Abstract
Background:
Impaired cerebral autoregulation and cerebral hypoperfusion may play a critical role in the high morbidity and mortality in patients with sepsis-associated encephalopathy (SAE). Bedside assessment of cerebral autoregulation may help individualize hemodynamic targets that optimize brain perfusion. We hypothesize that near-infrared spectroscopy (NIRS)– derived cerebral oximetry can identify blood pressure ranges that enhance autoregulation in patients with SAE and that disturbances in autoregulation are associated with severity of encephalopathy.
Methods:
Adult patients with acute encephalopathy directly attributable to sepsis were followed using NIRS-based multimodal monitoring for 12 consecutive hours. We used the correlation in time between regional cerebral oxygen saturation and mean arterial pressure (MAP) to determine the cerebral oximetry index (COx) as a measure of cerebral autoregulation. Autoregulation curves were constructed for each patient with averaged COx values sorted by MAP in 3 sequential 4-hour periods; the optimal pressure (MAPOPT), defined as the MAP associated with most robust autoregulation (lowest COx), was identified in each period. Severity of encephalopathy was measured with Glasgow coma scale (GCS).
Results:
Six patients with extracranial sepsis met the stringent criteria specified, including no pharmacological sedation or neurologic premorbidity. Optimal MAP was identified in all patients and ranged from 55 to 115 mmHg. Additionally, MAPOPT varied within individual patients over time during monitoring. Disturbed autoregulation, based on COx, was associated with worse neurologic status (GCS < 13) both with and without controlling for age and severity of sepsis (adjusted odds ratio [OR]: 2.11; 95% confidence interval [CI]: 1.77–2.52; P < .001; OR: 2.97; 95% CI: 1.63–5.43; P < .001).
Conclusions:
In this high-fidelity group of patients with SAE, continuous, NIRS-based monitoring can identify blood pressure ranges that improve autoregulation. This is important given the association between cerebral autoregulatory function and severity of encephalopathy. Individualizing blood pressure goals using bedside autoregulation monitoring may better preserve cerebral perfusion in SAE than current practice.
Keywords: sepsis, cerebral autoregulation, hemodynamics, sepsis-associated encephalopathy, oximetry, critical care, multimodal monitoring, near-infrared spectroscopy
Introduction
Sepsis is a common condition in the intensive care unit (ICU), which affects a clinically diverse population and carries a high risk of morbidity and mortality.1,2 Sepsis-associated encephalopathy (SAE), estimated to occur in up to 70% of patients with sepsis, is independently associated with increased mortality and long-term cognitive disability among survivors.3,4 This form of encephalopathy is an acute change in mental status in the setting of sepsis without direct evidence of central nervous system (CNS) infection. As in sepsis, the manifestation of SAE, the patients affected, and the course of illness are notoriously heterogeneous. Symptoms of SAE can vary from delirium to coma, with mortality rates over 60% in comatose patients with sepsis.5,6 Detection of SAE is particularly challenging in the ICU, where pharmacological sedation can cloud the neurologic picture. Unfortunately, there are no precise, well-established clinical markers to guide cardiovascular therapy if SAE is suspected.7,8
The etiology of SAE is multifactorial, and the pathophysiology is not fully understood. Recent studies in both humans and animals suggest that altered regulation of the cerebral circulation may play a role in SAE.9,10 In health, cerebral autoregulation maintains constant brain perfusion in the face of fluctuating blood pressure by adjusting the diameter of cerebral blood vessels, a process known as cerebrovascular reactivity. Higher and lower blood pressure limits of functional cerebral autoregulation vary among individuals, with cerebral blood flow passive to changes in arterial pressure outside these limts.11,12 Cerebral ischemic lesions can be seen on magnetic resonance imaging (MRI) of patients with SAE and on autopsy of patients who have died from septic shock, suggesting that a reduction in cerebral blood flow may precipitate SAE.13,14 Such MRI and postmortem findings may be due to impaired cerebrovascular reactivity and subsequent pressure-passive cerebral blood flow or a consequence of cerebral hypoperfusion below the lower limit of autoregulation.
One of the hallmarks of sepsis is impaired systemic vasoreactivity, which manifests as hypotension as sepsis progresses to septic shock. Therapy is focused on fluid resuscitation and judicious use of vasopressors, with a recommended mean arterial pressure (MAP) target of greater than 65 mm Hg to maintain organ perfusion.15 Restoring satisfactory organ perfusion is essential to surviving sepsis, and preserving adequate cerebral perfusion may be the key to improving outcomes in SAE. Present guidelines for hemodynamic targets in sepsis, however, rely on historical concepts of cerebral autoregulation thresholds, which have evolved alongside technology to be more precise.16–18 Furthermore, universal blood pressure targets may not be equally efficacious in all patients given the marked heterogeneity in sepsis and the complexity of the host response to sepsis.
Studies in critically ill patients with primary neurologic injury such as traumatic brain injury (TBI) and in patients at risk of cerebral hypoperfusion such as those undergoing cardiopulmonary bypass have demonstrated that individualized autoregulation-guided perfusion pressure management (termed “optimal blood pressure”) is feasible and may improve outcomes.19–23 The primary objective of this pilot study was to test the hypothesis that continuous autoregulation monitoring using near-infrared spectroscopy (NIRS)–based cerebral oximetry can identify optimal blood pressure in individual ICU patients with SAE. Pre-existing neuropathology and pharmacological sedation may influence the diagnosis of SAE and perhaps autoregulation.24,25 Therefore, we selected a set of patients with acute encephalopathy directly attributable to sepsis without neurological premorbidities or alternative causes of delirium, such as pharmacological sedation. Autoregulation measures included optimal MAP (MAPOPT) for most robust autoregulation and hourly mean cerebral oximetry index (COx), a measure of autoregulatory function. Our secondary objective was to test the hypothesis that higher COx, that is, reduced cerebral autoregulatory capacity, is associated with lower Glasgow coma scale (GCS) in this high-fidelity group.
Methods
Patient Selection and Management
Six patients who met specified criteria for SAE were selected from a prospective observational study, commenced in 2015 and approved by the institutional review board of Johns Hopkins University (JHU, Baltimore, Maryland). Adult patients with sepsis from different etiologies were monitored with NIRS within the first 48 hours of admission to the ICU after stabilization. All patients had an arterial catheter to measure blood pressure continuously, which was placed at the discretion of the patients’ treating physicians. Written informed consent was obtained from all patients or their legally authorized representatives. All diagnostic and therapeutic procedures were performed as clinically indicated, and primary providers were blinded to the NIRS-derived autoregulation index.
