Abstract
Background
Parent-of-origin gene expression and its role in seed development have drown a great attention in recent years. Genome-wide analysis has identified hundreds of candidate imprinted genes, a major type of parent-of-origin genes, in rice hybrid endosperms at the stage of 5 days after pollination (dap). However, the expression of these genes in early endosperm have been never confirmed due to technique limitations and the behavior of the imprinted genes in different rice hybridizations are still largely unknown.
Results
Here, based on our elaborate technique established previously, the expression patterns of PcG genes in the early stages of endosperm development (within 3 dap), were comprehensively analyzed. We revealed that the free nucleus stage of endosperm development is critical for parent-of-origin gene analysis. The expression of the imprinted genes are highly dynamic, likely corresponding to the critical developmental events during this period. Hybridizations between Oryza sativa japonica and indica showed that the expression patterns of the same imprinted gene could be varied by crossing with different parental cultivars, indicative of their parent-dependent character. There are strong alleles that often showed predominant expression over other alleles regardless of the parental origin, which provides a possible explanation for the cultivar-dependent predominant phenotype in crop hybridizations. In addition, we found that the transcripts of the same gene behave differently, with imprinting or non-imprinting patterns, suggesting the existence of not only imprinted and non-imprinted genes but also imprinted or non-imprinted transcripts, which reveals new aspects of the genomic imprinting.
Conclusions
These findings on the characters of parent-of-origin genes shed light on the understanding the real role of gene imprinting in endosperm development.
Electronic supplementary material
The online version of this article (10.1186/s12284-019-0306-x) contains supplementary material, which is available to authorized users.
Keywords: Polycomb group genes, Rice, Imprinting gene
Background
Genomic imprinting is a universal epigenetic phenomenon evolved independently in animals and plants, which results in the biased expression of mono allele dependent on their parent-of-origin. In animals, a subset of imprinted genes have been identified and considered to be involved in the regulation of nutrient transfer from maternal tissue to embryo for embryo development (Frost and Moore 2010; Reik et al. 2003; Tycko and Morison 2002; Wood and Oakey 2006). In plants, although several imprinting genes have been identified in early embryos, genomic imprinting are primarily confined to the triploid endosperm, a transient tissue nourishing the developing embryo as placenta in animals. A series of imprinting genes have been identified in endosperms from Arabidopsis thaliana, Oryza sativa and Zea maize through a genome-wide survey (Guo et al. 2003; Hsieh et al. 2011; Luo et al. 2011; Zhang et al. 2011b). Among them, Polycomb Group (PcG) genes were explored more intensively due to their important roles in early endosperm development (Hermon et al. 2007; Ingouff et al. 2005; Kinoshita et al. 1999; Nallamilli et al. 2013; Yadegari et al. 2000).
In A. thaliana, PcG genes were shown to play critical roles in several important transition phases (e.g. from gametophyte to sporophyte), coordinating the development of endosperm, embryo proper and surrounded maternal tissues. Among them, two core components of PRC2 complex, MEA and FIS2, were revealed as imprinted genes and only transcribed from the maternal allele in endosperm (Kinoshita et al. 1999; Luo et al. 2000). The SET domain of MEA interacts directly with FIE to form a PcG complex, which is crucial for endosperm formation by controlling the activity of a number of imprinted genes in the endosperm (Baroux et al. 2006; Kohler et al. 2005; Spillane et al. 2000; Wang et al. 2006).
In O. sativa, great attention has also been paid on the PcG genes in endosperm due to the contribution of endosperm to the quality and yield of rice production. Six PcG genes including OsFIE1, OsFIE2, OsEMF2a, OsEMF2b, OsCLF, OsiEz1 have been identified according to the sequence similarity to known PcG genes (Luo et al. 2009). The transcripts of all PcG genes could be detected in endosperms. Among them, OsFIE1 and OsFIE2 were shown to play critical roles in endosperm development (Folsom et al. 2014; Li et al. 2014; Nallamilli et al. 2013). Overexpression of OsFIE1 result in the precocious cellularization and reduced seed size, whereas down regulation of OsFIE1 expression resulted in the reduced fertility and delayed embryo development (Folsom et al. 2014). OsFIE2 has also been reported to play essential roles in the regulation of rice vegetative and reproductive development, especially in the endosperm development and grain filling (Li et al. 2014).
However, whether these developmental defects in endosperm are attributed to the imprinting effect of PcG genes is still largely unknown. OsFIE1 was shown as a maternally expressed imprinting gene in 5 days after pollination (dap) hybrid endosperms from the cross between Nipponbare and IR64 (Luo et al. 2009). And both maternal and paternal transcripts of other five PcG genes (OsiEZ1, OsCLF, OsEMF2a, OsEMF2b, and OsFIE2) in 5 dap hybrid endosperms could be detected simultaneously (Luo et al. 2009). Since several elaborated events of endosperm development such as the formation of primary endosperm nucleus, the initiation of primary endosperm nucleus division and the onset of endosperm cellularization usually occur in the early stage before 5 dap (Brown et al. 1996), and the expression of imprinted genes may be developmental-stage-dependent, it is necessary to screen and confirm the imprinting pattern of these PcG genes in very early stages of endosperm development to figure out the role of the imprinted PcG genes in seed development. Here, the imprinting pattern of PcG genes in the entire process of endosperm development, especially in the early stages of endosperm development (within 3 dap), were analyzed. In addition, the influences of parental genetic background, alterative splicing form on the expression of parental alleles were also discussed in the present study.
