Abstract
Fundamental electronic principles underlying all transition metal compounds are the symmetry and filling of the d-electron orbitals and the influence of this filling on structural configurations and responses. Here we use a sensitive local structural technique, x-ray atomic pair distribution function analysis, to reveal the presence of fluctuating local-structural distortions at high temperature in one such compound, CuIr2S4. We show that this hitherto overlooked fluctuating symmetry-lowering is electronic in origin and will modify the energy-level spectrum and electronic and magnetic properties. The explanation is a local, fluctuating, orbital-degeneracy-lifted state. The natural extension of our result would be that this phenomenon is likely to be widespread amongst diverse classes of partially filled nominally degenerate d-electron systems, with potentially broad implications for our understanding of their properties.
Subject terms: Electronic properties and materials, Structure of solids and liquids
A common feature of many transition metal materials is global symmetry breaking at low temperatures. Here the authors show that such materials are characterized by fluctuating symmetry-lowering distortions that exist pre-formed in higher temperature phases with greater average symmetry.
Introduction
Broken symmetry ground states are often found in transition metal systems exhibiting emergent properties such as metal-insulator transitions1–3, charge-ordered and charge-density wave states4, colossal magnetoresistive effects5, frustrated magnetism6,7, pseudogap8 and high-temperature superconductivity9,10. These are generally driven by electronic interactions understood as Fermi-surface nesting11–13, Peierls distortions14,15, and cooperative Jahn-Teller effects16. These phenomena have energy scales of hundreds to thousands of meV17, corresponding to thousands of Kelvin, yet the broken symmetries tend to appear at much lower temperatures, typically 101–102 K. The symmetry-broken states at low temperature, have been extensively studied. Fewer details are known about what happens when these materials transition to crystallographically higher symmetry structures upon warming.
Here we present a study that reveals critical insights into the unaccounted for separation in energy scales by applying a quantitative local structure probe, atomic pair distribution function analysis (PDF), to a model material system that exemplifies this behavior. The material system, CuIr2S4, has rich broken symmetries in its ground state18, including the formation of magnetic singlet Ir–Ir pairs, which disappear on warming through a structural transition that is also, concurrently, a metal-insulator transition (MIT). The PDF analysis reveals difficult to detect but important local-structural distortions that exist at high temperatures, something that has been seen before in other systems (see for example Billinge et al.19). However, the special scattering characteristics of this system, together with our detailed temperature and doping dependent study with multiple dopant species, exposes fine details of the phenomenon establishing it as a robust but fluctuating d-orbital-degeneracy-lifted (ODL) state that is observed to the highest temperatures measured. Much lower elastic energies govern the long-range ordering of the pre-formed local symmetry-broken ODL objects in these structurally compliant materials20 which therefore occurs at temperatures much lower than the electronic energies of ODL formation. Interestingly, in CuIr2S4 it is not the formation of the ODL objects, but their ordering that precipitates the MIT and magnetic dimer formation of the ground state. The fluctuating ODL state is stabilized electronically by breaking d-electron orbital degeneracies and as such is likely to be a phenomenon that is widespread, though not widely appreciated, among the many materials with incompletely filled d-electron manifolds21,22, many of which have important emergent low-temperature electronic and magnetic behaviors, from classics such as manganites11,23,24, cuprates4,9,25, and iron chalcogenides/pnictides7,10,26–29, to materials featuring exotic low-temperature orbital molecules30,31. It may also explain the unexpected observation of phonon-glass-like thermal conductivity in various transition metal oxides32.
Results
Long-range orbital and charge order and spin dimerization
The low-temperature insulating state in CuIr2S433–35 consists of ordered Ir3+ (5d6) and Ir4+ (5d5) ions36, with a four-fold periodicity, an example of tetrameric charge ordering37. Concurrently, spin dimerization of Ir4+ pairs occurs within the tetramer, with large associated structural distortions as they move towards each other, making this charge order particularly amenable to detection using structural probes18. Notwithstanding the complexities of the insulating state, including formation of remarkable three-dimensional S24 and S24 molecule-like assemblies embedded in the lattice, its quasi-one-dimensional character was unmasked and MIT attributed to an orbital-selective Peierls mechanism, postulated from topological considerations38. The global symmetry lowering at the MIT was declared to lift the existing t2g d-orbital degeneracies38. Although the high-temperature crystallographically cubic metallic state18,33 appears to be undistorted, with nominally Ir3.5+ (5d5.5) partially filled delocalized bands39, CuIr2S4 does not behave like a band-metal, as evidenced by anomalous transport and spectroscopic signatures40,41. Despite early speculations to the contrary40–42, it was established that the structural dimers disappear on warming through the transition on all length scales, leaving the mystery of poor metallicity unresolved39. Curiously, the isostructural and isoelectronic sister compound, CuIr2Se4, has an order of magnitude higher conductivity and no MIT down to 0.5 K40, which is also difficult to rationalize within the current understanding of these systems.
In our high-sensitivity x-ray pair distribution function (xPDF) analysis of the high-temperature metallic state of CuIr2S4 we uncover a previously unobserved local symmetry lowering of the Ir pyrochlore sublattice, associated with an orbital liquid-like state that is present to the highest measured temperature. Through judicious chemical substitutions, we demonstrate that the effect is electronic and that it involves a symmetry lowering of the molecular orbitals, or Ir metal-metal bonds, on the pyrochlore sublattice. This is related to, but qualitatively different from, the dimer state observed in the insulating phase. It is Jahn-Teller like, in that the symmetry lowering breaks the degeneracy of partially filled Ir d states, which results in orbital selectivity, with charges preferrentially selecting a subset of Ir–Ir metal–metal orbitals. At high temperature the selected orbitals do not order and are presumably fluctuating. This orbital liquid-like precursor state crystallizes upon approaching the Peierls-like MIT, testifying to the crucial role of orbital physics38.
