Abstract
Household air pollution from combustion of solid fuels is an important risk factor for morbidity and mortality, causing an estimated 2.6 million premature deaths globally in 2016. Self-reported health symptoms are a meaningful measure of quality of life, however, few studies have evaluated symptoms and quantitative measures of exposure to household air pollution. We assessed the cross-sectional association of self-reported symptoms and exposures to household air pollution among women in rural Honduras using stove type (traditional [n=76]; cleaner-burning Justa [n=74]) and 24-hour average personal and kitchen fine particulate matter (PM2.5) concentrations. The odds of prevalent symptoms were higher among women using traditional stoves vs Justa stoves (e.g., headache: odds ratio = 2.23; 95% confidence interval = 1.13-4.39). Associations between symptoms and measured PM2.5 were generally consistent with the null. These results add to the evidence suggesting reduced exposures and better health-related quality of life among women using cleaner-burning biomass stoves.
Keywords: particulate matter, health, respiratory
Introduction
Household air pollution from combustion of solid fuels for cooking and heating is one of the leading causes of death and morbidity around the world (Gakidou et al. 2017). In 2016, household air pollution was the 8th leading contributor to global disability adjusted life-years in women, and responsible for an estimated 2.6 million premature deaths worldwide. Lower- and middle-income countries bear the brunt of this burden due to the abundant use of solid-fuel cookstoves, with exposure to pollutants disproportionally affecting women and children (Bruce et al. 2000). Cookstove emissions contain numerous harmful pollutants including fine particulate matter (PM2.5; particles with aerodynamic diameter less than 2.5 μm), carbon monoxide, nitrous oxides, and carcinogens such as benzene, benzo[a]pyrene and formaldehyde (Bruce et al. 2000; Naeher et al. 2007). Previous studies have attempted to quantify these exposures and their association with health outcomes such as cardiovascular and chronic respiratory diseases (McCracken et al. 2007; Romieu et al. 2009; Baumgartner et al. 2011; Smith Kirk R. et al. 2011; Clark, Bachand, et al. 2013; Oluwole et al. 2013; Smith K. R. et al. 2014; Guarnieri et al. 2015; Pope et al. 2015; Mortimer et al. 2017). PM2.5 is often used as the indicator of exposure to household air pollution due to its association with numerous health endpoints (Naeher et al. 2007; Gordon et al. 2014).
Studies assessing the health impact of cookstove use often focus on measuring health outcomes that have well-established clinical importance (e.g., blood pressure, pneumonia, birth weight). However, health endpoints such as self-reported symptoms can also provide meaningful information. Although self-reported symptoms are potentially subject to various forms of information bias, they are important to consider because they can serve as an indicator of health-related quality of life that may not be captured using clinical endpoints (Sarna et al. 2004; Wheaton et al. 2013; Wong et al. 2013; Alexander et al. 2014; Cook et al. 2015). Symptoms are also important markers of common obstructive respiratory diseases such as chronic obstructive pulmonary disease and asthma (Gordon et al. 2014), and can be an indicator of overall lung function (Wheaton et al. 2013). Several studies have evaluated exposure to household air pollution and health symptoms using proxies of exposure such as stove type (Schei et al. 2004; Regalado et al. 2006; Diaz et al. 2008; Singh et al. 2012; Jamali et al. 2017). For example, women in rural Mexico had lower risk of self-reported respiratory symptoms following an improved cookstove intervention (Romieu et al. 2009). Other studies have included quantitative pollution measurements such as carbon monoxide or PM2.5 (Singh et al. 2012; Kurmi et al. 2014; Pope et al. 2015). A previous study in Honduras reported some associations between indoor PM2.5 and self-reported symptoms, although the sample size was limited to 79 women and quantitative exposure values were limited to a single eight-hour measurement for each household (Clark et al. 2009). Other studies assessing household air pollution and self-reported symptoms share similar limitations such as small sample size or exposure assessment during single cooking events (Ellegard 1996; Regalado et al. 2006; Oluwole et al. 2013; Rumchev et al. 2015).
Our objective was to evaluate the cross-sectional association of exposure to household air pollution and self-reported symptoms among women in rural Honduras using traditional and cleaner-burning Justa stoves. We evaluated exposure to household air pollution using information on stove type (including age of the Justa stove and information on primary and secondary stoves in the same household) and using quantitative measurements of personal exposure to PM2.5.
Materials and Methods
Study location and population
The study population was recruited from 11 rural communities near La Esperanza, Honduras, with elevations ranging from 1,730 to 2,200 meters above sea level. The local economy relies heavily on agriculture, and households almost exclusively use biomass fuels for energy needs.
Participant recruitment
A group of 500 households was screened during initial recruitment efforts in 2014. Potential participants were identified from community meetings in which study coordinators spoke about the objectives of the study to assess interest in participation from the community at large. Between February and April of 2015, a convenience sample of 150 participants (74 using Justa stoves for at least four months and 76 using traditional stoves) was selected based on the study eligibility criteria: female, primary cook of the household, non-pregnant, non-smoking, and age from 25 to 56 years. Participants provided verbal informed consent and received food items worth approximately $5 USD after completing the assessment. The study protocol was approved by the Institutional Review Board at Colorado State University.
Exposure to Household Air Pollution
We assessed exposure to household air pollution using stove type and measured PM2.5. The primary stove type was a dichotomous variable indicating traditional or Justa stove. Traditional stoves (Figure 1) were defined as being self-constructed, with or without a chimney (n = 5 traditional stoves without chimney), but with no improved combustion chamber. Justa stoves (Figure 1) have an improved ceramic combustion chamber, chimney, a steel-framed griddle embedded into the stove body, and a side compartment to remove soot (Kshirsagar and Kalamkar 2014). Participants self-reported the date in which their Justa stove was installed. In separate analyses we also assessed a three-level stove type variable categorizing the Justa stoves based on the median age of the stove (traditional; Justa 4 - 19 months old; Justa > 19 months old) and a four-level stove type variable indicating primary and secondary stoves (traditional stove only; traditional stove with a traditional secondary stove; Justa stove only; or Justa stove with use of a traditional secondary stove).
