Abstract
BACKGROUND
We have previously proved that treatment of thick/deep infantile hemangiomas (IHs) with a long-pulse Alexandrite laser was clinically effective and safe. This article aims to investigate the efficiency of long-pulse Alexandrite laser use in treating thick and high-risk IHs located in particular anatomic areas and provides some new data on this issue.
CASE SUMMARY
A two-month-old girl with a thick and high-risk IH covering most of the right labia majora was examined in this study. The infant received four treatment sessions at 4- to 6-wk intervals with a long-pulse Alexandrite laser with settings as follows: 3 ms pulse duration, 8 mm spot size, 45 to 50 J/cm2 fluences, and dynamic cooling device (DCD) spray duration of 90 ms with a delay of 80 ms. Following each of the four treatment sessions, the IH showed a remarkable reduction in thickness and size without any sign of relapse. Ten months after the last treatment, the IH had completely regressed without adverse effects. During the laser treatment, no severe side effects were observed; blistering occurred only immediately after treatment and then scabbed over the next day, gradually improving in the following days.
CONCLUSION
Long-pulse Alexandrite laser treatment may be considered one of the first-line noninvasive therapeutic options for the treatment of thick IH.
Keywords: Alexandrite laser, Infantile hemangiomas, Treatment, Case report
Core tip: Infantile hemangiomas (IHs) are the most common benign tumors of infancy. In this paper, we describe a two-month-old female who presented with a thick IH covering most of the right labia majora and was treated using a long-pulse Alexandrite laser, and we review the literature to further understand IH, including its definition, diagnosis, and treatment.
INTRODUCTION
Infantile hemangiomas (IHs), affecting approximately 10% of infants younger than 1 year old, are true neoplastic proliferations of endothelial cells and the most common type of congenital benign vascular tumors during infancy[1,2]. IHs have a typically characteristic growth curve with an initial proliferation phase for several months followed by a spontaneous regression phase for several years[3-5]. However, involution does not mean disappearance; children may be left with a residual deformity, such as scarring, anetoderma, fibrofatty residuum, destroyed anatomical structures, red-undant skin, and telangiectasias[6]. In addition, owing to the risk of complications, including discomfort or pain, infection, ulceration, and permanent discoloration or scarring[7,8], IHs located in particular anatomic areas (such as in the perineum, nose, ear, lip, periorbital, oropharyngeal, and parotid regions), those with complications, and those which are ulcerated need rapid and active treatment[3,9].
There is no standardized management strategy for IHs, but several treatment modalities have been used to manage the growth of IHs and to increase the speed of involution, including steroids, interferon-α, vincristine, propranolol, surgical excision, and laser therapy. With regard to laser therapy, it was reported that pulsed dye laser (PDL) treatment, as a safe and effective option that has been used for many years, limits superficial IH proliferation and increases complete clearance rates[7,10,11]. However, because of its limited penetration, PDL treatment was not very effective for thick/deep IHs[12,13]. In our previous study, we proved that treatment of thick/deep IHs with a long-pulse Alexandrite laser was clinically effective and safe[14]. In the current study, one infant with a rapidly growing and thick IH located on the right labia majora was treated using a long-pulse Alexandrite laser because of the particular location that makes this IH harbor a high risk of ulceration, infection, and even scarring. Finally, a noteworthy effect was obtained without severe complications. We also briefly review the literature, emphasizing the pros and cons of other options for the treatment of IHs. Because most patients with IH present with superficial or non-organ dysfunctional lesions, clinicians should be noted the indications for treatment. Generally, accepted indications of treatment for IH are summarized by the acronym GLUT-ONE and include liver and/or other visceral organ involvement, giant infantile hemangiomas, and ulcerated or bleeding infantile hemangiomas; this acronym can help clinicians make rapid treatment decisions.
CASE PRESENTATION
Chief complaints
A purplish spot located on the right labia majora for 2 months.