Structural fingerprint of the ODL state
The PDF consists of peaks whose position is at interatomic distances in a material. It is therefore sensitive to any structural perturbation, because sharp single-valued PDF peaks in a high symmetry structure become broadened or multicomponent when the symmetry is lowered. In the low-temperature state of CuIr2S4, long-range orbital and charge order results in Ir4+–Ir4+ pairs forming structural and magnetic dimers, which have been established crystallographically18. The Ir–Ir dimer pair distance is ~0.5 Å shorter than that of the Ir–Ir non-dimer pairs, creating two well-resolved peaks in the low-temperature PDF. In fact, the PDF dimer-peaks can be clearly seen by eye in the stack of PDFs shown as a function of temperature in Fig. 1a as a vertical ridge in the waterfall plot at ≈3 Å, labeled with the red arrow.
Fig. 1.
Observation of high-temperature fluctuating ODL state in CuIr2S4. a Temperature waterfall stack of xPDFs measured on warming from 10 K (bottom) to 500 K (top) in 2 K increments. TMI is the MIT transition temperature (226 K). The dimer peak at ~3 Å (marked by arrow) is only seen in the insulating phase, and disappears above TMI. b Fit of the undistorted cubic Fdm model (red line) to the 500 K data (blue open circles) and their difference (green line, offset for clarity) unmask the footprint of the localized ODL state at ~3.5 Å. c Simulated Fdm total xPDF of CuIr2S4 (blue line), decomposed into Ir–Ir partial xPDF (green line) and its complement (red line). Shaded peaks in total xPDF are sensitive to t2g orbital overlaps (sketched) and their spatial correlations. Inset: t2g-derived molecular orbitals discussed in the main text. d, e Section of Ir pyrochlore sublattice of corner-shared Ir4 tetrahedra for undistorted (cubic) and distorted (tetragonal) spinel structure, respectively. The strongest t2g orbital overlaps (e.g. xy with xy, etc.) are along the chains formed by the tetrahedral edges of the Ir sublattice38
The dimers disappear in the average structure at TMI18, but they also disappear in the local structure, as first reported in ref. 39, and which can be seen directly in the data in Fig. 1a. There is no dimer-liquid state at high temperature in CuIr2S4, and the dimers themselves disappear at TMI, which rules out fluctuating dimers as the culprit behind the poor metallicity at high temperature.
We have approached the question of the anomalous metallic state by measuring a new, more complete and higher precision set of xPDF data from CuIr2S4 (Fig. 1a), where we now focus on the high-temperature metallic state above TMI. The average crystal structure in this regime is cubic spinel, space-group Fdm, in which the iridium ions make a pyrochlore sublattice that consists of a network of regular corner-shared tetrahedra illustrated in Fig. 1d. The high symmetry of this cubic structure results in an apparent sharpening of peaks in the PDF, as is evident in the waterfall plot in Fig. 1a, where PDF peak sharpening is observed on warming through TMI (normally PDF peaks broaden on warming due to increased atomic thermal motion). This occurs because the higher symmetry (cubic) phase has fewer PDF peaks than the lower symmetry (triclinic) phase. Indeed, fits of the cubic structure model to the high-T data result in excellent agreement (e.g., for T = 500 K, rw = 5.1%, Fig. 1b) Under normal circumstances this would be considered a highly satisfactory PDF fit. However, careful inspection of the residual curve in green in Fig. 1b reveals a feature at around 3.5 Å, indicating a shift in intensity to higher-r in the data compared to the model.
The PDF peak centered at 3.5 Å originates almost exclusively from the Ir–Ir nearest neighbor atomic pair on the pyrochlore sublattice, as shown in Fig. 1c. The total PDF consists of the weighted sum of partial PDFs between pairs of each type of atom, and Fig. 1c shows that the Ir–Ir partial-PDF contributes more than 95% of the signal to the 3.5 Å peak in the total PDF. The residual signal therefore clearly originates from deviation of the structural geometry from the regular pyrochlore lattice implied by the cubic model. Importantly, maximal t2g overlaps of the orbitals of the same type (xy with xy, yz with yz, and zx with zx) are precisely along the directions defined by the edges of the pyrochlore lattice38, as sketched in the inset to Fig. 1c, implying that the orbital sector is involved.
Temperature evolution and characterization of the ODL state
To explore the temperature dependence, the same analysis is carried out on PDFs measured at temperatures up to 780 K and representative fits are shown in Fig. 2a–f. The result of the fitting for the 500 K dataset is reproduced in Fig. 2a over the entire r-range, and then on a narrower r-scale in Fig. 2c, with the residual signal highlighted. The same signal in the residual is also evident at 232 K, 300 K and in the 780 K data (Fig. 2d–f, respectively). The 232 K dataset is from immediately above the MI transition temperature (226 K on warming).
Fig. 2.
