Abstract
Plexin D1 belongs to a family of transmembrane proteins called plexins. It was characterized as a receptor for semaphorins and is known to be essential for axonal guidance and vascular patterning. Mutations in Plexin D1 have been implicated in pathologic conditions such as truncus arteriosus and Möbius syndrome. Emerging data show that expression of Plexin D1 is deregulated in several cancers; it can support tumor development by aiding tumor angiogenesis and metastasis; and conversely, it can act as a dependence receptor and stimulate cell death in the absence of its canonical ligand, semaphorin 3E. The role of Plexin D1 in tumor development and progression is thereby garnering research interest for its potential as a biomarker and as a therapeutic target. In this review, we describe its discovery, structure, mutations, role in cancer, and therapeutic potential.
Keywords: angiogenesis, cancer, metastasis, plexin, Plexin D1
1.0. Discovery
Takagi and colleagues [1] discovered plexins in 1987 in the Xenopus laevis tadpole model. The authors were screening molecules that were helping to regulate neuronal interactions between the retinal axons and the optic tectum by using monoclonal antibodies (MAb). The optic tectum of an amphibian has a laminar structure with 9 defined layers. One MAb, MAb-B2, preferentially bound to the plexiform layers in the deeper part of the optic tectum. Molecular cloning indicated that the antigen for MAb-B2, a peptide with a molecular weight of about 200–220 kDa, was a new type-one membrane glycoprotein [2]. This new calcium-dependent, single-pass membrane protein was renamed plexin [3] in accordance with its preferential binding of retinal plexiform layers [4]. In 1996, Maestrini and colleagues [5] identified the human plexins. In 1999, Tamagnone et al. [6] reported 4 classes of plexins in vertebrates: A to D. They showed that human plexins belonging to the subfamilies B and C associated with membrane-bound classes 4 and 7 of semaphorins and postulated that the plexins belonging to the A and D subfamilies would function as receptors for other subclasses of semaphorins [6].
2.0. Structure of Plexin D1
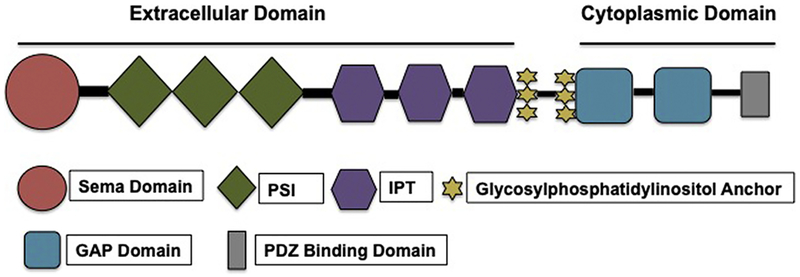
Plexins, type-1 transmembrane proteins, are categorized into 4 subfamilies on the basis of sequence similarity [7]: classes A (A1, A2, A3, and A4), B (B1, B2, and B3), C (C1), and D (D1) [8]. Among the 4 classes of plexins, Plexin D1 is considered the most structurally diverse. Plexin D1 and Plexin C1 have some features in their extracellular domains that are different from the other plexin family members [6]. The structure of Plexin D1 is shown in Figure 1.
Figure 1.
Structure of Plexin D1. GAP indicates GTPase-activating protein; IPT, immunoglobulin-plexin-transcription; PSI, plexin, semaphorin, and integrin; sema, semaphorin.
Plexins are composed of an extracellular segment that typically has 10 domains; 1 membrane-spanning region, which is thought to be helical and conserved; and a cytoplasmic domain that has a GTPase-activating protein (GAP) domain and a Rho GTPase-binding domain insert [7]. Plexins have an N-terminal Sema domain that is trailed by a cysteine-rich plexin, semaphorin, and integrin (PSI) domain and an immunoglobulin-plexin-transcription (IPT) domain [8]. The Sema domain’s β-propeller structure has 7 blades and 2 extensive inserts; the latter is also known as an “extrusion region” [9]. The difference between the Sema domain of plexins and semaphorins is that the Sema domains of plexins do not dimerize [10].
The PSI domains are composed of about 50 amino acid residues and generally have 8 cysteine residues [9]. The structure of Plexin D1 differs from other plexins in having only 6 of the conserved cysteine residues instead of 8 [11]. PSI domains can be positioned between the Sema and IPT domains or between IPT domains. This positioning aids in creating a segment between the comparatively stiff structures that helps appropriately orient the ligand and receptor-binding sites [9]. The IPT domains consist of immunoglobulin folds and share homology with several transcription factors. The immunoglobulin folds are relatively firm, and the loops within them and the linkers in between them provide a favorable binding site. The PSI and IPT domains have been postulated to be essential for receptor activation [9]. Several hypotheses have been advanced regarding an understanding of how plexins are activated: dimerization and GTPase binding, allosteric changes, and association with other proteins. However, no decisive conclusions have been reached [9]. Another hypothesis is that binding of semaphorins causes plexin monomers to form active dimers [12].