History of present illness
A two-month-old girl presented to our department with her parents. She was born at full-term (38 wk) and weighed pproximately 3300 g. Three weeks after birth, she was noted to have a light purplish spot located on the right labia majora. The vascular lesion promptly grew in size over a period of 5 wk. No ulcers or hemorrhagic areas were noted externally. No prior treatment had been administered.
History of past illness
Unremarkable.
Personal and family history
There were no similar patients in the family.
Physical examination upon admission
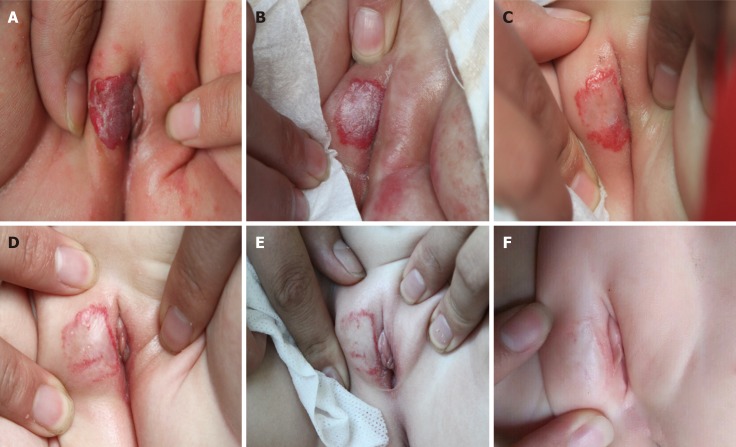
Physical examination showed that the right labia majora was covered with a thick hemangioma (Figure 1A).
Figure 1.
The patient before, during, and after treatment with a long-pulse Alexandrite laser. A: A two-month-old girl with a thick and high-risk infantile hemangioma (IH) covering most of the right labia majora before treatment; B: The IH 4 wk after the first treatment session; C: The IH 5 wk after the second treatment session; D: The IH 4 wk after the third treatment session; E: The IH 4 mo after the fourth treatment session; and F: The IH 10 mo after the fourth treatment session.
Laboratory examinations
There were no suitable laboratory examinations.
Imaging examinations
There were no suitable imaging examinations.
FINAL DIAGNOSIS
Because of the characteristic clinical course, such as appearing in the first few weeks following birth and exhibiting a characteristic sequence of growth, a diagnosis of IH was supported.
TREATMENT
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and the family was informed in detail of the likely risks, benefits, and potential complications of the treatment and about the other therapeutic alternatives available. Written informed consent was obtained from the patient's parents for publication of this case report and any accompanying images.
Treatment with a long-pulse Alexandrite laser was administered to the IH at 4-to 6-wk intervals with laser settings as follows: 3 ms pulse duration; 8 mm spot size; 45 to 50 J/cm2 fluences, adjusted as needed to achieve the correct treatment endpoint at each treatment session; and concurrent dynamic cooling device (DCD) cryogen spray spurt duration of 90 ms with 80 ms delay. The target treatment endpoint with the long-pulse Alexandrite laser is transient gray discoloration of the skin followed by lasting purpura, and it was attempted and achieved in nearly all treatment session. No areas were double-pulsed. For posttreatment care, a topical antibiotic cream (Fusidic acid, Bright Future Pharm, Hong Kong) was applied once a day for approxi-mately 1 wk.
OUTCOME AND FOLLOW-UP
As described in the previous study[14], the patient had taken photographs before the first treatment, at regular intervals during treatment, and at four months and ten months after the final treatment. The infant was examined for clinical assessment, treatment compliance, and tolerance. Treatment with the long-pulse Alexandrite laser was initiated on the day of the evaluation. Improvement was noted after the first treatment session, and the IH showed a reduction in thickness (Figure 1B). After two treatment sessions, the IH showed a remarkable reduction in size, thickness, and appearance with part of the IH nearly completely regressing (Figure 1C). Following the next two treatment sessions, the size of the IH decreased dramatically, and the appearance improved further without any sign of relapse (Figure 1D and E). Ten months after the last treatment, the patient was reexamined, and the IH had completely regressed without adverse effects such as hypopigmentation, dyspigmen-tation, or scarring (Figure 1F).