Temperature evolution and character of the ODL state associated distortion. a–f Fit of the cubic Fdm model (red line) to the CuIr2S4 data at various temperatures as indicated (blue open circles). Difference curve between the data and the model (green line) is offset for clarity in all cases. g Simulated Ir–Ir partial xPDFs for undistorted cubic Fdm (red line) and distorted tetragonal I41/amd (blue line) structures, with the associated difference between the tetragonal and cubic models (green line) underneath, offset for clarity. h Short range 1.5 < r < 6 Å fit of the distorted tetragonal model (red line) to the 500 K data (blue open circles), with associated difference curve underneath. The model and residual curves for r > 6 Å represent a result of extending the calculation range without altering the fitted parameters. Insets to a, b show evolution with reduced temperature of the cubic model fit residual, and the estimated local ODL distortion correlation length, respectively. Solid lines are guides to the eye
To explore the structural origin of this residual signal we utilized structural models that allowed for distortions to the pyrochlore sublattice, and focused on a model in the I41/amd space group that was implicated in the early studies of the low-temperature phase33. In particular, the tetragonal distortion lowers the symmetry of the regular Ir4 pyrochlore tetrahedra, yielding 2 short and 4 long Ir-pair distances (Fig. 1e). Comparison of PDFs computed from the distorted and undistorted models, as seen in Fig. 2g, results in a difference curve that qualitatively reproduces the residual signal observed at 3.5 Å, Fig. 1b, when fitting with an undistorted cubic model. However, the tetragonal distortion leads to additional features in the PDF which are not seen in the measured data, suggesting that the tetragonal distortion is not appropriate at all length scales. Here we take advantage of the real-space nature of the PDF, and fit a tetragonal model over the narrow range 1.5 < r < 6 Å. This removes the residual signal at 3.5 Å (Fig. 2h) and introduces only a single additional refinement parameter (tetragonal axis). The resulting fit produced a tetragonal distortion of 0.08(1) Å, which corresponds to long Ir–Ir bonds of 3.52 Å and short Ir–Ir bonds of 3.44 Å.
This disparity in symmetry paints a clear picture where Ir4 tetrahedra, each with a local tetragonal distortion, are oriented in a disordered manner, such that individual distortions do not accumulate over long length scales, but rather average to a cubic symmetry. This is consistent with the observation that the low-r portion of the PDF can be reproduced well only when using a tetragonally distorted model, but that this model fails to reproduce higher-r features. Notably, the magnitude of the structural distortion at high temperature is 7× smaller than the distortion corresponding to the dimer state.
The correlation length of the ordering of such distorted tetrahedra can in principle be extracted from the PDF. Inspection of the residual curve in the 232 K data (Fig. 2b) suggests that at lower temperatures, though still above TMI, the fit of the cubic model is worse than at higher temperature and that the residual signal extends over a wider-range of r, up to 40 Å. This would be the case if the short bonds were beginning to short-range order with some correlation length. The temperature dependence of the correlation length, ξ, can be estimated using previously reported protocols43 and further described in Methods Section. The result is shown in the inset to Fig. 2b. The correlation length is 6 Å at high temperature, but smoothly increases to 20 Å as the MI transition is approached. This divergent behavior is mimicked if we consider the cubic model fit residual, rw, as a function of temperature (inset to Fig. 2a).
The symmetry breaking implies a breaking of the degeneracy of orbitals42, which we refer to as an orbital-degeneracy-lifted state, dubbed ODL, on at least some of the Ir tetrahedra. The charge selects and preferrentially enters the lower energy orbitals, which may fluctuate among all the possible edges of the pyrochlore tetrahedra at high temperature (Fig. 3h). Such a phenomenon could be caused by various driving forces, including Jahn-Teller effects, covalency, or spin-orbit coupling44,45, and we do not speculate on the origin yet. However, we note that orbital selectivity impacts phenomena in diverse systems from VO21, K2Cr8O1615, and Sr2−xCaxRuO446, to FeSe21,22,47, but the persistence of orbital selectivity to such high temperatures in a disordered orbital liquid state has not been widely observed. Our data show that the structural and metal-insulator transition on cooling corresponds not to the formation of an orbitally ordered state, but to the phase coherence and resulting long-range ordering of the pre-formed ODL objects.
Fig. 3.