The cytoplasmic domains of plexins are highly conserved and can interact with signaling molecules such as p21-activated kinase, Rho family GTPases, and others [8]. The intracellular domains share homology with Ras GAP, and their GTPase-activating ability has been confirmed by several groups [9]. An unusual feature is the insertion of an approximately 200-residue segment in the GAP homologous region. This segment was named the Rho GTPase binding domain after it was found to bind many Rho GTPases in plexin families A and B. Binding of small Rho GTPase to this domain is thought to cause dissociation of the 2 GAP homologous regions and thereby activate the receptor [9]. The Rho GTPase-binding domain structure is similar to that of a ubiquitin fold [9]. All plexins exhibit GAP activity towards Rap GTPases. Many GAPs can stimulate guanosine triphosphate hydrolysis in both Ras and Rap GTPases, and their activities against these 2 substrates could be regulated in different ways. Plexins are thought to be a part of the family of dual-specificity GTPases. The Rap GAP function of plexins is likely activated by the dimerization of the intracellular region [9]. There is a possibility that Plexin D1 sequesters R-Ras [13]. The C terminal of Plexin D1 has a type I PDZ-domain-binding motif, serine-glutamate-alanine [14], that facilitates its binding to proteins such as GIPC [15]
Understanding the structure of Plexin D1 is essential for gaining insight into its different domains and their roles and its binding partners, as well as for understanding how binding occurs and how these interactions are coordinated and regulated. This knowledge would help reveal how Plexin D1 stimulates various signaling pathways and could ultimately lead to new therapeutic targets.
3.0. Mutations of Plexin D1
Mutations in human Plexin D1 have been associated with various pathologic conditions. For example, Ta-Shma et al. [16] reported a homozygous mutation in the PLEXND1 gene that affected a highly conserved residue. The resulting protein had an Argl299Cys change and is thought to have an unstable intracellular region that in turn impairs its anchoring and catalytic activity. This mutation is implicated in truncus arteriosus [16]. Toma-Roca et al. [17] reported that de novo mutations in the gene PLEXND1 were a cause of Möbius syndrome (MBS). Many patients with MBS whom they studied had various developmental abnormalities that occurred in PLEXND1 mutant models. For example, 2 patients had craniofacial bone abnormalities. Craniofacial bone abnormalities have also been reported in other patients with MBS. The authors described 2 de novo mutations in PLEXND1: one affected an arginine residue in the GTPase-activating domain of the protein, and another affected a leucine residue in the IPT domain. Both of these mutations likely resulted in the reported loss of function [17].
4.0. Plexin D1 in Cancer
The expression of plexins is often deregulated in cancer [18]. Typically, the expression of Plexin D1 is low in adult tissues [19] and is thought to be limited to a subset of activated fibroblasts and macrophages [20]. However, in several types of cancer, Plexin D1 is overexpressed in both tumor cells and their vasculature, including pancreatic [21], melanoma [20], ovarian [22], and colon cancer [23]. Thus, Plexin D1 is being considered as a marker for tumor vasculature [24] and is gaining prominence in cancer research [9]. Furthermore, to our knowledge, Plexin D1 is the only protein that is expressed in both tumor vessels and tumor cells [24]. The canonical ligand of Plexin D1 is semaphorin 3E (Sema3E) [14]. Overexpression of Sema3E in pancreatic cancer has been associated with poor survival [25].
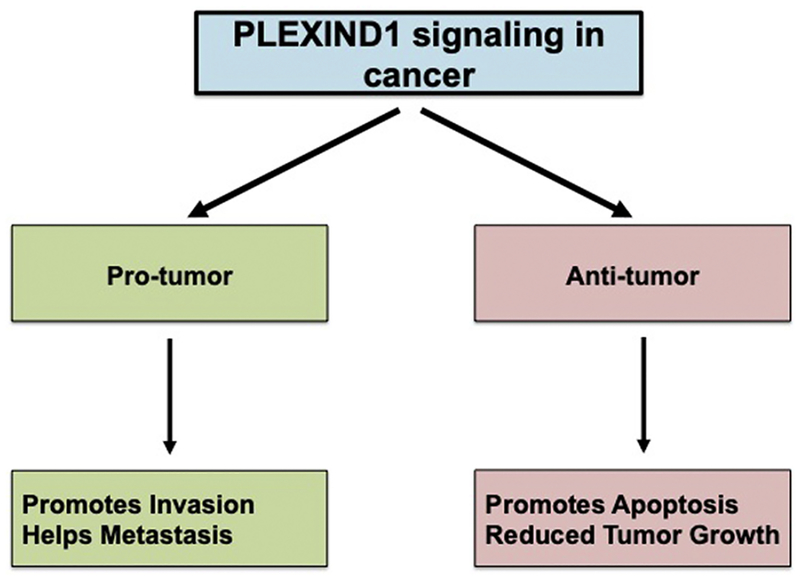
Plexin D1 can act as both a tumor promoter and a tumor suppressor (Figure 2). Plexin D1 has been shown to promote tumor growth in several studies [19, 22, 23]. Casazza et al. [23] reported that in colon cancer, p61-Sema3E/Plexin D1 signaling promoted invasive and metastatic behavior of cancer cells. Tseng et al. [22] reported that Sema3E/Plexin D1 signaling in ovarian endometrioid cancer cells induced epithelial-mesenchymal transition (EMT) through nuclear localization of Snail1 and thereby promoted tumor development by facilitating migration and EMT [22]. In addition, Plexin D1 acted as a transcriptional target in prostate cancer, which was stimulated by Notch signaling, and helped in cancer cell migration and mediated Slug-dependent down regulation of E-cadherin [19]. Furthermore, reduced metastasis, as compared with controls, has been reported for mice xenograft models that were implanted with cancer cells with Plexin D1 knockdown [23].
