Abstract
Single-walled carbon nanotubes (SWCNTs) are a class of one-dimensional nanomaterials that exhibit extraordinary electrical and optical properties. However, many of the fundamental studies and practical applications are stymied by sample polydispersity. SWCNTs are synthesized in bulk with broad structural (chirality) and geometrical (length and diameter) distributions; problematically, all known post-synthetic sorting methods rely on ultrasonication, which cuts SWCNTs into short segments (typically <1 μm). Here we demonstrate that ultralong (>10 μm) SWCNTs can be efficiently separated from the shorter ones through a solution-phase “self-sorting”. We show that thin film transistors fabricated from long semiconducting SWCNTs exhibit a carrier mobility as high as ~90 cm2·V−1·s−1, which is ~10 times higher than the shorter counterparts and well exceeding other known materials such as organic semiconducting polymers (<1 cm2·V−1·s−1), amorphous silicon (~1 cm2·V−1·s−1) and nanocrystalline silicon (~50 cm2·V−1·s−1). Mechanistic studies suggest that this self-sorting relies on the equilibrium between isotropic and liquid crystalline SWCNT phases, which is driven by the solution phase behavior above the cloud point of the isotropic phase, and inversely scales with the SWCNT length. This length-dependent self-sorting technique opens a path to attain the long-sought ultralong, electronically pure carbon nanotube materials through scalable solution processing.
Keywords: Superacid-surfactant exchange, ultralong carbon nanotubes, nanomaterials processing, carrier mobility, thin-film transistor, phase behavior
Graphical Abstract
ToC Text
Ultralong carbon nanotubes self-sort from the short ones in a superacid-surfactant exchange process. This “self-sorting” effect is driven by the length-dependent phase behaviors of rigid rods in solution. The attained nanotubes, with the longest approaching 13 μm, exhibit a carrier mobility as high as ~90 cm2·V−1·s−1 in thin-film transistors, which is ~10 times higher than the shorter counterparts and approaches that of the polycrystalline silicon.
Single-walled carbon nanotubes (SWCNTs) are one-dimensional nanomaterials that feature extraordinary optical,[1, 2] electronic,[3–5] and mechanical properties[4, 6] depending on chirality and length. The last decade has witnessed significant progress in post-synthetic sorting techniques for separating SWCNTs based on their chiralities and electronic types.[7–10] In addition to chirality, nanotube length is another critical factor that governs the properties of SWCNTs and hence the performance of SWCNT-based devices. While short and ultrashort SWCNTs are preferable for bioimaging and drug delivery purposes,[11, 12] electrical, mechanical, and many other optical applications require long nanotubes. For electronic applications, when assembled into macroscale objects, short tubes lead to high resistance, low on-currents, and low carrier mobility.[13] The mechanical properties of macroscale aligned objects are determined by inter-nanotube friction, which scales with CNT length and limits the tensile strength far below that of an individual tube.[14, 15] Optically, short nanotubes typically fluoresce less brightly than long nanotubes due to the quenching of excitons at the nanotube ends. In extreme scenarios, unfunctionalized ultrashort nanotubes with lengths of less than ~100 nm completely lose their intrinsic fluorescence.[16, 17] To this end, sorting SWCNT mixtures by length is an important step to harness the full potential of electrical, mechanical, and optical properties of these remarkable nanomaterials.
Several length sorting techniques including size exclusion chromatography,[18] density gradient ultracentrifugation,[19, 20] cross-flow filtration,[21] and polymer precipitation[22] have been reported. However, the sorted nanotubes are still short due to the ultrasonication at the initial sample preparation step, which cuts long SWCNTs into smaller pieces. The longest portion attained thus far has an average length (Lavg) less than ~1.5 μm[20] and a considerable population (~35% to ~75%) are short nanotubes (<500 nm).[18, 21] Moreover, all of these length sorting methods have relatively low throughput. Therefore, it remains an unmet challenge to obtain long SWCNTs in a scalable manner and study their collective properties.
In this work, we demonstrate that the sorting of ultralong nanotubes (>10 μm) can be realized by “self-sorting” in aqueous solutions. In contrast to other reported mechanisms, which all rely on SWCNT interactions with other media, such as gels,[18, 23] density gradients,[19, 20] porous membranes,[21] or polymers,[22] the separation here occurs solely due to the length-dependent interactions between the SWCNTs themselves. This process is highly scalable, yielding a significant fraction (~10% based on atomic force microscopy measurements after the 6th sorting cycle) of ultralong (>10 μm) nanotubes featuring a narrow distribution. Importantly, the sorted SWCNTs are individually stabilized in water without additives, such as density gradient media, making them well compatible with established aqueous-based SWCNT sorting (e.g., by diameter, electronic type, or chirality) and other processing methods, allowing us to produce aligned thin films from long SWCNTs with high semiconducting purity (> 99%). We further demonstrate that thin film transistors (TFTs) fabricated from these films exhibit a current ON/OFF ratio of more than 1000 with the carrier mobility exceeding 90 cm2·V−1·s−1, which is ~10 times better than short tube controls, exceeding the conventional TFT materials such as organic semiconducting polymers (<1 cm2·V−1·s−1),[24] nanocrystalline silicon (~50 cm2·V−1·s−1)[25] and approaching that of polycrystalline silicon TFTs (~100 cm2·V−1·s−1).[26, 27]
We start this self-sorting process by dissolving raw SWCNT materials into chlorosulfonic acid (CSA, a superacid), followed by adding the SWCNT/CSA dispersion into an aqueous solution containing sodium deoxycholate (DOC) as the surfactant and sodium hydroxide (NaOH; see the Experimental Section for details). The aqueous dispersion is then centrifuged to separate bundled nanotubes from those individually dispersed in the supernatant.