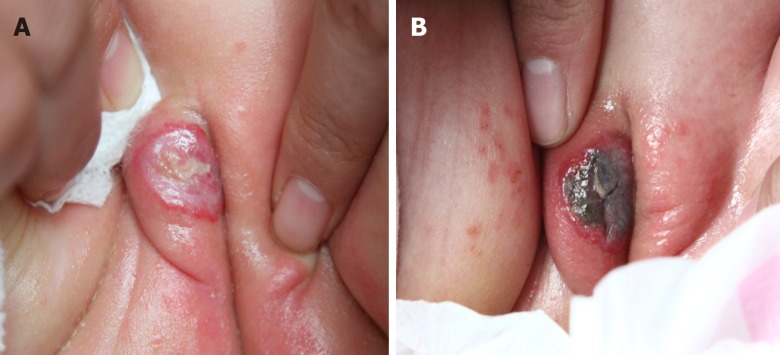
During the laser treatment, no severe side effects were observed; blistering occurred only immediately after treatment and then scabbed over the next day, gradually improving in the following days (Figure 2A and B).
Figure 2.
Side effects observed during laser therapy. A: Blistering observed immediately after the first treatment; B: The blistering scabbed the next day and improved gradually in the following days.
DISCUSSION
IH is the most commonly observed benign vascular tumor in infants with a reported incidence of approximately 10%[1,15]. The incidence is three times higher in females than in males and is especially prevalent in premature infants because lower birth weight and younger gestational age are associated with tumor development[16]. How-ever, the patient in this case report is a female, but full-term infant.
Most IHs (70% to 80%) can be left untreated and allowed to follow their natural course without negative consequences[17,18]. Unfortunately, in the other cases, it is now possible to find residual changes such as fibrofatty tissue, scarring, or simply telan-giectasia, wrinkling, and dyspigmentation, which significantly affect the patients and their families both physically and psychologically[16]. Moreover, it is difficult to predict the possible occurrence of functional impairments and complications[19,20]. As a result, the timing of IH treatment is important but difficult to determine. Many IHs do not require any treatment, but only a close observation of the infant made through periodic visits. Specialists should continuously monitor IHs through measurements and photographs, not only with the aim to check on the situation but also to help the patient’s parents better understand that the “wait and see” approach is feasible and to provide them with emotional support. The factors leading to aggressive treatment are essentially as follows[16]: Specific anatomical location with high aesthetic risks, ulceration/infection or likeliness of ulceration/infection/scarring, potential of the IH to limit some important functions, and presence of life-threatening risks. In this case report, the particular location (labia majora) of the IH caused it to be high risk for ulceration, infection, and even scarring, so active treatment was needed.
When treatment is needed, the best choice involves many factors that physicians must take into consideration: Depth, size, anatomic location, phase of the IH, age of the patient, and physician’s experience[16]. Treatment modalities can include medical management, laser therapy, or a surgical approach.
Since the first case reported by Zarem and Edgerton, systemic corticosteroids are commonly used for treatment of orbital IHs, segmental IHs, and other large and (or) aggressive IHs elsewhere in the patient’s body. However, the efficacy of systemic steroids is limited by numerous potential side effects, such as behavior changes, Cushingoid appearance and growth delay, irritability, and increased susceptibility to infection[21-24]. Although reversible, these side effects can be too difficult for parents to endure. Fortunately, systemic steroids are now the second-line treatment for IH in the senior author’s practice.
