Highlights
-
•
Stomatal conductance is a major determinant of crop yield.
-
•
The speed of stomatal response to changing environmental conditions greatly impacts photosynthesis and water use.
-
•
Existing natural variation in the magnitude and rapidity of stomatal conductance is a potential target for future breeding.
-
•
Genetic manipulation of stomatal conductance has the potential to improve crop performance.
Abstract
Rising global temperatures and more frequent episodes of drought are expected to drive reductions in crop yield, therefore new avenues for improving crop productivity must be exploited. Stomatal conductance (gs) balances plant CO2 uptake and water loss, therefore, greatly impacting the cumulative rate of photosynthesis and water use over the growing season, which are key determinants of crop yield and productivity. Considerable natural variation exists in stomatal anatomy, biochemistry and behavioural characteristics that impact on the kinetics and magnitude of gs and thus gaseous exchange between the plant and atmosphere. Exploiting these differences in stomatal traits could provide novel breeding targets for new crop varieties that are potentially more water use efficient and have the ability to maintain and/or maximize yield in a range of diverse environments. Here we provide an overview of variation in stomatal traits and the impact these have on gs behaviour, as well as the potential to exploit such variation and genetic manipulation for crop improvement.
Current Opinion in Plant Biology 2019, 49:1–7
This review comes from a themed issue on Physiology and metabolism
Edited by Elizabeth A Ainsworth and Elizabete Carmo-Silva
For a complete overview see the Issue and the Editorial
Available online 7th March 2019
https://doi.org/10.1016/j.pbi.2019.01.003
1369-5266/© 2019 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Stomatal conductance influences crop photosynthesis and yield
Stomata govern gaseous diffusion between the leaf and the external atmosphere, regulating CO2 assimilation, water loss and evaporative cooling. Stomata continually adjust aperture in response to external environmental cues (e.g. light), plant water status [1], and internal signals, that may be hormonal (e.g. ABA) [2], circadian [3], and/or a currently unidentified ‘mesophyll signal’ [4,5], to maintain an appropriate balance between CO2 uptake and water loss. Over the long-term and under steady-state, non-limiting conditions, stomatal conductance (gs) has been reported to correlate strongly with the rate of photosynthesis (A) [6], with high gs generally associated with high A and yield [7]. However, short-term dynamic changes in the environment result in a lack of synchrony between gs and A, as stomatal responses to changing environmental cues are often substantially slower than those observed in A, resulting in a temporal disconnect between A and gs that can limit photosynthetic carbon assimilation and reduce plant water use efficiency (Wi, carbon assimilation as a ratio of water lost) [5,8,9••]. Stomatal conductance is determined by both anatomical and behavioural characteristics, yet both vary greatly between and within species, as well as between [10] and within leaves [11], resulting in significant variation in stomatal behaviour and absolute gs [12].
Anatomical characteristics determine the rate of gs
Anatomical features such as stomatal density (SD), size and maximum pore area, determine the calculated theoretical maximum stomatal conductance (gsmax) [13], whilst the control of stomatal opening and closure determine ‘operational’ or measured gs, that is the fraction of gsmax at which the leaf operates [14]. A positive relationship between SD and gs has been reported within species [15], which often, but not always [16] translates into high A [17,18]. For example, [19] reported that increased SD in two near isogenic lines of barley did not result in increased gs due to a concurrent decrease in stomatal size. Stomatal density is also positively related to photosynthetic capacity, with several studies illustrating increases in operational and maximum gs with increases in photosynthetic potential [20,21]. Furthermore, it is well established that significant natural variation in photosynthetic capacity exists between [22] and within species [23•,24•]. Stomatal size and SD also vary greatly within and between plant species [10], with differences often driven by changes in the growth environment [25], including [CO2] [26], light intensity and spectral quality [27]. There are numerous studies that have also demonstrated significant variation in stomatal anatomical characteristics within species, cultivars, genotypes and ecotypes. For example, [28] examined 62 wild Arabidopsis accessions and reported significant variation in SD that was also related to other epidermal traits, including cell size, stomatal index and patterning, suggesting a common genetic basis. In [29] varietal differences in SD and aperture in rice genotypes were shown, which [16] demonstrated the importance of variation in stomatal length that resulted in genotypic variation in gs. Variation in SD has also been associated with differences in drought resistance, as well as photosynthetic rates in wheat cultivars [30]. Therefore, natural variation in stomatal characteristics represents an unexploited genetic resource for improving gs, A and plant performance. Although variation in SD is well-established there is limited information on the impact of stomatal behaviour and/or kinetics on A, Wi and plant productivity.