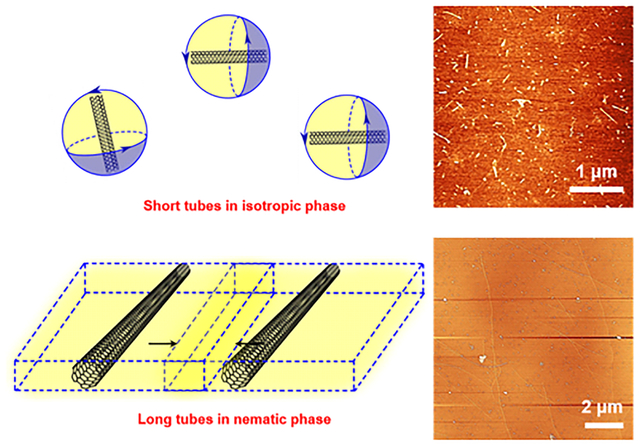
SWCNTs spontaneously dissolve in CSA to form an optically homogenous solution at high concentrations (~0.61 vol%, or ~8.8 mg/mL).[28] In this superacid, SWCNTs behave as a weak base that can be protonated. The protonation induces Coulombic repulsion between nanotubes, which overcomes the strong tube-tube van der Waals interaction, allowing the SWCNTs to individually dissolve in the superacid to form a thermodynamically stable solution.[28] Unlike many other acids such as nitric acid,[29, 30] nitric/sulfuric acid mixture[31] or hydrogen peroxide/sulfuric acid mixture,[32] which can oxidize SWCNTs, chlorosulfonic acid is a true solvent that dissolves SWCNTs without oxidation.[28, 33] The sidewall protonation effect is completely reversible and the structures of SWCNTs can be fully recovered as evidenced by spectroscopy studies.[28, 33, 34] Once dissolved in CSA, the SWCNTs behave like rigid rods with ultrahigh aspect ratios (length/diameter, L/d). The phase behavior for rods of monodisperse length was first described by Onsager[35] and Flory,[36] then extended to polydisperse rods by Wensink and Vroege,[37] and finally applied to SWCNTs by Green.[38] These theories have been shown to well describe the SWCNTs dissolved in CSA, which are polydisperse in aspect ratio and exhibit distinct phase behavior at different concentrations.[28, 39, 40] At sufficiently low SWCNT concentration, the solutions are isotropic and all the SWCNTs freely rotate as Brownian rods. Increasing the SWCNT concentration beyond a critical value (the isotropic cloud point, φiso) leads to the formation of a nematic liquid crystalline phase that coexists within an isotropic phase. This transition into a biphasic system occurs as the SWCNTs sacrifice rotational entropy to maximize translational entropy. Further increasing the concentration beyond the nematic point (φnem) causes all of the SWCNTs to transit into a fully liquid crystalline nematic phase.
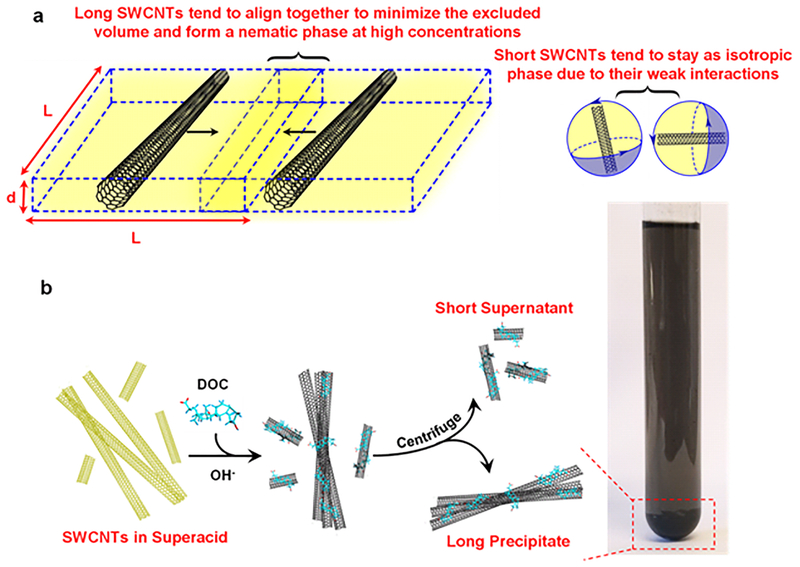
Both φiso and φnem are linearly related to the inverse aspect ratio of the rods (here φiso = 3.29(L/d)−1 and φnem = 4.19(L/d)−1) and these scaling laws have been applied to polydisperse CNT solutions[38] and confirmed experimentally.[41] Indeed, the polydispersity in aspect ratio of the SWCNTs leads to a self-partitioning of the longest SWCNTs into the nematic phase while the shortest nanotubes remain in the isotropic phase (Figure 1a). We have previously demonstrated that the CSA molecules on SWCNTs can be spontaneously replaced by DOC molecules in a straightforward superacid-surfactant exchange (S2E) process to produce surfactant-stabilized individual SWCNTs.[34, 42] In this context, we hypothesize that during S2E, the individual SWCNTs in the isotropic phase are maintained far apart from each other such that the DOC molecules can diffuse to their surfaces before re-bundling can occur as the CSA is neutralized. On the other hand, the SWCNTs in the nematic phase readily bundle and precipitate before DOC can stabilize them individually in the aqueous dispersion. Therefore, by tuning the SWCNT concentration, it should be possible to preferentially bundle long nanotubes during S2E because long CNTs concentrate in the liquid crystalline phase due to their higher excluded volume, and once bundled, they precipitate during centrifugation, leaving short SWCNTs individually dissolved in the aqueous solution (Figure 1b).