Propranolol, a nonselective oral beta-blocker, was serendipitously discovered to be clinically effective for the treatment of cutaneous IH by Léauté-Labrèze et al in 2008[25]. Since then, the large-scale use of propranolol for the treatment of IH has been prompted, and its promising effectiveness in IH has been demonstrated. In the following years, investigators confirmed that propranolol was effective in the treatment of IH at all sites on the body[26-28]. Regression of IHs and improvement of ulcerated ones with the administration of propranolol in patients younger than 12 mo were documented[29]. The drug works well if started at 2 to 3 mg/kg/d and divided into 2 or 3 doses, and it can be used for 3 to 9 mo. The results can be very rapid, and gradual discontinuation can take 3 to 4 wk[25,30]. Currently, propranolol is a first-line treatment option for IH. Despite its widespread use, several adverse events of propranolol should be considered, such as transient hypoglycemia, insomnia, bronch-ospasm, bradycardia, and hypotension.
Additionally, topical timolol maleate, a nonselective β-blocker that comes as a solution or gel-forming solution, is used alone or in conjunction with oral propranolol for the treatment of IH. However, laser therapy provides better results than timolol solution because it penetrates deeply enough to reach the deep dermal blood vess-els[31,32]. Other pharmacologic agents, including interferon-α and vincristine, were used occasionally for the treatment of IH. Owing to their potential severe side effects, such as neurotoxicity with use of interferon-α and peripheral and autonomic neuropathies with use of vincristine[33-35], the use of interferon-α and vincristine for the treatment of IH was not recommended as first-line therapy.
The aim of laser treatment of IH is to maximize vascular damage while minimizing epidermal and dermal injury. The efficacy and safety of laser treatment in dermatology was completely improved in 1983 due to the introduction of the selective photothermolysis theory by Anderson and Parrish[36]. Numerous studies have confirmed the efficacy of PDL treatment, especially for the treatment of superficial IHs[37,38]. However, it was not very effective for thick/deep IHs, because of the limited penetration of the laser. In our previous study, we treated IHs, including superficial and thick/deep ones, using a long-pulse Alexandrite laser with the following settings: 3 ms pulse duration, 6-8 mm spot size, and 45-70 J/cm2 fluences. As expected, very significant improvement accompanied by relatively few complications was observed in the group with thick/deep IHs[14]. These results indicated that the long-pulse Alexandrite laser may be more efficacious than the PDL for the treatment of IHs with a prominent deep or hypertrophic component because of the greater depth of pene-tration of the Alexandrite laser. However, the disadvantage of the long-pulse Alexandrite laser is that higher fluences are needed compared with the PDL, and this significantly increases the risk of deep dermal heating and associated side effects, such as tissue breakdown, ulceration, dyspigmentation, and scarring[14]. In this case report, a decision to treat IH with a long-pulse Alexandrite laser was made after an extensive discussion with parents about the risks and benefits of different treatment modalities. On the other hand, due to the significantly greater tissue penetration of the laser, the risk of scarring with Alexandrite lasers appears to be greater than the risk with PDLs reported in the treatment of port wine stains (PWS)[39,40]. To prevent the occurrence of scarring, relatively conservative parameters were recommended in the treatment of IH using a long-pulse Alexandrite laser. In the current study, relatively conservative fluences of 45 to 50 J/cm2 were adopted. In addition, a higher cooling setting of the DCD was used to provide more protection.
A neodymium:Yttrium-aluminum-garnet (Nd:YAG) laser has also been used occasionally for treating IHs with a prominent deep component[31,41,42]. However, the challenge of the Nd:YAG laser for the treatment of vascular lesions is the narrow therapeutic window. Forthermore, increasing the fluences to greater than 20% beyond the minimal purpuric dose can cause dermal blisters and scarring and deep dermal damage[43-45].