Figure 1.
Examples of traditional (left) and Justa stoves (right), rural Honduras
We calculated time-weighted average concentrations of 24-hour personal and kitchen PM2.5 samples. Participants were asked to wear personal PM2.5 monitors near their breathing zone during normal activities, and to place the monitors close by while bathing and sleeping. Exposure monitors for kitchen PM2.5 measurements were hung in front of the stoves in an area that represented the typical breathing zone for a participant while also avoiding cooking interference and drafts from windows and doorways. Measured distances for the kitchen monitors ranged from a minimum of 76 centimeters to a maximum of 127 centimeters from the front edges of the stoves.
Personal and kitchen PM2.5 was selected using cyclones (Triplex, BGI Inc., NJ, USA) and collected on 37-mm filters (Fiberfilm, Pall Corporation, NY, USA) using pumps (AirChek XR5000, SKC Inc., PA, USA) that were run for 24 hours. Pumps were calibrated to a flow rate of 1.5 liters per minute (DryCal Lite, BGI Inc., NJ, USA). Samples that ran for less than 24 hours were removed from the analysis. Flow rates stayed within 10% of pre- and post-sampling calibrations.
Filters were stored at −22°C before being transporting to Colorado State University, where they were stored at −80 °C before analysis. Filters were equilibrated for 24 hours before being pre-weighed and post-weighed (MX5, Mettler, OH, USA). The limit of detection (LOD) was calculated using field and travel blanks (LOD = 54 μg) (MacDougall et al. 1980); values below the LOD (four personal filters and seven kitchen filters) were replaced with values of LOD/(square root of two). The substitution method LOD/(square root of two) was based on sources which state that when frequency of censored data is low (in our data censored frequency was less than 10% for personal and kitchen PM2.5), and data are not highly skewed, using LOD/(square root of two) is an appropriate method for estimating censored data (Hornung and Reed 1990; Hewett and Ganser 2007; Nieuwenhuijsen 2015).
Self-Reported Symptoms
We assessed nine dichotomous (yes or no) self-reported health symptoms, typically experienced while cooking, using questionnaires during in-home visits with the participants: eye irritation, blurred vision, nose irritation, mucus or phlegm, difficulty breathing, headache, chest wheezing, throat irritation, and cough.
Sociodemographic Assessment
We collected sociodemographic and health data through questionnaires developed specifically for the study population with the help of local leaders. Questionnaires were administered in Spanish, and responses were entered into Open Data Kit (ODK Collect 1.4.5, UK, https://opendatakit.org/).
Age was confirmed by reviewing each participant’s date of birth on their national identification card. Indicators of socioeconomic status included beds per person in each household, possession of household assets common to the area (bicycle, motorcycle, television, radio, refrigerator, sewing machine, working electricity), education (reported as the highest grade completed in school), and dietary diversity score (a sum of ten food groups categorized from 19 commonly eaten food items reported from a 24-hour dietary recall) (Arps 2011).
We measured weight with an electronic scale and height with a measuring tape and level against a wall. We then calculated body mass index (BMI) by dividing weight (kilograms) by height (meters squared). Waist-to-hip ratio was calculated by dividing waist circumference (measured at the smallest circumference of the natural waist) by hip circumference (measured at the widest point of the hips). Past history of exposure to cookstove smoke, in cooking years, was calculated by subtracting the self-reported age at which the women started cooking from their current age. Second hand smoke exposure was assessed by asking participants if anyone currently smoked in their household.
Statistical Analysis
Statistical analysis was performed using SAS statistical software (SAS 9.4, SAS Institute, Inc., Cary, NC, USA) and R (version 3.4.1, The R Foundation for Statistical Computing). We conducted descriptive analyses of all variables. For analyses using PM2.5 concentrations, the sample sizes were 106 for kitchen PM2.5 and 105 for personal PM2.5. We were unable to collect personal and kitchen PM2.5 from the first 41 households enrolled in the study due to pump calibration equipment malfunction. Among the PM2.5 measurements completed in the field from the remaining 109 households, three kitchen filters and four personal filters were excluded due to missing data on post-sample filter weights. Logistic regression was conducted for each combination of exposure and symptom variables. Based on previous literature, potential confounders included: age, BMI, waist-to-hip ratio, years of education, household assets, and beds per person. Because some potential confounders were proxies for each other (e.g., BMI and waist-to-hip ratio), we did not include all variables in our final model. Covariates were included in final models if they meaningfully changed model estimates (i.e., by more than 10%) compared to crude estimates with no covariates; final models included household assets (≤ 2 vs. > 2), BMI (continuous), and age (continuous). We conducted sensitivity analyses replacing the household assets variable with other indicators of socioeconomic status (beds per person in the household, education, dietary diversity score), replacing BMI with waist-to-hip ratio, and examining models with subsets of the potential confounders. We also did a sensitivity analysis removing participants who reported exposure to second hand smoke in their home (n=5). Prevalence odds ratios and 95% confidence intervals were calculated using a reference category (for categorical stove type variables) or per interquartile range increase (for continuous pollution concentrations).
Results
Study population characteristics are presented in Table 1. The mean age of study participants was 37 years, with around half having less than six years of education (Table 1). Average BMI of the study participants was 25.8 kg/m2, and the average time cooking with a biomass cookstove was nearly 26 years (Table 1). Women with traditional and Justa stoves were generally similar to one another, with the exception of education. Women with a Justa stove were more likely to have had six or more years of education (Table 1).
Table 1.