Results
Identification of DNA polymorphisms of PcG genes among Nipponbare, 9311 and Zhonghua 11
To explore which allele of PcG gene in endosperm was transcribed, the expression pattern of each PcG gene in endosperms at different stages (from 1 dap to 7 dap) were carefully examined. The endosperm at different stages were isolated according to our previous method (Kuang et al. 2015). cDNA was prepared using mRNA extracted from endosperm at different stages. RT-PCR analysis results revealed that the transcripts of all PcG genes could be detected in endosperms from 1 to 7 dap (Detection results from 3 dap endosperm shown in Fig. 1), giving a possibility to elucidate the dynamics of the expression of parental alleles in endosperms at different stages.
Fig. 1.
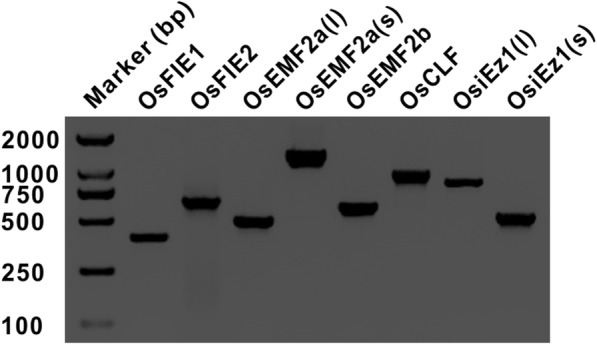
The expression of PcG genes in endosperm confirmed by RT-PCR. Distinction between OsEMF2a(l) and OsEMF2a(s) based on different amplification primers and sequencing primers (See Additional file 1: Table S1)
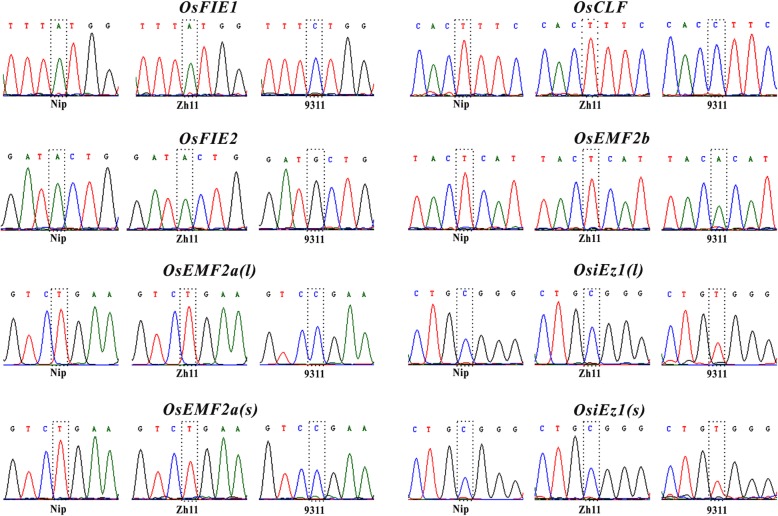
To distinguish two alleles of PcG genes in the genome, the DNA polymorphisms among three cultivated species including Nipponbare (Nip), 9311 and Zhonghua 11 (Zh11) were investigated firstly. The genomic sequences of each PcG gene were confirmed by the specific PCR amplification and subsequent Sanger sequencing. Comparing the PcG gene sequences from Nip, 9311 and Zh11, the single nucleotide polymorphisms (SNPs) were identified (Fig. 2 and Table 1). There is at least one base polymorphism located in the exon of each PcG gene between two subspecies, which could be used to distinguish parental transcript efficiently.
Fig. 2.
Identification of single nucleotide polymorphisms (SNPs) of PcG genes among Nip, 9311 and Zh11. SNPs for each transcript was labeled with dashed box
Table 1.