Data from the first half-day (12 hours) of uninterrupted autoregulation monitoring was analyzed from the six selected patients, who all exhibited an acute change in mental status within 2 days of study enrollment. Inclusion and exclusion criteria for the current study are outlined in Table 1. Importantly, patients were excluded from the current analysis based on the following: CNS source of infection; pharmacologic sedation, drug toxicity, or pharmacological agent–related side effects; pre-existing intracranial pathology; pre-existing suspicion or diagnosis of dementia; and delirium or altered consciousness attributable to causes other than sepsis. Documentation in the electronic medical record (EMR) of altered mental status congruent with SAE was required; this included reduced attention, disorientation, agitation, change in cognition, perceptual disturbances, stupor, and coma.26 Since sedation with hypnotic, anesthetic, or anxiolytic agents can affect the ability to accurately assess mental status and may contribute to delirium and signs of stupor or coma,27,28 patients receiving propofol, benzodiazepines, and other forms of pharmacologic sedation within 72 hours of study monitoring were excluded. The Informant Questionnaire on Cognitive Decline in the Elderly, an assessment of cognitive decline independent of premorbid ability, was administered to family members upon study enrollment to ensure exclusion of patients with pre-existing neurocognitive deficits.29 Severity of encephalopathy was graded using the GCS.6,30,31 Glasgow coma scale was assessed by bedside critical care nurses trained in performing this evaluation and scores were extracted from the EMR. Patient characteristics, such as source of infection, pathogen type, and significant comorbidities, as well as clinically rele vant data routinely collecting during the monitoring period, such as temperature, arterial partial pressure of carbon dioxide (PaCO2), and hemoglobin level, were also extracted from the EMR. The severity of sepsis was measured at time of admission to the ICU using the Acute Physiology and Chronic Health Evaluation (APACHE) II score.32
Table 1.
Inclusion/Exclusion Criteria for Study Patient Selection Into Case Series.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Men and women age > 18 years | Central nervous system source of infection |
| Sepsis or septic shock with an acute change in mental status | Patients receiving propofol, benzodiazepines, or other forms of pharmacological sedation within 72 hours of study monitoring |
| Arterial line pressure monitoring | Drug toxicity or pharmacological agent–related side effects |
| Acute change in mental status (included reduced attention, disorientation, agitation, change in cognition, perceptual disturbances, stupor, and coma) | History of cerebrovascular or intracranial neurological disease (including stroke or tumor) |
| Pre-existing suspicion or diagnosis of dementia, cognitive decline or psychiatric disease Delirium attributable to a cause other than sepsis |
Autoregulation Monitoring
Two self-adhesive cerebral oximetry sensors (INVOS; Covidien, Boulder, Colorado) were placed bilaterally on the patients’ foreheads upon study enrollment and connected to the NIRS monitor (INVOS 5100C; Covidien, Mansfield, Massachusetts) to measure changes in regional cerebral oxygen saturation (rScO2). The NIRS rScO2 signals along with analog outputs from arterial pressure monitoring were transferred to a laptop computer via an analog-to-digital converter (DT9800; Data Translation, Inc., Marlboro, Massachusetts) and processed using ICM Software (University of Cambridge, Cambridge, United Kingdom). The first uninterrupted 12-hour period of monitoring was analyzed using methodology previously described.33,34 Briefly, NIRS and arterial pressure signals were filtered as nonoverlapping 10-second mean values that were time integrated, which is equivalent to having a moving average filter with a 10-second time window and resampling at 0.1 Hz. This eliminates high-frequency oscillations from respiration and pulse waveforms to focus on the slow-frequency (0.33–3 cycles per minute) vasogenic waves mediating cerebral autoregulation, known as Lundberg’s B-waves.35–40 A continuous, moving Pearson’s correlation coefficient between changes in MAP and fluctuations in rScO2 was calculated, rendering the variable COx. Consecutive average COx within 10-second windows was collected at 30 data points to monitor each COx in a 300-second window and continuously updated, resulting in 360 COx calculations per hour. The autoregulation index, COx, is based on the assumption that changes in tissue oxygen saturation measured with cortical reflectance oximetry are proportional to changes in cerebral blood flow over brief periods with steady cerebral metabolic rate.36,41 When autoregulation is active, rScO2 is either not correlated or negatively correlated with arterial blood pressure, yielding near-zero or negative COx values. Without autoregulation, rScO2 positively correlates with arterial pressure, resulting in positive COx values42 (Figure 1). Average hourly COx values were derived for the entire 12-hour monitoring period, with the exception of one subject who was monitored for only 10 hours because of arterial cannula removal. Thus, the total analysis time across all six patients amounted to 70 hours. Artifacts in the NIRS and MAP signals (eg, arterial line flushes and arterial blood gas sampling) were removed manually.
Figure 1.
A, B, Calculation of the cerebral oximetry index (COx) in a 71-year-old man with septic shock from disseminated Nocardia. When mean arterial pressure (MAP) was approximately 55 to 90 mm Hg, MAP and regional cerebral oxygen saturation (rScO2) measured by near-infrared spectroscopy were positively correlated. The positive correlation yielded a COx value of 0.78, indicating pressure-passive cerebral blood flow with impaired autoregulation. The linear regression line is illustrated (E[Y] = 48.1+0.16X; 95% confidence interval for slope: 0.15, 0.17; P < .001). C, D, Calculation of COx in a 69-year-old patient with septic shock from Clostridium difficile colitis. When MAP was approximately 80–100 mm Hg, MAP and rScO2 were negatively correlated. This negative correlation resulted in a COx value of ‒0.42, indicating pressure-reactive cerebral blood flow with functional autoregulation. The linear regression line is illustrated (E[Y] = 74.54–0.29X; 95% confidence interval for slope: ‒0.35, ‒0.22; P < .001).
Optimal Blood Pressure
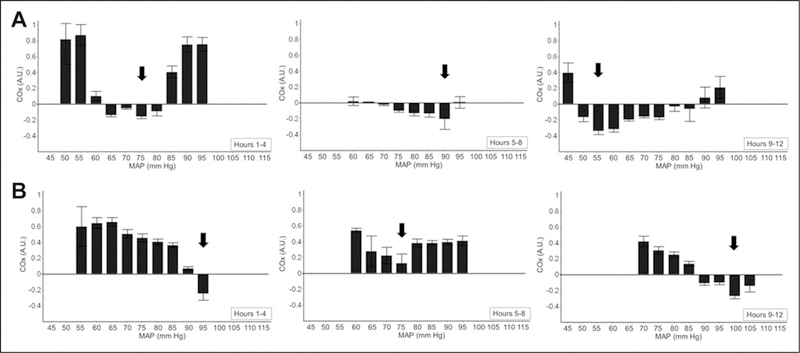
Autoregulation-guided “optimal MAP” (MAPOPT) is the arterial pressure at which an individual’s cerebral autoregulation is functioning at its best. This is the MAP associated with the lowest COx. Specifically, MAPOPT represents the 5 mm Hg range of MAP in which autoregulation is most responsive to changes in perfusion pressure, and consequently, rScO2 is least influenced by systemic blood pressure. We used standard methods to identify this range.19–22 First, each patient’s monitoring period was divided into three 4-hour sequential periods of time, based on commonly measured time periods in autoregulation studies of TBI and coma patients.43 Mean arterial pressure values were then divided into groups of 5-mmHg bins (eg, 60–65 mm Hg) for each 4-hour interval. Next, COx was averaged within these binned groups separately for the first, second, and third interval to generate three bar graphs. The value for MAPOPT was accepted if the graph during each 4-hour period showed a distinct minimum value for mean COx, ie, mean values for COx in both adjacent MAP bins were higher (Figure 2).19 If a distinct valley in the graph was not easily identified or if there was more than 1 valley, MAPOPT was defined as the MAP with the absolute lowest hourly average COx (nadir) in each of the 4-hour periods (eg, see Figure 3).21,22 The MAP associated with the lowest COx was chosen when the left and right hemisphere differed.