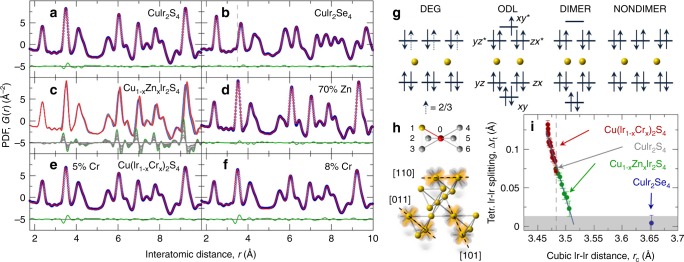
Manipulation of the ODL state. a Fit of the cubic model (red line) to the 300 K CuIr2S4 data (blue open circles). b Fit of the cubic model (red line) to the 300 K CuIr2Se4 data (blue open circles). c Compositional stack of 300 K data for Zn-substituted CuIr2S4 with Zn content ranging from 0% (blue line) to 70% (red line) in 10% increments (gray lines). The differences between the CuIr2S4 parent and all other datasets are stacked underneath, offset for clarity. The largest difference between the 0% Zn and 70% Zn datasets is shown in green, other differences in gray, evolving uniformly with Zn content. d Fit of the cubic model (red line) to the 300 K 70% Zn-substituted CuIr2S4 data (blue open circles). e, f Fit of the undistorted cubic model (red lines) to the 300 K 5% and 8% Cr-substituted CuIr2S4 data (blue open circles), respectively. g Molecular-orbital (MO) view, from left to right, of degenerate MO, degeneracy-lifted MO, dimerized, and non-dimerized Ir–Ir contacts. In the legend, DEG (Ir3.5+), ODL (Ir3.5+), DIMER (Ir4+), and NONDIMER (Ir3+). h Sketch of [1, 1, 0]-type Ir t2g overlaps (bottom) and six choices for each Ir to form an ODL state (top). i Evolution of the ODL distortion, defined as the difference of the Ir–Ir nearest neighbor distance on a pyrochlore lattice extracted from local tetragonal model, with the average Ir–Ir separation in the cubic structure. These are extracted from fits to 300 K data of Cr-substituted (red circles, 0 < x < 0.6) and Zn-substituted (green circles, 0 < x < 0.7) samples, as well as pure CuIr2S4 (gray circle) and CuIr2Se4 (blue circle). Error bars represent estimated standard uncertainties on the refined parameters. Gray shaded region marks 2σ uncertainty for detecting small tetragonal distortions by the approach utilized here. In CuIr2S4 there are 0.5 t2g holes per Ir (one hole per pair)38. Vertical gray dashed lines in b, i refer to CuIr2S4
Electronic manipulation of the ODL state
We now establish an electronic driving force for this ODL effect. Iridium takes on a nominal 5d5.5 average electron configuration. In the cubic pyrochlore lattice the t2g orbitals are well separated in energy from the eg orbitals due to crystal field effects, and the t2g orbital of one Ir points directly towards the neighboring Ir ion38,45. The large spatial extent of the 5d-states suggests a significant overlap of these orbitals and considerable covalency45, though this is not required for the discussion. We could then consider the orbital selectivity17, to happen on a basis of molecular orbitals48 (inset to Fig. 1c). In this case there would be a symmetry breaking into short and long edges on the pyrochlore tetrahedra depending on the electron filling of the molecular orbital but the incomplete filling of the t2g manifold provides a Jahn-Teller-like driving force for the distortion that lifts the orbital degeneracy. Each Ir has six neighbors to choose from and randomize orbital selectivity. These concepts are illustrated in Fig. 3g, h top.
Altering the charge state of the Ir ions offers a method by which we can test this hypothesis. We have done this by doping Zn2+ on the Cu1+ site. The zinc doping increases the electron count in the Ir sublattice, without significantly disrupting the lattice49. The details are provided in Supplementary Note 1 and Supplementary Fig. 1, and the results are summarized in Fig. 3. Zn doping increases the electron count in an anti-bonding ODL state, marked with an asterisk in Fig. 3g. If the observed structural distortion is driven by a local Jahn-Teller effect, the feature in the residual should diminish with increasing Zn content as doping electrons in an anti-bonding band destabilizes the ODL state. This is exactly what is observed (Fig. 3c, d), establishing the electronic driving force for the effect.
We also consider the substitution of chromium on the iridium sublattice. Chromium is a small ion and introduces a compressive chemical pressure. It also introduces quenched defects into the Ir sublattice, disrupting the ability of Ir orbitals to order over long range at low temperature and suppressing the low-temperature orbital order state43. Fits of the cubic model to two of the Cr doped data-sets are shown in Fig. 3e, f. They clearly show that the signal in the residual at 3.5 Å remains robustly up to x = 8%, and indeed is stronger than in the CuIr2S4 endmember, despite the absense of a symmetry-broken ground state. The compressive chemical pressure has the effect of stabilizing the ODL state, possibly due to an increasing Ir–Ir t2g orbital overlap, suggesting that the symmetry lowering is among molecular orbitals rather than atomic d-states.
Finally, we consider the sister compound CuIr2Se4. In this case the ODL signature in the fit residual is absent, Fig. 3b. In CuIr2Se4 the electron counting arguments are the same as in CuIr2S4, with the Ir t2g states being at the Fermi-level, implying similar physics. However, the Se ion is considerably larger than the S ion, which would result in a larger inter-Ir distance and reduced Ir–Ir t2g orbital overlap in the case of CuIr2Se4. In a traditional site-centered Jahn-Teller picture this would not affect the driving force, indeed it may even make the Jahn-Teller distortion larger by lowering the elastic stiffness of the material. However, if covalency between neighboring Ir ions is important, as the data suggest is the case here, we speculate that the reduced orbital overlap of the Ir t2g orbitals would reduce the splitting of the bonding and anti-bonding orbitals, which would reduce the driving force for the distortion. Indeed, we see an anticorrelation between the size of the tetragonal distortion and the magnitude of the average Ir–Ir distance as we vary the composition by Zn doping and Cr doping (Fig. 3i), consistent with a stronger t2g orbital overlap strengthening the ODL effect. The Ir–Ir distance in CuIr2Se4 is also shown in Fig. 3i, and it is much higher. Notably, we do not observe any tetragonal distortion even in the local structure in CuIr2Se4, again supporting the importance of the Ir–Ir covalency in the ODL mechanism in this case. The importance of covalency would also suggest that the orbital-degeneracy lifting may be stabilized by pressure, since pressure would increase the overlap of neighboring t2g orbitals. Indeed, under pressure CuIr2Se4 does undergo a metal-insulator transition as reflected in transport measurements50. This observation also provides an explanation of why the MIT temperature increases with pressure in CuIr2S451, a trend opposite to that seen in conventional Fermi-surface nesting driven charge-density-wave systems.