Figure 1. Schematic of the self-sorting process of ultralong SWCNTs.
a) Long SWCNTs dissolved in CSA tend to align together to minimize the excluded volume (V) and form a nematic phase. The excluded volume scales with dL2, where d and L are the SWCNT diameter and length respectively (). Short SWCNTs in superacid tend to stay in an isotropic phase due to their limited interactions. b) The SWCNTs are dissolved in superacid and subsequently neutralized with NaOH and DOC to produce short, individual nanotubes that are stabilized by surfactant in the aqueous solution. Meanwhile, long carbon nanotubes bundle and form precipitates, which can be separated from the dispersion by centrifugation.
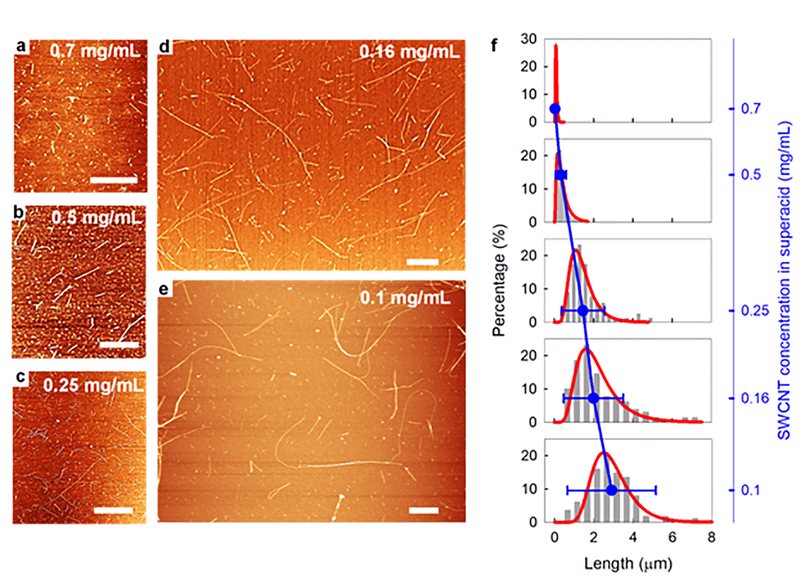
We first studied the impact of SWCNT/CSA concentration on S2E to understand how it could potentially be employed to separate the nanotubes by length. Atomic force microscopy (AFM) was used to characterize the length distribution of the DOC-stabilized SWCNTs in the aqueous supernatant after centrifugation (see the Experimental Section for details). Figure 2a–e shows representative AFM topographic images of the SWCNTs as a function of the initial nanotube concentration in CSA. At a high concentration of ~0.7 mg/mL, only short SWCNTs with an Lavg of ~0.1 μm were found in the supernatant. As the concentration decreases, we began to observe longer nanotubes in the supernatant, and at a dilute concentration of ~0.1 mg/mL, a maximum Lavg of ~3.1 μm was reached. Additionally, the height profiles of these AFM images (Figure S1) unambiguously demonstrate that all the samples consist mainly of individual SWCNTs with diameters ranging from ~0.6 to 1.5 nm, indicating that SWCNTs are separated by length rather than the diameter or bundle size.
Figure 2. ength separation occurs effectively at different nanotube concentrations.
L a-e) Representative AFM images and f) corresponding length distributions (grey bars) of individual DOC-stabilized SWCNTs from decreasing SWCNT-CSA solution concentrations of ~0.70, ~0.50, ~0.25, ~0.16, and ~0.10 mg/mL, respectively. All scale bars are 1 μm. The distributions are fitted using log-normal functions (red lines). The average length of the DOC-stabilized SWCNTs increases as the concentration of SWCNTs in CSA decreases, as indicated by the blue line.