SNPs used to distinguish paternal and maternal transcripts in endosperm
OsFIE1 is a maternally expressed imprinting gene at 7 DAP endosperm
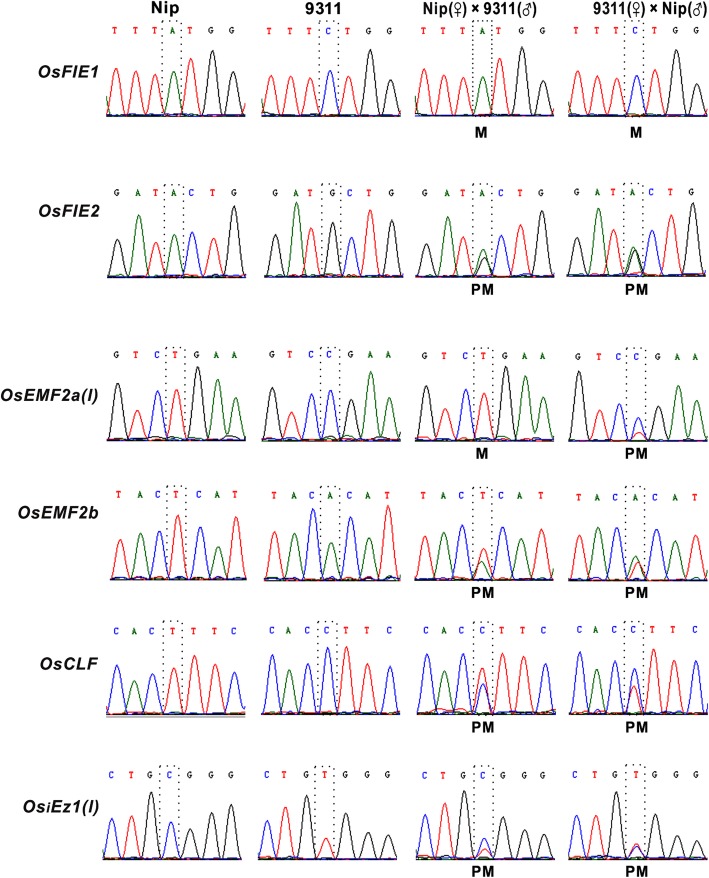
Early allelic specific expression pattern analysis PcG genes in rice endosperm revealed that only OsFIE1 is maternally expressed imprinting gene among six PcG genes (Luo et al. 2009). To confirm allelic expression patterns of PcG genes in endosperm, three independent 7 dap hybrid endosperm cDNAs by crossing the Nip and the 9311 reciprocally were produced. Each cDNA sample from hybrid endosperm was subsequently used to amplify the SNP-containing sequence of each PcG transcript by RT-PCR with primer sequence flanking SNP. The PCR products were sequenced by Sanger sequencing and assessed for their parent-of-origin biased expression according to SNPs. Then, allele-specific expression analyses of PcG genes in endosperm were performed as shown in Fig. 3. Among six PcG genes, only OsFIE1 showed monoallelic expression, confirming that OsFIE1 is a maternally expressed imprinting gene (Luo et al. 2009). Four PcG genes show biallelic expression in 7 dap endosperm, while OsEMF2a display either maternal expression pattern or a biallelic expression pattern depending on the direction of the cross between Nip and 9311.
Fig. 3.
OsFIE1 is a maternally expressed imprinting gene at 7 DAP endosperm. SNPs for each transcript was labeled with dashed box. Each data has been confirmed by three independent experiments
Dynamics of allele-specific expression of PcG genes during endosperm development
Early researches indicated that several key endosperm developmental events including the onset of primary endosperm nucleus division, the compartmentalization of endosperm nuclei and the initiation of endosperm cellularization occur within 3 dap (Brown et al. 1996; Kuang et al. 2015). In addition, the effect of imprinting gene on endosperm development in A. thaliana is majorly on early endosperm development (Baroux et al. 2006; Jullien et al. 2006; Luo et al. 2000; Yadegari et al. 2000). Hence, to analyze allelic expression patterns of PcG genes comprehensively, hybrid endosperms were isolated at six key stages of endosperm development including stage 1 (1 dap), stage 2 (2 dap), stage 3 (3 dap), stage 4 (7 dap), stage 5 (10 dap) and stage 6 (15 dap), and cDNA samples from hybrid endosperms were prepared according to the previous protocol (Kuang et al. 2015).
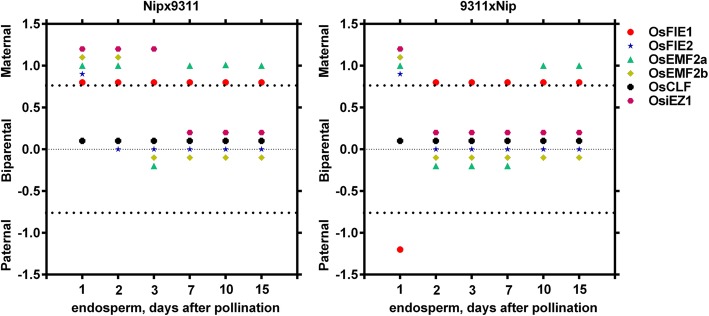
Allele-specific expression analyses of PcG genes in the process of endosperm development revealed that the expression pattern of two alleles of PcG genes show dynamic changes as endosperm development, especially within 3 dap. Among six PcG genes, only OsCLF display a stable expression pattern, both transcripts from paternal allele and maternal allele could be detected in endosperms at six stages, indicating OsCLF is not an imprinting gene in rice endosperm at these stages tested (Fig. 4). However, parent alleles of other five PcG genes show diverse transcriptional activities in endosperms at different stages, especially in stage 1 and stage 2, demonstrating a stage-specific manner. At stage 1, only maternal allele of four PcG genes (OsFIE2, OsEMF2a, OsEMF2b and OsiEZ1) had been transcribed its transcripts.