Figure 2.
Representative graph of cerebral autoregulation by near-infrared spectroscopy (NIRS)–derived cerebral oximetry (COx) monitoring during a 4-hour period. The top graph represents the time series of blood pressure, and the bottom bar-graph represents the percentage of the 4-hour monitoring time spent at each 5-mm Hg bin. Optimal mean arterial pressure (MAPOPT) was defined as that MAP with the lowest COx. In this example, MAPOPT is 65 mm Hg (arrow). AU indicates arbitrary unit; MAP, mean arterial pressure.
Figure 3.
Cerebral oximetry monitoring for 12 hours during sepsis in 2 patients. Mean values of COx were sorted into 5-mm Hg bins of mean arterial pressure (MAP) to determine optimal mean arterial pressure (MAPOPT) for most active autoregulation based on a valley in the graph or the lowest (nadir) COx. A, Patient 2. Cerebral autoregulation was most robust at a MAP of 75, 90, and 55 mm Hg during the first, second, and third 4-hour periods, respectively, as indicated by the valley in each bar graph (arrows). B, Patient 3. The blood pressure at which autoregulation was most functional MAPOPT was 95, 75, and 100 mm Hg during the first, second, and third 4-hour periods, respectively (arrows). In the first 4-hour period of monitoring in patient 3, a valley in the graph was not apparent; instead, the lowest (nadir) COx indicated MAPOPT. Error bars represent the standard error of the mean.
Autoregulation and Encephalopathy
In addition to identifying the optimal blood pressure for best autoregulation, we sought to determine if poor autoregulation, and thus pressure-dependent cerebral blood flow, was associated with SAE. To do this, we examined the relationship between average hourly COx, an index of cerebral autoregulatory function measured with NIRS, and severity of encephalopathy, measured with GCS. The exposure variable, COx, is a moving, linear (Pearson’s) correlation coefficient between MAP and rScO2, using a sliding 300-second window updated every 10 seconds. Cerebral oximetry index approaches 0 or is negative when cerebral autoregulation is active and COx approaches 1 when cerebral autoregulation is lost. The highest average COx was chosen for each patient when values from the right and left hemisphere differed during any given hour. The response variable was worst (lowest) GCS during monitoring. Other exposure variables were mean hourly MAP and mean hourly NIRS rScO2 signals to evaluate the independent effect of hypotension and frontal cortex oxyhemoglobin saturation, respectively, on neurologic status.
Data Analysis
The current study was designed to generate pilot and feasibility data for autoregulation monitoring of patients with SAE in order to inform the development of a larger cohort study. Descriptive statistics and graphical displays were used to evaluate the primary hypothesis that we can identify an MAPOPT to preserve cerebral autoregulation in patients with SAE. Specifically, cerebral autoregulation was quantified by constructing an autoregulation curve for each patient by binning and averaging discrete COx values according to the average arterial blood pressure (5-mm Hg bins; Figures 2 and 3). The individual MAPOPT was defined for each patient by systematically reviewing individual histograms. The Shapiro–Wilk test was used to assess the normality of distribution of investigated parameters. All COx values in our study were normally distributed and are reported as mean values and standard errors. Bar graphs of binned COx values identified using linear regression, as detailed earlier, were used to evaluate our primary hypothesis. Mean arterial pressure and rScO2 were recorded every 10 seconds by the bedside software. Average hourly MAP and rScO2 values were calculated from their normally distributed recordings. However, hourly MAP and rScO2 values were not normally distributed within or between patients across the 12 hours of monitoring and thus are reported as medians and interquartile ranges (IQRs). Age, gender, PaCO2, hemoglobin level, temperature, and APACHE II scores are reported as medians and IQRs or absolute values, ranges and percentages when appropriate. Glasgow coma scale scores were dichotomized using a median split with a cutoff score of 13 to indicate mild (GCS≥13) versus moderate to severe (GCS < 13) encephalopathy.6,30,44 We used logistic regression models fit with generalized estimating equations and robust variance estimation to examine the association of mean hourly COx with the worst GCS, assuming an independence working model for the within-cluster correlation structure. Mean arterial pressure and rScO2 were examined independently through uni-variable analyses of their individual association with the primary outcome, worst GCS. Comparisons between low and high GCS groups were made using the Mann–Whitney U test. A 2-tailed P < .05 was used as the cutoff for statistical significance. All analyses were conducted using Stata Version 15 (StataCorp, College Station, Texas).
Results
Eighty-three patients admitted with sepsis were screened and six patients were selected who met the stringent criteria outlined in Table 1; most patients were excluded for failure to receive no sedation and failure to exclude causes of encephalopathy other than sepsis such as hepatic encephalopathy and structural brain lesions. All patients were resuscitated with vasopressors either prior to or during monitoring. The median age was 70 years (range 54–76) and two-third of the patients were male. The median APACHE II score at admission was 34 (range 31–37) and the worst GCS during monitoring ranged from 6 to 14. Two of the six patients died before 60 days from complications of septic shock and the families’ decision to initiate comfort measures. Clinical characteristics of the six patients are summarized in Table 2.
Table 2.
Clinical Characteristics of the Selected Cohort of Patients.
| Pt no. | Age (years) | Sex | LOS ICU | Source of Infection | Inciting Pathogen | APACHE II | GCS | Significant Comorbidity |
|---|---|---|---|---|---|---|---|---|
| 1 | 69 | M | 4 | Abdominal | Clostridium difficile | 34 | 14 | Cancer, COPD, CRI, DM, HTN |
| 2 | 71 | F | 5 | Urinary | Klebsiella + MRSA | 31 | 13 | Cancer, COPD, CRI, DM, HTN |
| 3 | 71 | M | 42 | Pneumonia | Nocardia | 34 | 11 | AFIB, COPD, CRI, HTN, HLD |
| 4 | 58 | F | 11 | Pneumonia | Group B streptococcus | 37 | 8 | AFIB, cancer, CHF, CRI, COPD, DM, HLD, HTN |
| 5 | 54 | M | 9 | Unknown | MRSA | 33 | 6 | AFIB, HTN |
| 6 | 76 | M | 3 | Abdominal/urinary | Enterobacter cloacae | 34 | 13 | COPD, CRI, DM, HLD, HTN |
Abbreviations: AFIB, atrial fibrillation; APACHE II, Acute Physiology and Chronic Health Evaluation; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CRI, chronic renal insufficiency; DM, diabetes mellitus; GCS, Glasgow coma scale; HLD, hyperlipidemia; HTN, hypertension; LOS ICU, length of stay in intensive care unit in days; Pt no, patient number.