Analogous to the molecular weight polydispersity index in polymer physics, we calculated the length polydispersity index (LPDI) as a ratio of the number-averaged lengths (Lavg) and length average lengths (Ll) to quantify the length distribution (eq 1–3)[41]:
| eq 1 |
| eq 2 |
| eq 3 |
LPDI is a unitless coefficient that enables the direct comparison of the length distribution of SWCNT samples regardless of their different Lavg values. We compiled the Lavg, Ll, and LPDI values of DOC-stabilized SWCNTs from different initial CSA concentrations in Table 1. A clear trend of increasing Lavg was observed as the SWCNT concentration in CSA decreases (Figure 2f). In addition, LPDI increased from 1.27 to 1.58, indicating that as the concentration of SWCNTs in CSA decreases, nanotubes of a wider range of lengths are stabilized as individual nanotubes during the S2E process.
Table 1.
Correlation of Lavg, Ln, and LPDI of SWCNTs stabilized in the aqueous phase by S2E with the SWCNT concentration in CSA.
| SWCNT concentration in CSA (mg/mL) | |||||
|---|---|---|---|---|---|
| ~0.70 | ~0.50 | ~0.25 | ~0.16 | ~0.10 | |
| Lavg (μm) | 0.11 | 0.41 | 1.53 | 2.13 | 3.10 |
| Ln (μm) | 0.14 | 0.59 | 2.29 | 3.28 | 4.84 |
| LPDI | 1.27 | 1.44 | 1.50 | 1.54 | 1.58 |
The strong inverse correlation between SWCNT/CSA concentration with the Lavg of DOC-stabilized SWCNTs motivated us to selectively remove the shorter nanotubes by lowering the initial concentrations stepwise. Through repeated application of this self-sorting process, followed by separation of the bundled (longer nanotube) component, and subsequent re-dissolution of this precipitate in the CSA to repeat the process at a lower concentration, we were able to successively reduce the population of shorter nanotubes in the precipitated material (Figure S2). SWCNTs were first dispersed at a high concentration (~0.7 mg/mL) so that only the shortest nanotubes were extracted from the bulk materials by the S2E process (defined as 1 cycle). The precipitates were then collected and re-dissolved into the CSA at a lower concentration (see the Experimental Section). Applying the same S2E process again, a portion of slightly longer SWCNTs were removed (Figure S3), further increasing the average length of the separated nanotubes. Since the overall length of the SWCNTs can be maintained throughout this iterative process, short nanotubes can be extracted repeatedly until a satisfactory long nanotube length is achieved.
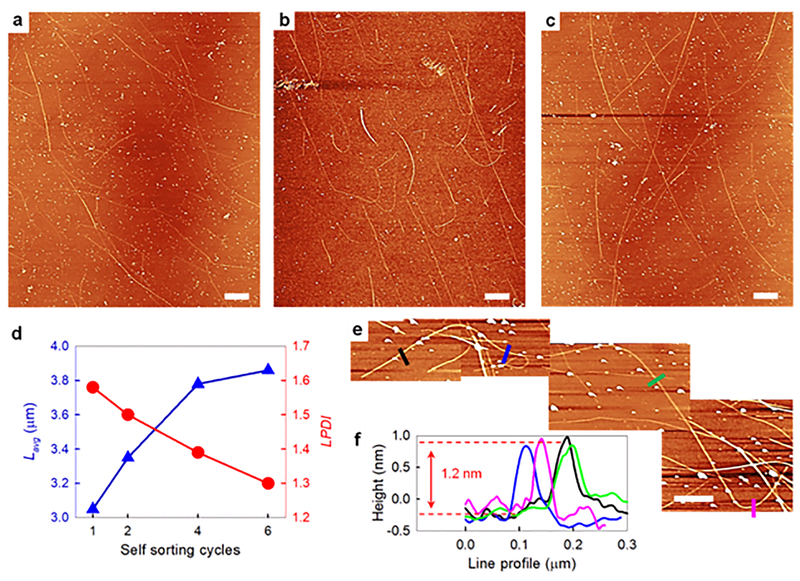
Figure 3a–c shows representative AFM images of the sorted long tubes from the 2nd, 4th and 6th cycles of self-sorting. Note that the corresponding height profiles can be found in Figure S4. The removal of short nanotubes is clearly evident by the absence of nanotubes <1 μm in length. Moreover, the length distribution becomes progressively narrower (with a LPDI decreasing from 1.58 in the starting material to 1.30, approaching the ideal limit of 1) while the corresponding Lavg values increasing from an initial value of ~3.1 μm to ~3.3 μm after the 2nd cycle, and eventually to ~3.8 μm after the 6th cycle (Figure 3d). We note that a significant amount of ultralong nanotubes (>10 μm) can be easily found after 4 cycles. Figure 3e shows tiled AFM topography images of a ~12 μm ultralong SWCNT. Analysis of the AFM height profile unambiguously confirmed that it is an individual nanotube with a diameter of ~1.2 nm (Figure 3f). AFM images of additional ultralong SWCNTs (>10 μm) can be found in Figure S5.