Figure 2.
Role of Plexin D1–Mediated Signaling in Cancer.
Conversely, Plexin D1 signaling has also been shown to be antitumor. In a breast cancer mouse model, Plexin D1 promoted apoptosis through association with the orphan nuclear receptor NR4A1 in the absence of Sema3E [18]. In this case, Plexin D1 acted as a dependence receptor. To our knowledge, Plexin D1 is the only plexin family member that has been characterized as a dependence receptor [18]. Dependence receptors are known to mediate negative signaling. They trigger apoptosis when the ligand stimuli are absent but inhibit cell death in the presence of the respective stimulus [26]. In the breast cancer model, the authors showed that in the absence of Sema3E, Plexin D1 interacted with NR4A1, which caused the release of cytochrome c that in turn, activated caspase 9 and subsequently resulted in mitochondria-mediated apoptosis. However, in the presence of the ligand, the association of Plexin D1 and NR4A1 was disturbed, perhaps because of changes in the structure of the receptor or oligomeric state, or because of the simultaneous binding of other molecules such as Rho GTPase Rnd2, or a combination of these causes [18]. This finding also suggests that cancer cells need Sema3E/Plexin D1–mediated signaling for their survival in this scenario.
A potential mechanism by which Plexin D1 promotes tumor growth is by helping to form neovasculature through angiogenesis. It is known that during the early phases of development, Plexin D1 is present in the vasculature; however, adult vasculature lacks its expression. The roles of Plexin D1 in developmental angiogenesis and its expression in tumor vasculature have led investigators to study whether it promotes tumor angiogenesis [24]. Kim et al. [27] showed that vascular endothelial growth factor (VEGF) directly controlled the expression of Plexin D1 and that Sema3E/Plexin D1 signaling negatively regulated the activity of the VEGF-induced Delta-like 4 (D114)–Notch signaling pathway [27]. In a mouse model of ischemic retinopathy, Fukushima et al. [28] showed that increased Plexin D1 expression in the extraretinal vessels inhibited disoriented projections of the endothelial filopodia induced by VEGF. Zygmunt et al. [29] showed that Sema3E/Plexin D1 signaling confined angiogenic ability along the aorta and restricted angiogenic responses in segmental arteries. Sakurai et al. [13] reported that Sema3E/Plexin D1 mediated antiangiogenic signaling by regulating Arf6 and R-Ras.
A recent study in mouse pancreatic microvascular endothelial cells reported that the microRNA-27b acts as a positive mediator of transforming growth factor β–mediated endothelial-mesenchymal transition (EndMT) and regulates the expression of Plexin D1 [30]. It would be interesting to extend this study to cancer cells and investigate if and how the regulation of Plexin D1 contributes to EndMT and, thereby, cancer.
A recent study reported the involvement of Plexin D1 in the regulation of the type V collagen microenvironment in visceral adipose tissue and, thereby, body fat distribution and insulin sensitivity [31].
Furthermore, it has been postulated that Plexin D1 might have a role in cell motility and cytoskeletal rearrangement through its involvement in Rac/RhoA signaling and, thus, may likely be participating in tumor angiogenesis and metastasis [20].