Fig. 4.
Dynamics of allele-specific expression of PcG genes during endosperm development. The dots above the first dash line indicated only transcripts derived from maternal allele could be detected. The dots nearby the middle dash line indicated both transcripts from paternal allele and maternal allele could be detected simultaneously. The dots below the third dash line indicated only transcripts derived from paternal allele could be detected. Each data has been confirmed by three independent experiments
In more details, maternal allele of OsFIE1 was always expressed in endosperm from 1 to 15 dap in Nip × 9311. By contrast, paternal allele of OsFIE1 was only expressed at 1 dap endosperm in 9311 × Nip. Maternal allele of OsFIE2 was first expressed in 1 dap endosperm and then parental alleles of OsFIE2 were both expressed in endosperm at following stages in both Nip× 9311 and 9311 × Nip. More interestingly, OsEMF2a was maternally expressed in early and late endosperm, while paternal allele of OsEMF2a were only expressed in the middle stage in Zh11 × 9311.With similar expression pattern as OsFIE2, both OsEMF2b and OSiEZ1 were maternally expressed in early endosperm and were parentally expressed in following stages. All these data implied that endosperm at free nucleus stage is critical for parent-of-origin gene analysis in rice and the expression of the imprinted genes are highly dynamic or stage-dependent.
The imprinting pattern of PcG genes are influenced by genetic background
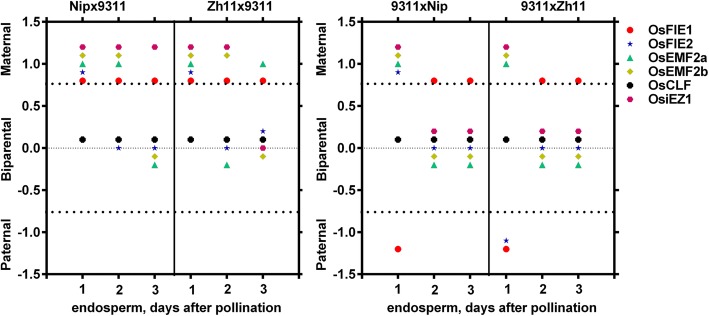
As mentioned above, parent alleles of PcG genes display different transcriptional activities based on the direction of the cross between Nip and the 9311, suggesting that the imprinting pattern of PcG genes are influenced by different parent combinations. To address this hypothesis, the imprinting pattern of PcG genes in two reciprocal cross combinations (Nip or Zh11 with 9311) was compared. The results revealed that the PcG genes exhibit notable variation in parental allele activation in the process of endosperm development and the transcriptional activities of two alleles of PcG genes are different dependent on the combination of two parents. Among six PcG genes, OsCLF showed a consistent bi-allelic expression pattern in endosperms in two independent reciprocal cross combinations. Maternal allele of OsEMF2b was firstly activated in endosperms (within 2 dap), and parental allele was activated in 3 dap endosperm subsequently. However, other PcG genes display notable variation in parental allele activation in two different reciprocal cross combinations. For example, only OsiEZ1 transcript transcribed from the maternal allele could be detected in 3 dap hybrid endosperm derived from the cross between Nip and 9311, but the transcripts both from maternal allele and paternal allele could be detected simultaneously in 3 dap hybrid endosperm derived from the cross between Zh11 and 9311 (Fig. 5). In addition, the maternal allele of OsFIE1 was expressed at 1 dap in Zh11 × 9311, while paternal allele of OsFIE1 was also expressed in 1 dap endosperm 9311 × Zh11. Similar expression pattern of OsFIE2 is also observed at 1 dap in both Zh11 × 9311 and 9311 × Zh11. Interestingly, in Nip× 9311 reciprocal crosses, it was maternal allele of OsFIE2 expressed at 1 dap, whereas, in Zh11 × 9311 reciprocal crosses OsFIE2 seems a dominant allele at 1 dap and associated with Zh11 whenever Zh11 was used as paternal or maternal cultivar. These results indicated the expression patterns of certain gene could be varied by crossing with different parental cultivars and this dominant expression of the imprinted genes associated with specific cultivar is also stage-dependent.
Fig. 5.
