Abstract
BACKGROUND
Primary choriocarcinoma of the stomach (PCCS) is a rare tumor, with fewer than 60 cases published in the English-language literature up to December 2018. In this paper, we present the complex immunoprofile of one PCCS and a hypothesis regarding its histogenesis.
CASE SUMMARY
A 66-year-old previously healthy male underwent an emergency palliative gastrectomy for a gastric obstructive tumor. The histologic examination and immunoprofile of tumor cells showed a mixed tumor that consisted of choriocarcinoma (90%) and moderate differentiated adenocarcinoma (10%), with hepatic metastases (Stage pT2NxM1L1V1R0) and microsatelite stable status. The patient died one month after surgery. The tumor cells showed focal positivity for CDX2 (adenocarcinoma component), HCG (choriocarcinoma) and CD138 (plasmacytoid carcinoma component) and were negative for HER-2, α-fetoprotein, VEGF, maspin and markers of epithelial-mesenchymal transition. The gastric mucosa cells displayed positivity for CDX2, Hepar A and CD138. The complex immunoprofile and literature data synthesis prove that the gastric mucosa cells can present a multilineage differentiation.
CONCLUSION
PCCS should be considered as an aggressive variant of microsatellite stable gastric adenocarcinoma of an epithelial type, and not a germ cell tumor.
Keywords: Choriocarcinoma, Stomach, Gastrointestinal, Immunohistochemistry, Maspin, Case report
Core tip: In this paper we presented an extremely rare histological variant of aggressive gastric carcinomas. Few than 60 cases of primary choriocarcinoma of the stomach were published in English literature. The complex immunoprofile of tumor cells, correlated with literature data, showed that gastric mucosa cells can present a multilineage differentiation and choriocarcinoma is a primary microsatellite stable adenocarcinoma without germ cell origins.
INTRODUCTION
Choriocarcinoma is a malignant tumor that is characterized by the secretion of the beta subunit of human choriogonadotropin (β-HCG). It is a common tumor of the ovary and testis, being developed from trophoblastic or totipotent germ cells[1,2]. Choriocarcinoma can also occur in other organs such as the uterine body, brain (pineal gland), mediastinum, lung, retroperitoneum, urinary tract, liver, gallbladder, and gastrointestinal tract[1,3].
Primary choriocarcinoma of the stomach (PCCS) is an extremely rare (0.08% of all gastric cancers) but aggressive tumor (survival below six months) in which histogenesis and therapeutic management are still undefined[1,2]. PCCS was first described by Davidhon in 1905 and nominated as chorionepitheliom[4].
Until 2005, 142 cases of PCCS have been reported in total of which only 32 have been reported in English literature, with a male: female ratio of 2.3:1[1,2,5]. The reported cases were predominantly diagnosed in patients over 60 years old, with about 40% of cases occurring in the lower third of the stomach, with direct pancreatic or duodenal invasion[1,2]. Another 26 cases have been reported in English research literature between 2006 and 2018.
In this paper, we present a case of PCCS development in a male patient, and a hypothesis regarding the histogenesis of PCCS, based on the complex immunoprofile of the tumor cells and literature data.
CASE PRESENTATION
Chief complaints
A 66-year-old previously healthy male presented with epigastric pain and nausea.
History of present illness
Patient related two-week history of epigastric pain and nausea, without other signi-ficant symptoms.
History of past illness
He was an ex-smoker of four years and a social drinker. No other diseases were asso-ciated.
Personal and family history
No significant diseases were related.
Physical examination upon admission
Physical examination revealed scleral and cutaneous jaundice and hepatomegaly.
Laboratory examinations
All of the usually performed serum parameters were in normal limits. The serum value of tumor-specific markers such α-Fetoprotein (AFP), Carcinoembryonic Antigen (CEA), or HCG were not checked.
Imaging examinations
The CT scan showed a thickened posterior wall of the stomach at the pylorus, with periaortic lymphadenopathies and hepatic and pulmonary metastases. No other tumors of the breast, testis, mediastinum or peritoneum were described.
