI) | P value | ||
|---|---|---|---|---|---|
| Education (ref < high school) | High school /GED | 1.16 (0.79, 1.72) | 1.03 (0.64, 1.66)* | 0.92 | |
| >High school | 1.32 (0.85, 2.07) | 0.80 (0.51, 1.26)* | 0.34 | ||
| Race (ref White non-Hispanic) | Hispanic | Male | 0.59 (0.39, 0.89) | 0.59 (0.39, 0.90) + | 0.01 (p-value for interaction) |
| Female | 0.16 (0.06, 0.41) | 0.16 (0.06, 0.41) + | |||
| Black non-Hispanic | Male | 0.69 (0.48, 0.99) | 0.69 (0.48, 0.99) + | 0.25 (p-value for interaction) | |
| Female | 0.43 (0.21, 0.89) | 0.43 (0.21, 0.89) + | |||
| Insurance status (ref no insurance/ unknown) | Public (including Medicaid) | 1.95 (1.32, 2.90) | 1.75 (1.16, 2.65)* | 0.008 | |
| Medicare | 2.89 (1.80, 4.63) | 2.43 (1.45, 4.08)* | <0.001 | ||
| Private | 1.17 (0.81, 1.71) | 0.91 (0.60, 1.36)* | 0.63 |
Adjusted for sex, age, and race/ethnicity
Adjusted for age
GED, general education diploma or high school equivalency certificate
In models adjusted for age, both Hispanic and Black individuals remained significantly less likely to take psychotropic medications, with sex differences remaining significant in the comparisons between Hispanic and white, non-Hispanic individuals. In models adjusted for age and race/ethnicity, the odds of taking psychotropic medications remained significantly greater for publicly-insured participants (OR=1.75 [1.16, 2.65], p= 0.008) and participants on Medicare (OR=2.43 [1.45, 4.08], p < 0.001) compared with those without insurance (Table 3).
Psychotropic Medication Use and Clinical Outcomes
At study entry, 419 (41%) participants had slow gait speed and 166 (17%) had NCI. Women were significantly more likely to have slower gait speed (54% vs 38%, p<0.001) and NCI (24% vs 15%, p=0.002) than men.
Gait speed
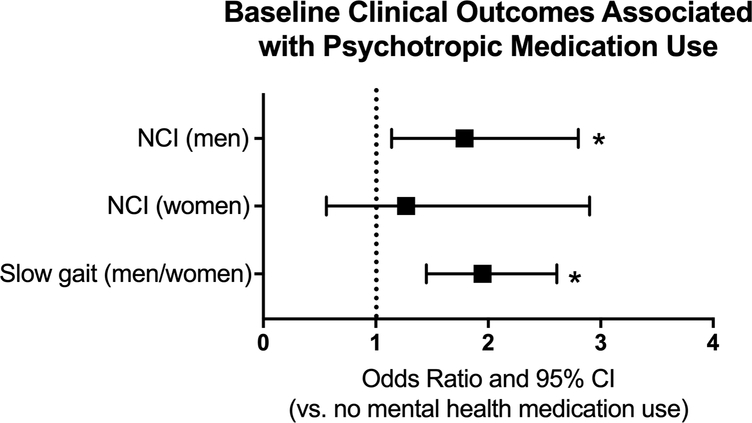
In models adjusted for age only, participants taking psychotropic medications had greater odds of baseline slow gait (OR=1.61 [1.23, 2.10], p<0.001) and greater odds of developing slow gait speed over time (HR=1.52 [1.10, 2.10], p=0.01) than those not on psychotropic medications. In sex-stratified models, the associations between psychotropic medication use and baseline gait speed were similar among men and women (OR=1.51 vs 2.09, respectively; p-value interaction=0.36). In the final multivariable models, psychotropic medication use remained significantly associated with slower baseline gait speed in both sexes (OR=1.95 [1.45, 2.61], p<0.001, Figure 1).
Figure 1:
Odds of baseline neurocognitive impairment (NCI), stratified by sex, and odds of baseline slow gait (men and women combined) associated with psychotropic medication use. NCI models are adjusted for age, race/ethnicity, substance abuse and an interaction between sex and medication use; gait speed models are adjusted for age and race/ethnicity; *p<0.05; separation of outcomes by sex is included only if there was a sex interaction in the outcome.