Participant characteristics and symptoms, total and by stove type, rural Honduras
| All households n=150 |
Traditional stove owners n=76 |
Justa stove owners n=74 |
|
|---|---|---|---|
| Mean (SD; min; median; max) or n (%) | Mean (SD; min; median; max) or n (%) | Mean (SD; min; median; max) or n (%) | |
| Age (years) | 37.3 (9.0; 25.0; 35.0; 56.0) | 38.5 (9.9; 25.0; 37.5; 56.0) | 36.1 (7.9; 25.0; 34.0; 56.0) |
| Years of cooking with biomass cookstoves | 25.8 (9.9; 7.0; 23.5; 50.0) | 26.9 (10.8; 7.0; 25.0; 49.0) | 24.7 (8.7; 9.0; 23.0; 50.0) |
| Beds per person in the household | 0.52 (0.18; 0.23; 0.50; 1.00) | 0.51 (0.17; 0.23; 0.50; 1.00) | 0.54 (0.19; 0.25; 0.50; 1.00) |
| Education | |||
| Less than six years | 70 (47%) | 40 (53%) | 30 (41%) |
| Six or more years | 78 (53%) | 35 (47%) | 43 (59%) |
| Household assets1 (dichotomous) | |||
| Two or fewer household assets | 115 (77%) | 56 (74%) | 59 (80%) |
| More than two household assets | 35 (23%) | 20 (26%) | 15 (20%) |
| Dietary diversity score2 | 6.1 (1.7; 2.0; 6.0; 10.0) | 6.1 (1.7; 3.0; 6.0; 10.0) | 6.0 (1.6; 2.0; 6.0; 10.0) |
| Body mass index (kg/m2) | 25.8 (4.2; 17.1; 25.2; 37.5) | 25.6 (4.6; 17.1; 25.0; 37.5) | 26.0 (3.8; 18.2; 25.5; 33.6) |
| Waist-to-hip ratio | 0.88 (0.06; 0.74; 0.87; 1.09) | 0.86 (0.06; 0.74; 0.87; 1.09) | 0.87 (0.05; 0.77; 0.87; 0.99) |
| Exposure to second hand smoke | 5 (3.3%) | 5 (6.6%) | 0 (0%) |
| Self-reported symptoms experienced while cooking | |||
| Eye Irritation | 80 (53%) | 51 (67%) | 29 (39%) |
| Blurred Vision | 50 (33%) | 36 (47%) | 14 (19%) |
| Nose Irritation | 45 (30%) | 29 (38%) | 16 (22%) |
| Mucus or Phlegm | 23 (15%) | 15 (20%) | 8 (11%) |
| Difficulty Breathing | 26 (17%) | 16 (21%) | 10 (14%) |
| Headache | 82 (55%) | 48 (63%) | 34 (46%) |
| Chest Wheezing or Whistling | 28 (19%) | 19 (25%) | 9 (12%) |
| Throat Irritation | 40 (27%) | 27 (36%) | 13 (18%) |
| Cough | 43 (29%) | 30 (39%) | 13 (18%) |
SD, standard deviation
Sum of household assets: car, bike, motorcycle, television, radio, refrigerator, sewing machine, electricity.
Sum of items in normal diet: cereal, nuts, roots, vegetables, fruit, sweets, eggs, dairy, meat, non-water beverages.
There was a high prevalence of self-reported symptoms in the study population. Eighty-two percent of the women reported at least one symptom (95% of traditional stove users and 69% of Justa stove users). Headache and eye irritation had the highest prevalence of the nine symptoms, with 55% and 53% of participants reporting them while cooking, respectively. Blurred vision, nose irritation, throat irritation, and cough were also reported during typical cooking events by over 25% of the women (Table 1).
PM2.5 concentrations are presented in Table 2 and Figure 2. Women with Justa stoves were exposed to lower levels of pollution on average, although there was large variation within both groups (Table 2 and Figure 2). The mean personal 24-hour average PM2.5 concentration (standard deviation; median) across traditional stove users was 125 μg/m3 (76; 114), compared to 66 μg/m3 (38; 53) across Justa stove users. Kitchen 24-hour PM2.5 concentrations showed similar patterns to personal concentrations (Table 2 and Figure 2); the Spearman correlation coefficient for personal and kitchen PM2.5 concentrations was 0.80. Median quantitative pollution levels decreased as the quality of the stove increased (highest exposure to lowest: traditional, older Justa, newer Justa) (Table 2). A similar trend was observed using a four-level stove exposure variable (highest exposure to lowest: traditional + traditional, traditional only, Justa + traditional, Justa only) (Table 2).
Table 2.
Personal and kitchen 24-hour average fine particulate matter concentrations for all households and by stove type
| 24-hour average personal PM2.5 (μg/m3) | 24-hour average kitchen PM2.5 (μg/m3) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | 25th – 75th percentile | Median | n | Mean (SD) | 25th – 75th percentile | Median | n | |
| All households | 101 (70) | 51-135 | 79 | 105 | 264 (328) | 62-374 | 132 | 106 |
| Stove Type | ||||||||
| Traditional stove | 125 (76) | 65-155 | 114 | 62 | 354 (373) | 91 – 511 | 181 | 62 |
| Justa stove | 66 (38) | 39-81 | 53 | 43 | 137 (194) | 38-159 | 71 | 44 |
| Stove Type, incorporating age of Justa stove | ||||||||
| Traditional stove | 125 (76) | 65-155 | 114 | 62 | 354 (373) | 91-511 | 181 | 62 |
| Justa 19 months old or older | 61 (28) | 41-77 | 59 | 16 | 102 (76) | 39-176 | 77 | 15 |
| Justa less than 19 months old | 69 (43) | 39-81 | 52 | 27 | 155 (232) | 37-155 | 70 | 29 |
| Stove Type, incorporating primary and secondary stoves in household | ||||||||
| Traditional primary stove plus traditional secondary stove | 133 (85) | 65-166 | 115 | 22 | 318 (405) | 91-394 | 136 | 21 |
| Traditional stove only | 120 (72) | 63-150 | 111 | 40 | 372 (359) | 105-538 | 243 | 41 |
| Justa primary stove plus traditional secondary stove | 68 (35) | 39-84 | 68 | 22 | 148 (245) | 39-150 | 67 | 23 |
| Justa stove only | 63 (42) | 41-69 | 52 | 21 | 124 (119) | 37-176 | 71 | 21 |
PM2.5, fine particulate matter; SD, standard deviation
Figure 2.