Histopathological assessment
Macroscopic examination of the 40 × 35 × 15 gastric specimen revealed a 33 mm × 33 mm × 15 mm ulcero-vegetative tumor, without lymph nodes in the surgical specimen. The 30 × 20 × 15 mm hepatic specimen was replaced by a whitish ill-defined tumor mass with necrosis and hemorrhages on cut section.
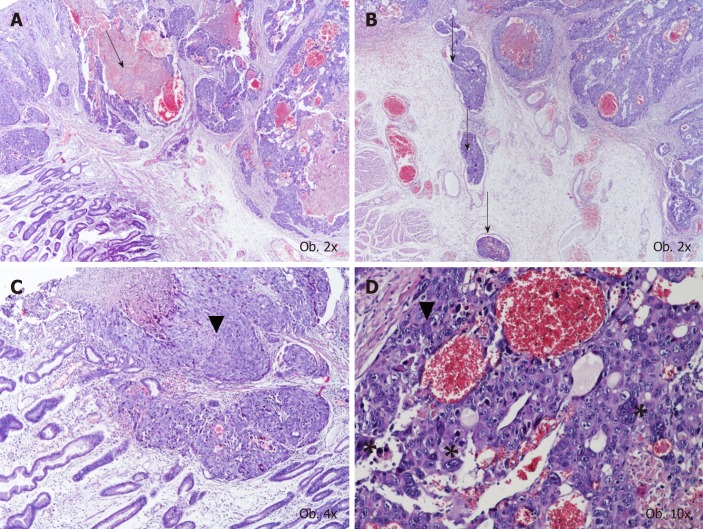
Under microscope, in the mucosa, submucosa and muscularis propria of the stomach, proliferation of large dyscohesive and pleomorphic tumor cells and large hemorrhagic areas were seen. These cells proliferated, predominately surrounding large blood vessels. Multiple vascular emboli were also shown. Two types of cells were identified: Giant cells with lobulated or bizarre nuclei, similar to syncytio-trophoblastic cells, and oval cells with pale cytoplasm, with a similar aspect to cytotrophoblastic cells (Figure 1).
Figure 1.
Histological examination results. A, B: The histological aspect of choriocarcinoma, with large hemorrhagic area (A), multiple vascular emboli (B), and characteristic proliferation of oval cells with pale cytoplasm with similar aspect to cytotrophoblastic cells (marked by ▼) and giant cells with lobulated or bizarre nuclei (marked by *); C, D: Similar to syncytiotrophoblastic cells.
Immunohistochemical profile
Both types of tumor cells were diffusely marked by cytokeratin 7, MLH-1, MSH-2, PMS-2 and c-MET. The syncitiotrophoblastic- but not cytotrophoblastic cells showed diffuse positivity for inhibin and the plasma-cell-specific marker CD138 (Figure 2).
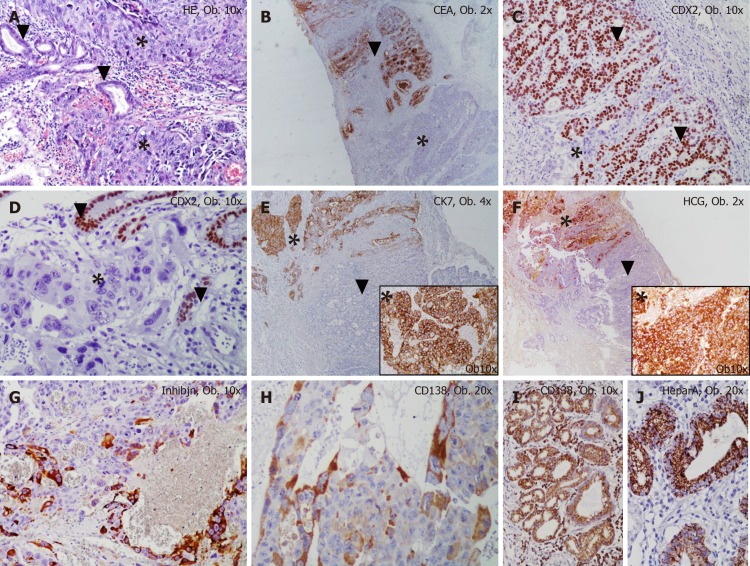
Figure 2.