Influence of genetic background on allele-specific expression of PcG genes. The dots above the first dash line indicated only transcripts derived from maternal allele could be detected. The dots nearby the middle dash line indicated both transcripts from paternal allele and maternal allele could be detected simultaneously. The dots below the third dash line indicated only transcripts derived from paternal allele could be detected. Each data has been confirmed by three independent experiments
Different alternative splicing forms of PcG genes display different imprinting pattern
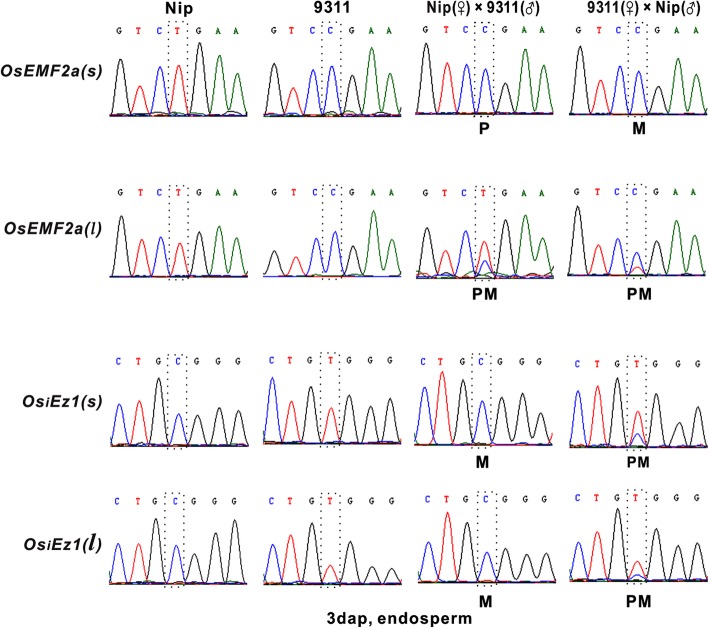
Alternative splicing is an important gene regulatory mechanism in eukaryotes, which results in a single gene coding for multiple proteins. In plants, about least 20% multi-exon genes are alternatively spliced (Estrada et al. 2015). However, whether the two alleles of different alternative splicing forms display similar imprinting pattern is still largely unknown. To address this question, two representative PcG genes (OsEMF2a and OsiEZ1) with distinguishable splicing forms were selected to analyze the imprinting pattern of different splicing forms. OsEMF2a and OsiEZ1 could generate different splicing isoforms with different size. In order to distinguish alternative splicing forms more efficiently, two representative splicing forms, the longest (l) and the shortest (s) transcripts of OsEMF2a and OsiEZ1, were selected to analyze the expression of two alleles (Fig. 2 and Table 1). The results revealed that OsiEZ1(l) and OsiEZ1(s), two alternative splicing forms OsiEZ1 displayed the same allele-specific expression pattern in the 3 dap endosperm from reciprocal crosses between Nip and 93–11 (Fig. 6). However, two isoforms of OsEMF2a display different parent-of-origin expression pattern in the 3 dap endosperm (Fig. 6), providing a clear example of parent of origin-biased transcript isoforms arising from the same gene.
Fig. 6.
Different alternative splicing form of PcG genes display different imprinting pattern. SNPs for each transcript was labeled with dashed box. Each data has been confirmed by three independent experiments
Discussion
Uniparental expression pattern of PcG genes is developmental stage-dependent during endosperm formation
One clear finding in the present study is that uniparental expression pattern of PcG genes displays a developmental stage-dependent manner. OsFIE1 is only a maternally expressed imprinting gene in the whole process of hybrid endosperm (Nip × 9311) development, and no biased expression of OsCLF alleles was found in any developmental stages of hybrid endosperm (Nip × 9311). However, the activation or silencing of two alleles of other four PcG genes shows dynamic changes at different stages of endosperm development, suggesting that the expression pattern of parental-origin genes changes as endosperm development, especially in early stages of endosperm development. Similar stage-specific manner of parental-origin genes has also been reported in hybrid embryos. In tobacco, only transcripts from paternal allele of EB426694 and CN744644 could be detected in hybrid zygote, whereas both paternal and maternal transcripts could be detected simultaneously in eight-celled embryo (Zhang et al. 2011a). In maize, allele-specific expression assays of 90 genes in endosperms at different stages revealed that only eight of them exhibited persistent maternally or paternally biased expression at multiple stages of endosperm development (Stupar et al. 2007). In A. thaliana, only paternal allele of FUSCA3 was activated at the 2–4 cell embryo stage, but either maternal or biallelic was activated at the globular embryo stage depending on the direction of the cross between Columbia-0 (Col-0) and Landsbergerecta (Ler) (Raissig et al. 2013). These data revealed that expression pattern of allele specific genes both in embryo and endosperm display a developmental stage-dependent manner, and thus parental and maternal transcripts may have specific contribution to the embryo or endosperm development at specific stage.
Endosperm development in rice experience several critical early stages, including the fusion of the sperm cell with central cell, first cell division of primary endosperm nucleus or promotion of endosperm development, free nuclear movement and oriented distribution, the initialization of cellularization. The molecular mechanism to regulate these critical stages are not well understood. We speculate that there was a correlation between gene imprinting and these critical developmental events. Different imprinted genes may involve in a specific developmental event, not all these events. In addition, parents may differentially contribute to the same developmental events. That’s probably why their expression is highly dynamic and in a stage-dependent manner.