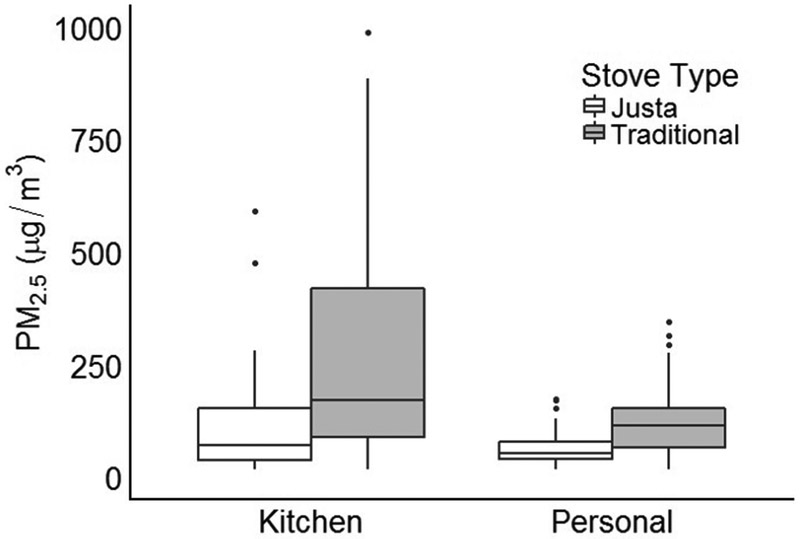
Concentrations of kitchen and PM2.5 by stove type. Boxplots are reporting minimum value, 1st quartile, median, 3rd quartile, maximum value, and outliers (four highest outliers removed from kitchen PM2.5 plots to improve scale).
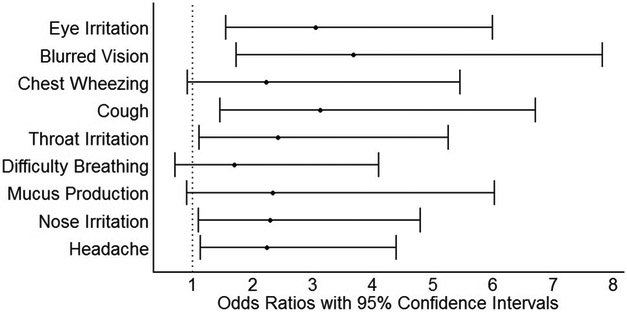
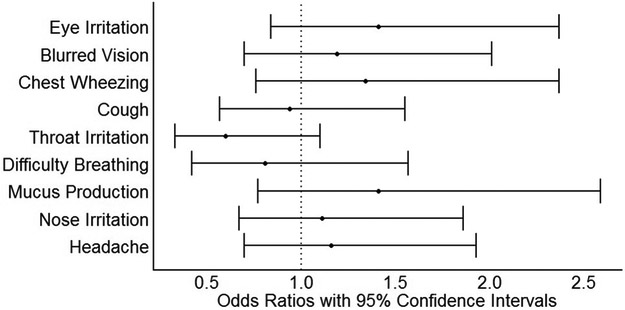
Adjusted prevalence odds ratios and 95% confidence intervals are presented in Table 3 and in Figures 3 (stove type) and 4 (personal 24-hour PM2.5). The odds of prevalent symptoms was generally higher among women using traditional stoves compared to those using Justa stoves. These results were most pronounced in models with the dichotomous stove-type variable (Justa vs. traditional) as the exposure metric, which consistently indicated two to four times higher odds of prevalent symptoms in traditional stove users compared to Justa users. For several of the symptoms, a trend of increasing odds ratios was seen as exposure levels increased in the three- and four-level stove exposure variables (Table 3). There was less evidence of an association with 24-hour measures of personal and kitchen PM2.5 (Table 3, Figure 4). We did not observe meaningful differences for results in any of the sensitivity analyses.
Table 3.