Surgical treatment can be seen from two different points of view: Early surgery and late surgery[16]. Early surgery has a relevant role for IH during the proliferative phase, which is drug-resistant and/or function-impairing and/or complicated by ulcerations and persistent bleedings. Late surgery can be useful to repair residual deformities, typically observed after regression, including: Anetoderma, destroyed anatomical structures, fibrofatty residuum, redundant skin, wrinkles, scarring, dyspigmentations, and telangiectasias. The main risk correlated with the surgical a approach is the indu-ction of post-healing scarring.
CONCLUSION
Despite decades of development, treatment of IH remains a major challenge. Recently, the combination of laser therapy and drugs in IH treatment in various approaches for increased efficacy is a growing trend. Combination treatments may have potential benefits, including possibilities of synergistic effects, greater efficacy, and lower toxicity of drugs. Several researchers have reported that IH treatment using a combi-nation of propranolol and PDL therapy resulted in significantly better improvement compared to either treatment alone[46,47]. Whether a PDL is the optimal laser for use in combination with propranolol remains to be investigated. We speculate that it would be more effective to treat the IH reported in this study with a combination of a long-pulse Alexandrite laser and propranolol. We are now performing a clinical trial of propranolol-Alexandrite laser combination therapy compared with monotherapy to further advance the treatment of IH.
Footnotes
Informed consent statement: Consent was obtained from relatives of the patient for publication of this report and the accompanying images.
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: February 15, 2019
First decision: March 14, 2019
Article in press: May 23, 2019
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aksoy B, Kupeli S S-Editor: Gong ZM L-Editor: Wang TQ E-Editor: Wang J
Contributor Information
Wen-Ting Su, Medical Cosmetology Department, Wenzhou Hospital of Integrated Traditional Chinese and Western Medicine, Wenzhou Skin Disease and Plastic Surgery Hospital, Wenzhou 325000, Zhejiang Province, China.
Ji-Xin Xue, Department of Hand and Plastic Surgery, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China.
You-Hui Ke, Medical Cosmetology Department, Wenzhou Hospital of Integrated Traditional Chinese and Western Medicine, Wenzhou Skin Disease and Plastic Surgery Hospital, Wenzhou 325000, Zhejiang Province, China. 672673450@qq.com.
References
- 1.Drolet BA, Swanson EA, Frieden IJ Hemangioma Investigator Group. Infantile hemangiomas: an emerging health issue linked to an increased rate of low birth weight infants. J Pediatr. 2008;153:712–715, 715.e1. doi: 10.1016/j.jpeds.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 2.Harter N, Mancini AJ. Diagnosis and Management of Infantile Hemangiomas in the Neonate. Pediatr Clin North Am. 2019;66:437–459. doi: 10.1016/j.pcl.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Admani S, Krakowski AC, Nelson JS, Eichenfield LF, Friedlander SF. Beneficial effects of early pulsed dye laser therapy in individuals with infantile hemangiomas. Dermatol Surg. 2012;38:1732–1738. doi: 10.1111/j.1524-4725.2012.02487.x. [DOI] [PubMed] [Google Scholar]