Optimal Blood Pressure
The median MAP during the 10 to 12 hours of monitoring between all six patients was 86 mm Hg (IQR: 72–92) and the median rScO2 was 54% (IQR: 46–59). Optimal MAP values were identified in all six patients. Optimal MAP ranged from 55 to 115 mm Hg with a median value of 85 mm Hg (IQR: 70–100) across all six patients (Table 3). Notably, MAPOPT varied widely within individual patients during the half-day of monitoring. Among the three 4-hour intervals, MAPOPT differed as much as 35 mm Hg (patient 2) and as little as 10 mmHg (patient 5). For example, MAPOPT in patient 5 was 65 mm Hg in the first and second 4 hours of monitoring and 55 mm Hg in the third 4 hours of monitoring. In contrast, MAPOPT in patient 2 was 75 mm Hg in the first 4 hours, 90 mm Hg in the second 4 hours, and 55 mm Hg in the third 4 hours. Determination of MAPOPT for patients 2 and 3 are demonstrated in Figure 3. Plots for all six patients are shown in the Supplemental Figures.
Table 3.
Optimal Mean Arterial Pressure (MAPOPT) for Best Autoregulation. Data are from three sequential 4-hour periods of monitoring.
| MAPOPT |
|||
|---|---|---|---|
| Pt no. | 1–4 Hours | 5–8 Hours | 9–12 Hours |
| 1 | 80 | 80 | 95a |
| 2 | 75 | 90 | 55 |
| 3 | 95 | 75 | 100 |
| 4 | 90 | 70 | 100 |
| 5 | 65 | 65 | 55 |
| 6 | 115 | 100 | 100 |
Abbreviation: Pt no., patient number.
Hours 9–10 only.
Autoregulation and Encephalopathy
Compared to patients with mild encephalopathy (GCS≥13), patients with moderate to severe SAE (GCS < 13) did not differ on measures of average hourly MAP (P = .52) or rScO2 as assessed with NIRS (P =.79). However, average hourly COx was higher in patients with moderate to severe SAE (0.19±0.13; 95% confidence interval [CI]: 0.14–0.23) compared with patients with mild SAE (0.00 ± 0.13; 95% CI: ‒0.05 to 0.05; Figures 4 and 5). Higher COx values were associated with significantly worse neurologic status (GCS < 13; odds ratio [OR]: 2.97; 95% CI: 1.63–5.43; P < .001). The association between COx and GCS remained statistically significant even after adjusting for age and APACHE II scores (adjusted OR: 2.11; 95% CI: 1.77–2.52; P < .001). Admission APACHE II score, PaCO2 (median 40 mmHg [IQR: 32–45]), temperature (median 36.9○C [IQR: 35.7–38.3]), and hemoglobin level (median 8.4 g/dL [IQR: 6.3–9.2]) did not differ between patients with mild encephalopathy and moderate to severe encephalopathy. There were no significant correlations between COx and APACHE II score, COx and PaCO2, COx and temperature, nor COx and hemoglobin level.
Figure 4.
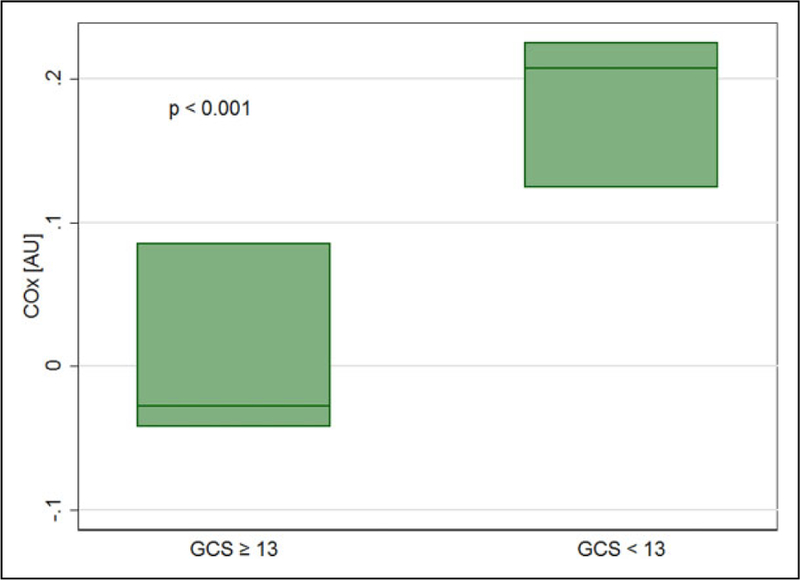
Cerebral autoregulation is significantly different in sepsis patients with moderate to severe encephalopathy and mild encephalopathy. Boxes show median (line inside boxes) and interquartile range (box limits). COx indicates cerebral oximetry index; GCS, Glasgow coma scale.
Figure 5.
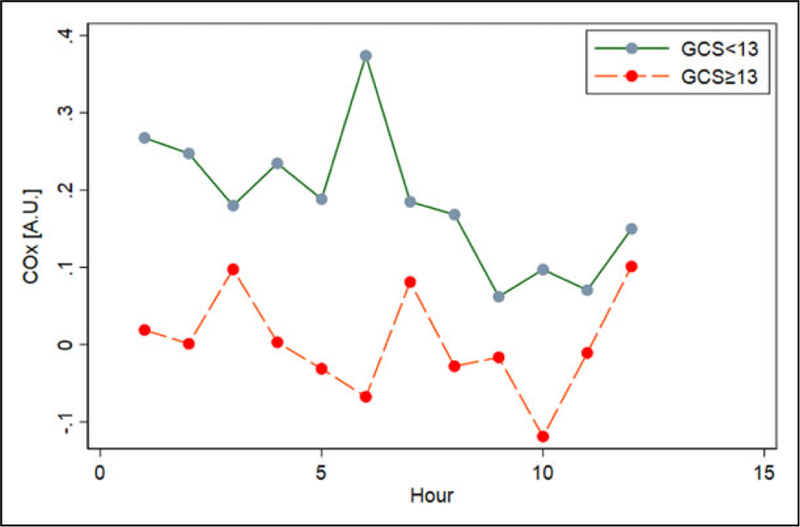
Cerebral oximetry index (COx) for 10 to 12 consecutive hours during sepsis grouped according to Glasgow coma scale (GCS).
Discussion
In our small group of patients with SAE, we demonstrate that autoregulation monitoring with NIRS-derived COx can identify optimal blood pressure for cerebral autoregulatory function. We also show that optimal blood pressure varies between patients and within patients over time during sepsis. Furthermore, in these six well-screened, nonpharmacologically sedated patients, we showed a strong association between cerebral autoregulatory function and neurological status. Hourly COx measurements were consistently higher in patients with a lower GCS and lower in patients with a higher GCS, suggesting that autoregulatory dysfunction plays a role in the pathophysiology of SAE. This association was not observed between NIRS-measured rScO2 and GCS nor arterial pressure and GCS, further suggesting that thresholds delineating detrimental cerebral hypoperfusion differ between individuals. In light of the wide variability in optimal blood pressure identified between and within our study patients, these secondary findings support the aforementioned hypothesis that impairment of autoregulation in SAE may be due to blood pressure below the optimal range.