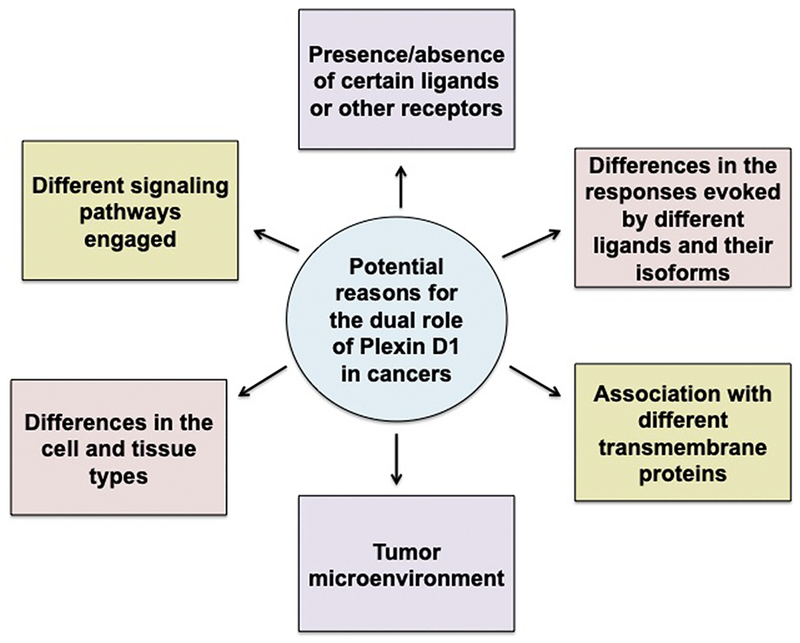
Several factors may be responsible for the ability of Plexin D1 both to promote and inhibit cancer development and progression (Figure 3). One of them is the difference in the signaling mechanisms engaged. For example, Casazza et al. [23] reported that in endothelial cells, paracrine Sema3E/Plexin D1 signaling caused cell repulsion, leading to decreased blood vessel density and tumor growth, whereas autocrine signaling in tumor cells expressing Sema3E, transactivation of Plexin D1–associated ErbB2/Neu oncogenic kinase promoted invasion and metastasis.
Figure 3.
Potential Reasons for the Dual Role of Plexin D1 in Cancer.
A second possibility for this contrasting role could be differences in cell and tissue types. Plexin D1 can evoke different responses in cancer vs. endothelial cells by activating divergent signaling pathways, even when it is stimulated by the same ligand [23]. In cancer cells, Sema3E/Plexin D1 promoted cell invasiveness and metastasis via transactivation of ErbB2/Neu oncogenic kinase. However, Plexin D1 has a cell repelling activity in endothelial cells [23]. These differing responses suggest the involvement of either the tumor microenvironment or some other factor(s) in deciding the outcome of the signaling cascade. Plexins and semaphorins are known to promote metastasis through autocrine signaling loops and by regulating communication amidst the various cell types present within the tumor [10]. This is one way in which the tumor microenvironment is potentially contributing to the signaling outcome.
Third, differences in the signaling responses evoked by different ligands and their various isoforms could also be a possibility for the divergent roles. For instance, the proteolytic fragment p61 of secreted Sema3E facilitates cell invasiveness and metastatic spreading, whereas an uncleavable variant of Sema3E does not promote metastatic spreading but rather hinders it by competing with endogenous p61-Sema3E [32].
Furthermore, the association of Sema3E/Plexin D1 with different transmembrane proteins can evoke different responses. Plexins engage tissue and cell lineage–specific coreceptors and cytoplasmic protein kinases that might be contributing to the diverse functions and outcomes [8]. For instance, in corticofugal and striatonigral neurons expressing Plexin D1 but lacking NRP1, Sema3E acts as a repellent; however, in subiculo-mammillary neurons, where both Plexin D1 and NRP1 are expressed, Sema3E signals changes to attraction or stimulation of axonal growth, or both [11].
NRP1 is a coreceptor for Plexin D1 [15]. Plexins and NRP1 could influence each other’s functioning [9]. For example, Chauvet et al. [33] showed that the association of NRP1 with Plexin D1 converted axonal repulsion by Plexin D1 to attraction during brain development. There is a possibility that the role of Plexin D1 in cancer is also influenced by the presence or absence of its coreceptors. However, in-depth studies are needed to validate this idea.
5.0. Therapeutic Importance of Plexin D1
The expression of Plexin D1 is upregulated in several clinical solid tumors, particularly in tumor vasculature and malignant cells in addition to fibroblasts and macrophages. However, in the corresponding normal tissues, Plexin D1 expression is limited to fibroblasts and macrophages that have likely been activated [20]. This suggests that Plexin D1 could potentially serve as a marker for tumor vessels [20, 24]. Shalaby et al. [34] have proposed that Plexin D1 could be a biomarker for cervical cancer. Taken together, all of these findings suggest that targeting Plexin D1 or the signaling it mediates may have substantial therapeutic implications.
Most tumors require angiogenesis mediated by neovasculature to grow over 2 to 3 mm3 [24], which has generated substantial interest in antiangiogenic therapies. Most studies that have focused on inhibiting angiogenesis were directed at blocking the VEGF-A pathway [20, 35, 36]. Although several therapeutic studies in animal tumor models had positive results, these therapies did not have curative effects in clinical studies [20, 37, 38]. Various reasons have been attributed for these results, including heterogeneity of the tumor vasculature. Typically, the patients who are candidates for these therapies have tumors in which all maturation stages are represented, thereby rendering only a small portion of the tumor susceptible to angiogenesis inhibition [20]. In addition, in organs such as the brain and liver, where the vascular density is intrinsically high, tumor and metastasis can be facilitated by preexisting vessels [39–41]. Thus, these tumors are relatively less susceptible to antiangiogenic therapies. Furthermore, antiangiogenic therapies could promote the tumor microenvironment to adapt or continue tumor growth, or both, by co-option of an existing capillary bed [42–44]. Thus, markers that specifically differentiate tumor vasculature from normal vasculature must be found for effective vascular targeting. Plexin D1 is a promising marker for tumor vessels and cells for some cancers because it is highly expressed in tumor cells [20] but not in adult vasculature [24]. However, further in-depth investigations are needed to fully validate this possibility because in certain cancers, such as breast carcinomas and vulvar squamous cell carcinoma, its expression is very low or absent [20]. It would be essential to extend the analysis of Plexin D1 expression to other cancer types as well as to stages and grades to reach decisive conclusions.