Variation in stomatal anatomy impacts on dynamic gs responses
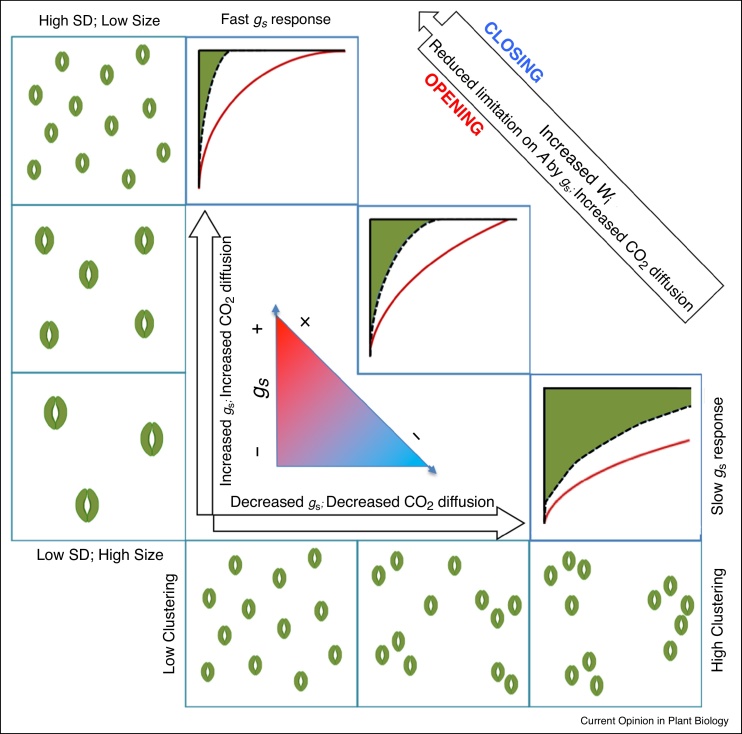
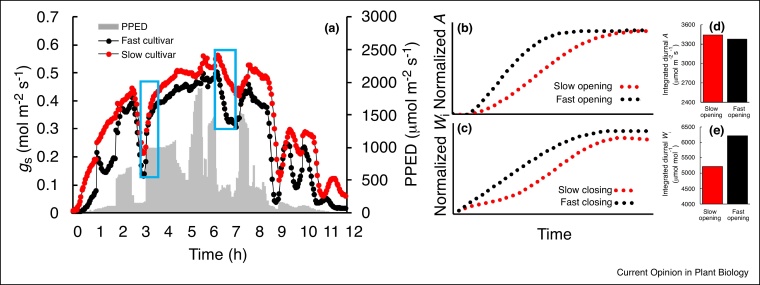
Modifications in SD have been reported to negatively correlate with stomatal size [25], which influences not only gs but also the speed at which stomata respond to changing environmental conditions [31,9••]. Several recent studies have demonstrated that stomatal kinetics are determined by anatomical attributes including stomatal morphology and shape [31,9••], size and density [32], patterning [33] and the presence or absence of subsidiary cells [9••,34], and that manipulation of these features could have positive effects on the efficiency of carbon assimilation and water use [35,36•]. Figure 1 shows the predicted impact of anatomical characters such as stomatal density and size on the magnitude and rapidity of the gs response to a step increase in light intensity, based on the literature [9••,31,32,33]. Leaves with a greater number of smaller stomata would be expected to have more rapid stomatal responses and a higher overall gs compared with leaves that had lower density and larger stomata. Additionally, stomatal patterning defects (i.e. stomatal clustering) have been reported to result in slower gs responses and lower gs values. [32] illustrated that the maximum rate of stomatal opening is driven by the surface-to-volume ratio of stomata, attributed to changes in SD and size, as species with higher stomatal densities and smaller stomata exhibited more rapid gs kinetics [31]. [9••] Quantified the impact of slow stomatal opening, in a range of species including crops, and determined on average a 10% limitation on carbon assimilation, which could equate to substantial losses in carbon gain over the course of the day, potentially negatively impacting productivity and yield [37,38]. In contrast, slow stomatal closure results in a significant decrease in intrinsic water use efficiency (Wi) and resource use [9••,39••] thus potentially accellerating early soil water exhaustion [40]. Figure 2 highlights the impact on A of variation in the speed of stomatal opening and closure, between two wheat varieties (Figure 2a). Slow increases in gs limit CO2 diffusion, reducing A (Figure 2b + d); whilst slow decreases in gs result in lower Wi (Figure 2c + e). Synchronized behaviour and close coupling of A and gs, therefore, have the potential to enhance carbon gain and Wi, and in turn improve performance, productivity and yield [17,39••]. The wheat cultivars measured in Figure 2 showed little difference in A (Figure 2d) between the fast and slow gs responding cultivars, (most likely due to the greater gs in the slower responding cultivar), whilst Wi (Figure 2e) was much greater in the cultivar with the faster gs responses.