We have shown that in CuIr2S4 the origin of the orbital-degeneracy lifting is a local symmetry lowering of Ir–Ir t2g molecular orbitals. We briefly note here that 5d ions such as Ir are also susceptible to an orbital-degeneracy lifting due to spin-orbit coupling. ODL due to spin-orbit effects are prevalent in predominately Ir4+ oxides such as Sr2IrO452,53 resulting in an isospin-1/2 relativistic Mott insulating ground-state. We can speculate that in the current Ir3.5+ case, and with sufficient orbital overlap, valence electron itinerancy may dominate over the atomic picture that is the basis for the SOC38. Learning what factors determine whether SOC or Jahn-Teller effects govern the orbital-degeneracy lifting will be an interesting line of inquiry.
Implications
The characterization of the high-temperature state of CuIr2S4 as being an ODL state, made up of local symmetry-broken objects stabilized by orbital-degeneracy lifting, presents a potential unifying framework and a new lens through which to view multiple material systems. Calculations that derive from the crystal structure, such as density functional theory calculations, should therefore be modified to account for the very different (tenths of an angstrom) bond-lengths that may be present in the material54. This would not be necessary if the ground state consisted simply of ODL objects whose orbitals become ordered over long-range at low temperature. However, often the ground state is quite different from this, as in CuIr2S4 where it consists of charge order, with structural and magnetic dimers, none of which persist above the MIT and into the ODL state39. Likewise, if we view the insulating polaronic state in the colossal magnetoresistant 30% doped La1−xCaxMnO3 manganites55 as an ODL state, the low-temperature ground state has been shown to be absent structural distortions19 and is a non-ODL state. In the CuIr2S4 case long-range ordering may be suppressed due to the geometric frustration of disordering a short Ir–Ir bond over the six edges of the tetrahedron in the pyrochlore lattice, a problem that maps onto the Pauling ice rules56,57. The high-temperature ODL state is in general not just a disordered form of the ground state and needs to be studied independently and in its own right. This is not straightforward, requiring probes of local structure and the local electronic system. Because the objects are local and fluctuating, they are not observable in the crystal structure. The ODL objects are also, in general, fluctuating in the disordered ODL state thus requiring probes that are also faster than any fluctuation dynamics. However, because the nature of the ODL formation is electronic, we expect that optically pumped ultrafast time-resolved measurements of local structure should be a powerful approach to investigate ODL58,59.
Methods
Sample preparation and characterization
Polycrystalline samples of CuIr2S4, CuIr2Se4, (Cu1−xZnx)Ir2S4, and Cu(Ir1−xCrx)2S4 were prepared following standard solid state routes in sealed, evacuated quartz ampoules. Stoichiometric quantities of the metals and elemental sulfur or selenium were thoroughly mixed, pelletized, and sealed under vacuum. The ampoules were slowly heated to various temperatures in 650–1100 °C range, as appropriate to targeted compositions, and held at these temperatures for several weeks with intermediate grinding and pressing. All products were found to be single phase based on laboratory x-ray powder diffraction. Standard characterization of DC susceptibility and four-terminal resistivity of the samples were carried out in Quantum Design PPMS-9 and MPMS-XL5, and found to be in excellent agreement with other studies18,33,35,40,49,60.
PDF data collection and analysis
PDF data for 10 K ≤ T ≤ 780 K were obtained using standard protocols61 from synchrotron x-ray total scattering experiments carried out at the 28-ID-2 x-ray powder diffraction (XPD) beamline of the National Synchrotron Light Source II at Brookhaven National Laboratory. The setup utilized a 67.7 keV x-ray beam (λ = 0.183 Å), a Perkin Elmer amorphous silicon detector, a closed cycle Cryoindustries of America helium refrigerator, and a gas flow reactor with flexible coil heater. Two dimensional (2D) diffraction data were collected in rapid acquisition mode62, with 60 s exposure time for each data set. The raw 2D data (collected on warming) were integrated and converted to intensity versus Q using the software Fit2D63, where Q is the magnitude of the scattering vector. Data reduction and Sine Fourier transform of measured total scattering structure functions up to a momentum transfer of Qmax = 25 Å−1 was carried out using the PDFgetX364 program. PDF structure refinements and simulations were carried out using the PDFgui program suite65.
Correlation length estimate was based on a protocol utilizing a 4-Å wide box car window integration of the residual difference between the data and the cubic Fdm model. ξ is then defined as the r value at which the integral drops by a factor of 2 from its low-r limit, with uncertainty of the estimate conservatively set to half of the window size, similar to correlation length estimates carried out in past PDF studies43,66.
Supplementary information
Acknowledgements
Work at Brookhaven National Laboratory was supported by US DOE, Office of Science, Office of Basic Energy Sciences under contract DE-SC0012704. Work in the Materials Science Division of Argonne National Laboratory, was sponsored by the U.S. Department of Energy Office of Science, Basic Energy Sciences, Materials Science and Engineering Division. This research used 28-ID-2 beamline of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory.
Author contributions
E.S.B., J.F.M. and S.J.L.B. conceived and designed the research. C.P., H.C.L., J.F.M., Y.S.H., and H.Z. developed and carried out the synthesis and did material characterizations. E.S.B., R.J.K. and M.A. carried out PDF measurements and analysis. W.G.Y. provided theoretical inputs. E.S.B., S.J.L.B. and J.F.M. wrote the manuscript with contributions from all the authors.
Data availability
The data supporting the findings of this study are within the Article and its Supplementary Information files and are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
E. S. Bozin, Email: bozin@bnl.gov
S. J. L. Billinge, Email: sb2896@columbia.edu
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-11372-w.