Plexin D1 has a role in developmental angiogenesis. Therefore, a possibility exists that inhibiting Plexin D1 could eventually lead to antiangiogenic effects [24]. One possible approach to avoiding this problem would be to target ligands engaged by Plexin D1. Plexin D1 signaling seems to be cell-type specific. For instance, it negatively regulates angiogenesis when it is expressed in the tips of endothelial cells [10]. In endothelial cells, the GAP domain of Plexin D1 is activated and adhesion mediated by integrins is suppressed [10]. It would be beneficial to understand and test if this antiangiogenic signaling mediated by Plexin D1 could be used for therapeutic purposes.
Targeting Plexin D1 has also been shown to allow for concurrent targeting of 2 different tumor compartments—tumor cells and tumor vessels [24]. After intravenous injections in mice, phages that had single domain antibodies against Plexin D1 on their surface were specifically observed in cerebral melanoma metastases and were associated with the tumor vessels. Roodink et al., the authors of this study, observed that the injected single-domain antibodies accumulated in the tumor vasculature and less so, possibly nonspecifically, in the tumor interstitium. The authors suggested that antibodies against Plexin D1 might be a viable route for delivery of cytotoxic molecules to tumors because the tumor vessels would probably allow extravasation of the antibodies [24]. However, these hypotheses need to be validated experimentally.
A few concerns surround the therapeutic targeting of Plexin D1. A few normal adult tissues like heart, testis, and liver express Plexin D1 transcripts at low levels. As Plexin D1 expression is not restricted to tumors, it might be important to target only cancer tissue therapeutically. Plexin D1 could be targeted directly by administering antibodies against it or by using a soluble form that could act as a decoy receptor [10].
Plexin D1, as a membrane-bound receptor, has a crucial role in axonal guidance, nervous system development, and vascular patterning. Its homozygous deletion in mice results in neonatal death, defects in axial skeletal morphogenesis and structure of the cardiac outflow tract, and deformities in the peripheral vasculature [45]. Therefore, some studies have been focused on the possibility of mitigating Plexin D1 signaling by targeting its canonical ligand, Sema3E. The results were variable. A few studies have shown that Sema3E [13] and its mutant form, noncleavable Uncl-Sema3E [32], could decrease tumor angiogenesis. Another study showed that Sema3E could inhibit tumor development [46]. In contrast, Casazza et al. [23] reported that overexpression of Sema3E resulted in fewer tumor vessels and less tumor growth, but it enhanced the invasiveness of the cancer cells, as well as transendothelial migration and metastasis. Similarly, Luchino et al. [18] showed that Sema3E facilitated survival of tumor cells by inhibiting the apoptotic pathway stimulated by Plexin D1. These various observations of the therapeutic potential of Sema3E/Plexin D1 could be attributed to differences in cell and tumor types, models used, and the way Sema3E is overexpressed. Furthermore, Sema3E differs from other class 3 semaphorins because it binds to Plexin D1 and not to neuropilins [46]. In addition, Roodink et al. [47] showed that in human melanoma, Plexin D1 contributed to the invasive and metastatic nature of the cancer, but Sema3E was not the stimulating ligand. Collectively, all of these reports suggest that Plexin D1 seems to be a strong candidate as a therapeutic target. However, more extensive studies need to be done on the pathways it engages to mediate its role(s) in tumor growth and progression. This knowledge is vital for understanding how Plexin D1 influences tumor progression and for identifying other key players that could either serve as biomarkers or be therapeutically targeted.
6.0. Future Directions: Questions That Need Answers
Most research on Plexin D1 signaling centers on semaphorin-mediated pathways. Plexin D1 can bind several members of class 3 semaphorins in an NRP1-dependent fashion and 2 members, Sema3E and Sema4A, independent of NRP1 [10]. Another exciting area of study would be to determine whether Plexin D1 could bind other cytokines and function as a coreceptor for receptors besides NRP1. Results of such studies would likely yield information about the various pathways engaged by Plexin D1 to mediate its functions. In addition, the surface of interactions between Plexin D1 and other molecules could be explored as potential therapeutic targets.
Some studies have shown that Plexin D1 helps promote tumor development, particularly metastasis. We believe that in-depth studies on its role in cancer and details on the pathways it engages need to be elucidated. Unraveling new mechanisms employed by Plexin D1 in cancer will potentially uncover new therapeutic targets. One study has shown that Plexin D1 is a dependence receptor [18]. Determining whether Plexin D1 is a dependence receptor in other cancer types is another critical area of study.