Figure 3. Ultralong SWCNTs from different iterations of the self-sorting process.
a-c) Representative AFM images of individual, long SWCNTs separated after the 2nd, 4th, and 6th iterative S2E cycles. All scale bars are 1 μm. d) The Lavg and LPDI of the extracted DOC-stabilized SWCNTs as a function of the self-sorting cycles. e) Tiled AFM images of a 12 μm long SWCNT. Scale bar: 1 μm. f) Height profiles extracted along the nanotube, as marked in (e) by the black, blue, green, and purple lines.
We further confirmed the concentration-dependent phase behaviors by cross-polarized microscopy (Figure 4a). Clearly, as the SWCNT concentration in the CSA decreases from ~0.7 mg/mL to ~0.1 mg/mL, the relative proportions of the nematic and isotropic phases changes, and the birefringent liquid-crystalline structures disappear completely at φiso ~0.1 mg/mL. When SWCNT samples with polydisperse length are dissolved in the CSA at a sufficiently high concentration, shorter nanotubes stay as isotropic individual rigid rods while longer nanotubes assemble into nematic ordered phases (Figure 4b). Therefore, when neutralizing a SWCNT/CSA solution at a concentration above φiso, only short nanotubes that stay as isotropic individual rods can be effectively converted into DOC-stabilized SWCNTs by S2E. As the concentration of SWCNTs in CSA decreases, longer SWCNTs partition in the isotropic phase, and eventually with the same S2E procedure, they can be “captured” by the surfactant molecules and stabilized as individual nanotubes in aqueous solution. The fitting between Lavg with SWCNT concentration in CSA (c) yield an exponential relationship of , which closely follows the prediction from Onsager’s Theory (, Figure S6). We note the Lavg of the pristine sample measured by capillary thinning extensional viscosity[41] is ~4.5 μm, which is ~45% longer than the Lavg of ~3.1 μm for the DOC-stabilized SWCNTs before length sorting (see the Experimental Section for details). This discrepancy may be simply due to the approximations intrinsic in the extensional viscosity length determination, or may indicate that even at the optically dilute isotropic regime of ~0.1 mg/mL, ultralong tubes (>20 μm for instance) are still not well-stabilized by DOC molecules during the S2E process. Lowering the concentration of SWCNTs further in the CSA may allow the attainment of even longer SWCNTs by this self-sorting process.
Figure 4. Proposed mechanism of the length dependent SWCNT self-sorting effect.
a) Polarized light microscopy shows the solution morphology of SWCNTs at different CSA concentrations. The cross arrows indicate the relative orientation between the polarizer and analyzer. Scale bars: 200 μm. b) Phase behavior of SWCNTs in CSA solvent as a function of SWCNT length and concentration. The correlation of φiso and φnem with polydispersed SWCNT length are plotted according to the Onsager theory (axes are plotted in logarithmic scale). Above φiso the short tubes are selectively extracted by S2E while long tubes collapse into bundles.
Although our experiments unambiguously show that the observed “self-sorting” phenomena originate from nanotube phase separation in the CSA, directly centrifuging the SWCNT/CSA solution to separate the long nanotubes from the short ones does not work because the isotropic and nematic phases occur at very similar concentrations (about a factor of 1.5). Hence, the density difference and interfacial tension between the two phases are expected to be minimal. As a result, the small density difference would make it difficult to separate SWCNTs of different length at low centrifugal force, and the low interfacial tension would allow the two phases to mix during centrifugation.
It is also interesting to note that the quality of the acid is another factor that can influence the length of the DOC-stabilized SWCNT fractions. By adding just 10% (by volume) of 98% sulfuric acid into the SWCNT/CSA solution to reduce the overall acidity of the system, we observed a significant reduction of the length of the DOC-stabilized SWCNTs after S2E (Figure S7). This observation is related to the previous studies showing that a reduction of the acidity of the solvent can effectively decrease the surface charges of the SWCNTs and downshift the φiso.[28] Decreased surface charges can be translated into reduced repulsive forces between nanotubes, which decreases the length cutoff for CNTs that transition into the nematic phase.
Because our long sorted S2E-SWCNTs are suspended in water, we can further purify the materials according to their electronic properties. We demonstrate this possibility by using the well-established aqueous two-phase (ATP) extraction method[43] with minimum modifications. An ATP system is comprised of two polymer-modified immiscible aqueous phases with slightly different hydrophobicities, which can spontaneously partition SWCNTs according to their electronic types and chirality.[10] Figure 5a shows a photo of three sorted fractions of long nanotubes with different chirality distributions, as indicated by the different colors of the solutions and their distinct UV-vis-NIR absorption spectra (Figure 5b). From the left to right in Figure 5a, the three solutions manifest different electronic properties and are categorized as metallic SWCNTs, large-diameter semiconducting SWCNTs, and small-diameter semiconducting SWCNTs. Photoluminescence (PL) microscopy was used to confirm the long length of the sorted semiconducting SWCNTs enriched fraction (Figure 5c). We also used resonance Raman spectroscopy with different excitation lasers to further validate the enrichment of SWCNTs with specific electronic types after multiple cycles of ATP extraction (Figure S8).