- 4.Chang LC, Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW, Nopper AJ, Frieden IJ Hemangioma Investigator Group. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360–367. doi: 10.1542/peds.2007-2767. [DOI] [PubMed] [Google Scholar]
- 5.Ramtohul P, Beylerian M, Dambricourt L, Matonti F, Denis D. Secondary Congenital Glaucoma Associated With Retro-orbital Infantile Hemangioma: A Masquerade Syndrome. J Glaucoma. 2019 doi: 10.1097/IJG.0000000000001227. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Couto RA, Maclellan RA, Zurakowski D, Greene AK. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619–624. doi: 10.1097/PRS.0b013e31825dc129. [DOI] [PubMed] [Google Scholar]
- 7.Rizzo C, Brightman L, Chapas AM, Hale EK, Cantatore-Francis JL, Bernstein LJ, Geronemus RG. Outcomes of childhood hemangiomas treated with the pulsed-dye laser with dynamic cooling: a retrospective chart analysis. Dermatol Surg. 2009;35:1947–1954. doi: 10.1111/j.1524-4725.2009.01356.x. [DOI] [PubMed] [Google Scholar]
- 8.Tu JB, Ma RZ, Dong Q, Jiang F, Hu XY, Li QY, Pattar P, Zhang H. Induction of apoptosis in infantile hemangioma endothelial cells by propranolol. Exp Ther Med. 2013;6:574–578. doi: 10.3892/etm.2013.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz RA, Sidor MI, Musumeci ML, Lin RL, Micali G. Infantile haemangiomas: a challenge in paediatric dermatology. J Eur Acad Dermatol Venereol. 2010;24:631–638. doi: 10.1111/j.1468-3083.2010.03650.x. [DOI] [PubMed] [Google Scholar]
- 10.Leonardi-Bee J, Batta K, O'Brien C, Bath-Hextall FJ. Interventions for infantile haemangiomas (strawberry birthmarks) of the skin. Cochrane Database Syst Rev. 2011;(5):CD006545. doi: 10.1002/14651858.CD006545.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Batta K, Goodyear HM, Moss C, Williams HC, Hiller L, Waters R. Randomised controlled study of early pulsed dye laser treatment of uncomplicated childhood haemangiomas: results of a 1-year analysis. Lancet. 2002;360:521–527. doi: 10.1016/S0140-6736(02)09741-6. [DOI] [PubMed] [Google Scholar]
- 12.Ashinoff R, Geronemus RG. Failure of the flashlamp-pumped pulsed dye laser to prevent progression to deep hemangioma. Pediatr Dermatol. 1993;10:77–80. doi: 10.1111/j.1525-1470.1993.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 13.Poetke M, Philipp C, Berlien HP. Flashlamp-pumped pulsed dye laser for hemangiomas in infancy: treatment of superficial vs mixed hemangiomas. Arch Dermatol. 2000;136:628–632. doi: 10.1001/archderm.136.5.628. [DOI] [PubMed] [Google Scholar]
- 14.Su W, Ke Y, Xue J. Beneficial effects of early treatment of infantile hemangiomas with a long-pulse Alexandrite laser. Lasers Surg Med. 2014;46:173–179. doi: 10.1002/lsm.22221. [DOI] [PubMed] [Google Scholar]
- 15.Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med. 1999;341:173–181. doi: 10.1056/NEJM199907153410307. [DOI] [PubMed] [Google Scholar]
- 16.Bruscino N, Bonan P, Cannarozzo G, Moretti S, Lotti T, Campolmi P. Laser use in infantile hemangiomas, when and how. Dermatol Ther. 2012;25:314–321. doi: 10.1111/j.1529-8019.2012.01466.x. [DOI] [PubMed] [Google Scholar]
- 17.Léauté-Labrèze C, Prey S, Ezzedine K. Infantile haemangioma: part II. Risks, complications and treatment. J Eur Acad Dermatol Venereol. 2011;25:1254–1260. doi: 10.1111/j.1468-3083.2011.04105.x. [DOI] [PubMed] [Google Scholar]