Autoregulation is traditionally considered impaired or absent when blood pressure is below or above its functional thresholds. Following injury and possibly in sepsis, the capacity for autoregulation may be lost completely or the thresholds for autoregulation may be reset. The complex pathophysiology of SAE is thought to involve direct and indirect toxicity to the brain parenchyma, disruption of the blood brain barrier, mitochondrial and endothelial dysfunction, and neurotransmission disturbances.7,45,46 Based on the results of our small study, the effect of these mechanisms on cerebral autoregulation and brain perfusion may be substantial.5,47
Both intact48 and disrupted10,49 cerebral autoregulation have been demonstrated in patients with sepsis, but few studies have examined autoregulation specifically in patients with SAE.10,49 This paucity of knowledge is justified by the challenge of studying SAE in the ICU; it is difficult to distinguish brain dysfunction as a result of sepsis from other causes of altered mental status in critically ill patients.4 There are no precise clinical or biological markers of injury that identify SAE,7,8 and radiological and electrophysiological parameters are not optimal for delineating diagnoses.50–53 Sepsis-associated encephalopathy, thus, remains a diagnosis of exclusion and can be underdiagnosed, or sometimes, misdiagnosed in the ICU, where pharmacological sedation may be necessary to assist mechanical ventilation and other invasive treatments.
A recent review of cerebral blood flow autoregulation in adults with sepsis found that autoregulation is impaired more often in patients with SAE than in those without SAE and more often in early versus late sepsis.54 Impairment of autoregulation during sepsis, or pressure-passive (vs pressure-reactive) cerebral blood flow, may be due to cerebrovascular dysfunction and microcirculatory changes or merely from profound hypotension with cerebral hypoperfusion below the lower limit of autoregulation. Of the studies reviewed, those specifically looking at cerebrovascular reactivity found that cerebral vasculature maintains vasodilatory ability, which makes vascular dysfunction a less likely explanation for impaired autoregulation in SAE.48,55–58 Additionally, blood pressure in all the studies was maintained, on average, above 70 mm Hg,54 suggesting that hypotension, based on traditional definitions (MAP < 65 mm Hg), is also an unlikely explanation for impairment. Alternatively, impairment during sepsis may be due to shifts in functional thresholds of autoregulation with associated cerebral hypoperfusion (or hyperperfusion) occurring at uncharacteristic blood pressure values. In this scenario, it is hypothesized that cerebral autoregulation is functional in sepsis, but only during a narrow window of optimal blood pressure values, which may change during the course of sepsis. To date, studies evaluating autoregulatory changes over time in sepsis are lacking.
In our small study, optimal blood pressure varied over the monitoring period, supporting the hypothesis that limits of autoregulation shift over time during sepsis. In addition, we address some of the limitations of prior investigations, primarily the confounding effect of sedation – a rigorously screened for alternate cause of delirium and altered mental status.10,49,59,60 In a prospective study of 16 patients with sepsis, Pfister et al found a significant association between delirium and cerebral autoregulation disturbance using a transcranial Doppler (TCD)–derived autoregulation index (Mx).10 In a prospective study of 30 patients with severe sepsis or septic shock, Schramm et al also reported a significant association between delirium and cerebral autoregulation impairment using Mx.49 These studies were groundbreaking in that they support a hypothesized mechanism for brain dysfunction in patients with sepsis. However, six of the 16 total patients in the former study and all of the patients in the latter study received propofol sedation, with temporary dose reductions during delirium assessment.10,49 During sepsis, propofol has a prolonged anesthetic effect due to an increase in the free concentration at steady state and a decrease in clearance.61,62 Propofol sedation may influence autoregulation and affect the precision and accuracy of diagnosing SAE.24,25 Our study supports and confirms the findings of these previous studies in a population free from exogenous sedation. Further, TCD is operator-dependent and subject to motion artifact, which limits its use for long monitoring periods, especially in septic patients with hyperactive delirium. Near-infrared spectroscopy–based autoregulation monitoring is less subject to user discrepancy than TCD, can be monitored continuously, and has been satisfactorily validated in sepsis patients.40
Previous work in cerebral autoregulation in brain-injured patients and those undergoing cardiopulmonary bypass have demonstrated that optimal MAP for autoregulation varies from individual to individual and in a single individual over time.11,63 Bindra et al recently investigated the effects of cerebral autoregulation on mortality in pharmacologically sedated patients with septic shock and demonstrated a range of over 30 mm Hg in optimal MAP for autoregulation in 28 study patients using an NIRS-derived tissue oxygenation index (TOx).59 The results of our small study in patients with SAE support these findings; the continuous 12-hour NIRS monitoring revealed optimal MAP values that varied widely between patients and within patients during the day. The substantial variability in MAPOPT within study patients may reflect severely disturbed autoregulation, which would be expected in the most severely injured patients. Fittingly, 2 of the 3 patients with severe encephalopathy (GCS ≤ 8) had an MAPOPT range greater than 30 mm Hg.
Perhaps this pattern represents shifts in functional limits of autoregulation during sepsis. Endogenous oscillatory behavior, such as heart rate variability (HRV) driven by the sympathetic and parasympathetic nervous system, is a normal biologic function and can be seen in multiple organ systems. Altered HRV in sepsis has been demonstrated,64 and several studies have shown that a reduction in HRV is proportional to the severity of critical illness65 and associated with worse outcomes.66 In contrast, other studies have revealed that an increase in systolic blood pressure variability is related to the severity of sepsis and associated with worse outcomes.67,68 Optimal MAP variability seen within individual study patients is an interesting finding considering these previous studies and warrants further investigation, possibly in comparison to individual blood pressure and HRV in patients with SAE.
Moreover, the high individual variability in MAPOPT over time stresses the value of continuous real-time cerebral autoregulation monitoring in identifying optimal pressure. Each patient’s monitoring session was divided into 4-hour periods because this is the time window most commonly used to construct autoregulation curves in brain-injured patients.43,63 However, unlike previous studies, we investigated discrete sequential periods of time rather than a moving average of the data. While this is not the conventional method, if we averaged data over the whole monitoring period, fluctuations in MAPOPT over brief periods of time would be missed.19 Additionally, 4 hours is the traditional time interval for serial delirium and coma assessments in our institute’s medical and surgical ICUs. Thus, this 4-hour time window is clinically relevant for potential autoregulation-based management decisions for future patients with SAE.
Based on our finding of a median optimal MAP value of 85 mm Hg for best autoregulation in our small study, a universal MAP goal of ≥ 65 mm Hg in sepsis may result in inadequate brain perfusion in some patients. In other patients, empiric MAP targets might lead to cerebral hyperperfusion when the limits of autoregulation are unintentionally surpassed. Individualizing blood pressure to optimize autoregulation would more likely ensure adequate brain perfusion than does the current practice of empirically targeting a MAP ≥ 65 mm Hg for all patients with sepsis. With continuous real-time NIRS-based cerebral autoregulation monitoring, therapeutic interventions can be targeted to each patient’s underlying pathophysiological process with timely feedback on its effectiveness.