Prevalence odds ratios and 95% confidence intervals for the association between exposure to household air pollution and self-reported symptoms experienced while cooking in rural Honduras1
| Self-reported Symptoms Experienced While Cooking | |||
|---|---|---|---|
| Exposure to Household Air Pollution |
Headache | Eye Irritation | Nose Irritation |
| Stove Type | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Traditional stove (n=76) Justa stove (n=74) | 2.23 (1.13-4.39) Ref | 3.04 (1.55-5.99) Ref | 2.29 (1.09-4.79) Ref |
| Traditional stove (n=76) | 1.57 (0.69-3.59) | 3.23 (1.41-7.40) | 2.66 (1.02-6.95) |
| Older Justa stove (n=37) | 0.50 (0.19-1.28) | 1.12 (0.44-2.88) | 1.33 (0.43-4.08) |
| Newer Justa stove (n=37) | Ref | Ref | Ref |
| P-Value for trend | 0.14 | <0.01 | 0.03 |
| Traditional primary plus traditional secondary stove (n=28) | 6.53 (2.06-20.66) | 4.51 (1.50-13.56) | 2.31 (0.74-7.25) |
| Traditional stove only (n=48) | 2.22 (0.92-5.37) | 2.63 (1.10-6.31) | 2.60 (0.98-6.92) |
| Justa plus Traditional stove (n=35) | 2.02 (0.78-5.22) | 1.07 (0.42-2.74) | 1.20 (0.39-3.68) |
| Justa stove only (n=39) | Ref | Ref | Ref |
| P-Value for trend | <0.01 | <0.01 | 0.05 |
| Measured Particulate Matter | |||
| 24-hour average personal PM2.5 (μg/m3) (n=105) | 1.16 (0.70-1.93) | 1.41 (0.84-2.37) | 1.11 (0.67-1.86) |
| Odds ratio per IQR increase (85 μg/m3) | |||
| 24-hour average kitchen PM2.5 (μg/m3) (n=106) | 0.91 (0.63-1.33) | 1.79 (1.09-2.95) | 1.19 (0.81-1.76) |
| Odds ratio per IQR increase (310 μg/m3) | |||
| Difficulty Breathing | Throat Irritation | Cough | |
| Stove Type | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Traditional stove (n=76) | 1.69 (0.70-4.09) | 2.41 (1.11-5.25) | 3.12 (1.45-6.70) |
| Justa stove (n=74) | Ref | Ref | Ref |
| Traditional stove (n=76) | 0.92 (0.35-2.47) | 1.81 (0.71-4.59) | 1.77 (0.74-4.24) |
| Older Justa stove (n=37) | 0.19 (0.04-1.00) | 0.53 (0.16-1.84) | 0.23 (0.06-0.94) |
| Newer Justa stove (n=37) | Ref | Ref | Ref |
| P-Value for trend | 0.82 | 0.10 | 0.06 |
| Traditional plus Traditional stove (n=28) | 1.16 (0.27-5.00) | 2.60 (0.79-8.63) | 2.74 (0.77-9.82) |
| Traditional stove only (n=48) | 2.24 (0.71-7.08) | 3.03 (1.04-8.79) | 5.79 (1.93-17.38) |
| Justa plus Traditional stove (n=35) | 1.20 (0.31-4.60) | 1.42 (0.42-4.77) | 2.05 (0.60-7.00) |
| Justa stove only (n=39) | Ref | Ref | Ref |
| P-Value for trend | 0.44 | 0.04 | 0.02 |
| Measured Particulate Matter | |||
| 24-hour average personal PM2.5 (μg/m3) (n=105) | 0.81 (0.42-1.57) | 0.60 (0.33-1.10) | 0.94 (0.57-1.55) |
| Odds ratio per IQR increase (85 μg/m3) | |||
| 24-hour average kitchen PM2.5 (μg/m3) (n=106) | 0.93 (0.56-1.54) | 0.67 (0.41-1.11) | 0.88 (0.59-1.31) |
| Odds ratio per IQR increase (310 μg/m3) | |||
| Mucus Production | Chest Wheezing | Blurred Vision | |
| Stove Type | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Traditional stove (n=76) | 2.33 (0.90-6.02) | 2.22 (0.91-5.45) | 3.67 (1.72-7.82) |
| Justa stove (n=74) | Ref | Ref | Ref |
| Traditional stove (n=76) | 2.32 (0.69-7.79) | 1.92 (0.63-5.79) | 2.24 (0.93-5.41) |
| Older Justa stove (n=37) | 0.99 (0.22-4.40) | 0.73 (0.18-3.05) | 0.31 (0.08-1.12) |
| Newer Justa stove (n=37) | Ref | Ref | Ref |
| P-Value for trend | 0.12 | 0.15 | 0.01 |
| Traditional plus Traditional stove (n=28) | 6.20 (1.03-37.28) | 2.12 (0.58-7.71) | 6.88 (2.11-22.46) |
| Traditional stove only (n=48) | 5.18 (1.05-25.65) | 2.06 (0.63-6.72) | 3.69 (1.27-10.72) |
| Justa plus Traditional stove (n=35) | 4.22 (0.78-22.92) | 0.87 (0.21-3.60) | 1.59 (0.48-5.25) |
| Justa stove only (n=39) | Ref | Ref | Ref |
| P-Value for trend | 0.03 | 0.13 | <0.01 |
| Measured Particulate Matter | |||
| 24-hour average personal PM2.5 (μg/m3) (n=105) | 1.41 (0.77-2.59) | 1.34 (0.76-2.37) | 1.19 (0.70-2.01) |
| Odds ratio per IQR increase (85 μg/m3) | |||
| 24-hour average kitchen PM2.5 (μg/m3) (n=106) | 0.93 (0.55-1.58) | 1.19 (0.79-1.81) | 1.10 (0.75-1.63) |
| Odds ratio per IQR increase (310 μg/m3) | |||
PM, particulate matter; CI, confidence interval; OR, odds ratio; IQR, interquartile range
All results are adjusted for age (continuous), body mass index (continuous) and household assets (dichotomous, split at median).
Figure 3.
Prevalence odds ratios and 95% confidence intervals for the association between symptoms and stove type exposure (reference = Justa), n = 150. All results are adjusted for age (continuous), body mass index (continuous) and household assets (dichotomous, split at median).
Figure 4.
Prevalence odds ratios and 95% confidence intervals for the association between symptoms and 24-hour average personal PM2.5 exposure (per IQR increase [85 μg/m3]), n = 105. All results are adjusted for age (continuous), body mass index (continuous) and household assets (dichotomous, split at median).
Discussion
This cross-sectional study assessed the association between exposure to solid fuel cookstoves and self-reported health symptoms among women in rural Honduras. Mean personal PM2.5 exposures, expressed as a 24-hr average, in the study population were 125 μg/m3 for traditional stove users and 66 μg/m3 for Justa stove users (Table 2, Figure 2). Prevalence of each of the nine self-reported symptoms was lower in Justa stove users than in traditional stove users (Table 1), and adjusted logistic regression models showed lower odds of all nine symptoms in Justa stove users compared to traditional stove users (Table 3, Figure 3).