7.0. Conclusions
Plexin D1 has several fundamental and important functions, such as the development of the nervous system and vascular patterning. Results of recent studies suggest its involvement in various cancers, and its expression pattern in certain cancers indicates that it may be a strong candidate for therapeutic targeting. Several reports strongly indicate its involvement in tumor angiogenesis and tumor metastasis. Taken together, all of these studies support a valuable role for Plexin D1 in both cancer diagnosis and therapy. Nevertheless, more in-depth studies are needed to fully delineate the mechanisms by which Plexin D1 mediates its role in tumor development. Such studies will provide crucial information on what roles Plexin D1 has in cancer development and progression, how it mediates its effects, and the pathways it engages. These findings could ultimately be used to uncover new molecular targets for cancer therapy.
Acknowledgments
This work was supported by National Institutes of Health grants CA78383 and CA150190, Florida Department of Health Cancer Research Chair’s Fund (FLORIDA #3J-02–04) and Development Grant (MCKENZIE16-PAN) to D. Mukhopadhyay, and the Mayo Clinic College of Medicine and Sciences to S. Vivekanandhan. The authors thank Nisha C. Durand, PhD, for her editorial help.
Abbreviations
- Dll4
Delta-like 4
- EMT
epithelial-mesenchymal transition
- EndMT
endothelial-mesenchymal transition
- GAP
GTPase-activating protein
- IPT
immunoglobulin-plexin-transcription
- MAb
monoclonal antibody, monoclonal antibodies
- MBS
Möbius syndrome
- PSI
plexin, semaphorin, and integrin
- Sema3E
semaphorin 3E
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Both authors declare no conflicts of interest.
Portions of this manuscript were used in the dissertation thesis submitted by Sneha Vivekanandhan, PhD; however, the thesis has not been published.
References
- [1].Takagi S, Tsuji T, Amagai T, Takamatsu T, Fujisawa H, Specific cell surface labels in the visual centers of Xenopus laevis tadpole identified using monoclonal antibodies, Dev. Biol 122(1) (1987) 90–100 [DOI] [PubMed] [Google Scholar]
- [2].Fujisawa H, Discovery of semaphorin receptors, neuropilin and plexin, and their functions in neural development, J. Neurobiol 59(1) (2004) 24–33. [DOI] [PubMed] [Google Scholar]
- [3].Ohta K, Mizutani A, Kawakami A, Murakami Y, Kasuya Y, Takagi S, Tanaka H, Fujisawa H, Plexin: a novel neuronal cell surface molecule that mediates cell adhesion via a homophilic binding mechanism in the presence of calcium ions, Neuron 14(6) (1995) 1189–99. [DOI] [PubMed] [Google Scholar]
- [4].Ohta K, Takagi S, Asou H, Fujisawa H, Involvement of neuronal cell surface molecule B2 in the formation of retinal plexiform layers, Neuron 9(1) (1992) 151–61. [DOI] [PubMed] [Google Scholar]
- [5].Maestrini E, Tamagnone L, Longati P, Cremona O, Gulisano M, Bione S, Tamanini F, Neel BG, Toniolo D, Comoglio PM, A family of transmembrane proteins with homology to the MET-hepatocyte growth factor receptor, Proc. Natl. Acad. Sci. U. S. A 93(2) (1996) 674–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, Tessier-Lavigne M, Comoglio PM, Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates, Cell 99(1) (1999) 71–80. [DOI] [PubMed] [Google Scholar]
- [7].Kong Y, Janssen BJ, Malinauskas T, Vangoor VR, Coles CH, Kaufmann R, Ni T, Gilbert RJ, Padilla-Parra S, Pasterkamp RJ, Jones EY, Structural Basis for Plexin Activation and Regulation, Neuron 91(3) (2016) 548–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kumanogoh A, Kikutani H, Immunological functions of the neuropilins and plexins as receptors for semaphorins, Nat. Rev. Immunol 13(11) (2013) 802–14. [DOI] [PubMed] [Google Scholar]
- [9].Hota PK, Buck M, Plexin structures are coming: opportunities for multilevel investigations of semaphorin guidance receptors, their cell signaling mechanisms, and functions, Cell. Mol. Life Sei 69(22) (2012)3765–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Worzfeld T, Offermanns S, Semaphorins and plexins as therapeutic targets, Nat Rev Drug Discov 13(8) (2014) 603–21. [DOI] [PubMed] [Google Scholar]