This study has important limitations. The primary limitation is the small sample size. Although a significant association was observed between COx and GCS, our model relies on the large number of individual hourly measurements of autoregulation indices in each subject. In the future, our findings will be validated in a larger cohort of patients. Second, there was substantial heterogeneity in both the etiology and clinical manifestation of sepsis among these patients. A diverse study population allows us to generalize the applicability of our findings and more accurately reflects the true population of sepsis patients. However, in our case, the small sample size prohibits formal accounting of the clinical heterogeneity in the analysis and therefore limits the interpretation of our results. Third, sedation in the ICU is commonplace. Our study is an exploratory study with inherent selection bias that limits external validity. Further investigations into the relationship between neurologic status and cerebral autoregulation are warranted in a broader sepsis population. Fourth, although the use of GCS is routinely used for grading encephalopathy in sepsis patients, it is far from complete and may have limited accuracy as a measure in intubated patients.6,15,69 Alternative measures will be considered in future studies. Fifth, factors such as temperature and PaCO2 influence how cerebral blood flow responds to changes in cerebral metabolism.70–72 We could not control PaCO2 nor temperature in this strictly observational study. All patients were breathing spontaneously, or if tracheally intubated, with a ventilator-assisted mode of spontaneous breathing. While temperature and PaCO2 during monitoring were not significantly different between patients with mild versus more severe encephalopathy, continuous temperature and capnography monitoring are warranted and may shed new light on important parameters affecting changes in autoregulation over time in sepsis.
Lastly, while all six patients were diagnosed with septic shock upon ICU admission, their MAP values reflected aggressive early resuscitation. The higher blood pressure values in some of our patients do not truly reflect the initial hypotension experienced by sepsis patients prior to resuscitation. Moreover, blood pressure augmentation with vasopressors may have contributed to the wide fluctuations in MAPOPT in individual patients during the monitoring period. In a population of sepsis patients with lower blood pressures, autoregulation indices may have reflected worse disturbance and MAPOPT may have varied less over time within patients. It is also possible that the type of vasopressor management influences the relationship between COx and MAP.73,74 Furthermore, the extent of blood brain barrier disruption may lead to unpredictable vasopressor effects on cerebral perfusion, cerebral metabolism, and neurovascular coupling.75,76 This may produce high COx values and an assumption of dysfunctional autoregulation when vasopressor-supported increases in MAP occur alongside vasopressor-induced metabolic neural activation with appropriate increases in rScO2.77,78 Our small sample size does not allow us to explore this potential confounder, and it is the subject of further prospective study. More research is needed to understand the effect of profound hypotension on autoregulatory capacity and the usefulness of NIRS in detecting optimal blood pressure ranges during active resuscitation of the patient in acute septic shock.
Conclusions
In conclusion, noninvasive NIRS-based COx is a feasible bedside tool that can identify cerebral autoregulation as a therapeutic target in patients with SAE. The results of our study of six well-screened, nonpharmacologically sedated patients with SAE demonstrate an association between cerebral autoregulatory function and neurologic status based on GCS. More research is needed to define the relationship between cerebral autoregulation and long-term neurological outcomes in patients with SAE.
Supplementary Material
Acknowledgments
The authors thank Dr. Marie Diener-West, Professor of Biostatistics at Johns Hopkins Bloomberg School of Public Health, for her statistical advice and support.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Brown consulted for and received grant support from Medtronic (Minneapolis, Minnesota). This study was supported in part by grant 532GM075774 from the National Institutes of Health/National Institute of General Medical Sciences Ruth L. Kirschstein National Research Service Award (T32).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013;41(5):1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 2.Paoli CJ, Reynolds MA, Sinha M, Gitlin M, Crouser E. Epidemiology and costs of sepsis in the united states-an analysis based on timing of diagnosis and severity level. Crit Care Med 2018; 46(12):1889–1897. doi: 10.1097/CCM.0000000000003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol 2012;8(10):557–566. doi: 10.1038/nrneurol.2012.183. [DOI] [PubMed] [Google Scholar]
- 4.Ziaja M Septic encephalopathy. Curr Neurol Neurosci Rep 2013;13(10):383. doi: 10.1007/s11910-013-0383-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonneville R, Verdonk F, Rauturier C, et al. Understanding brain dysfunction in sepsis. Ann Intensive Care 2013;3(1):15. doi: 10.1186/2110-5820-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eidelman LA, Putterman D, Putterman C, Sprung CL. The spectrum of septic encephalopathy: definitions, etiologies, and mortalities. JAMA 1996;275(6):470–473. doi: 10.1001/jama.1996.03530300054040. [DOI] [PubMed] [Google Scholar]
- 7.Iacobone E, Bailly-Salin J, Polito A, Friedman D, Stevens RD, Sharshar T. Sepsis-associated encephalopathy and its differential diagnosis. Crit Care Med 2009;37(suppl 10):S331–S336. doi: 10.1097/CCM.0b013e3181b6ed58. [DOI] [PubMed] [Google Scholar]
- 8.Kress JP. The complex interplay between delirium, sepsis and sedation. Crit Care 2010;14(3):164. doi: 10.1186/cc9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molnár L, Németh N, Berhés M, et al. Assessment of cerebral circulation in a porcine model of intravenously given E. coli induced fulminant sepsis. BMC Anesthesiol 2017;17(1):98. doi: 10.1186/s12871-017-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfister D, Siegemund M, Dell-Kuster S, et al. Cerebral perfusion in sepsis-associated delirium. Crit Care 2008;12(3):R63. doi: 10.1186/cc6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joshi B, Ono M, Brown C, et al. Predicting the limits of cerebral autoregulation during cardiopulmonary bypass. Anesth Analg 2012;114(3):503–510. doi: 10.1213/ANE.0b013e31823d292a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ono M, Arnaoutakis GJ, Fine DM, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury*. Crit Care Med 2013;41(2):464–471. doi: 10.1097/CCM.0b013e31826ab3a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharshar T, Polito A, Checinski A, Stevens RD. Septic-associated encephalopathy-everything starts at a microlevel. Crit Care 2010;14(5):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janz DR, Abel TW, Jackson JC, Gunther M, Heckers S, Ely EW. Brain autopsy findings in ICU patients previously suffering from delirium: a pilot study. J Crit Care 2010;25(3):538 e7–e12. doi: 10.1016/j.jcrc.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev 1959;39(2):183–238. [DOI] [PubMed] [Google Scholar]