References
- 1.Aetukuri NB, et al. Control of the metal-insulator transition in vanadium dioxide by modifying orbital occupancy. Nat. Phys. 2013;9:661–666. doi: 10.1038/nphys2733. [DOI] [Google Scholar]
- 2.Tian Z, et al. Field-induced quantum metal-insulator transition in the pyrochlore iridate Nd2Ir2O7. Nat. Phys. 2016;12:134–138. doi: 10.1038/nphys3567. [DOI] [Google Scholar]
- 3.Liang T, et al. Orthogonal magnetization and symmetry breaking in pyrochlore iridate Eu2Ir2O7. Nat. Phys. 2017;13:599–603. doi: 10.1038/nphys4051. [DOI] [Google Scholar]
- 4.Achkar AJ, et al. Orbital symmetry of charge-density-wave order in La1.875Ba0.125CuO4 and YBa2Cu3O6.67. Nat. Mater. 2016;15:616–620. doi: 10.1038/nmat4568. [DOI] [PubMed] [Google Scholar]
- 5.Savitzky BH, et al. Bending and breaking of stripes in a charge ordered manganite. Nat. Commun. 2017;8:1883. doi: 10.1038/s41467-017-02156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zorko A, Adamopoulos O, Komelj M, Arčon D, Lappas A. Frustration-induced nanometre-scale inhomogeneity in a triangular antiferromagnet. Nat. Commun. 2014;5:3222. doi: 10.1038/ncomms4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glasbrenner JK, et al. Effect of magnetic frustration on nematicity and superconductivity in iron chalcogenides. Nat. Phys. 2015;11:953–958. doi: 10.1038/nphys3434. [DOI] [Google Scholar]
- 8.Borisenko SV, et al. Pseudogap and charge density waves in two dimensions. Phys. Rev. Lett. 2008;100:196402. doi: 10.1103/PhysRevLett.100.196402. [DOI] [PubMed] [Google Scholar]
- 9.Keimer B, Kivelson SA, Norman MR, Uchida S, Zaanen J. From quantum matter to high-temperature superconductivity in copper oxides. Nature. 2015;518:179–186. doi: 10.1038/nature14165. [DOI] [PubMed] [Google Scholar]
- 10.Wang F, Kivelson SA, Lee D-H. Nematicity and quantum paramagnetism in FeSe. Nat. Phys. 2015;11:959–963. doi: 10.1038/nphys3456. [DOI] [Google Scholar]
- 11.Chuang Y, Gromko A, Dessau D, Kimura T, Tokura Y. Fermi surface nesting and nanoscale fluctuating charge/orbital ordering in colossal magnetoresistive oxides. Science. 2001;292:1509–1513. doi: 10.1126/science.1059255. [DOI] [PubMed] [Google Scholar]
- 12.Johannes MD, Mazin II. Fermi surface nesting and the origin of charge density waves in metals. Phys. Rev. B. 2008;77:165135. doi: 10.1103/PhysRevB.77.165135. [DOI] [Google Scholar]
- 13.Terashima K, et al. Fermi surface nesting induced strong pairing in iron-based superconductors. Proc. Natl Acad. Sci. USA. 2009;106:7330–7333. doi: 10.1073/pnas.0900469106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee PA, Rice TM, Anderson PW. Fluctuation effects at a peierls transition. Phys. Rev. Lett. 1973;31:462–465. doi: 10.1103/PhysRevLett.31.462. [DOI] [Google Scholar]
- 15.Bhobe PA, et al. Electronic structure evolution across the Peierls metal-insulator transition in a correlated ferromagnet. Phys. Rev. X. 2015;5:041004. [Google Scholar]
- 16.Huang HY, et al. Jahn-Teller distortion driven magnetic polarons in magnetite. Nat. Commun. 2017;8:15929. doi: 10.1038/ncomms15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khomskii, D. I. Transition Metal Compounds (Cambridge University Press, Cambridge, UK, 2014). .
- 18.Radaelli PG, et al. Formation of isomorphic Ir3+ and Ir4+ octamers and spin dimerization in the spinel CuIr2S4. Nature. 2002;416:155–158. doi: 10.1038/416155a. [DOI] [PubMed] [Google Scholar]
- 19.Billinge SJL, DiFrancesco RG, Kwei GH, Neumeier JJ, Thompson JD. Direct observation of lattice polaron formation in the local structure of La1−xCaxMnO3. Phys. Rev. Lett. 1996;77:715–718. doi: 10.1103/PhysRevLett.77.715. [DOI] [PubMed] [Google Scholar]
- 20.Billinge SJL, Duxbury PM. Structural compliance, misfit strain and stripe nanostructures in cuprate superconductors. Phys. Rev. B. 2002;66:064529. doi: 10.1103/PhysRevB.66.064529. [DOI] [Google Scholar]
- 21.Sprau PO, et al. Discovery of orbital-selective Cooper pairing in FeSe. Science. 2017;357:75–80. doi: 10.1126/science.aal1575. [DOI] [PubMed] [Google Scholar]
- 22.Kostin A, et al. Imaging orbital-selective quasiparticles in the Hund’s metal state of FeSe. Nat. Mater. 2018;17:869–874. doi: 10.1038/s41563-018-0151-0. [DOI] [PubMed] [Google Scholar]
- 23.Massee F, et al. Bilayer manganites reveal polarons in the midst of a metallic breakdown. Nat. Phys. 2011;7:978–982. doi: 10.1038/nphys2089. [DOI] [Google Scholar]