- 18.Satterfield KR, Chambers CB. Current Treatment and Management of Infantile Hemangiomas. Surv Ophthalmol. 2019 doi: 10.1016/j.survophthal.2019.02.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Finn MC, Glowacki J, Mulliken JB. Congenital vascular lesions: clinical application of a new classification. J Pediatr Surg. 1983;18:894–900. doi: 10.1016/s0022-3468(83)80043-8. [DOI] [PubMed] [Google Scholar]
- 20.Huang SA, Tu HM, Harney JW, Venihaki M, Butte AJ, Kozakewich HP, Fishman SJ, Larsen PR. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med. 2000;343:185–189. doi: 10.1056/NEJM200007203430305. [DOI] [PubMed] [Google Scholar]
- 21.Bennett ML, Fleischer AB, Jr, Chamlin SL, Frieden IJ. Oral corticosteroid use is effective for cutaneous hemangiomas: an evidence-based evaluation. Arch Dermatol. 2001;137:1208–1213. doi: 10.1001/archderm.137.9.1208. [DOI] [PubMed] [Google Scholar]
- 22.Sadan N, Wolach B. Treatment of hemangiomas of infants with high doses of prednisone. J Pediatr. 1996;128:141–146. doi: 10.1016/s0022-3476(96)70446-8. [DOI] [PubMed] [Google Scholar]
- 23.Boon LM, MacDonald DM, Mulliken JB. Complications of systemic corticosteroid therapy for problematic hemangioma. Plast Reconstr Surg. 1999;104:1616–1623. doi: 10.1097/00006534-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 24.George ME, Sharma V, Jacobson J, Simon S, Nopper AJ. Adverse effects of systemic glucocorticosteroid therapy in infants with hemangiomas. Arch Dermatol. 2004;140:963–969. doi: 10.1001/archderm.140.8.963. [DOI] [PubMed] [Google Scholar]
- 25.Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 26.Durr ML, Meyer AK, Huoh KC, Frieden IJ, Rosbe KW. Airway hemangiomas in PHACE syndrome. Laryngoscope. 2012;122:2323–2329. doi: 10.1002/lary.23475. [DOI] [PubMed] [Google Scholar]
- 27.Moss HB, Sines DT, Blatt J, Dutton JJ, Proia AD. Epithelioid hemangioma responsive to oral propranolol. Ophthalmic Plast Reconstr Surg. 2012;28:e88–e90. doi: 10.1097/IOP.0b013e31823645f9. [DOI] [PubMed] [Google Scholar]
- 28.Mazereeuw-Hautier J, Hoeger PH, Benlahrech S, Ammour A, Broue P, Vial J, Ohanessian G, Léauté-Labrèze C, Labenne M, Vabres P, Rössler J, Bodemer C. Efficacy of propranolol in hepatic infantile hemangiomas with diffuse neonatal hemangiomatosis. J Pediatr. 2010;157:340–342. doi: 10.1016/j.jpeds.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Küpeli S. Use of propranolol for infantile hemangiomas. Pediatr Hematol Oncol. 2012;29:293–298. doi: 10.3109/08880018.2011.622833. [DOI] [PubMed] [Google Scholar]
- 30.Arneja JS, Pappas PN, Shwayder TA, Cullen ML, Becker CJ, Hamzavi FH, Roarty JD, Madgy DN, Baker JD. Management of complicated facial hemangiomas with beta-blocker (propranolol) therapy. Plast Reconstr Surg. 2010;126:889–895. doi: 10.1097/PRS.0b013e3181e5f8b6. [DOI] [PubMed] [Google Scholar]
- 31.Tawfik AA, Alsharnoubi J. Topical timolol solution versus laser in treatment of infantile hemangioma: a comparative study. Pediatr Dermatol. 2015;32:369–376. doi: 10.1111/pde.12542. [DOI] [PubMed] [Google Scholar]
- 32.Yu J, Keuter T, Schilter K, Seefeldt M, Bates B, Mueller K, Drolet BA. Variability of Delivery of Timolol for the Treatment of Infantile Hemangiomas. Pediatr Dermatol. 2017;34:458–460. doi: 10.1111/pde.13189. [DOI] [PubMed] [Google Scholar]
- 33.Barlow CF, Priebe CJ, Mulliken JB, Barnes PD, Mac Donald D, Folkman J, Ezekowitz RA. Spastic diplegia as a complication of interferon Alfa-2a treatment of hemangiomas of infancy. J Pediatr. 1998;132:527–530. doi: 10.1016/s0022-3476(98)70034-4. [DOI] [PubMed] [Google Scholar]