- 17.LeDoux D, Astiz ME, Carpati CM, Rackow EC. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med 2000;28(8):2729–2732. [DOI] [PubMed] [Google Scholar]
- 18.Bourgoin A, Leone M, Delmas A, Garnier F, Albanèse J, Martin C. Increasing mean arterial pressure in patients with septic shock: effects on oxygen variables and renal function. Crit Care Med 2005;33(4):780–786. [DOI] [PubMed] [Google Scholar]
- 19.Steiner LA, Czosnyka M, Piechnik SK, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med 2002;30(4):733–738. [DOI] [PubMed] [Google Scholar]
- 20.Hori D, Ono M, Rappold TE, et al. Hypotension after cardiac operations based on autoregulation monitoring leads to brain cellular injury. Ann Thorac Surg 2015;100(2):487–493. doi: 10.1016/j.athoracsur.2015.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hori D, Hogue C, Adachi H, et al. Perioperative optimal blood pressure as determined by ultrasound tagged near infrared spectroscopy and its association with postoperative acute kidney injury in cardiac surgery patients. Interact CardioVasc Thorac Surg 2016;22(4):445–451. doi: 10.1093/icvts/ivv371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aries MJH, Czosnyka M, Budohoski KP, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med 2012;40(8):2456–2463. doi: 10.1097/CCM.0b013e3182514eb6. [DOI] [PubMed] [Google Scholar]
- 23.Le Roux P, Menon DK, Citerio G, et al. Consensus summary statement of the international multidisciplinary consensus conference on multimodality monitoring in neurocritical care: a statement for healthcare professionals from the neurocritical care society and the European society of intensive care medicine. Intensive Care Med 2014;40(9):1189–1209. doi: 10.1007/s00134-014-3369-6. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa Y, Iwasaki K, Aoki K, et al. The different effects of midazolam and propofol sedation on dynamic cerebral autoregulation. Anesth Analg 2010;111(5):1279–1284. doi: 10.1213/ANE.0b013e3181f42fc0. [DOI] [PubMed] [Google Scholar]
- 25.Steiner LA, Johnston AJ, Chatfield DA, et al. The effects of large-dose propofol on cerebrovascular pressure autoregulation in head-injured patients. Anesth Analg 2003;97(2):572–576. table of contents. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhry N, Duggal AK. Sepsis associated encephalopathy. Adv Med 2014;2014:762320. doi: 10.1155/2014/762320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009;301(5):489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 28.Pisani MA, Murphy TE, Ness PHV, Araujo KLB, Inouye SK. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med 2007;167(15): 1629–1634. doi: 10.1001/archinte.167.15.1629. [DOI] [PubMed] [Google Scholar]
- 29.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med 1989;19(4): 1015–1022. [DOI] [PubMed] [Google Scholar]
- 30.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;2(7872):81–84. [DOI] [PubMed] [Google Scholar]
- 31.Healy RJ, Zorrilla-Vaca A, Ziai W, et al. Glasgow coma scale score fluctuations are inversely associated with a NIRS-based index of cerebral autoregulation in acutely comatose patients. J Neurosurg Anesthesiol 2018. doi: 10.1097/ANA.0000000000000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knaus WA, Draper EAM, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985;13(10):818–829. [PubMed] [Google Scholar]
- 33.Brady K, Joshi B, Zweifel C, et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke 2010;41(9):1951–1956. doi: 10.1161/STROKEAHA.109.575159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brady KM, Mytar JO, Lee JK, et al. Monitoring cerebral blood flow pressure autoregulation in pediatric patients during cardiac surgery. Stroke 2010;41(9):1957–1962. doi: 10.1161/STROKEAHA.109.575167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czosnyka M, Smielewski P, Kirkpatrick P, Menon DK, Pickard JD. Monitoring of cerebral autoregulation in head-injured patients. Stroke 1996;27(10):1829–1834. [DOI] [PubMed] [Google Scholar]
- 36.Brady KM, Lee JK, Kibler KK, Easley RB, Koehler RC, Shaffner DH. Continuous measurement of autoregulation by spontaneous fluctuations in cerebral perfusion pressure: comparison of 3 methods. Stroke 2008;39(9):2531–2537. doi: 10.1161/STROKEAHA.108.514877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundberg N, Kjallquist A, Bien C. Reduction of increased intracranial pressure by hyperventilation. A therapeutic aid in neuro-logical surgery. Acta Psychiatr Scand Suppl 1959;34(139):1–64. [PubMed] [Google Scholar]
- 38.Momjian S, Czosnyka Z, Czosnyka M, Pickard JD. Link between vasogenic waves of intracranial pressure and cerebrospinal fluid outflow resistance in normal pressure hydrocephalus. Br J Neurosurg 2004;18(1):56–61. doi: 10.1080/02688690410001660481. [DOI] [PubMed] [Google Scholar]
- 39.Weerakkody RA, Czosnyka M, Zweifel C, et al. Slow vasogenic fluctuations of intracranial pressure and cerebral near infrared spectroscopy—an observational study. Acta Neurochir 2010; 152(10):1763–1769. doi: 10.1007/s00701-010-0748-9. [DOI] [PubMed] [Google Scholar]
- 40.Steiner LA, Pfister D, Strebel SP, Radolovich D, Smielewski P, Czosnyka M. Near-infrared spectroscopy can monitor dynamic cerebral autoregulation in adults. Neurocrit Care 2008;10(1): 122–128. doi: 10.1007/s12028-008-9140-5. [DOI] [PubMed] [Google Scholar]
- 41.Brady KM, Lee JK, Kibler KK, et al. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke 2007;38(10):2818–2825. doi: 10.1161/STROKEAHA.107.485706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moerman AT, Vanbiervliet VM, Van Wesemael A, Bouchez SM, Wouters PF, De Hert SG. Assessment of cerebral autoregulation patterns with near-infrared spectroscopy during pharmacological- induced pressure changes. Anesthesiology 2015;123(2):327–335. doi: 10.1097/ALN.0000000000000715. [DOI] [PubMed] [Google Scholar]
- 43.Rivera-Lara L, Zorrilla-Vaca A, Geocadin RG, Healy RJ, Ziai W, Mirski MA. Cerebral autoregulation-oriented therapy at the bedside: a comprehensive review. Anesthesiology 2017;126(6): 1187–1199. doi: 10.1097/ALN.0000000000001625. [DOI] [PubMed] [Google Scholar]
- 44.Barbosa RR, Jawa R, Watters JM, et al. Evaluation and management of mild traumatic brain injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg 2012;73(5 suppl 4):S307–S314. doi: 10.1097/TA.0b013e3182701885. [DOI] [PubMed] [Google Scholar]
- 45.Tsuruta R, Oda Y. A clinical perspective of sepsis-associated delirium. J Intensive Care 2016;4:18. doi: 10.1186/s40560-016-0145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ebersoldt M, Sharshar T, Annane D. Sepsis-associated delirium. Intensive Care Med 2007;33(6):941–950. doi: 10.1007/s00134-007-0622-2. [DOI] [PubMed] [Google Scholar]
- 47.Rosengarten B, Hecht M, Auch D, et al. Microcirculatory dysfunction in the brain precedes changes in evoked potentials in endotoxin-induced sepsis syndrome in rats. Cerebrovasc Dis 2007;23(2–3):140–147. doi: 10.1159/000097051. [DOI] [PubMed] [Google Scholar]