These results are similar to those from previous household air pollution studies that assessed the association between stove types and health symptoms. An intervention study in rural Guatemala reported reduced exposure to household air pollution and lower prevalence of sore eyes and headache in improved biomass cookstove users (Diaz et al. 2007). A randomized intervention trial in Guatemala showed reduced prevalence of chronic respiratory symptoms in participants who received the improved wood-burning intervention stove (Smith-Sivertsen et al. 2009). A cross-sectional study in Nepal showed higher associations between respiratory symptoms and biomass stove use than respiratory symptoms and non-biomass (primarily liquid petroleum gas) stove use (Kurmi et al. 2014). Women in the Nepal study had 3.90 times higher odds of reporting prevalent breathlessness (95% CI 2.00 – 7.79) and 3.55 times higher odds of reporting prevalent wheezing (95% CI 2.06 – 6.13) if using a biomass stove (Kurmi et al. 2014). An intervention study in Pakistan reported lower risk of cough, phlegm, shortness of breath and chest tightness in the improved biomass cookstove intervention group compared to traditional stove users (Jamali et al. 2017). Few studies have assessed associations between health symptoms and quantitative measures of fine particulate matter. Those that have done so report similar results to the current study, showing consistently weaker associations with measured pollution than with stove type (Regalado et al. 2006; Clark et al. 2009; Kurmi et al. 2014).
Self-reported health symptoms are important to assess due to their possible influence on health-related quality of life. One study observed strong associations between self-reported respiratory symptoms and self-reported overall mental and physical health, which the authors stated indicates a high impact on quality of life for the study population of nearly 1,000 Russian men (Cook et al. 2015). Other studies have reported findings that respiratory symptoms are associated with decreased quality of life in cancer survivors (Sarna et al. 2004) and among the general population in the United States (Wheaton et al. 2013). A cookstove intervention study in rural Bolivia reported improved respiratory health-related quality of life following implementation of ventilated biomass cookstoves by using a previously established respiratory questionnaire that incorporates quality of life measures (Alexander et al. 2014).
There are possible biologic mechanisms to support an association between lower exposure to household air pollution and lower prevalence of adverse health symptoms. Previous studies have reported associations between woodsmoke exposure and pulmonary and systemic inflammation through a number of pathways including oxidative stress and coagulation factors (Barregard et al. 2008; Ghio et al. 2012). Inflammation could be a mechanism behind some of the self-reported health symptoms in this population, such as difficulty breathing, nose and throat irritation, and cough. Similar inflammatory pathways could also cause eye irritation (Cao et al. 2015). Headache, another common symptom in this population, can be caused by carbon monoxide (CO) exposure (Carbon Monoxide’s Impact on Indoor Air Quality [Internet] 2017), which, along with PM2.5, is common among users of solid-fuel cookstoves (Carter et al. 2017).
There are limitations to cross-sectional study designs, such as the inability to distinguish whether exposure came before the symptoms we evaluated. However, this study was designed to include only participants who had been using their current stoves for at least four months, potentially attenuating the issue of temporality. Selection bias was also a potential limitation during study recruitment. However, selection bias likely had minimal impact on the observed results, as study participation was likely not based on both exposure (stove type or measured PM2.5) and symptoms of interest.
We did not observe consistent evidence of an association between the nine health symptoms and measured 24-hour average PM2.5 as we did between the symptoms and stove type. This discrepancy may be due to the influence of recall bias on the results for stove type. Women in the study may have reported symptoms they expected to experience based on their stove type, which could lead to a misclassification of the reported symptoms. If women with Justa stoves misreported that they had fewer symptoms on a consistent basis as compared to traditional stove users, it could potentially have a large influence on study results and bias estimates away from the null. In addition, household air pollution is made up of a very complex combination of hundreds of pollutants (Naeher et al. 2007). It is possible that the associations we saw between symptoms and stove type were partially caused by other unmeasured pollutants such as gases or ultrafine particles or by peaks and sub-daily patterns of PM2.5 that were not captured in our time-weighted average PM2.5 measurements (Clark, Peel, et al. 2013). However, it is also possible that stove type may have less measurement error than a single, 24-hour measurement of PM2.5, giving a better representation of a typical exposure and more accurately describing the true association between household air pollution and self-reported symptoms.
Even with the uncertainty in a single exposure measurement, a 24-hour collection of personal and kitchen PM2.5 is an important strength in this study. Personal PM2.5 is considered the most accurate exposure measure of household air pollution (Balakrishnan et al. 2014), but is used in few field studies due to cost and required resources. In this study, both personal and kitchen levels of PM2.5 were substantially lower in Justa stove users compared to traditional stove users (Table 2, Figure 2), demonstrating that improved Justa stoves did on average result in lower exposures in this population.
In conclusion, we observed lower exposure to PM2.5 and lower odds of self-reported health symptoms for women using Justa biomass stoves compared to those using traditional biomass stoves in rural Honduras. Although there are several potential limitations in these results, the lower levels of pollution and symptoms suggest improved quality of life in women cooking with improved Justa biomass cookstoves. This study adds to the body of evidence that improved cookstove technology using biomass can lead to reductions in exposure to household air pollution and potentially to meaningful improvements in health and quality of life.
Acknowledgments
We thank Jonathan Stack and Gloribel Bautista Cuellar for their excellent contributions to the data collection. We are grateful to our community leaders for helping guide us during this project.
This work was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under grant number R21ES022810. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Interest Statement
In accordance with Taylor & Francis policy and my ethical obligation as a researcher, I am reporting that two of the authors, Sebastian Africano of Trees, Water & People (TWP), and Anibal Benjamin Osorto of Asociación Hondureña para el Desarrollo (AHDESA), are members of the implementing non-governmental organizations that deploy the cookstove technology studied in this paper. Results of research like this are often shown as evidence of the effectiveness of this particular cookstove technology in TWP and AHDESA publications, including blogs, articles, and grant proposals, which may lead to future funding of these initiatives by individual and/or institutional supporters of the respective organizations. I have disclosed those interests fully to Taylor & Francis, and have in place an approved plan for managing any potential conflicts arising from this arrangement.