Figure 5. Sorting ultralong SWCNTs with specific electrical properties and their applications in TFTs.
a) A photograph of aqueous solutions of chirality-sorted SWCNTs with increasing bandgaps by ATP. b) UV-vis-NIR absorption spectra of the 3 solutions shown in (a). c) A broadband (900–1600 nm) PL image of the sorted semiconducting DOC-stabilized SWCNTs. d-f) False-color SEM images of thin films fabricated from (d) random, long semiconducting SWCNTs, (e) aligned, long semiconducting SWCNTs, and (f) random, short semiconducting SWCNTs. All scale bars are 500 nm. g) Electrical transport characteristic curves and h) mobility vs. the current ON/OFF ratio of thin film TFTs fabricated from the random, long semiconducting SWCNTs (red, d), aligned, long semiconducting SWCNTs (cyan, e), and random, short semiconducting SWCNTs (grey, f).
To understand the influence of nanotube length and alignment on their macroscale electrical performances, we fabricated thin-film transistors from large-diameter semiconducting SWCNTs with different lengths and alignment, including aligned long semiconducting SWCNTs, random long semiconducting SWCNTs, and random short semiconducting SWCNTs (Figure 5d–f and Figure S9), in a back-gated configuration. Electrical transport characteristic curves and the plot of mobility vs. the current ON/OFF ratio of TFTs fabricated from different SWCNT films are shown in Figure 5g and Figure 5h, respectively. All TFTs showed similar p-type semiconducting behaviors with all ON/OFF ratios larger than 1000. However, TFTs fabricated from long aligned semiconducting SWCNTs show the highest mobility up to ~91 cm2·V−1·s−1 (average ~61.0 ± 15.6 cm2·V−1·s−1), while long random semiconducting SWCNTs and short random semiconducting SWCNTs exhibited much lower average mobilities of 30.1 ± 3.7 cm2·V−1·s−1 and 10.7 ± 2.4 cm2·V−1·s−1, respectively. To the best of our knowledge, the mobility from the long aligned semiconducting SWCNTs are among the highest values reported for SWCNT-based TFTs and is significantly higher than transistor devices made from soluble organic semiconductors (<1 cm2·V−1·s−1),[24] amorphous Si (~1 cm2·V−1·s−1) and nanocrystalline Si (~50 cm2·V−1·s−1),[25] even approaching that of p-type Si TFTs (~100 cm2·V−1·s−1).[26, 27] The relatively low ON/OFF ratios (~1000–4000) of our multilayer thin-film devices can be explained by the charge screening effect, which is typically observed for relatively thick films of semiconducting nanotubes. Generally, the back-gate potential only has good control on the SWCNT-layer that is in contact with the dielectric. The charge carriers generated on this contact layer can screen the overlying SWCNT-layers, making it difficult for them to be fully turned off, resulting in higher off currents and lower ON/OFF ratios.[44, 45] This charge screen effect can be circumvented by decreasing the film thickness. We therefore fabricated TFTs comprising a sub-monolayer of long random semiconducting SWCNTs. The ON/OFF ratio increased up to ~22000 while the mobility remained at ~50 cm2·V−1·s−1. The output characteristic curves exhibit strong field-effect behaviors (Id is linear under small Vd and saturated under large Vd), further indicating the electronic purity of our long semiconducting SWCNTs (Figure S10). Strategies for further improving the ON/OFF ratio include using SWCNTs with larger bandgaps,[44] using single-chirality semiconducting SWCNTs[46] or using high-κ dielectric materials.[45] Our study here shows that with long semiconducting SWCNTs as the channel material for long-channel TFTs, the mobilities of charge carriers can be improved by more than 10 times, demonstrating the significant influence of SWCNT-length on the performance of SWCNT-based TFTs.
In conclusion, we demonstrate that ultralong SWCNTs can be self-sorted by exploiting their length-dependent phase partitioning in solution, which depends on nanotube length. We obtained an Lavg ~3.8 μm with a narrow length distribution (LPDI = 1.30), with a significant fraction of ultralong SWCNTs longer than 10 μm. We further sorted SWCNTs with different lengths into semiconducting and metallic fractions using aqueous two-phase extraction. TFTs fabricated from the long semiconducting nanotubes show ON/OFF ratios greater than 1000 and a significant improvement of carrier mobilities up to ~90 cm2·V−1·s−1, well-exceeding those of short semiconducting SWCNTs, organic semiconducting polymers or polycrystalline silicon. Our approach provides a pathway to attain ultralong SWCNTs with defined electrical properties, opening opportunities to significantly improve the transport properties and performance of SWCNT-based devices, such as TFTs and sensors.