- [11].Oh WJ, Gu C, The role and mechanism-of-action of Sema3E and Plexin-D1 in vascular and neural development, Semin. Cell Dev. Biol 24(3) (2013) 156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pascoe HG, Wang Y, Zhang X, Structural mechanisms of plexin signaling, Prog. Biophys. Mol. Biol 118(3) (2015) 161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sakurai A, Gavard J, Annas-Linhares Y, Basile JR, Amornphimoltham P, Palmby TR, Yagi H, Zhang F, Randazzo PA, Li X, Weigert R, Gutkind JS, Semaphorin 3E initiates antiangiogenic signaling through plexin D1 by regulating Arf6 and R-Ras, Mol. Cell. Biol 30(12) (2010) 3086–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gay CM, Zygmunt T, Torres-Vazquez J, Diverse functions for the semaphorin receptor Plexin D1 in development and disease, Dev. Biol 349(1) (2011) 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Burk K, Mire E, Bellon A, Hocine M, Guillot J, Moraes F, Yoshida Y, Simons M, Chauvet S, Mann F, Post-endocytic sorting of Plexin-D1 controls signal transduction and development of axonal and vascular circuits, Nat Commun 8 (2017) 14508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ta-Shma A, Pierri CL, Stepensky P, Shaag A, Zenvirt S, Elpeleg O, Rein AJ, Isolated truncus arteriosus associated with a mutation in the plexin-D1 gene, Am. J. Med. Genet. A 161A(12) (2013)3115–20. [DOI] [PubMed] [Google Scholar]
- [17].Tomas-Roca L, Tsaalbi-Shtylik A, Jansen JG, Singh MK, Epstein JA, Altunoglu U, Verzijl H, Soria L, van Beusekom E, Roscioli T, Iqbal Z, Gilissen C, Hoischen A, de Brouwer AP, Erasmus C, Schubert D, Brunner H, Perez Aytes A., Marin F, Aroca P, Kayserili H, Carta A, de Wind N, Padberg GW, van Bokhoven H, De novo mutations in PLXND1 and REV3L cause Mobius syndrome, Nat Commun 6 (2015) 7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Luchino J, Hocine M, Amoureux MC, Gibert B, Bernet A, Royet A, Treilleux I, Lecine P, Borg JP, Mehlen P, Chauvet S, Mann F, Semaphorin 3E suppresses tumor cell death triggered by the plexin D1 dependence receptor in metastatic breast cancers, Cancer Cell 24(5) (2013) 673–85. [DOI] [PubMed] [Google Scholar]
- [19].Rehman M, Gurrapu S, Cagnoni G, Capparuccia L, Tamagnone L, PlexinD1 Is a Novel Transcriptional Target and Effector of Notch Signaling in Cancer Cells, PLoS One 11(10) (2016) e0164660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Roodink I, Verrijp K, Raats J, Leenders WP, Plexin D1 is ubiquitously expressed on tumor vessels and tumor cells in solid malignancies, BMC Cancer 9 (2009) 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Huang HY, Cheng YY, Liao WC, Tien YW, Yang CH, Hsu SM, Huang PH, SOX4 transcriptionally regulates multiple SEMA3/plexin family members and promotes tumor growth in pancreatic cancer, PLoS One 7(12) (2012) e48637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tseng CH, Murray KD, Jou MF, Hsu SM, Cheng HJ, Huang PH, Sema3E/plexin-D1 mediated epithelial-to-mesenchymal transition in ovarian endometrioid cancer, PLoS One 6(4) (2011) e19396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Casazza A, Finisguerra V, Capparuccia L, Camperi A, Swiercz JM, Rizzolio S, Rolny C, Christensen C, Bertotti A, Sarotto I, Risio M, Trusolino L, Weitz J, Schneider M, Mazzone M, Comoglio PM, Tamagnone L, Sema3E-Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice, J. Clin. Invest 120(8) (2010) 2684–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Roodink I, Raats J, van der Zwaag B, Verrijp K, Kusters B, van Bokhoven H, Linkels M, de Waal RM, Leenders WP, Plexin D1 expression is induced on tumor vasculature and tumor cells: a novel target for diagnosis and therapy?, Cancer Res. 65(18) (2005) 8317–23. [DOI] [PubMed] [Google Scholar]
- [25].Yong LK, Lai S, Liang Z, Poteet E, Chen F, van Buren G, Fisher W, Mo Q, Chen C, Yao Q, Overexpression of Semaphorin-3E enhances pancreatic cancer cell growth and associates with poor patient survival, Oncotarget 7(52) (2016) 87431–87448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gibert B, Mehlen P, Dependence Receptors and Cancer: Addiction to Trophic Ligands, Cancer Res. 75(24) (2015) 5171–5. [DOI] [PubMed] [Google Scholar]
- [27].Kim J, Oh WJ, Gaiano N, Yoshida Y, Gu C, Semaphorin 3E-Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism, Genes Dev. 25(13) (2011) 1399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fukushima Y, Okada M, Kataoka H, Hirashima M, Yoshida Y, Mann F, Gomi F, Nishida K, Nishikawa S, Uemura A, Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice, J. Clin. Invest 121(5) (2011) 1974–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zygmunt T, Gay CM, Blondelle J, Singh MK, Flaherty KM, Means PC, Herwig L, Krudewig A, Belting HG, Affolter M, Epstein JA, Torres-Vazquez J, Semaphorin-PlexinD1 signaling limits angiogenic potential via the VEGF decoy receptor sFlt1, Dev. Cell 21(2) (2011)301–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Suzuki