Figure 1.
Diagram representing the influence of changes in stomatal anatomy (density and size; left panels, stomatal clustering; lower panels) on stomatal conductance (gs, arrows) and the rate of gs response (red lines). The impact of anatomical traits on carbon gain (A, dashed lines), the limitation of A by gs (green area) and water use efficiency (Wi) are illustrated. The influence of stomatal density and size (vertical arrow) and stomatal clustering (horizontal arrow) on the rate of gs response and the maximum or operational value of gs is highlighted.
Figure 2.
Diurnal time course of gs in two wheat cultivars with contrasting rapidity (a) under a dynamic light regime. Examples (blue sections) of the impact of slow and fast gs responses on A after a step increase in light (b); and Wi after a step decrease in light (c). The integrated daily values of A(d) and Wi(e) for cultivars with fast and slow stomatal responses is illustrated.
Although substantial progress has been made in linking stomatal anatomy to function, the size and density of stomata are not the only determinants of the speed of response [9••], with stomatal patterning [33,41•] and guard cell biochemistry [17] also playing key roles. In fact, stomatal clustering has been shown to decrease gs and, therefore A, without any change in overall SD and size [33], and was attributed to reduced guard cell function and increased hydraulic competition with neighbouring guard cells [33,41•] (see Figure 1). Guard cell movement is the cumulative sum of net solute fluxes (e.g. K+, Cl− and Malate) integrated over time and transported across the plasma membrane and the tonoplast [17,36•]. The density and the activity of the guard cell membrane transporters determine solute transport capacity and, inevitably, the speed and magnitude of stomatal movement [42]. Inter-specific variation in guard cells solute flux has been previously shown [17], corroborating the idea that stomatal movement is not only dependent on anatomical factors. Optimization of solute fluxes in guard cells has the potential to enhance stomatal rapidity and provides another unexploited target for crop breeding and should be given greater consideration in future research efforts.
Genetic manipulation of gs
As A is strongly correlated with gs a greater emphasis should be placed on recognising gs as a major target to improve crop yields and optimize water use. There are multiple examples of the genetic manipulation of SD successfully altering gs and influencing plant performance. Work by Gray et al. produced mutants with altered stomatal density by manipulating epidermal patterning factor genes [43]. Overexpression of the epidermal patterning factor EPF2 has been shown to improve long-term Wi without adversely affecting photosynthetic capacity [44] whilst also improving drought tolerance [35]. This model has been successfully applied to improve drought tolerance in barley [45•]. In contrast, [46] manipulated another member of the EPF family, the mesophyll driven EPF9 (STOMAGEN), which increased SD and gs resulting in a 30% increase in A, although a 40% decrease in Wi and no significant increase on growth was reported [47]. The above findings highlight that manipulation of stomatal anatomy could be a potential mechanism to increase gs and improve crop productivity and yield. However, it is worth bearing in mind that gs is fundamentally determined by stomatal behaviour and pore width and compensatory mechanism between density and behaviour can exist. For example work by [48] showed that reducing SD (by overexpressing the STOMATAL DENSITY AND DISTRIBUTION (SDD1) gene) in Arabidopsis, did not reduce gs as expected, because an increase in stomatal aperture compensated for the lower SD and, therefore, there was no difference in gs between the mutants and controls.
Overcoming the stomatal aperture/stomatal density trade-off was successfully shown by [49], whereby downregulation of either the α-subunit or β-subunit of farnesyltransferase (ERA1) increased stomatal sensitivity to ABA in canola. The increased ABA sensitivity reduced gs, and facilitated yield maintenance in plants subjected to drought conditions through improved resource use. Increased gs has been achieved through a number of metabolic manipulations, for example, silencing a mitogen-activated protein kinase MPK4 in Nicotiana attenuata increased gs and A threefold, as well as increased sensitivity to water stress [50]. In rice [51], tomato [52] and grapevine [53] aquaporin overexpression increased gs and A, both under optimal and stress conditions. These studies clearly demonstrate the potential of manipulating stomatal characteristics to improve carbon assimilation and resource use. However, restrictions on growing GM crops in many countries (particular in Europe) mean that alternative methods for manipulating gs need to be realised. This could be achieved by exploiting the significant natural variation in stomatal characteristics and behaviour that is known to exist. However, in order to achieve this, a greater understanding of the underlying genetics that control variation as well as the compensatory mechanisms between stomatal anatomy and behaviour need to be fully understood.
Natural variation in gs and genetic control for selection
Large natural variation in gs under optimal, steady-state light conditions has been shown for a range of crops. In Table 1, some of the most significant and recently reported work on the variation in gs is summarized.
Table 1.