Histological examination results. A: The mixed gastric carcinoma shows two components: choriocarcinoma (marked by *) and adenocarcinoma (marked by ▼); B-F: The choriocarcinoma cells are negative for CEA (B) and CDX2 (C, D) and show positivity for Cytokeratin 7 (E) and HCG (F); G, H: The syncytiotrophoblast-like cells are marked by inhibin (G) and CD138 (H). The adenocarcinoma component is positive for CEA (B) and CDX2 (C, D) and to not present positivity for Cytokeratin 7 (E) and HCG (F); I, J: The normal gastric mucosa cells are marked by CD138 (I) and Hepar A (J).
In one of the resection margins, a differentiated (G2) adenocarcinoma component was identified, which accounted for below 10% of the tumor and showed diffuse positivity for CDX2, cytokeratin 7, CEA and c-MET and focal positivity for cytokeratin 20 and CD138. A gradual transition between the two components was seen (Figure 2). The characteristics of the biomarkers used and complete immunohistochemical (IHC) profile of normal gastric mucosa and the two components of the tumor can be seen in Table 1. It is necessary to mention the progressive loss of E-cadherin in both carcinoma components and tumor emboli. In emboli, CD138 and β-catenin positivity was displayed by the tumor cells but inhibin was negative. In emboli and hepatic metastasis, the choriocarcinoma component was identified.
Table 1.
The immunoprofile of gastric mucosa versus adenocarcinoma and choriocarcinoma component of a primary gastric tumor
| Antibody | Normal gastric mucosa | Adenocarcinoma component | Choriocarcinoma component | |
| Markers for diagnosis | CK7 | Positive | Positive | Positive |
| CK20 | Positive | Positive | Negative | |
| CK19 | Negative | Negative | Negative | |
| CDX2 | Positive | Positive | Negative | |
| HCG | Negative | Negative | Positive | |
| Inhibin | Negative | Negative | Positive - syncytiotrophoblast cells | |
| CEA | Negative | Positive | Negative | |
| Ki67 index | Internal control | 100% | 100% | |
| Synaptophysin | Internal control | Negative | Negative | |
| CD138 | Positive-membrane | Positive-membrane+cytoplasm | Positive- syncytiotrophoblast cells | |
| Hep Par 1 | Positive | Negative | Negative | |
| PLAP | Negative | Negative | Negative | |
| ALK | Negative | Negative | Negative | |
| AFP | Negative | Negative | Negative | |
| HER-2 | Negative | Negative | Negative | |
| c-KIT | Negative | Negative | Negative | |
| P63 | Negative | Negative | Negative | |
| CK5/6 | Negative | Negative | Negative | |
| S100 | ||||
| Markers for microsatelite status | MLH-1 | Positive | Positive | Positive |
| MSH-2 | Positive | Positive | Positive | |
| PMS-2 | Positive | Positive | Positive | |
| EMT markers | E-cadherin | Positive | Negative | Negative |
| β-catenin | Positive-membrane | Positive-membrane | Positive-membrane | |
| Vimentin | Negative | Negative | Negative | |
| SLUG | Negative | Negative | Negative | |
| Adhesion molecules | VSIG1 | Positive-membrane | Negative | Negative |
| CD44 | ||||
| Markers of angiogenesis | Maspin | Negative | Negative | Negative |
| VEGF-A | Negative | Negative | Negative | |
| c-met | Positive | Positive | Positive |
AFP: Alpha fetoprotein; ALK: Anaplastic lymphoma kinase; CEA: Carcinoembryonic antigen; CD: Cluster of differentiation; CK: Cytokeratin; EMT: Epithelial-mesenchymal transition; HCG: Human choriogonadotropin; Hep Par 1: Hepatocyte paraffin 1; PLAP: Placental alkaline phosphatase; VEGF: Vascular endothelial growth factor; VSIG: V-set and immunoglobulin domain containing 1.