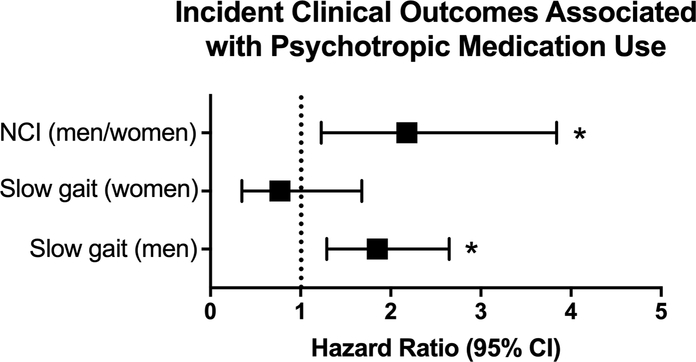
The age-adjusted association between psychotropic medication use and incident slow gait speed varied significantly by sex. When compared to men not taking psychotropic medications, men who took at least one psychotropic medication had higher incidence of developing slow gait speed (HR=1.85 [1.29, 2.65], p=0.002) -- a finding not seen among women (HR=0.77 [0.35, 1.68], p=0.15; p-value interaction=0.045; Figure 2).
Figure 2:
Hazard of developing neurocognitive impairment (NCI; men and women combined), or slow gait (sex stratified) in association with psychotropic medication use. NCI models are adjusted for age, alcohol abuse, substance abuse, and smoking status; gait speed adjusted for age and interaction between sex and medication use; *p<0.05; separation of outcomes by sex is included only if there was a sex interaction in the outcome.
Neurocognitive impairment
In models combining men and women, psychotropic medication use was not associated with NCI at baseline (OR=1.31 [0.92, 1.87], p= 0.13). In sex-stratified models, however, the association between medication use and baseline NCI was stronger among men (OR=1.60 [1.07, 2.41] than women (OR=0.71 [0.34, 1.48]; p-value interaction=0.059). In multivariable models including an interaction term for sex, psychotropic medication use was associated with baseline NCI in men (OR=1.79 [1.14, 2.80], p=0.01), but not women (OR=1.27 [0.56, 2.90], p=0.57; Figure 1); however, this effect modification was no longer statistically significant (p-value interaction=0.47).
Psychotropic medication use was also associated with incident NCI (adjusted HR=2.18 [1.23, 3.84], p=0.007; Figure 2), with no significant differences by sex. Efavirenz use did not change the effect estimate by ≥10% and was not kept in the multivariable model.
Effects of Medication Class on Outcomes
In multivariable analysis including the same variables, we next explored the effect of sedating or stimulating psychotropic medications. Participants on stimulating (OR=1.95 [1.21, 3.14], p=0.006), neutral (OR=2.44 [1.42, 4.22], p=0.001), and a combination of stimulating and sedating medications (OR=3.20 [1.64, 6.21], p<0.001) all had higher odds of having slow gait speed at study entry compared with those not taking psychotropic medications. Higher odds of baseline NCI were seen in participants taking sedating medications (OR=1.96 [1.07, 3.59], p=0.03) and participants taking a combination of stimulating and sedating medications (OR=2.69 [1.27, 5.65], p=0.009).
Opioid Medication Use
Lastly, we explored the additional effect that opioids may have as mood altering medications. Women were more likely than men to be prescribed an opioid medication (13% vs 5%, p <0.001). When analyses were repeated including opioids as a psychotropic medication, the number of participants taking one or more psychotropic medications remained similar (330 without opioids and 356 with opioids). Black participants no longer had lower odds of being on psychotropic medications compared to white participants (OR=0.81, [0.60, 1.09] p=0.16). The addition of opioids resulted in similar effects on baseline gait speed or NCI, and incident slow gait or NCI. The addition of opioids to sedating medications also resulted in similar outcomes.
Discussion
We have explored demographic factors associated with psychotropic medication use and clinical impact on functional outcomes of adults with well-suppressed HIV. Overall, with the exception of opioids, the class and number of prescribed of psychotropic medications were similar by sex, which is surprising in the context of literature suggesting higher rates of major depressive disorder among women than men with HIV.11 Some differences in psychotropic medication use by demographics were identified, including higher use of medications in Medicare and publicly-insured participants and lower use in racial and ethnic minorities. Both men and women taking ≥1 psychotropic medications were more likely to have slower baseline gait speed and to develop NCI over time; in contrast, only men taking one or more psychotropic medications were more likely to have NCI at baseline and to develop slow gait speed than those men not on psychotropic medications.