Examples of variation assessed and the range of gs detected in cultivars or populations of different crops. The experimental design and methods for gs estimation are shown
| Authors | Crop | gs range (mol m−2 s−1) | Experimental material and analysis |
|---|---|---|---|
| [54] | Wheat | 0.15–0.55 | Chromosome substitution lines grown under field conditions with and without supplementary irrigation. gs analysed with Li-Cor 6400 at saturating light |
| [55] | Wheat | 0.10–0.42 | Field experiment. Double haploid population grown under supplementary irrigation and no irrigation treatment. gs estimated with CI-340 portable gas-exchange system at saturating light |
| [7] | Spring wheat | 0.34–0.57 | Historical selection of wheat cultivars grown over three field seasons. gs analysed with steady state porometry on both adaxial and abaxial surface |
| [56] | Durum wheat | 0.25–0.42 | Historical selection of Italian cultivars grown over two growing seasons. gs estimated with CIRAS-1 under natural light conditions |
| [16] | Rice | 0.25–0.85 | 64 accessions from a rice diversity research set of germplasm and 3 high-yielding cultivars grown under field conditions. gs estimated with Li-Cor 6400 at saturating light |
| [63] | Rice | 0.12–0.21 | Field screening under optimal and water stress condition of a BC3F6 mapping population. gs analysed with Li-Cor 6400 at near-saturating light |
| [62] | Soybean | 0.40–0.65 | Greenhouse experiments including VPD manipulation and water stress application on eleven cultivars. gs analysed with Li-Cor 6400 at saturating light |
| [65] | Cotton | 0.51–0.82 | Field grown segregating population. gs analysed with steady-state porometer |
| [57] | Cotton | 0.70–0.85 | Field grown historical selection of cotton. gs estimated during sunny days with Li-Cor 1600 steady state porometry |
| [67] | Cotton | 0.25–0.75 | Field experiment on obverse and reverse F1 lines. gs analysed with Li-Cor 6400 diurnally and at different light intensities and temperatures. |
| [58] | Tomato | 0.80–1.20 | Historical selection of tomatoes cultivars grown in the field and the greenhouse. gs was analysed in the field with a Li-cor 6400 at saturating light |
Potentially useful genomic regions have been identified that could provide crucial information for future breeding programmes. For example in cereals, variation in radiation use-efficiency [59], canopy temperature and yield [7] have been attributed to differences in gs, signifying the importance of this trait for possible further yield progress. Indeed, [7] showed that the year of release and yield genetic gain in wheat were linearly related with gs thus illustrating that the increase in yield was achieved by inadvertently selecting for high gs, cooler canopy and inevitably higher A. A large normally distributed phenotypic variation for gs was reported in two segregating populations of wheat, illustrating potential quantitative inheritance and a heritability on a family mean basis of up to 73% [60]. Subsequently, it has been shown that gs is subject to a polygenic control which was in turn associated with QTLs for yield under stress conditions [61]. Therefore, there is strong evidence that variation in gs is present in wheat and that marker-assisted selection could be carried out if more accurate genomic regions controlling gs are detected.
Genotypic differences in gs have also been detected in eleven soybean genotypes analysed under saturating light with different soil water conditions, which lead to variation in Wi in response to water stress [62]. Anatomy-driven variation in gs was shown to be present in elite rice cultivars [16], and QTLs for steady-state gs at saturating light in introgression lines under water stress conditions were identified on chromosomes 3 and 9 [63]. Other QTLs related to gs were identified in rice [64] and cotton [65], thus suggesting the possibilities of selection for gs through marker-assisted selection in several crops. Other sources of potential variation in gs (and thus A) include inter-specific and inter-generic crosses within the Triticeae [66]. The use of F1 hybrids in crops where heterosis for gs is present (e.g. cotton; [67]) has also been shown to be successful. Hence, variation in gs is already present in many crops with potential to be included in breeding programmes for both yield potential and enhancement in stress tolerance. Moreover, although previous research has put a great deal of emphasis on assessing the variation in stomatal anatomical characteristics or steady-state gs, there is limited information regarding potential intra-specific variation in the rapidity of stomata responses in major food crops, with some information available in rice only [39••]. Further work needs to focus on detecting the genetic basis of stomatal rapidity, thus enhancing the ability for selection of more efficient crops under naturally dynamic environmental conditions.