HI, Katsura A, Mihira H, Horie M, Saito A, Miyazono K, Regulation of TGF-beta-mediated endothelial-mesenchymal transition by microRNA-27, J. Biochem 161(5) (2017) 417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Minchin JE, Dahlman I, Harvey CJ, Mejhert N, Singh MK, Epstein JA, Arner P, Torres-Vazquez J, Rawls JF, Plexin D1 determines body fat distribution by regulating the type V collagen microenvironment in visceral adipose tissue, Proc. Natl. Acad. Sci. U. S. A 112(14) (2015)4363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Casazza A, Kigel B, Maione F, Capparuccia L, Kessler O, Giraudo E, Mazzone M, Neufeld G, Tamagnone L, Tumour growth inhibition and anti-metastatic activity of a mutated furin-resistant Semaphorin 3E isoform, EMBO Mol. Med 4(3) (2012) 234–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chauvet S, Cohen S, Yoshida Y, Fekrane L, Livet J, Gayet O, Segu L, Buhot MC, Jessell TM, Henderson CE, Mann F, Gating of Sema3E/PlexinD1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development, Neuron 56(5) (2007) 807–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shalaby MA, Hampson L, Oliver A, Hampson I, Plexin D1: new potential biomarker for cervical cancer, J. Immunoassay Immunochem. 33(3) (2012) 223–33. [DOI] [PubMed] [Google Scholar]
- [35].Presta LG, Chen H, O’Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N, Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders, Cancer Res. 57(20) (1997) 4593–9. [PubMed] [Google Scholar]
- [36].Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, Murray LJ, Carver J, Chan E, Moss KG, Haznedar JO, Sukbuntherng J, Blake RA, Sun L, Tang C, Miller T, Shirazian S, McMahon G, Cherrington JM, In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship, Clin. Cancer Res 9(1) (2003) 327–37. [PubMed] [Google Scholar]
- [37].Eichhorn ME, Strieth S, Dellian M, Anti-vascular tumor therapy: recent advances, pitfalls and clinical perspectives, Drug Resist Updat 7(2) (2004) 125–38. [DOI] [PubMed] [Google Scholar]
- [38].Narayana A, Kelly P, Golfinos J, Parker E, Johnson G, Knopp E, Zagzag D, Fischer I, Raza S, Medabalmi P, Eagan P, Gruber ML, Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival, J. Neurosurg 110(1) (2009) 173–80. [DOI] [PubMed] [Google Scholar]
- [39].Kusters B, Leenders WP, Wesseling P, Smits D, Verrijp K, Ruiter DJ, Peters JP, van Der Kogel AJ, de Waal RM, Vascular endothelial growth factor-A(165) induces progression of melanoma brain metastases without induction of sprouting angiogenesis, Cancer Res. 62(2) (2002) 341–5. [PubMed] [Google Scholar]
- [40].Leenders WP, Kusters B, de Waal RM, Vessel co-option: how tumors obtain blood supply in the absence of sprouting angiogenesis, Endothelium 9(2) (2002) 83–7. [DOI] [PubMed] [Google Scholar]
- [41].Vermeulen PB, Colpaert C, Salgado R, Royers R, Hellemans H, Van Den Heuvel E, Goovaerts G, Dirix LY, Van Marck E, Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia, J. Pathol 195(3) (2001) 336–42. [DOI] [PubMed] [Google Scholar]
- [42].van Kempen LC, Leenders WP, Tumours can adapt to anti-angiogenic therapy depending on the stromal context: lessons from endothelial cell biology, Eur. J. Cell Biol 85(2) (2006) 61–8. [DOI] [PubMed] [Google Scholar]
- [43].Leenders WP, Kusters B, Verrijp K, Maass C, Wesseling P, Heerschap A, Ruiter D, Ryan A, de Waal R, Antiangiogenic therapy of cerebral melanoma metastases results in sustained tumor progression via vessel co-option, Clin. Cancer Res 10(18 Pt 1) (2004) 6222–30. [DOI] [PubMed] [Google Scholar]
- [44].Claes A, Wesseling P, Jeuken J, Maass C, Heerschap A, Leenders WP, Antiangiogenic compounds interfere with chemotherapy of brain tumors due to vessel normalization, Mol. Cancer Ther 7(1) (2008)71–8. [DOI] [PubMed] [Google Scholar]
- [45].Zhang Y, Singh MK, Degenhardt KR, Lu MM, Bennett J, Yoshida Y, Epstein JA, Tie2Cre-mediated inactivation of plexinD1 results in congenital heart, vascular and skeletal defects, Dev. Biol 325(1) (2009) 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sabag AD, Bode J, Fink D, Kigel B, Kugler W, Neufeld G, Semaphorin-3D and semaphorin-3E inhibit the development of tumors from glioblastoma cells implanted in the cortex of the brain, PLoS One 7(8) (2012) e42912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Roodink I, Kats G, van Kempen L, Grunberg M, Maass C, Verrijp K, Raats J, Leenders W, Semaphorin 3E expression correlates inversely with Plexin D1 during tumor progression, Am. J. Pathol 173(6) (2008) 1873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]