- 24.Panopoulos N, et al. Polaron freezing and the quantum liquid-crystal phase in the ferromagnetic metallic La0.67Ca0.33MnO3. npj Quantum Mater. 2018;3:20. doi: 10.1038/s41535-018-0093-4. [DOI] [Google Scholar]
- 25.Scagnoli V, et al. Observation of orbital currents in CuO. Science. 2011;332:696–698. doi: 10.1126/science.1201061. [DOI] [PubMed] [Google Scholar]
- 26.Wen Y-C, et al. Gap opening and orbital modification of superconducting FeSe above the structural distortion. Phys. Rev. Lett. 2012;108:267002. doi: 10.1103/PhysRevLett.108.267002. [DOI] [PubMed] [Google Scholar]
- 27.Kasahara S, et al. Electronic nematicity above the structural and superconducting transition in BaFe2(As1−xPx)2. Nature. 2012;486:382–385. doi: 10.1038/nature11178. [DOI] [PubMed] [Google Scholar]
- 28.Baek S-H, et al. Orbital-driven nematicity in FeSe. Nat. Mater. 2015;14:210–214. doi: 10.1038/nmat4138. [DOI] [PubMed] [Google Scholar]
- 29.Frandsen BA, et al. Widespread orthorhombic fluctuations in the (Sr, Na)Fe2As2 family of superconductors. Phys. Rev. B. 2018;98:180505. doi: 10.1103/PhysRevB.98.180505. [DOI] [Google Scholar]
- 30.Radaelli PG. Orbital ordering in transition-metal spinels. New J. Phys. 2005;7:53. doi: 10.1088/1367-2630/7/1/053. [DOI] [Google Scholar]
- 31.Attfield JP. Orbital molecules in electronic materials. APL Mater. 2015;3:041510. doi: 10.1063/1.4913736. [DOI] [Google Scholar]
- 32.Rivas-Murias B, Zhou HD, Rivas J, Rivadulla F. Rapidly fluctuating orbital occupancy above the orbital ordering transition in spin-gap compounds. Phys. Rev. B. 2011;83:165131. doi: 10.1103/PhysRevB.83.165131. [DOI] [Google Scholar]
- 33.Furubayashi T, Matsumoto T, Hagino T, Nagata S. Structural and magnetic studies of metal-insulator transition in thiospinel CuIr2S4. J. Phys. Soc. Jpn. 1994;63:3333–3339. doi: 10.1143/JPSJ.63.3333. [DOI] [Google Scholar]
- 34.Matsuno J, et al. Photoemission study of the metal-insulator transition in cuir2s4. Phys. Rev. B. 1997;55:R15979. doi: 10.1103/PhysRevB.55.R15979. [DOI] [Google Scholar]
- 35.Nagata S, et al. Metal-insulator transition in the spinel-type CuIr2(S1−xSex)4 system. Phys. Rev. B. 1998;58:6844–6854. doi: 10.1103/PhysRevB.58.6844. [DOI] [Google Scholar]
- 36.Takubo K, et al. X-ray photoemission study of CuIr2S4: Ir3+-Ir4+ charge ordering and the effect of light illumination. Phys. Rev. Lett. 2005;95:246401. doi: 10.1103/PhysRevLett.95.246401. [DOI] [PubMed] [Google Scholar]
- 37.Croft M, et al. Metal-insulator transition in CuIr2S4: XAS results on the electronic structure. Phys. Rev. B. 2003;67:201102. doi: 10.1103/PhysRevB.67.201102. [DOI] [Google Scholar]
- 38.Khomskii DI, Mizokawa T. Orbitally induced Peierls state in spinels. Phys. Rev. Lett. 2005;94:156402. doi: 10.1103/PhysRevLett.94.156402. [DOI] [PubMed] [Google Scholar]
- 39.Božin ES, Masadeh AS, Hor YS, Mitchell JF, Billinge SJL. Detailed mapping of the local Ir4+ dimers through the metal-insulator transitions of CuIr2S4 thiospinel by x-ray atomic pair distribution function measurements. Phys. Rev. Lett. 2011;106:045501. doi: 10.1103/PhysRevLett.106.045501. [DOI] [PubMed] [Google Scholar]
- 40.Burkov AT, et al. Anomalous resistivity and thermopower of the spinel-type compounds CuIr2S4 and CuIr2Se4. Phys. Rev. B. 2000;61:10049–10056. doi: 10.1103/PhysRevB.61.10049. [DOI] [Google Scholar]
- 41.Takubo K, Mizokawa T, Matsumoto N, Nagata S. In-gap state and effect of light illumination in CuIr2S4 probed by photoemission spectroscopy. Phys. Rev. B. 2008;78:245117. doi: 10.1103/PhysRevB.78.245117. [DOI] [Google Scholar]
- 42.Yagasaki K, et al. Hopping conductivity in CuIr2S4 spinel compound: I. empirical model for electronic configuration and mechanism of metal-insulator transition. J. Phys. Soc. Jpn. 2006;75:074706. doi: 10.1143/JPSJ.75.074706. [DOI] [Google Scholar]
- 43.Božin ES, et al. Cu(Ir1−xCrx)2S4: a model system for studying nanoscale phase coexistence at the metal-insulator transition. Sci. Rep. 2014;4:4081. doi: 10.1038/srep04081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Streltsov SV, Khomskii DI. Covalent bonds against magnetism in transition metal compounds. Proc. Natl Acad. Sci. USA. 2016;113:10491–10496. doi: 10.1073/pnas.1606367113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Streltsov SV, Khomskii DI. Orbital physics in transition metal compounds: new trends. Phys. Uspekhi. 2017;60:1121–1146. doi: 10.3367/UFNe.2017.08.038196. [DOI] [Google Scholar]