Conclusions
Stomatal conductance is a major determinant of photosynthesis, and there is clear evidence that manipulating gs can improve crop performance and yield. Natural variation in gs exists in crops, with several genomic regions identified that could provide unexploited targets for ongoing breeding programmes. Additionally the rapidity and kinetics of stomatal responses to changing environmental conditions have been demonstrated to greatly impact A and water use, and are the result of differences in anatomical and biochemical stomatal components [9••]. As higher stomatal density is often correlated with smaller stomata, and smaller stomata have been reported to respond more rapidly to changing environmental cues, a future priority could be the selection of cultivars with these anatomical features or the identification of the genomic regions that correspond to such traits of interest. Guard cell biochemistry and the density and activity of membrane transporters play a key role in both the magnitude and rapidity of gs responses, representing novel targets for improving crop productivity, although little is known regarding natural intra-specific variation in these functional traits. Future breeding programmes should consider the integration of both density and behavioural beneficial traits so that equal consideration is given to the magnitude and rapidity of gs responses, as well as the overall steady state gs value. In conclusion intra-specific variation in the key components governing stomatal dynamics and overall gs represent an unexploited target for improving A and Wi for increased plant productivity.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgement
This work was funded by Biotechnology and Biological Sciences Research Council (BBSRC, UK) grants to TL (BB/NO16831/1; BB/S005080/1).
References
- 1.Lin Y.S., Medlyn B.E., Duursma R.A., Prentice I.C., Wang H., Baig S. Optimal stomatal behaviour around the world. Nat Clim Change. 2015;5:459. [Google Scholar]
- 2.Haworth M., Marino G., Cosentino S.L., Brunetti C., De Carlo A., Avola G. Increased free abscisic acid during drought enhances stomatal sensitivity and modifies stomatal behaviour in fast growing giant reed (Arundo donax L.) Environ Exp Bot. 2018;147:116–124. [Google Scholar]
- 3.Hassidim M., Dakhiya Y., Turjeman A., Hussien D., Shor E., Anidjar A. CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and the circadian control of stomatal aperture. Plant Physiol. 2017 doi: 10.1104/pp.17.01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawson T., Terashima I., Fujita T., Wang Y. The Leaf: A Platform for Performing Photosynthesis. Springer; Cham: 2018. Coordination between photosynthesis and stomatal behaviour; pp. 141–161. [Google Scholar]
- 5.Matthews J.S., Vialet-Chabrand S.R., Lawson T. Acclimation to fluctuating light impacts the rapidity and diurnal rhythm of stomatal conductance. Plant Physiol. 2018 doi: 10.1104/pp.17.01809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong S.C., Cowan I.R., Farquhar G.D. Stomatal conductance correlates with photosynthetic capacity. Nature. 1979;282:424. [Google Scholar]
- 7.Fischer R.A., Rees D., Sayre K.D., Lu Z.M., Condon A.G., Saavedra A.L. Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci. 1998;38:1467–1475. [Google Scholar]
- 8.Lawson T., Simkin A.J., Kelly G., Granot D. Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytol. 2014;203:1064–1081. doi: 10.1111/nph.12945. [DOI] [PubMed] [Google Scholar]
- 9••.McAusland L., Vialet-Chabrand S., Davey P., Baker N.R., Brendel O., Lawson T. Effects of kinetics of light‐induced stomatal responses on photosynthesis and water‐use efficiency. New Phytol. 2016;211:1209–1220. doi: 10.1111/nph.14000. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article highlights the inter-specific variation in the rapidity of response and steady state stomatal conductance in a range of crops and other plant functional types. Particular attention is given to the impact of changes in stomatal kinetics on carbon gain and intrinsic water use efficiency.
- 10.Tichá I. Photosynthetic characteristics during ontogenesis of leaves. 7. Stomata density and sizes. Photosynthetica. 1982;16:375–471. [Google Scholar]
- 11.Lawson T., Weyers J. Spatial and temporal variation in gas exchange over the lower surface of Phaseolus vulgaris L. primary leaves. J Exp Bot. 1999;50:1381–1391. [Google Scholar]
- 12.Lawson T., James W., Weyers J. A surrogate measure of stomatal aperture. J Exp Bot. 1998;49:1397–1403. [Google Scholar]
- 13.Franks P.J., Beerling D.J. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc Nat Acad Sci U S A. 2009;106:10343–10347. doi: 10.1073/pnas.0904209106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franks P.J., Farquhar G.D. The effect of exogenous abscisic acid on stomatal development, stomatal mechanics, and leaf gas exchange in Tradescantia virginiana. Plant Physiol. 2001;125:935–942. doi: 10.1104/pp.125.2.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muchow R.C., Sinclair T.R. Epidermal conductance, stomatal density and stomatal size among genotypes of Sorghum bicolor (L.) Moench. Plant Cell Environ. 1989;12:425–431. [Google Scholar]