FINAL DIAGNOSIS
Based on the histological aspect and immunoprofile of the tumor cells, the final diagnosis was gastric adenocarcinoma with choriocarcinomatous component, pT2NxM1L1V1R0 (Stage IV).
TREATMENT
Due to the obstructive effect of the hepatic metastases, urgent palliative gastrectomy with partial hepatic resection was performed, without preoperative biopsy. Signed informed consent for surgical intervention and publication of data was obtained before surgery.
OUTCOME AND FOLLOW-UP
The patient died one month after surgery. No chemotherapy was administered.
DISCUSSION
There are at least three theories regarding the histogenesis of PCCS. It was postulated that PCCS might originate from putative displaced gonadal anlage, from a teratoma or by retro-differentiation of adenocarcinoma[1] through the direction of embryonal ectoderm, retaining the ability to form trophoblasts[2]. As an adenocarcinoma com-ponent can be identified in 70% of PCCS[1], such as in our case, and as an adenocar-cinoma component can be infrequently founded in nodal metastasis[2], we consider that PCCS is a dedifferentiated component of adenocarcinoma. In our case, CD138 positivity indicated a plasmacytoid component, as a possible multilineage differentiation (Table 1). Although no hepatoid component was identified and AFP was negative, gastric mucosa showed positivity for both Hepar A and CD138. This aspect indicates the possible stemness features of gastric epithelial cells. In line with our hypothesis, the possible multilineage differentiation is proved by co-existence of other histological particular tumor types such as PCCS and small cell carcinoma[5].
Diagnosis of PCCS is difficult to conduct and is usually established in surgical specimens only. In bioptic specimens, the diagnosis of PCCS was reported in less than 15% of cases[1].
In serum, several markers might be elevated, such as HCG (reported preoperative values between 246 and 15500 mIU/mL) and/or AFP (approximately 50 mU/mL)[1-3]. Postoperative decreasing HCG value is seen in most of the cases[1-3] and can be used as a potential marker of recurrence[3]. Although the choriocarcinomatous component is CEA negative, such as in our case, the serum value of CEA can be higher than 20 ng/mL[3], probably due to the adenocarcinoma component.
The differential diagnosis should take into account both primary and metastatic tumors, based on imagistic investigation of the patient and the histological aspect, combined with the IHC profile. Metastases from a primary tumor of gonads (ovary, testes), endometrium or retroperitoneum must be excluded.
As approximately 70% of PCCS showed an adenocarcinoma component[1], as in the present case, other rare variants of gastric carcinomas should be taken into account. The hepatoid variant was excluded based on negativity for hepatocyte paraffin 1 (Hep Par 1) and α-Fetoprotein[3]. Negativity of tumor cells for markers such as Cytokeratin 5/6 and p63 exclude the diagnosis of adenosquamous carcinoma, whereas negativity for vimentin and c-KIT (CD117) exclude a gastrointestinal stromal tumor[1].
The PCCS shows predominately systemic spread in the lungs, liver and peritoneum, but lymph node metastases are also associated[1]. Over 30% of the cases are diagnosed in the metastatic stage[3], with direct pancreatic invasion[2], such as in the present case.
As the histogenetic pathway is still unknown, there is no consensus regarding therapeutic strategies[6]. In the reported cases, the chemotherapics used for gestational choriocarcinoma were recommended[1-3,6]. They include a BEP (Bleomycin, Etoposide, Cisplatin) regimen[1], EMA/CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine) regimen[3], DCF (docetaxel, cisplatin, 5-FU) regimen[6], association of Cisplatin and Carboplatin[2] or Vinblastine, Ifosfomide and Cisplatin[1]. Folinic acid was also reported to be used[1,2] but no complete answer can be obtained with the above nominated drugs[6].