Several of our findings merit further discussion: our finding that psychotropic medication use was associated with baseline slow gait speed is similar to prior studies linking depression32,33 and psychotropic medications7,8 to slow gait speed. The sex differences supporting more pronounced impact of these medications on men with regards to baseline NCI and incident slow gait are intriguing; sedating medication use was similar between men and women, and our models controlled for substance use. One possibility is a neuroprotective effect of estrogen in cognition-altering mental health disorders, especially schizophrenia.34 This effect may be the reason that women with schizophrenia generally have later onset and less severe disease, and evidence suggests that treating schizophrenic patients with estrogen can improve their psychotic symptoms.35 The association between baseline NCI and psychotropic medication use among men may explain the decline in gait speed of these men over time as well; previous studies have shown that baseline neurocognitive status predicts decline in gait speed and other physical function tests.36,37
The increased rate of psychotropic medication use among Medicare and publicly insured participants may reflect the high burden of mental health disorders, more consistent prescription medication coverage, or increased use of psychotropic medications due to a lack of counseling services. Indeed, evidence suggests that psychiatrists are less likely to take patients with Medicare and Medicaid than physicians of other specialties38. By contrast, lower use of psychotropic medications among Hispanic and African-American participants, and among Hispanic women compared to men may be due to differences in treatment preferences and rates of depressive disorders. Hispanic-Americans and African-Americans with depressive disorders may be less likely than their white counterparts to find psychotropic medications acceptable for treatment.39 Atypical presentations of mental health disorders among Hispanic women (e.g., somatization of depressive symptoms) may underestimate mental health disorders in this population.40,41 With increased rates of depressive disorders in the poor and in the elderly42 and worse clinical outcomes among racial minorities43 and impoverished PWH,44 the access to mental healthcare, cultural sensitivity in diagnosis of mental health disorders, and stigma associated with mental health treatment should be considerations among HIV primary care providers.
Lastly, we found that the combination of sedating and stimulating medications was associated with slow gait and NCI, with similar but attenuated associations with sedating medications or stimulating medications alone. Several of these medications can be found in the Beers Criteria for Potentially Inappropriate Medication Use in Older Adults.45 In our aging population, these medications can put patients at risk for adverse medication reactions and decreased clearance of medications.45 The stronger negative effect on clinical outcomes among the participants on a combination of medications is not surprising: with at least two prescribed psychotropic medications in addition to at least three ART medications, participants met the definition for polypharmacy,46 which has been associated with physical impairments47 and functional decline.48 The risk of polypharmacy holds especially true among older PWH, who are more likely than younger PWH to be prescribed medications that can cause dangerous drug-drug interactions with ART.49
Several limitations of this analysis should be acknowledged: our study population was predominantly men and has been consistently participating in clinical trials and observational studies for several years, and thus may not be representative of the overall population of PWH. With a limited proportion of participants with negative clinical outcomes, we were limited in the number of covariates that could be included in our models. Symptoms of mood disorders were not routinely assessed; thus, we were unable to assess the adequacy of treatment. Regarding NCI, medications used to treat the neurocognitive disorders other than stimulants were not included. Also, the ALLRT Neuroscreen is brief and does not provide comprehensive neurological testing. Finally, the rates of psychotropic medication use do not necessarily reveal the prevalence of mental health disorders, as participants may have declined medical therapy, may have utilized non-pharmacologic therapy, or may have stopped therapy due to cost, side effects, or stigma.
In conclusion, we found that psychotropic medication use may confer worse outcomes in terms of gait speed and neurocognition, among middle- and older-aged PWH, and especially among men. What remains unclear is whether mental health disorders, unmeasured confounders associated with mental health disorders, or the psychotropic medications were associated with poorer outcomes. Further research is needed to determine the adequacy of mental health treatment on clinical outcomes, and whether these effects differ by sex and among PWH.
Acknowledgements
We thank the AIDS Clinical Trials Group, Statistical Data and Management Center, participating sites, and most importantly, the study participants for the ongoing involvement in this and other studies.