- 16.Ohsumi A., Kanemura T., Homma K., Horie T., Shiraiwa T. Genotypic variation of stomatal conductance in relation to stomatal density and length in rice (Oryza sativa L.) Plant Prod Sci. 2007;10:322–328. [Google Scholar]
- 17.Lawson T., Blatt M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014;164:1556–1570. doi: 10.1104/pp.114.237107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi S., Monda K., Negi J., Konishi F., Ishikawa S., Hashimoto-Sugimoto M. Natural variation in stomatal responses to environmental changes among Arabidopsis thaliana ecotypes. PloS One. 2015;10 doi: 10.1371/journal.pone.0117449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones H.G. Transpiration in barley lines with differing stomatal frequencies. J Exp Bot. 1977;28:162–168. [Google Scholar]
- 20.Frank D.C., Poulter B., Saurer M., Esper J., Huntingford C., Helle G. Water-use efficiency and transpiration across European forests during the Anthropocene. Nat Clim Change. 2015;5:579. [Google Scholar]
- 21.McElwain J.C., Yiotis C., Lawson T. Using modern plant trait relationships between observed and theoretical maximum stomatal conductance and vein density to examine patterns of plant macroevolution. New Phytol. 2016;209:94–103. doi: 10.1111/nph.13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawson T., Kramer D.M., Raines C.A. Improving yield by exploiting mechanisms underlying natural variation of photosynthesis. Curr Opin Biotechnol. 2012;23:215–220. doi: 10.1016/j.copbio.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 23•.Driever S.M., Lawson T., Andralojc P.J., Raines C.A., Parry M.A.J. Natural variation in photosynthetic capacity, growth, and yield in 64 field-grown wheat genotypes. J Exp Bot. 2014;65:4959–4973. doi: 10.1093/jxb/eru253. [DOI] [PMC free article] [PubMed] [Google Scholar]; Driever et al. highlight the intra-specific variation in photosynthetic traits that exist across a panel of wheat varieties. Emphasizing the manipulation of key genes within electron transport and the Calvin cycle, the authors discuss the potential for crop improvements through breeding for this unexploited genetic resource. No evidence of a correlation between photosynthetic capacity and yield traits was reported.
- 24•.Carmo-Silva E., Andralojc P.J., Scales J.C., Driever S.M., Mead A., Lawson T. Phenotyping of field-grown wheat in the UK highlights contribution of light response of photosynthesis and flag leaf longevity to grain yield. J Exp Bot. 2017;68:3473–3486. doi: 10.1093/jxb/erx169. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article details the natural variation of photosynthetic traits in wheat. With specific attention given to large scale phenotyping of physiological and agronomic traits, and how these may be used to inform breeding programs. A correlation between yield and operational photosynthetic carbon assimilation under natural light conditions was reported.
- 25.Hetherington A.M., Woodward F.I. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- 26.Woodward F.I., Bazzaz F.A. The responses of stomatal density to CO2 partial pressure. J Exp Bot. 1988;39:1771–1781. [Google Scholar]
- 27.Gay A.P., Hurd R.G. The influence of light on stomatal density in the tomato. New Phytol. 1975;75:37–46. [Google Scholar]
- 28.Delgado D., Alonso-Blanco C., Fenoll C., Mena M. Natural variation in stomatal abundance of Arabidopsis thaliana includes cryptic diversity for different developmental processes. Ann Bot. 2011;107:1247–1258. doi: 10.1093/aob/mcr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maruyama S., Tajima K. Leaf conductance in japonica and indica rice varieties: I. Size, frequency, and aperture of stomata. Jpn J Crop Sci. 1990;59:801–808. [Google Scholar]
- 30.Liao J.X., Chang J., Wang G.X. Stomatal density and gas exchange in six wheat cultivars. Cereal Res Commun. 2005;33:719–726. [Google Scholar]
- 31.Franks P.J., Farquhar G.D. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol. 2007;143:78–87. doi: 10.1104/pp.106.089367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drake P.L., Froend R.H., Franks P.J. Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance. J Exp Bot. 2013:495–505. doi: 10.1093/jxb/ers347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dow G.J., Bergmann D.C. Patterning and processes: how stomatal development defines physiological potential. Curr Opin Plant Biol. 2014;21:67–74. doi: 10.1016/j.pbi.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Chen Z.H., Chen G., Dai F., Wang Y., Hills A., Ruan Y.L. Molecular evolution of grass stomata. Trend Plant Sci. 2017;22:124–139. doi: 10.1016/j.tplants.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Doheny-Adams T., Hunt L., Franks P.J., Beerling D.J., Gray J.E. Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Phil Trans R Soc B. 2012;367:547–555. doi: 10.1098/rstb.2011.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Lawson T., Vialet‐Chabrand S. Speedy stomata, photosynthesis and plant water use efficiency. New Phytol. 2018;221:93–99. doi: 10.1111/nph.15330. [DOI] [PubMed] [Google Scholar]; This article reviews the determinants of the rapidity of stomatal conductance, including anatomical and biochemical components, and the impact of variation in these traits on photosynthesis and intrinsic water use.