Although no HER-2 positivity was seen in our case, the adenocarcinoma or choriocarcinoma component can show HER-2 gene amplification[3,5] and targeted therapy with Trastuzumab combined with docetaxel and carboplatin can be done, with possible complete response[6]. In these cases, maintenance therapy with trastuzumab and capecitabine is indicated[6]. As positivity for PD-L-1 was shown in choriocarcinoma cells, these rare carcinomas might benefit by anti–PD-1 or anti–PD-L1 agents used for immunotherapy, such as nivolumab and pembrolizumab[7].
CONCLUSION
Primary gastric choriocarcinoma is a rare variant of gastric adenocarcinoma that can show multilineage differentiation. Targeted therapy of this histological type still remains unknown.
Footnotes
Informed consent statement: Signed consent of patient was obtained.
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Invited manuscript
Peer-review started: February 26, 2019
First decision: April 18, 2019
Article in press: June 10, 2019
Specialty type: Medicine, Research and Experimental
Country of origin: Romania
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kato J, Isik A S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ
Contributor Information
Simona Gurzu, Department of Pathology, University of Medicine, Pharmacy, Sciences and Technology, Targu Mures 530149, Romania. simona.gurzu@umftgm.ro; Research Center (CCAMF), University of Medicine, Pharmacy, Sciences and Technology, Targu Mures 540139, Romania.
Constantin Copotoiu, Department of Surgery, University of Medicine, Pharmacy, Sciences and Technology, Targu Mures 530149, Romania.
Alexandra Tugui, Department of Pathology, University of Medicine, Pharmacy, Sciences and Technology, Targu Mures 530149, Romania.
Cedric Kwizera, Department of Surgery, University of Medicine, Pharmacy, Sciences and Technology, Targu Mures 530149, Romania.
Rita Szodorai, Department of Pathology, University of Medicine, Pharmacy, Sciences and Technology, Targu Mures 530149, Romania.
Ioan Jung, Department of Pathology, University of Medicine, Pharmacy, Sciences and Technology, Targu Mures 530149, Romania.
References
- 1.Raghavapuram R, Veerankutty FH, Anandakumar M. Primary Choriocarcinoma of the Stomach. A Case Report and Review of the Literature. Indian J Surg Oncol. 2016;7:119–123. doi: 10.1007/s13193-016-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kobayashi A, Hasebe T, Endo Y, Sasaki S, Konishi M, Sugito M, Kinoshita T, Saito N, Ochiai A. Primary gastric choriocarcinoma: two case reports and a pooled analysis of 53 cases. Gastric Cancer. 2005;8:178–185. doi: 10.1007/s10120-005-0332-9. [DOI] [PubMed] [Google Scholar]
- 3.Baraka BA, Al Kharusi SS, Al Bahrani BJ, Bhathagar G. Primary Gastric Chorioadenocarcinoma. Oman Med J. 2016;31:381–383. doi: 10.5001/omj.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidsohn C. Chorion-epitheliom and magenkrebs, eine seltene verschmelzung zweizer bosartiger geschwulste. Charite Annal. 1905;29:426–436. [Google Scholar]
- 5.Fukuda S, Fujiwara Y, Wakasa T, Inoue K, Kitani K, Ishikawa H, Tsujie M, Yukawa M, Ohta Y, Inoue M. Collision tumor of choriocarcinoma and small cell carcinoma of the stomach: A case report. Int J Surg Case Rep. 2017;37:216–220. doi: 10.1016/j.ijscr.2017.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunduz S, Elpek GO, Uysal M, Goksu SS, Tatli M, Arslan D, Coskun HS, Bozcuk H, Savas B, Ozdogan M. Coexistence of gastric adenocarcinoma and choriocarcinoma: complete response to trastuzumab and chemotherapy. Case Rep Oncol. 2012;5:394–399. doi: 10.1159/000341662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu B, Teng X, Fu G, Bao L, Tang J, Shi H, Lu W, Lu Y. Analysis of PD-L1 expression in trophoblastic tissues and tumors. Hum Pathol. 2019;84:202–212. doi: 10.1016/j.humpath.2018.10.001. [DOI] [PubMed] [Google Scholar]