References:
- Alexander D, Linnes JC, Bolton S, Larson T. 2014. Ventilated cookstoves associated with improvements in respiratory health-related quality of life in rural Bolivia. J Public Health. 36(3):460–466. eng. [DOI] [PubMed] [Google Scholar]
- Arps S 2011. Socioeconomic status and body size among women in Honduran Miskito communities. Ann Hum Biol. 38(4):508–519. [DOI] [PubMed] [Google Scholar]
- Balakrishnan K, Sankar S, Ghosh S, Thangavel G, Mukhopadhyay K, Ramaswamy P, Johnson P, Thanasekaraan V. 2014. Household Air Pollution Related to Solid Cookfuel Use: The Exposure and Health Situation in Developing Countries. Berlin. 1867-979X 1616-864X. [Google Scholar]
- Barregard L, Sallsten G, Andersson L, Almstrand AC, Gustafson P, Andersson M, Olin AC. 2008. Experimental exposure to wood smoke: effects on airway inflammation and oxidative stress. Occup Environ Med. 65(5):319–324. [DOI] [PubMed] [Google Scholar]
- Baumgartner J, Schauer JJ, Ezzati M, Lu L, Cheng C, Patz JA, Bautista LE. 2011. Indoor air pollution and blood pressure in adult women living in rural China. Environ Health Perspect. 119(10):1390–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce N, Perez-Padilla R, Albalak R. 2000. Indoor air pollution in developing countries: a major environmental and public health challenge. Bull World Health Organ. 78(9):1078–1092. [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Bindslev DA, Kjaergaard SK. 2015. Estimation of the in vitro eye irritating and inflammatory potential of lipopolysaccharide (LPS) and dust by using reconstituted human corneal epithelium tissue cultures. Toxicol Mech Meth. 25(5):402–409. [DOI] [PubMed] [Google Scholar]
- Carbon Monoxide’s Impact on Indoor Air Quality [Internet]. 2017. US EPA; [accessed]. https://www.epa.gov/indoor-air-quality-iaq/carbon-monoxides-impact-indoor-air-quality.
- Carter E, Norris C, Dionisio KL, Balakrishnan K, Checkley W, Clark ML, Ghosh S, Jack DW, Kinney PL, Marshall JD et al. 2017. Assessing Exposure to Household Air Pollution: A Systematic Review and Pooled Analysis of Carbon Monoxide as a Surrogate Measure of Particulate Matter. Environ Health Perspect. 125(7):076002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark ML, Bachand AM, Heiderscheidt JM, Yoder SA, Luna B, Volckens J, Koehler KA, Conway S, Reynolds SJ, Peel JL. 2013. Impact of a cleaner-burning cookstove intervention on blood pressure in Nicaraguan women. Indoor air. 23(2):105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark ML, Peel JL, Balakrishnan K, Breysse PN, Chillrud SN, Naeher LP, Rodes CE, Vette AF, Balbus JM. 2013. Health and Household Air Pollution from Solid Fuel Use: The Need for Improved Exposure Assessment. Environmental Health Perspectives. 121(10):1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark ML, Peel JL, Burch JB, Nelson TL, Robinson MM, Conway S, Bachand AM, Reynolds SJ. 2009. Impact of improved cookstoves on indoor air pollution and adverse health effects among Honduran women. Int J Environ Health Res. 19(5):357–368. [DOI] [PubMed] [Google Scholar]
- Cook S, Quint JK, Vasiljev M, Leon DA. 2015. Self-reported symptoms of chronic cough and breathlessness in working-age men in the city of Izhevsk, Russia: associations with cardiovascular disease risk factors and comorbidities. BMJ open respiratory research. 2(1):e000104. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz E, Bruce N, Pope D, Diaz A, Smith KR, Smith-Sivertsen T. 2008. Self-rated health among Mayan women participating in a randomised intervention trial reducing indoor air pollution in Guatemala. BMC Int Health Hum Rights. 8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz E, Smith-Sivertsen T, Pope D, Lie RT, Diaz A, McCracken J, Arana B, Smith KR, Bruce N. 2007. Eye discomfort, headache and back pain among Mayan Guatemalan women taking part in a randomised stove intervention trial. J Epidemiol Community Health. 61(1):74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegard A 1996. Cooking Fuel Smoke and Respiratory Symptoms among Women in Low-income Areas in Maputo. Environ Health Perspect. 104(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gakidou E, Afshin A, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F. 2017. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 390:1345–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio AJ, Soukup JM, Case M, Dailey LA, Richards J, Berntsen J, Devlin RB, Stone S, Rappold A. 2012. Exposure to wood smoke particles produces inflammation in healthy volunteers. Occup Environ Med. 69(3):170–175. [DOI] [PubMed] [Google Scholar]
- Gordon SB, Bruce NG, Grigg J, Hibberd PL, Kurmi OP, Lam KB, Mortimer K, Asante KP, Balakrishnan K, Balmes J et al. 2014. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med. 2(10):823–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri M, Diaz E, Pope D, Eisen EA, Mann J, Smith KR, Smith-Sivertsen T, Bruce NG, Balmes JR. 2015. Lung Function in Rural Guatemalan Women Before and After a Chimney Stove Intervention to Reduce Wood Smoke Exposure Results From the Randomized Exposure Study of Pollution Indoors and Respiratory Effects and Chronic Respiratory Effects of Early Childhood Exposure to Respirable Particulate Matter Study. Chest. 148(5):1184–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett P, Ganser GH. 2007. A comparison of several methods for analyzing censored data. Ann Occup Hyg. 51(7):611–632. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. 1990. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl Occup Env Hyg. 5(1). [Google Scholar]