Funding: This research was supported by the National Institute of Aging (NIA) through K23AG050260 to KME, the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health under Award Numbers UM1 AI068634, UM1 AI068636 and UM1 AI106701. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Roehrig C Mental Disorders Top The List Of The Most Costly Conditions In The United States: $201 Billion. Health Aff (Millwood). 2016;35(6):1130–1135. [DOI] [PubMed] [Google Scholar]
- 2.Zibman C Expenditures for Mental Health among Adults, Ages 18–64, 2009–2011: Estimates for the U.S. Civilian Noninstitutionalized Population In: Statistical Brief (Medical Expenditure Panel Survey (US)). Rockville (MD)2001. [PubMed] [Google Scholar]
- 3.Kantor ED, Rehm CD, Haas JS, et al. Trends in Prescription Drug Use Among Adults in the United States From 1999–2012. JAMA. 2015;314(17):1818–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ensrud KE, Blackwell TL, Mangione CM, et al. Central nervous system-active medications and risk for falls in older women. J Am Geriatr Soc. 2002;50(10):1629–1637. [DOI] [PubMed] [Google Scholar]
- 5.Williams LJ, Pasco JA, Stuart AL, et al. Psychiatric disorders, psychotropic medication use and falls among women: an observational study. BMC Psychiatry. 2015;15:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolton JM, Morin SN, Majumdar SR, et al. Association of Mental Disorders and Related Medication Use With Risk for Major Osteoporotic Fractures. JAMA Psychiatry. 2017;74(6):641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landi F, Russo A, Liperoti R, et al. Anticholinergic drugs and physical function among frail elderly population. Clin Pharmacol Ther. 2007;81(2):235–241. [DOI] [PubMed] [Google Scholar]
- 8.Donoghue OA, O’Hare C, King-Kallimanis B, et al. Antidepressants are independently associated with gait deficits in single and dual task conditions. Am J Geriatr Psychiatry. 2015;23(2):189–199. [DOI] [PubMed] [Google Scholar]
- 9.Torrent C, Martinez-Aran A, Daban C, et al. Effects of atypical antipsychotics on neurocognition in euthymic bipolar patients. Compr Psychiatry. 2011;52(6):613–622 [DOI] [PubMed] [Google Scholar]
- 10.Paleacu D, Shutzman A, Giladi N, et al. Effects of pharmacological therapy on gait and cognitive function in depressed patients. Clin Neuropharmacol. 2007;30(2):63–71. [DOI] [PubMed] [Google Scholar]
- 11.Closson K, Osborne C, Smith DM, et al. Factors Associated with Mood Disorder Diagnosis Among a Population Based Cohort of Men and Women Living With and Without HIV in British Columbia Between 1998 and 2012. AIDS Behav. 2017. [DOI] [PubMed] [Google Scholar]
- 12.Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58(8):721–728. [DOI] [PubMed] [Google Scholar]
- 13.Kessler RC, Birnbaum HG, Shahly V, et al. Age differences in the prevalence and co-morbidity of DSM-IV major depressive episodes: results from the WHO World Mental Health Survey Initiative. Depress Anxiety. 2010;27(4):351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pence BW, Miller WC, Gaynes BN, et al. Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44(2):159–166. [DOI] [PubMed] [Google Scholar]
- 15.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. [DOI] [PubMed] [Google Scholar]
- 16.Anastos K, Schneider MF, Gange SJ, et al. The association of race, sociodemographic, and behavioral characteristics with response to highly active antiretroviral therapy in women. J Acquir Immune Defic Syndr. 2005;39(5):537–544. [PubMed] [Google Scholar]
- 17.Asch SM, Kilbourne AM, Gifford AL, et al. Underdiagnosis of depression in HIV: who are we missing? J Gen Intern Med. 2003;18(6):450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bess KD, Adams J, Watt MH, et al. Providers’ attitudes towards treating depression and self-reported depression treatment practices in HIV outpatient care. AIDS Patient Care STDS. 2013;27(3):171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenblatt DJ, von Moltke LL, Harmatz JS, et al. Short-term exposure to low-dose ritonavir impairs clearance and enhances adverse effects of trazodone. J Clin Pharmacol. 2003;43(4):414–422. [DOI] [PubMed] [Google Scholar]
- 20.Wynn GH, Cozza KL, Zapor MJ, et al. Med-psych drug-drug interactions update. Antiretrovirals, part III: antiretrovirals and drugs of abuse. Psychosomatics. 2005;46(1):79–87. [DOI] [PubMed] [Google Scholar]
- 21.Erlandson KM, Allshouse AA, Jankowski CM, et al. Risk factors for falls in HIV-infected persons. J Acquir Immune Defic Syndr. 2012;61(4):484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285(11):1466–1474. [DOI] [PubMed] [Google Scholar]
- 23.Antelman G, Kaaya S, Wei R, et al. Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania. J Acquir Immune Defic Syndr. 2007;44(4):470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan MS, Marks G, Mertens SB. Distress and coping among women with HIV infection: preliminary findings from a multiethnic sample. Am J Orthopsychiatry. 1997;67(1):80–91. [DOI] [PubMed] [Google Scholar]