- 46.de’ Medici L, Giovannetti G, Capone M. Selective Mott physics as a key to iron superconductors. Phys. Rev. Lett. 2014;112:177001. doi: 10.1103/PhysRevLett.112.177001. [DOI] [PubMed] [Google Scholar]
- 47.Yu R, Zhu J-X, Si Q. Orbital selectivity enhanced by nematic order in FeSe. Phys. Rev. Lett. 2018;121:227003. doi: 10.1103/PhysRevLett.121.227003. [DOI] [PubMed] [Google Scholar]
- 48.Lei H, Yin W-G, Zhong Z, Hosono H. Structural, magnetic, and electrical properties of Li2Ir1−xRuxO3. Phys. Rev. B. 2014;89:020409. doi: 10.1103/PhysRevB.89.020409. [DOI] [Google Scholar]
- 49.Cao G, et al. Suppression of metal-to-insulator transition and appearance of superconductivity in Cu1−xZnxIr2S4. Phys. Rev. B. 2001;64:214514. doi: 10.1103/PhysRevB.64.214514. [DOI] [Google Scholar]
- 50.Furubayashi T, et al. Pressure induced metal-insulator transition of selenospinel CuIr2Se4. J. Phys. Soc. Jpn. 1997;66:1563–1564. doi: 10.1143/JPSJ.66.1563. [DOI] [Google Scholar]
- 51.Ma L, et al. Opposite pressure effects in the orbitally-induced Peierls phase transition systems CuIr2S4 and MgTi2O4. Dalton Trans. 2017;46:6708–6714. doi: 10.1039/C7DT00527J. [DOI] [PubMed] [Google Scholar]
- 52.Martins, C., Aichhorn, M., Vaugier, L. & Biermann, S. Reduced effective spin-orbital degeneracy and spin-orbital ordering in paramagnetic transition-metal oxides: Sr2IrO4 versus Sr2 RhO4. Phys. Rev. Lett. 107, 266404 (2011). [DOI] [PubMed]
- 53.Kim, B. J. et al. Phase-sensitive observation of a spin-orbital Mott state in Sr2IrO4. Science323, 1329–1332 (2009). [DOI] [PubMed]
- 54.Varignon J, Bibes M, Zunger A. Origin of band gaps in 3d perovskite oxides. Nat. Commun. 2019;10:1658. doi: 10.1038/s41467-019-09698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Millis AJ. Lattice effects in magnetoresistive manganese perovskites. Nature. 1998;392:147–150. doi: 10.1038/32348. [DOI] [Google Scholar]
- 56.Thygesen PM, et al. Orbital dimer model for the spin-glass state in Y2Mo2O7. Phys. Rev. Lett. 2017;118:067201. doi: 10.1103/PhysRevLett.118.067201. [DOI] [PubMed] [Google Scholar]
- 57.Bramwell ST, Gingras MJP. Spin ice state in frustrated magnetic pyrochlore materials. Science. 2001;294:1495–1501. doi: 10.1126/science.1064761. [DOI] [PubMed] [Google Scholar]
- 58.Koch, R. et al. Room temperature local nematicity in FeSe superconductor. Preprint at https://arxiv.org/abs/1902.08732 (2019).
- 59.Konstantinova T, et al. Photoinduced dynamics of nematic order parameter in FeSe. Phys. Rev. B. 2019;99:180102. doi: 10.1103/PhysRevB.99.180102. [DOI] [Google Scholar]
- 60.Endoh R, Awaka J, Nagata S. Ferromagnetism and the metal-insulator transition in the thiospinel Cu(Ir1−xCrx)2S4. Phys. Rev. B. 2003;68:115106. doi: 10.1103/PhysRevB.68.115106. [DOI] [Google Scholar]
- 61.Egami, T. & Billinge, S. J. L. Underneath The Bragg Peaks: Structural Analysis Of Complex Materials. 2nd edn (Elsevier, Amsterdam, 2012).
- 62.Chupas PJ, et al. Rapid acquisition pair distribution function analysis (RA-PDF) J. Appl. Crystallogr. 2003;36:1342–1347. doi: 10.1107/S0021889803017564. [DOI] [Google Scholar]
- 63.Hammersley AP, Svenson SO, Hanfland M, Hauserman D. Two-dimensional detector software: from real detector to idealised image or two-theta scan. High Press. Res. 1996;14:235–248. doi: 10.1080/08957959608201408. [DOI] [Google Scholar]
- 64.Juhás P, Davis T, Farrow CL, Billinge SJL. PDFgetX3: a rapid and highly automatable program for processing powder diffraction data into total scattering pair distribution functions. J. Appl. Crystallogr. 2013;46:560–566. doi: 10.1107/S0021889813005190. [DOI] [Google Scholar]
- 65.Farrow CL, et al. PDFfit2 and PDFgui: computer programs for studying nanostructure in crystals. J. Phys. Condens. Mater. 2007;19:335219. doi: 10.1088/0953-8984/19/33/335219. [DOI] [PubMed] [Google Scholar]
- 66.Qiu X, Proffen T, Mitchell JF, Billinge SJL. Orbital correlations in the pseudocubic O and rhombohedral R-phases of LaMnO3. Phys. Rev. Lett. 2005;94:177203. doi: 10.1103/PhysRevLett.94.177203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are within the Article and its Supplementary Information files and are available from the corresponding author upon request.