Alternative splicing forms of a certain transcript displays different expression pattern
Another interesting question raised in the present study is that alternative splicing forms of PcG genes display different imprinting patterns. The imprinting pattern of two splicing forms of OsEMF2a and OsiEZ1 were compared in the experiment. Most obviously, the short transcript for OsEMF2a (AK069556) is uniparental expressed, but the long transcript (AK120470) is biparental expressed in hybrid endosperm. A similar phenomenon has also been found in inter-subspecies hybrid mice. Allele-specific polyadenylation sites were found at a novel murine imprinted gene (H13). Maternal allele preferentially produces the long transcripts, whereas the truncated transcripts are preferentially originated from paternally derived alleles (Wood et al. 2008). This indicates the different roles of the same gene as biparental expressed gene or imprinted genes. Different parentally biased isoforms could generate from same gene via alternative splicing, providing a new aspect of genomic imprinting. The same gene could play both imprinted and non-imprinted roles by produce different transcripts. In this case, it is difficult to name it as an imprinted gene or non-imprinted gene, probably, to be more accurate, we may describe it as imprinted transcript or non-imprinted transcript of the gene. This finding enhances our understanding of the complexity of genomic imprinting.
Uniparental expression pattern of PcG genes is influenced by parental genetic background
It was reported that it will suffer a very important effects to the offspring’s phenotypic and quality traits by using different male and female parents as hybrid material, or exchanging parents’ position for reciprocal cross. This phenomenon is usually considered to be the result from unbalanced dose ratio between maternal genome and paternal genome during the reciprocal-crossing processes (Dilkes et al. 2008; Scott et al. 1998). Our work provides new explanations for the phenomenon. The uniparental expression pattern of genes is obviously influenced by parental genetic background. Some imprinted genes of certain cultivars could predominantly expresses in the hybrids whenever the cultivar is used as maternal or paternal material in the crosses, indicating that the behavior of the imprinted genes could be associated with certain genotype. The regulatory mechanism underlying the phenomenon is interesting but not yet understood. However, it is clear that the imprinting effects of the genes will certainly influence the phenotype, e.g. endosperm development, therefore, it may enable some specific cultivar with superiority to control specific phenotypes or developmental characters in hybrids. Thus, it might be an alternative explanation for the parent-associated characters in crop crosses and provides new clue for the selection of the parents in crop breeding.
Methods
Plant materials
Three Oryza sativa species including two Japonica cultivars Nipponbare, Zhonghua 11 and one Indica cultivar 9311 were used in the present study, which were cultivated in the greenhouse with 13 h of illumination every day. The daytime temperature was 30 °C, and the night temperature was 25 °C.
Polymorphism detection between rice species
In order to detect DNA polymorphisms among Nip, 9311 and Zh11, the genomic sequences of PcG genes (OsFIE1, OsFIE2, OsEMF2a, OsEMF2b, OsCLF, OsiEz1) were downloaded from rice genomic database, which is from “http://www.ricedata.cn/gene/” or “https://rapdb.dna.affrc.go.jp/” in this experiment. In the meantime the sequences of PcG genes from Nip, 9311 and Zh11 were confirmed through PCR, respectively. And the genomic sequences of PcG genes from two different species were aligned and compared to detect DNA polymorphisms.
RNA extraction and RT-PCR
The collection of hybrid ovaries at the same stages, endosperm isolation and mRNA extraction were carried out according to the previous protocol (Kuang et al. 2015). For the crosses, the flowers were previously labeled, emasculated, and hand-pollinated. After emasculation and pollination, the flowers were covered by paper bag, therefore, self-pollination was totally excluded and the time after pollination is exactly controlled. The detection on SNP loci in OsCLF ensured the hybrid ovaries for the experiments. cDNA were synthesized using SuperScript III Reverse Transcriptase (Thermo fisher Scientific) under the conditions recommended by the manufacturer. RT-PCR was performed in a 20 μl PCR mixture containing 2 μl of 10× Ex Taq buffer (including Mg2+), 200 μM dNTPs, 0.2 μM of primers, 0.5 U Ex Taq DNA polymerase (Takara), and cDNA prepared from endosperms at different stages. PCR conditions are performed as follows: initial denaturation at 94 °C for 2 min; 40 amplification cycles with denaturation at 94 °C for 30 s, annealing at 56 °C for 30 s; extension at 72 °C for 1 min; and a final incubation at 72 °C for 5 min. Each PCR products were purified and sequenced to distinguish which allele was transcribed according to DNA polymorphisms.
For genes with multiple transcripts such as OsEMF2a, we designed primers on the specific sequences of different regions from different transcripts, which can amplify specific splices of different lengths (Table 1). In general, long transcripts can translate proteins, and short transcripts cannot translate proteins without initiation codon ATG.
Additional file
Table S1. Primers of Distinction between OsEMF2a(l) and OsEMF2a(s). (DOC 27 kb)
Acknowledgements
The author acknowledge the use of the facilities, and scientific and technical assistance of the State Key Laboratory of hybrid rice in Wuhan University of China. The author also thank Prof. Mengxiang Sun and Dr. Peng Zhao for constructive comments on the manuscript.