- 37.Taylor S.H., Long S.P. Slow induction of photosynthesis on shade to sun transitions in wheat may cost at least 21% of productivity. Phil Trans R Soc B. 2017;372 doi: 10.1098/rstb.2016.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deans R.M., Brodribb T.J., Busch F.A., Farquhar G.D. Plant water‐use strategy mediates stomatal effects on the light induction of photosynthesis. New Phytol. 2018 doi: 10.1111/nph.15572. [DOI] [PubMed] [Google Scholar]
- 39••.Qu M., Hamdani S., Li W., Wang S., Tang J., Chen Z. Rapid stomatal response to fluctuating light: an under-explored mechanism to improve drought tolerance in rice. Funct Plant Biol. 2016;43:727–738. doi: 10.1071/FP15348. [DOI] [PubMed] [Google Scholar]; Qu et al. demonstrate, using a panel of 204 rice accessions, that the rapidity of stomatal opening and closing is strongly linked to drought resistance, highlighting the trade-off between carbon uptake and water saving. Primary focus is given to the impact of the speed of stomatal opening and closing on biomass and yield. This is the only work available on intra-specific variation for stomatal rapidity.
- 40.Bodner G., Nakhforoosh A., Kaul H.P. Management of crop water under drought: a review. Agron Sustain Dev. 2015;35:401–442. [Google Scholar]
- 41•.Papanatsiou M., Amtmann A., Blatt M.R. Stomatal clustering in Begonia associates with the kinetics of leaf gaseous exchange and influences water use efficiency. J Exp Bot. 2017;68:2309–2315. doi: 10.1093/jxb/erx072. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article provides insights into the impact of natural stomatal clustering on stomatal kinetics and CO2 assimilation under environmentally limited conditions. Particular attention is given to alterations in guard cell dynamics under light and dark treatments.
- 42.Blatt M.R. Cellular signaling and volume control in stomatal movements in plants. Ann Rev Cell Dev Biol. 2000;16:221–241. doi: 10.1146/annurev.cellbio.16.1.221. [DOI] [PubMed] [Google Scholar]
- 43.Hunt L., Gray J.E. The signaling peptide EPF2 controls asymmetric cell divisions during stomatal development. Curr Biol. 2009;19:864–869. doi: 10.1016/j.cub.2009.03.069. [DOI] [PubMed] [Google Scholar]
- 44.Franks P.J., Doheny-Adams T., Britton-Harper Z.J., Gray J.E. Increasing water-use efficiency directly through genetic manipulation of stomatal density. New Phytol. 2015;207:188–195. doi: 10.1111/nph.13347. [DOI] [PubMed] [Google Scholar]
- 45•.Hughes J., Hepworth C., Dutton C., Dunn J.A., Hunt L., Stephens J. Reducing stomatal density in barley improves drought tolerance without impacting on yield. Plant Physiol. 2017 doi: 10.1104/pp.16.01844. [DOI] [PMC free article] [PubMed] [Google Scholar]; Provides strong evidence that reducing stomatal density confers drought tolerance under limited water availability in barley. The transformed plants showed significant conservative water-use under water stressed conditions due to restricted leaf stomatal conductance.
- 46.Kondo T., Kajita R., Miyazaki A., Hokoyama M., Nakamura-Miura T., Mizuno S. Stomatal density is controlled by a mesophyll-derived signaling molecule. Plant Cell Physiol. 2009;51:1–8. doi: 10.1093/pcp/pcp180. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka Y., Sugano S.S., Shimada T., Hara-Nishimura I. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytol. 2013;198:757–764. doi: 10.1111/nph.12186. [DOI] [PubMed] [Google Scholar]
- 48.Büssis D., von Groll U., Fisahn J., Altmann T. Stomatal aperture can compensate altered stomatal density in Arabidopsis thaliana at growth light conditions. Funct Plant Biol. 2006;33:1037–1043. doi: 10.1071/FP06078. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y., Ying J., Kuzma M., Chalifoux M., Sample A., McArthur C. Molecular tailoring of farnesylation for plant drought tolerance and yield protection. Plant J. 2005;43:413–424. doi: 10.1111/j.1365-313X.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- 50.Hettenhausen C., Baldwin I.T., Wu J. Silencing MPK4 in Nicotiana attenuata enhances photosynthesis and seed production but compromises abscisic acid-induced stomatal closure and guard cell-mediated resistance to Pseudomonas syringae pv tomato DC3000. Plant Physiol. 2012;158:759–776. doi: 10.1104/pp.111.190074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanba Y.T., Shibasaka M., Hayashi Y., Hayakawa T., Kasamo K., Terashima I., Katsuhara M. Overexpression of the barley aquaporin HvPIP2; 1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant Cell Physiol. 2004;45:521–529. doi: 10.1093/pcp/pch070. [DOI] [PubMed] [Google Scholar]