Supplementary Material
Acknowledgements
This work is not directly supported by a grant. However, we wish to acknowledge the University of Maryland and federal funding agencies for providing student supports to B.B. and C.Z. (NIH/NIGMS R01GM114167), and H.Q., who was supported by the Center for Enhanced Nanofluidic Transport (CENT), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under Award # DE-SC0019112). R.J.H. and M.P. were supported by the Air Force Office of Scientific Research (AFOSR) grant FA9550–15-01-0370, the Robert A. Welch Foundation grant C-1668, and the Department of Energy (DOE) award DE-EE0007865. AFM images were obtained using a shared system supported by the NSF MRI program (CHE1626288). We thank Dr. Ming Zheng from NIST for valuable suggestions on ATP sorting. P.W. gratefully acknowledges the Millard and Lee Alexander Fellowship in Chemistry from the University of Maryland.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Supporting Information
Supporting information is available from the Wiley Online Library or from the author.
References:
- [1].O’connell MJ, Bachilo SM, Huffman CB, Moore VC, Strano MS, Haroz EH, Rialon KL, Boul PJ, Noon WH, Kittrell C, Ma J, Hauge RH, Weisman RB, Smalley RE, Science 2002, 297, 593. [DOI] [PubMed] [Google Scholar]
- [2].Zhang XA, Yu S, Xu B, Li M, Peng Z, Wang Y, Deng S, Wu X, Wu Z, Ouyang M, Wang YH, Science 2019, 363, 619. [DOI] [PubMed] [Google Scholar]
- [3].Wu Z, Chen Z, Du X, Logan JM, Sippel J, Nikolou M, Kamaras K, Reynolds JR, Tanner DB, Hebard AF, Rinzler AG, Science 2004, 305, 1273. [DOI] [PubMed] [Google Scholar]
- [4].Behabtu N, Young CC, Tsentalovich DE, Kleinerman O, Wang X, Ma AW, Bengio EA, ter Waarbeek RF, de Jong JJ, Hoogerwerf RE, Fairchild SB, Ferguson JB, Marayama B, Kono J, Talmon Y, Cohen Y, Otto MJ, Pasquali M, Science 2013, 339, 182. [DOI] [PubMed] [Google Scholar]
- [5].Javey A, Guo J, Wang Q, Lundstrom M, Dai H, Nature 2003, 424, 654. [DOI] [PubMed] [Google Scholar]
- [6].Bai Y, Zhang R, Ye X, Zhu Z, Xie H, Shen B, Cai D, Liu B, Zhang C, Jia Z, Zhang S, Li X, Wei F, Nat. Nanotechnol 2018, 13, 589. [DOI] [PubMed] [Google Scholar]
- [7].Arnold MS, Green AA, Hulvat JF, Stupp SI, Hersam MC, Nat. Nanotechnol 2006, 1, 60. [DOI] [PubMed] [Google Scholar]
- [8].Tu X, Manohar S, Jagota A, Zheng M, Nature 2009, 460, 250. [DOI] [PubMed] [Google Scholar]
- [9].Liu H, Nishide D, Tanaka T, Kataura H, Nat. Commun 2011, 2, 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Khripin CY, Fagan JA, Zheng M, J. Am. Chem. Soc 2013, 135, 6822. [DOI] [PubMed] [Google Scholar]
- [11].Kostarelos K, Nat. Biotechnol 2008, 26, 774. [DOI] [PubMed] [Google Scholar]
- [12].Kolosnjaj-Tabi J, Hartman KB, Boudjemaa S, Ananta JS, Morgant G, Szwarc H, Wilson LJ, Moussa F, ACS Nano 2010, 4, 1481. [DOI] [PubMed] [Google Scholar]
- [13].Hecht DS, Hu L, Irvin G, Adv. Mater 2011, 23, 1482. [DOI] [PubMed] [Google Scholar]
- [14].Tsentalovich DE, Headrick RJ, Mirri F, Hao J, Behabtu N, Young CC, Pasquali M, ACS Appl. Mater. Interfaces 2017, 9, 36189. [DOI] [PubMed] [Google Scholar]
- [15].Headrick RJ, Tsentalovich DE, Berdegué J, Bengio EA, Liberman L, Kleinerman O, Lucas MS, Talmon Y, Pasquali M, Adv. Mater 2018, 30, 1704482. [DOI] [PubMed] [Google Scholar]
- [16].Kamalasanan K, Gottardi R, Tan S, Chen Y, Godugu B, Rothstein S, Balazs AC, Star A, Little SR, Angew. Chem. Int. Ed 2013, 52, 11308. [DOI] [PubMed] [Google Scholar]
- [17].Danne N, Kim M, Godin AG, Kwon H, Gao Z, Wu X, Hartmann NF, Doorn SK, Lounis B, Wang Y, Cognet L, ACS Nano 2018, 12, 6059. [DOI] [PubMed] [Google Scholar]