- Jamali T, Fatmi Z, Shahid A, Khoso A, Kadir MM, Sathiakumar N. 2017. Evaluation of short-term health effects among rural women and reduction in household air pollution due to improved cooking stoves: quasi experimental study. Air Qual Atmos Health. [Google Scholar]
- Kshirsagar MP, Kalamkar VR. 2014. A comprehensive review on biomass cookstoves and a systematic approach for modern cookstove design. Renew Sust Energ Rev. 30:580–603. [Google Scholar]
- Kurmi OP, Semple S, Devereux GS, Gaihre S, Lam KB, Sadhra S, Steiner MF, Simkhada P, Smith WC, Ayres JG. 2014. The effect of exposure to biomass smoke on respiratory symptoms in adult rural and urban Nepalese populations. Environ Health. 13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall D, Lal J, Amore F, Langner R, Cox G. 1980. Guidelines for data acquisition and data quality evaluation in environmental chemistry. Anal Chem. 52(14):2242–2249. [Google Scholar]
- McCracken JP, Smith KR, Diaz A, Mittleman MA, Schwartz J. 2007. Chimney stove intervention to reduce long-term wood smoke exposure lowers blood pressure among Guatemalan women. Environ Health Perspect. 115(7):996–1001. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer K, Ndamala CB, Naunje AW, Malava J, Katundu C, Weston W, Havens D, Pope D, Bruce NG, Nyirenda M et al. 2017. A cleaner burning biomass-fuelled cookstove intervention to prevent pneumonia in children under 5 years old in rural Malawi (the Cooking and Pneumonia Study): a cluster randomised controlled trial. The Lancet. 389(10065):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeher L, Brauer M, Lipsett M, Zelikoff J, Simpson C, Koenig J, Smith K. 2007. Woodsmoke Health Effects: A Review. Inhal Toxicol. 19:67–106. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijsen MJ. 2015. Exposure Assessment in Environmental Epidemiology. Oxford University Press. [Google Scholar]
- Oluwole O, Ana GR, Arinola GO, Wiskel T, Falusi AG, Huo DZ, Olopade OI, Olopade CO. 2013. Effect of stove intervention on household air pollution and the respiratory health of women and children in rural Nigeria. Air Qual Atmos Health. 6(3):553–561. [Google Scholar]
- Pope D, Diaz E, Smith-Sivertsen T, Lie RT, Bakke P, Balmes JR, Smith KR, Bruce NG. 2015. Exposure to Household Air Pollution from Wood Combustion and Association with Respiratory Symptoms and Lung Function in Nonsmoking Women: Results from the RESPIRE Trial, Guatemala. Environ Health Perspect. 123(4):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regalado J, Perez-Padilla R, Sansores R, Paramo Ramirez JI, Brauer M, Pare P, Vedal S. 2006. The effect of biomass burning on respiratory symptoms and lung function in rural Mexican women. Am J Respir Crit Care Med. 174(8):901–905. [DOI] [PubMed] [Google Scholar]
- Romieu I, Riojas-Rodriguez H, Marron-Mares AT, Schilmann A, Perez-Padilla R, Masera O. 2009. Improved biomass stove intervention in rural Mexico: impact on the respiratory health of women. Am J Respir Crit Care Med. 180(7):649–656. [DOI] [PubMed] [Google Scholar]
- Rumchev K, Win T, Bertolatti D, Dhaliwal S. 2015. Prevalence of respiratory symptoms among children in rural Myanmar-disease burden assessment attributable to household biomass smoke. Indoor Built Environ. 25(5):728–736. [Google Scholar]
- Sarna L, Evangelista L, Tashkin D, Padilla G, Holmes C, Brecht ML, Grannis F. 2004. Impact of Respiratory Symptoms and Pulmonary Function on Quality of Life of Long-term Survivors of Non-Small Cell Lung Cancer. Chest. 125:439–445. [DOI] [PubMed] [Google Scholar]
- Schei MA, Hessen JO, Smith KR, Bruce N, McCracken J, Lopez V. 2004. Childhood asthma and indoor woodsmoke from cooking in Guatemala. J Expo Anal Environ Epidemiol. 14 Suppl 1:S110–117. [DOI] [PubMed] [Google Scholar]
- Singh A, Tuladhar B, Bajracharya K, Pillarisetti A. 2012. Assessment of effectiveness of improved cook stoves in reducing indoor air pollution and improving health in Nepal. Energy Sustain Dev. 16(4):406–414. [Google Scholar]
- Smith-Sivertsen T, Diaz E, Pope D, Lie RT, Diaz A, McCracken J, Bakke P, Arana B, Smith KR, Bruce N. 2009. Effect of reducing indoor air pollution on women’s respiratory symptoms and lung function: the RESPIRE Randomized Trial, Guatemala. Am J Epidemiol. 170(2):211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Bruce N, Balakrishnan K, Adair-Rohani H, Balmes J, Chafe Z, Dherani M, Hosgood HD, Mehta S, Pope D et al. 2014. Millions dead: how do we know and what does it mean? Methods used in the comparative risk assessment of household air pollution. Annu Rev Public Health. 35:185–206. [DOI] [PubMed] [Google Scholar]
- Smith KR, McCracken JP, Weber MW, Hubbard A, Jenny A, Thompson LM, Balmes J, Diaz A, Arana B, Bruce N. 2011. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. The Lancet. 378(9804):1717–1726. [DOI] [PubMed] [Google Scholar]
- Wheaton AG, Ford ES, Thompson WW, Greenlund KJ, Presley-Cantrell LR, Croft JB. 2013. Pulmonary function, chronic respiratory symptoms, and health-related quality of life among adults in the United States--National Health and Nutrition Examination Survey 2007-2010. BMC public health. 13:854. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KO, Hunter Rowe B, Douwes J, Senthilselvan A. 2013. Asthma and wheezing are associated with depression and anxiety in adults: an analysis from 54 countries. Pulm Med. 2013:929028. [DOI] [PMC free article] [PubMed] [Google Scholar]