- 48.Matta BF, Stow PJ. Sepsis-induced vasoparalysis does not involve the cerebral vasculature: indirect evidence from autoregulation and carbon dioxide reactivity studies. Br J Anaesth 1996;76(6): 790–794. [DOI] [PubMed] [Google Scholar]
- 49.Schramm P, Klein KU, Falkenberg L, et al. Impaired cerebrovascular autoregulation in patients with severe sepsis and sepsis-associated delirium. Crit Care 2012;16(5):R181. doi: 10.1186/cc11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young GB, Bolton CF, Archibald YM, Austin TW, Wells GA. The electroencephalogram in sepsis-associated encephalopathy. J Clin Neurophysiol 1992;9(1):145–152. [DOI] [PubMed] [Google Scholar]
- 51.Zauner C, Gendo A, Kramer L, et al. Impaired subcortical and cortical sensory evoked potential pathways in septic patients. Crit Care Med 2002;30(5):1136–1139. [DOI] [PubMed] [Google Scholar]
- 52.Zauner C, Gendo A, Kramer L, Kranz A, Grimm G, Madl C. Metabolic encephalopathy in critically ill patients suffering from septic or nonseptic multiple organ failure. Crit Care Med 2000; 28(5):1310–1315. [DOI] [PubMed] [Google Scholar]
- 53.Straver JS, Keunen RW, Stam CJ, et al. Nonlinear analysis of EEG in septic encephalopathy. Neurol Res 1998;20(2):100–106. [DOI] [PubMed] [Google Scholar]
- 54.Goodson CM, Rosenblatt K, Rivera-Lara L, Nyquist P, Hogue CW. Cerebral blood flow autoregulation in sepsis for the intensivist: why its monitoring may be the future of individualized care. J Intensive Care Med 2018;33(2):63–73. doi: 10.1177/0885066616673973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bowie RA, O’Connor PJ, Mahajan RP. Cerebrovascular reactivity to carbon dioxide in sepsis syndrome. Anaesthesia 2003;58(3): 261–265. [DOI] [PubMed] [Google Scholar]
- 56.Berg RMG, Plovsing RR, Ronit A, Bailey DM, Holstein-Rathlou NH, Møller K. Disassociation of static and dynamic cerebral autoregulatory performance in healthy volunteers after lipopolysaccharide infusion and in patients with sepsis. Am J Physiol Regul Integr Comp Physiol 2012;303(11):R1127–R1135. doi: 10.1152/ajpregu.00242.2012. [DOI] [PubMed] [Google Scholar]
- 57.Vaskó A, Siró P, László I, et al. Assessment of cerebral tissue oxygen saturation in septic patients during acetazolamide provocation - a near infrared spectroscopy study. Acta Physiol Hung 2014;101(1):32–39. doi: 10.1556/APhysiol.101.2014.1.4. [DOI] [PubMed] [Google Scholar]
- 58.Berg RMG, Plovsing RR, Bailey DM, Holstein-Rathlou NH, Møller K. Dynamic cerebral autoregulation to induced blood pressure changes in human experimental and clinical sepsis. Clin Physiol Funct Imaging 2016;36(6):490–496. doi: 10.1111/cpf.12256. [DOI] [PubMed] [Google Scholar]
- 59.Bindra J, Pham P, Chuan A, Jaeger M, Aneman A. Is impaired cerebrovascular autoregulation associated with outcome in patients admitted to the ICU with early septic shock?. Crit Care Resusc 2016;18(2):95–101. [PubMed] [Google Scholar]
- 60.Pierrakos C, Attou R, Decorte L, et al. Cerebral perfusion alterations and cognitive decline in critically ill sepsis survivors. Acta Clinica Belgica 2017;72(1):39–44. doi: 10.1080/17843286.2016.1191851. [DOI] [PubMed] [Google Scholar]
- 61.De Paepe P, Belpaire FM, Van Hoey G, Boon PA, Buylaert WA. The influence of endotoxemia on the pharmacokinetics and the electroencephalographic effect of propofol in the rat. J Pharm Sci 2003;92(1):104–114. doi: 10.1002/jps.10275. [DOI] [PubMed] [Google Scholar]
- 62.Zamacona MK, Suárez E, Aguilera L, Rodŕıguez-Sasiáın, Aguirre C, Calvo R. Serum protein binding of propofol in critically ill patients. Acta Anaesthesiol Scand 1997;41(10):1267–1272. [DOI] [PubMed] [Google Scholar]
- 63.Aries MJH, Wesselink R, Elting JWJ, et al. Enhanced visualization of optimal cerebral perfusion pressure over time to support clinical decision making. Crit Care Med 2016;44(10):e996–e999. doi: 10.1097/CCM.0000000000001816. [DOI] [PubMed] [Google Scholar]
- 64.Korach M, Sharshar T, Jarrin I, et al. Cardiac variability in critically ill adults: influence of sepsis. Crit Care Med 2001;29(7): 1380–1385. [DOI] [PubMed] [Google Scholar]
- 65.Barnaby D, Ferrick K, Kaplan DT, Shah S, Bijur P, Gallagher EJ. Heart rate variability in emergency department patients with sepsis. Acad Emerg Med 2002;9(7):661–670. [DOI] [PubMed] [Google Scholar]
- 66.de Castilho FM, Ribeiro ALP, da Silva JLP, Nobre V, de Sousa MR. Heart rate variability as predictor of mortality in sepsis: a prospective cohort study. Plos One 2017;12(6):e0180060. doi: 10.1371/journal.pone.0180060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pandey NR, Bian Y, Shou S. Significance of blood pressure variability in patients with sepsis. World J Emerg Med 2014;5(1): 42–47. doi: 10.5847/wjem.j.issn.1920-8642.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang Y, Sorenson J, Lanspa M, Grissom CK, Mathews VJ, Brown SM. Systolic blood pressure variability in patients with early severe sepsis or septic shock: a prospective cohort study. BMC Anesthesiol 2017;17(1):82. doi: 10.1186/s12871-017-0377-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wijdicks EFM, Kramer AA, Rohs T, et al. Comparison of the full outline of unresponsiveness score and the Glasgow coma scale in predicting mortality in critically ill patients*. Crit Care Med 2015;43(2):439–444. doi: 10.1097/CCM.0000000000000707. [DOI] [PubMed] [Google Scholar]
- 70.Lavinio A, Timofeev I, Nortje J, et al. Cerebrovascular reactivity during hypothermia and rewarming. Br J Anaesth 2007;99(2): 237–244. doi: 10.1093/bja/aem118. [DOI] [PubMed] [Google Scholar]
- 71.Raichle ME, Posner JB, Plum F. Cerebral blood flow during and after hyperventilation. Arch Neurol 1970;23(5):394–403. [DOI] [PubMed] [Google Scholar]
- 72.Adatia K, Geocadin RG, Healy R, et al. Effect of body temperature on cerebral autoregulation in acutely comatose neurocritically ill patients. Crit Care Med 2018;46(8):e733–e741. doi: 10.1097/CCM.0000000000003181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pfister D, Strebel SP, Steiner LA. Effects of catecholamines on cerebral blood vessels in patients with traumatic brain injury. Eur J Anaesthesiol 2008;25(S42):98–103. doi: 10.1017/S0265021507003407. [DOI] [PubMed] [Google Scholar]
- 74.Sookplung P, Siriussawakul A, Malakouti A, et al. Vasopressor use and effect on blood pressure after severe adult traumatic brain injury. Neurocrit Care 2011;15(1):46–54. doi: 10.1007/s12028-010-9448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Edvinsson L, Hardebo JE, MacKenzie ET, Owman C. Effect of exogenous noradrenaline on local cerebral blood flow after osmotic opening of the blood-brain barrier in the rat. J Physiol 1978;274:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.MacKenzie ET, McCulloch J, O’Kean M, Pickard JD, Harper AM. Cerebral circulation and norepinephrine: relevance of the blood-brain barrier. Am J Physiol 1976;231(2):483–488. doi: 10.1152/ajplegacy.1976.231.2.483. [DOI] [PubMed] [Google Scholar]
- 77.Bola RA, Kiyatkin EA. Inflow of oxygen and glucose in brain tissue induced by intravenous norepinephrine: relationships with central metabolic and peripheral vascular responses. J Neurophysiol 2018;119(2):499–508. doi: 10.1152/jn.00692.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burkhart CS, Siegemund M, Steiner LA. Cerebral perfusion in sepsis. Crit Care 2010;14(2):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.