- 34.Michaud AP, Bauman NM, Burke DK, Manaligod JM, Smith RJ. Spastic diplegia and other motor disturbances in infants receiving interferon-alpha. Laryngoscope. 2004;114:1231–1236. doi: 10.1097/00005537-200407000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Boehm DK, Kobrinsky NL. Treatment of cavernous hemangioma with vincristine. Ann Pharmacother. 1993;27:981. doi: 10.1177/106002809302700735. [DOI] [PubMed] [Google Scholar]
- 36.Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524–527. doi: 10.1126/science.6836297. [DOI] [PubMed] [Google Scholar]
- 37.Hunzeker CM, Geronemus RG. Treatment of superficial infantile hemangiomas of the eyelid using the 595-nm pulsed dye laser. Dermatol Surg. 2010;36:590–597. doi: 10.1111/j.1524-4725.2010.01511.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Li F, Yang Y, Xue L, Cao M, Wang L. Hemangioma treatment with pulsed dye laser-distinct parameters used between neonatal and non-neonatal patients. J Cosmet Laser Ther. 2016;18:389–392. doi: 10.1080/14764172.2016.1197402. [DOI] [PubMed] [Google Scholar]
- 39.Seukeran DC, Collins P, Sheehan-Dare RA. Adverse reactions following pulsed tunable dye laser treatment of port wine stains in 701 patients. Br J Dermatol. 1997;136:725–729. [PubMed] [Google Scholar]
- 40.Stier MF, Glick SA, Hirsch RJ. Laser treatment of pediatric vascular lesions: Port wine stains and hemangiomas. J Am Acad Dermatol. 2008;58:261–285. doi: 10.1016/j.jaad.2007.10.492. [DOI] [PubMed] [Google Scholar]
- 41.Alcántara-González J, Boixeda P, Truchuelo-Díez MT, Pérez-García B, Alonso-Castro L, Jaén Olasolo P. Infantile hemangiomas treated by sequential application of pulsed dye laser and Nd:YAG laser radiation: a retrospective study. Actas Dermosifiliogr. 2013;104:504–511. doi: 10.1016/j.ad.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Zhong SX, Tao YC, Zhou JF, Liu YY, Yao L, Li SS. Infantile Hemangioma: Clinical Characteristics and Efficacy of Treatment with the Long-Pulsed 1,064-nm Neodymium-Doped Yttrium Aluminum Garnet Laser in 794 Chinese Patients. Pediatr Dermatol. 2015;32:495–500. doi: 10.1111/pde.12593. [DOI] [PubMed] [Google Scholar]
- 43.Vlachakis I, Gardikis S, Michailoudi E, Charissis G. Treatment of hemangiomas in children using a Nd:YAG laser in conjunction with ice cooling of the epidermis: techniques and results. BMC Pediatr. 2003;3:2. doi: 10.1186/1471-2431-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izikson L, Anderson RR. Treatment endpoints for resistant port wine stains with a 755 nm laser. J Cosmet Laser Ther. 2009;11:52–55. doi: 10.1080/14764170802524452. [DOI] [PubMed] [Google Scholar]
- 45.Jasim ZF, Handley JM. Treatment of pulsed dye laser-resistant port wine stain birthmarks. J Am Acad Dermatol. 2007;57:677–682. doi: 10.1016/j.jaad.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 46.Reddy KK, Blei F, Brauer JA, Waner M, Anolik R, Bernstein L, Brightman L, Hale E, Karen J, Weiss E, Geronemus RG. Retrospective study of the treatment of infantile hemangiomas using a combination of propranolol and pulsed dye laser. Dermatol Surg. 2013;39:923–933. doi: 10.1111/dsu.12158. [DOI] [PubMed] [Google Scholar]
- 47.Herschthal J, Wulkan A, George M, Waibel J. Additive effect of propranolol and pulsed dye laser for infantile hemangioma. Dermatol Online J. 2013;19:18570. [PubMed] [Google Scholar]