- 25.Himelhoch S, Josephs JS, Chander G, et al. Network HIVR. Use of outpatient mental health services and psychotropic medications among HIV-infected patients in a multisite, multistate study. Gen Hosp Psychiatry. 2009;31(6):538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook JA, Grey D, Burke J, et al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health. 2004;94(7):1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. Department of Health and Human Services NIoH, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.0. November 2014. http://rcc.tech-res.com/docs/default-source/safety/daids_ae_grading_table_v2_nov2014.pdf?sfvrsn=8. [Google Scholar]
- 28.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53(10):1675–1680. [DOI] [PubMed] [Google Scholar]
- 29.Robertson KR, Smurzynski M, Parsons TD, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. Aids. 2007;21(14):1915–1921. [DOI] [PubMed] [Google Scholar]
- 30.Ciccarelli N, Fabbiani M, Di Giambenedetto S, et al. Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology. 2011;76(16):1403–1409. [DOI] [PubMed] [Google Scholar]
- 31.Ma Q, Vaida F, Wong J, et al. Long-term efavirenz use is associated with worse neurocognitive functioning in HIV-infected patients. J Neurovirol. 2016;22(2):170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enright PL, McBurnie MA, Bittner V, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123(2):387–398. [DOI] [PubMed] [Google Scholar]
- 33.Busch Tde A, Duarte YA, Pires Nunes D, et al. Factors associated with lower gait speed among the elderly living in a developing country: a cross-sectional population-based study. BMC Geriatr. 2015;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blum M, McEwen BS, Roberts JL. Transcriptional analysis of tyrosine hydroxylase gene expression in the tuberoinfundibular dopaminergic neurons of the rat arcuate nucleus after estrogen treatment. J Biol Chem. 1987;262(2):817–821. [PubMed] [Google Scholar]
- 35.Kulkarni J, de Castella A, Fitzgerald PB, et al. Estrogen in severe mental illness: a potential new treatment approach. Arch Gen Psychiatry. 2008;65(8):955–960. [DOI] [PubMed] [Google Scholar]
- 36.Atkinson HH, Rapp SR, Williamson JD, et al. The relationship between cognitive function and physical performance in older women: results from the women’s health initiative memory study. J Gerontol A Biol Sci Med Sci. 2010;65(3):300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atkinson HH, Rosano C, Simonsick EM, et al. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2007;62(8):844–850. [DOI] [PubMed] [Google Scholar]
- 38.Bishop TF, Press MJ, Keyhani S, et al. Acceptance of insurance by psychiatrists and the implications for access to mental health care. JAMA Psychiatry. 2014;71(2):176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper LA, Gonzales JJ, Gallo JJ, et al. The Acceptability of Treatment for Depression among African-American, Hispanic, and White Primary Care Patients. Medical Care. 2003;41(4):479–489. [DOI] [PubMed] [Google Scholar]
- 40.General OotS, Services CfMH. Mental health: Culture, race, and ethnicity: A supplement to mental health: A report of the surgeon general. 2001. [PubMed]
- 41.Escobar JI, Gomez J, Tuason VB. Depressive phenomenology in North and South American patients. Am J Psychiatry. 1983;140(1):47–51. [DOI] [PubMed] [Google Scholar]
- 42.Weinberger AH, Gbedemah M, Martinez AM, et al. Trends in depression prevalence in the USA from 2005 to 2015: widening disparities in vulnerable groups. Psychol Med. 2017:1–10. [DOI] [PubMed] [Google Scholar]
- 43.Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: Final Data for 2014. Natl Vital Stat Rep. 2016;65(4):1–122. [PubMed] [Google Scholar]
- 44.Singh GK, Azuine RE, Siahpush M. Widening Socioeconomic, Racial, and Geographic Disparities in HIV/AIDS Mortality in the United States, 1987–2011. Adv Prev Med. 2013;2013:657961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campanelli CM. American Geriatrics Society Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults: The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. Journal of the American Geriatrics Society. 2012;60(4):616–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pugh MJ, Palmer RF, Parchman ML, et al. Association of suboptimal prescribing and change in lower extremity physical function over time. Gerontology. 2007;53(6):445–453. [DOI] [PubMed] [Google Scholar]
- 48.Lau DT, Mercaldo ND, Shega JW, et al. Functional decline associated with polypharmacy and potentially inappropriate medications in community-dwelling older adults with dementia. Am J Alzheimers Dis Other Demen. 2011;26(8):606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holtzman C, Armon C, Tedaldi E, et al. Polypharmacy and risk of antiretroviral drug interactions among the aging HIV-infected population. J Gen Intern Med. 2013;28(10):1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]