- [18].Khripin CY, Tu X, Heddleston JM, Silvera-Batista C, Hight Walker AR, Fagan J, Zheng M, Anal. Chem 2013, 85, 1382. [DOI] [PubMed] [Google Scholar]
- [19].Fagan JA, Becker ML, Chun J, Hobbie EK, Adv. Mater 2008, 20, 1609. [Google Scholar]
- [20].Fagan JA, Becker ML, Chun J, Nie P, Bauer BJ, Simpson JR, Hight-Walker A, Hobbie EK, Langmuir 2008, 24, 13880. [DOI] [PubMed] [Google Scholar]
- [21].Ohmori S, Saito T, Shukla B, Yumura M, Iijima S, ACS Nano 2010, 4, 3606. [DOI] [PubMed] [Google Scholar]
- [22].Gui H, Chen H, Khripin CY, Liu B, Fagan JA, Zhou C, Zheng M, Nanoscale 2016, 8, 3467. [DOI] [PubMed] [Google Scholar]
- [23].Miyata Y, Shiozawa K, Asada Y, Ohno Y, Kitaura R, Mizutani T, Shinohara H, Nano Res 2011, 4, 963. [Google Scholar]
- [24].Allard S, Forster M, Souharce B, Thiem H, Scherf U, Angew. Chem. Int. Ed 2008, 47, 4070. [DOI] [PubMed] [Google Scholar]
- [25].Sun Y, Rogers JA, Adv. Mater 2007, 19, 1897. [Google Scholar]
- [26].Franklin AD, Science 2015, 349, aab2750. [DOI] [PubMed] [Google Scholar]
- [27].Street RA, Adv. Mater 2009, 21, 2007. [Google Scholar]
- [28].Davis VA, Parra-Vasquez ANG, Green MJ, Rai PK, Behabtu N, Prieto V, Booker RD, Schmidt J, Kesselman E, Zhou W, Fan H, Adams WW, Hauge RH, Fischer JE, Cohen Y, Talmon Y, Smalley RE, Pasquali M, Nat. Nanotechnol 2009, 4, 830. [DOI] [PubMed] [Google Scholar]
- [29].Hu H, Zhao B, Itkis ME, Haddon RC, J. Phys. Chem. B 2003, 107, 13838. [Google Scholar]
- [30].Tchoul MN, Ford WT, Lolli G, Resasco DE, Arepalli S, Chem. Mater 2007, 19, 5765. [Google Scholar]
- [31].Karousis N, Tagmatarchis N, Tasis D, Chem. Rev 2010, 110, 5366. [DOI] [PubMed] [Google Scholar]
- [32].Ziegler KJ, Gu Z, Peng H, Flor EL, Hauge RH, Smalley RE, J. Am. Chem. Soc 2005, 127, 1541. [DOI] [PubMed] [Google Scholar]
- [33].Parra-Vasquez ANG, Behabtu N, Green MJ, Pint CL, Young CC, Schmidt J, Kesselman E, Goyal A, Ajayan PM, Cohen Y, Talmon Y, Hauge RH, Pasquali M, ACS Nano 2010, 4, 3969. [DOI] [PubMed] [Google Scholar]
- [34].Wang P, Kim M, Peng Z, Sun C-F, Mok J, Lieberman A, Wang Y, ACS Nano 2017, 11, 9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Onsager L, Ann. N. Y. Acad. Sci 1949, 51, 627. [Google Scholar]
- [36].Flory PJ, Proc. R. Soc. Lond. A 1956, 234, 73. [Google Scholar]
- [37].Wensink H, Vroege G, J. Chem. Phys 2003, 119, 6868. [Google Scholar]
- [38].Green MJ, Parra-Vasquez ANG, Behabtu N, Pasquali M, J. Chem. Phys 2009, 131, 084901. [DOI] [PubMed] [Google Scholar]
- [39].Ramesh S, Ericson LM, Davis VA, Saini RK, Kittrell C, Pasquali M, Billups W, Adams WW, Hauge RH, Smalley RE, J. Phys. Chem. B 2004, 108, 8794. [Google Scholar]
- [40].Davis VA, Ericson LM, Parra-Vasquez ANG, Fan H, Wang Y, Prieto V, Longoria JA, Ramesh S, Saini RK, Kittrell C, Macromolecules 2004, 37, 154. [Google Scholar]
- [41].Tsentalovich DE, Ma AW, Lee JA, Behabtu N, Bengio EA, Choi A, Hao J, Luo Y, Headrick RJ, Green MJ, Talmon Y, Pasquali M, Macromolecules 2016, 49, 681. [Google Scholar]
- [42].Wang P, Peng Z, Li M, Wang Y, Small 2018, 14, 1802625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fagan JA, Hároz EH, Ihly R, Gui H, Blackburn JL, Simpson JR, Lam S, Hight Walker AR, Doorn SK, Zheng M, ACS Nano 2015, 9, 5377. [DOI] [PubMed] [Google Scholar]
- [44].Chortos A, Pochorovski I, Lin P, Pitner G, Yan X, Gao TZ, To JW, Lei T, Will III JW, Wong H-SP, Bao Z, ACS Nano 2017, 11, 5660. [DOI] [PubMed] [Google Scholar]
- [45].Sangwan VK, Ortiz RP, Alaboson JM, Emery JD, Bedzyk MJ, Lauhon LJ, Marks TJ, Hersam MC, ACS Nano 2012, 6, 7480. [DOI] [PubMed] [Google Scholar]
- [46].Zhang L, Tu X, Welsher K, Wang X, Zheng M, Dai H, J. Am. Chem. Soc 2009, 131, 2454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.