Abbreviations
- 9311
Oryza sativa subsp. indica (9311)
- DAP/dap
Days after pollination
- Nip
Oryza sativa subsp. japonica (Nipponbare)
- PcG
Polycomb group
- PCR
polymerase chain reaction
- PRC2
Polycomb Repressive Complex 2
- RT-PCR
Reverse transcriptase polymerase chain reaction
- SNPs
Single nucleotide polymorphisms
- Zh11
Oryza sativa subsp. japonica (Zhonghua 11)
Authors’ contributions
QK conducted experiment; YHW analyzed the images and data; QK and SSL designed all experiments and wrote the manuscript. All authors read and approved the final manuscript.
Funding
The research reported in this publication was supported by funding from doctoral start-up fund and “11531” engineering construction projects of Nanchang Normal University. The work was financially supported by the National Natural Science Foundation of China (31430007) and “973 Program” (2013CB126900).
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Baroux U, Gagliardini V, Page DR, Grossniklaus U. Dynamic regulatory interactions of Polycomb group genes: MEDEA autoregulation is required for imprinted gene expression in Arabidopsis. Genes Dev. 2006;20:1081–1086. doi: 10.1101/gad.378106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE, Olsen OA. Development of the endosperm in rice (Oryza sativa L): Cellularization. J Plant Res. 1996;109:301–313. doi: 10.1007/BF02344477. [DOI] [Google Scholar]
- Dilkes BP, Spielman M, Weizbauer R, Watson B, Burkart-Waco D, Scott RJ, Comai L. The maternally expressed WRKY transcription factor TTG2 controls lethality in interploidy crosses of Arabidopsis. PLoS Biol. 2008;6:2707–2720. doi: 10.1371/journal.pbio.0060308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada AD, Freese NH, Blakley IC, Loraine AE. Analysis of pollen-specific alternative splicing in Arabidopsis thaliana via semi-quantitative PCR. PeerJ. 2015;3:e919. doi: 10.7717/peerj.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsom JJ, Begcy K, Hao X, Wang D, Walia H. Rice fertilization-independent Endosperm1 regulates seed size under heat stress by controlling early endosperm development. Plant Physiol. 2014;165:238–248. doi: 10.1104/pp.113.232413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost JM, Moore GE. The importance of imprinting in the human placenta. PLoS Genet. 2010;6:e1001015. doi: 10.1371/journal.pgen.1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Rupe MA, Danilevskaya ON, Yang X, Hu Z. Genome-wide mRNA profiling reveals heterochronic allelic variation and a new imprinted gene in hybrid maize endosperm. Plant J. 2003;36:30–44. doi: 10.1046/j.1365-313X.2003.01852.x. [DOI] [PubMed] [Google Scholar]
- Hermon P, Srilunchang KO, Zou J, Dresselhaus T, Danilevskaya ON. Activation of the imprinted Polycomb group Fie1 gene in maize endosperm requires demethylation of the maternal allele. Plant Mol Biol. 2007;64:387–395. doi: 10.1007/s11103-007-9160-0. [DOI] [PubMed] [Google Scholar]
- Hsieh TF, Shin JY, Uzawa R, Silva P, Cohen S, Bauer MJ, Hashimoto M, Kirkbride RC, Harada JJ, Zilberman D, Fischer RL. Regulation of imprinted gene expression in Arabidopsis endosperm. Proc Natl Acad Sci U S A. 2011;108:1755–1762. doi: 10.1073/pnas.1019273108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingouff M, Haseloff J, Berger F. Polycomb group genes control developmental timing of endosperm. Plant J. 2005;42:663–674. doi: 10.1111/j.1365-313X.2005.02404.x. [DOI] [PubMed] [Google Scholar]
- Jullien PE, Katz A, Oliva M, Ohad N, Berger F. Polycomb group complexes self-regulate imprinting of the Polycomb group gene MEDEA in Arabidopsis. Curr Biol. 2006;16:486–492. doi: 10.1016/j.cub.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Yadegari R, Harada JJ, Goldberg RB, Fischer RL. Imprinting of the MEDEA polycomb gene in the Arabidopsis endosperm. Plant Cell. 1999;11:1945–1952. doi: 10.1105/tpc.11.10.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler C, Page DR, Gagliardini V, Grossniklaus U. The Arabidopsis thaliana MEDEA Polycomb group protein controls expression of PHERES1 by parental imprinting. Nat Genet. 2005;37:28–30. doi: 10.1038/ng1495. [DOI] [PubMed] [Google Scholar]
- Kuang Q, Yu X, Peng X, Sun MX. The isolation of early nuclear endosperm of Oryza sativa to facilitate gene expression analysis and screening imprinted genes. Plant Methods. 2015;11:49. doi: 10.1186/s13007-015-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhou B, Peng X, Kuang Q, Huang X, Yao J, Du B, Sun MX. OsFIE2 plays an essential role in the regulation of rice vegetative and reproductive development. New Phytol. 2014;201:66–79. doi: 10.1111/nph.12472. [DOI] [PubMed] [Google Scholar]