- 52.Sade N., Gebretsadik M., Seligmann R., Schwartz A., Wallach R., Moshelion M. The role of tobacco Aquaporin1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress. Plant Physiol. 2010;152:245–254. doi: 10.1104/pp.109.145854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perrone I., Gambino G., Chitarra W., Vitali M., Pagliarani C., Riccomagno N. The grapevine root-specific aquaporin VvPIP2; 4N controls root hydraulic conductance and leaf gas exchange under well watered conditions but not under water stress. Plant Physiol. 2012:112. doi: 10.1104/pp.112.203455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aminia R., Mohammadi S., Hoshmand S., Khodombashi M. Chromosomal analysis of photosynthesis rate and stomatal conductance and their relationships with grain yield in wheat (Triticum aestivum L.) under water-stressed and well-watered conditions. Acta Physiol Plant. 2011;33:755–764. [Google Scholar]
- 55.Wang S.G., Jia S.S., Sun D.Z., Wang H.Y., Dong F.F., Ma H.X. Genetic basis of traits related to stomatal conductance in wheat cultivars in response to drought stress. Photosynthetica. 2015;53:299–305. [Google Scholar]
- 56.De Vita P., Nicosi O.L.D., Nigro F., Platani C., Riefolo C., Di Fonzo N., Cattivelli L. Breeding progress in morpho-physiological, agronomical and qualitative traits of durum wheat cultivars released in Italy during the 20th century. Eur J Agron. 2007;26:39–53. [Google Scholar]
- 57.De Vita P., Li Destri Nicosia O., Nigro F., Platani C., Riefolo C., Di Fonzo N., Cattivelli L. Stomatal conductance predicts yields in irrigated Pima cotton and bread wheat grown at high temperatures. J Exp Bot. 1998;49:453–460. [Google Scholar]
- 58.Barrios-Masias F.H., Jackson L.E. California processing tomatoes: morphological, physiological and phenological traits associated with crop improvement during the last 80 years. Eur J Agron. 2014;53:45–55. [Google Scholar]
- 59.Motzo R., Pruneddu G., Giunta F. The role of stomatal conductance for water and radiation use efficiency of durum wheat and triticale in a Mediterranean environment. Eur J Agron. 2013;44:87–97. [Google Scholar]
- 60.Rebetzke G.J., Condon A.G., Richards R.A., Read J.J. Phenotypic variation and sampling for leaf conductance in wheat (Triticum aestivum L.) breeding populations. Euphytica. 2001;121:335–341. [Google Scholar]
- 61.Pinto R.S., Reynolds M.P., Mathews K.L., McIntyre L., Olivares-Villegas J.J., Chapman S.C. Heat and drought adaptive QTL in a wheat population designed to minimize confounding agronomic effects. Theor Appl Genet. 2010;121:1001–1021. doi: 10.1007/s00122-010-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gilbert M.E., Zwieniecki M.A., Holbrook N.M. Independent variation in photosynthetic capacity and stomatal conductance leads to differences in intrinsic water use efficiency in 11 soybean genotypes before and during mild drought. J Exp Bot. 2011;62:2875–2887. doi: 10.1093/jxb/erq461. [DOI] [PubMed] [Google Scholar]
- 63.Gu J., Yin X., Struik P.C., Stomph T.J., Wang H. Using chromosome introgression lines to map quantitative trait loci for photosynthesis parameters in rice (Oryza sativa L.) leaves under drought and well-watered field conditions. J Exp Bot. 2011;63:455–469. doi: 10.1093/jxb/err292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Price A.H., Young E.M., Tomos A.D. Quantitative trait loci associated with stomatal conductance, leaf rolling and heading date mapped in upland rice (Oryza sativa) New Phytol. 1997;137:83–91. [Google Scholar]
- 65.Ulloa M., Cantrell R.G., Percy R.G., Zeiger E., Lu Z. QTL analysis of stomatal conductance and relationship to lint yield in an interspecific cotton. J Cotton Sci. 2000;4:10–18. [Google Scholar]
- 66.Reynolds M., Foulkes M.J., Slafer G.A., Berry P., Parry M.A., Snape J.W., Angus W.J. Raising yield potential in wheat. J Exp Bot. 2009;60:1899–1918. doi: 10.1093/jxb/erp016. [DOI] [PubMed] [Google Scholar]
- 67.Zeng B., Xu X., Zhou S., Zhu C., Tang C. Effects of temperature and light on photosynthetic heterosis of an upland cotton hybrid cultivar. Crop Sci. 2012;52:282–291. [Google Scholar]