- Luo M, Bilodeau P, Dennis ES, Peacock WJ, Chaudhury A. Expression and parent-of-origin effects for FIS2, MEA, and FIE in the endosperm and embryo of developing Arabidopsis seeds. Proc Natl Acad Sci U S A. 2000;97:10637–10642. doi: 10.1073/pnas.170292997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Platten D, Chaudhury A, Peacock WJ, Dennis ES. Expression, imprinting, and evolution of rice homologs of the polycomb group genes. Mol Plant. 2009;2:711–723. doi: 10.1093/mp/ssp036. [DOI] [PubMed] [Google Scholar]
- Luo M, Taylor JM, Spriggs A, Zhang H, Wu X, Russell S, Singh M, Koltunow A. A genome-wide survey of imprinted genes in rice seeds reveals imprinting primarily occurs in the endosperm. PLoS Genet. 2011;7:e1002125. doi: 10.1371/journal.pgen.1002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallamilli BR, Zhang J, Mujahid H, Malone BM, Bridges SM, Peng Z. Polycomb group gene OsFIE2 regulates rice (Oryza sativa) seed development and grain filling via a mechanism distinct from Arabidopsis. PLoS Genet. 2013;9:e1003322. doi: 10.1371/journal.pgen.1003322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raissig MT, Bemer M, Baroux C, Grossniklaus U. Genomic imprinting in the Arabidopsis embryo is partly regulated by PRC2. PLoS Genet. 2013;9:e1003862. doi: 10.1371/journal.pgen.1003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W, Constancia M, Fowden A, Anderson N, Dean W, Ferguson-Smith A, Tycko B, Sibley C. Regulation of supply and demand for maternal nutrients in mammals by imprinted genes. J Physiol Lond. 2003;547:35–44. doi: 10.1113/jphysiol.2002.033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RJ, Spielman M, Bailey J, Dickinson HG. Parent-of-origin effects on seed development in Arabidopsis thaliana. Development. 1998;125:3329–3341. doi: 10.1242/dev.125.17.3329. [DOI] [PubMed] [Google Scholar]
- Spillane C, MacDougall C, Stock C, Kohler C, Vielle-Calzada JP, Nunes SM, Grossniklaus U, Goodrich J. Interaction of the Arabidopsis Polycomb group proteins FIE and MEA mediates their common phenotypes. Curr Biol. 2000;10:1535–1538. doi: 10.1016/S0960-9822(00)00839-3. [DOI] [PubMed] [Google Scholar]
- Stupar RM, Hermanson PJ, Springer NM. Nonadditive expression and parent-of-origin effects identified by microarray and allele-specific expression profiling of maize endosperm. Plant Physiol. 2007;145:411–425. doi: 10.1104/pp.107.101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycko B, Morison IM. Physiological functions of imprinted genes. J Cell Physiol. 2002;192:245–258. doi: 10.1002/jcp.10129. [DOI] [PubMed] [Google Scholar]
- Wang D, Tyson MD, Jackson SS, Yadegari R. Partially redundant functions of two SET-domain polycomb-group proteins in controlling initiation of seed development in Arabidopsis. Proc Natl Acad Sci U S A. 2006;103:13244–13249. doi: 10.1073/pnas.0605551103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AJ, Oakey RJ. Genomic imprinting in mammals: emerging themes and established theories. PLoS Genet. 2006;2:e147. doi: 10.1371/journal.pgen.0020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AJ, Schulz R, Woodfine K, Koltowska K, Beechey CV, Peters J, Bourc'his D, Oakey RJ. Regulation of alternative polyadenylation by genomic imprinting. Genes Dev. 2008;22:1141–1146. doi: 10.1101/gad.473408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadegari R, Kinoshita T, Lotan O, Cohen G, Katz A, Choi Y, Katz A, Nakashima K, Harada JJ, Goldberg RB, Fischer RL, Ohad N. Mutations in the FIE and MEA genes that encode interacting polycomb proteins cause parent-of-origin effects on seed development by distinct mechanisms. Plant Cell. 2000;12:2367–2382. doi: 10.1105/tpc.12.12.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JE, Luo A, Xin HP, Zhao J, Li SS, Qu LH, Ma LG, Scholten S, Sun MX. Genes of both parental origins are differentially involved in early embryogenesis of a tobacco interspecies hybrid. PLoS One. 2011;6:e23153. doi: 10.1371/journal.pone.0023153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhao H, Xie S, Chen J, Xu Y, Wang K, Zhao H, Guan H, Hu X, Jiao Y, Song W, Lai J. Extensive, clustered parental imprinting of protein-coding and noncoding RNAs in developing maize endosperm. Proc Natl Acad Sci U S A. 2011;108:20042–20047. doi: 10.1073/pnas.1112186108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers of Distinction between OsEMF2a(l) and OsEMF2a(s). (DOC 27 kb)
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.