Abstract
Background and Aims
Methotrexate [MTX] is a well-known immunomodulator in the treatment of inflammatory bowel disease [IBD] and is often combined with biologic agents. The ideal MTX dose for combination therapy has not been determined. This study aimed to investigate the effect of varying doses of MTX on efficacy and safety outcomes when used with anti-TNF agents in IBD.
Methods
This study included patients with Crohn’s disease [CD] or ulcerative colitis [UC] receiving care between January 2005 and June 2018. Low-dose MTX was defined as ≤12.5 mg/week and high-dose as >12.5 mg/week. The primary efficacy outcome was a composite of need for IBD-related hospitalization or surgery, steroid initiation, or change of biologic agent within 1 year. Safety outcomes included side effects related to MTX, serious infections, malignancy, and need to discontinue MTX therapy within 1 year. Multivariable logistic regression models adjusting for relevant covariates were used to assess independent association between MTX dose and outcomes.
Results
Our study included 222 patients with IBD [163 CD, 59 UC]. Just under a third were receiving low-dose MTX [28%]. The primary efficacy composite outcome was noted in 75 patients [47%] in the high-dose MTX group compared with 23 patients [37%] in the low-dose MTX group [p = 0.15]. We found no significant associations between MTX dose and any side effect [odds ratio 1.59, 95% confidence interval 0.77–3.31, p = 0.21] or development of serious infections [odds ratio 1.19, 95% confidence interval 0.41–3.45, p = 0.76].
Conclusions
Low-dose and high-dose MTX combination therapy were equally effective, and no difference in infection or malignancy rates was observed.
Keywords: Methotrexate, anti-TNF therapy, inflammatory bowel disease
1. Introduction
The past two decades have witnessed an evolution in therapy of inflammatory bowel diseases (IBDs; Crohn’s disease [CD], ulcerative colitis [UC]). The availability of monoclonal antibodies initially against tumor necrosis factor α [anti-TNF], and subsequently anti-integrin, and anti-interleukin 12/23 [IL-12/23] therapies, substantially improved our ability to achieve remission and reduce morbidity due to these progressive diseases. However, an important concern that arose out of the use of such therapies was the associated immunogenicity, with formation of anti-drug antibodies leading to reduced efficacy, loss of response, and adverse effects.
Pivotal clinical trials established the efficacy of combination immunomodulator–anti-TNF therapy in both CD and UC in immunosuppression-naïve individuals.1,2 Observational evidence also demonstrated an efficacy of combination therapy, primarily with thiopurines (azathioprine or 6-mercaptopurine [6 MP]) in more established disease.3–5 An important mechanism proposed for the benefit conferred by such combination therapy is the reduction in rate of anti-drug antibody formation, and consequently the boost in biologic trough levels, resulting in improved efficacy. In addition, concomitant immunomodulator therapy may also impact biologic trough level by impacting non-immune clearance of the drug. However, the dose of immunomodulator required to achieve this benefit [rather than to achieve efficacy when used as monotherapy] remains uncertain. While the RCTs examining the efficacy of combination thiopurine therapy used the full weight-based dose of azathioprine [2.5 mg/kg], a single observational study suggested that dosing to half the therapeutic trough concentration was sufficient to achieve a similar infliximab trough level.6
While most of the studies of combination therapy have examined the role of azathioprine, concerns about the safety of thiopurines, particularly in young men and in older patients, has led to increasing use of methotrexate [MTX] in combination with a biologic. However, the appropriate dose of MTX sufficient to confer a benefit when used in combination therapy is unknown. When used as monotherapy, an MTX dose of 25 mg weekly subcutaneously was efficacious in CD but not in UC,7,8 and a lower dose of 12.5 mg weekly orally was ineffective in UC.9 Limited literature from the treatment of inflammatory arthritis with MTX suggests that a lower dose may be sufficient to achieve benefit.10 There is limited data examining the effect of varying doses of MTX when used with anti-TNF agents in IBD. Consequently, we performed this study with the aim of comparing the safety and efficacy of high-dose compared with low-dose MTX in combination with anti-TNF therapy in CD and UC.
2. Methods
2.1. Study population
This was a retrospective single-centre study from a tertiary referral hospital. The study population was comprised of patients using MTX in combination with an anti-TNF biologic agent during the study period 2005–2017. Potential patients were identified through the Research Patient Data Registry [RPDR], an electronic data warehouse comprising all inpatient and outpatient health-care encounters at Massachusetts General Hospital [MGH] and affiliated hospitals within the Partners Healthcare system. This data registry collects administrative [billing and coding], laboratory, procedure, and prescription information continuously from all outpatient and inpatient encounters. We first identified patients with a potential diagnosis of CD or UC based on the presence of relevant International Classification of Diseases [ICD-9] [CD: 555.x; UC 556.x] or ICD-10 [CD: K50.x; UC: K51.x] codes. From among these patients, we identified those who received overlapping prescriptions for an anti-TNF medication (infliximab, adalimumab [ADA], certolizumab pegol, or golimumab) and MTX. Manual chart review was performed of all eligible patients to confirm IBD diagnosis and status as concurrent users of anti-TNF and MTX therapy. Patients were further stratified into those starting MTX and anti-TNF therapy at the same time versus those who had MTX added within 90 days after anti-TNF therapy initiation. Patients were excluded if they were younger than 18 years of age, had no follow-up available, or if they were not receiving both therapies concurrently. We included patients who may have been receiving MTX for indications other than luminal IBD [such as inflammatory arthritis] as long as the dose was consistent with that used for IBD [5–25 mg MTX weekly].
2.2. Covariates and study groups
Review of the electronic medical records of included patients was conducted, and several covariates were extracted, including age, gender, smoking history, age at diagnosis, IBD type, and disease duration. Disease extent in UC and disease behavior and location in CD were noted according to the Montreal Classification. We also noted information on baseline serum albumin and C-reactive protein [CRP] at the time of initiation of MTX. For each patient, we noted the date of initiation of MTX and anti-TNF agent, as well as dose, route, and frequency of administration of each drug. The highest MTX dose was used if the MTX dose changed during the first year of combination therapy.
The primary comparison of interest was based on the dose of MTX [irrespective of route of administration]. Patients were classified as low-dose MTX users if the maximum MTX dose was ≤12.5 mg weekly, whereas high-dose MTX users comprised those with a weekly dose >12.5 mg.
2.3. Outcomes
Our primary outcomes pertained to both treatment efficacy and safety. The primary efficacy outcome was a composite of need for IBD-related hospitalization or surgery, steroid initiation, or change of biologic agent within 1 year of combination therapy. We also separately examined each of these outcomes. Additional efficacy outcomes including remission at 1 year, switch or stop of anti-TNF agent, and serum albumin and CRP 1 year after MTX therapy. Remission was based upon global physician impression; validated disease activity indices were not routinely used in clinical practice during the duration of the study. Similarly, endoscopic evaluation to assess for mucosal healing was also not systematically performed and thus could not be included as a study outcome.
Safety outcomes included side effects related to MTX, serious infections, malignancy, and need to discontinue MTX therapy within 1 year.
2.4. Statistical analyses
All data collected were analyzed using Stata 13.1 [StataCorp, College Station, TX]. Means and standard deviations were utilized to summarize continuous variables and compared using the t test or Wilcoxon test if appropriate. Proportions were used to express categorical variables, and compared using the chi-square test and if necessary the Fisher’s exact test. First, we performed univariate analysis to identify predictors of each of our primary outcomes. Significant variables [p < 0.05] or variables previously noted in the literature to be important were included in a multivariable logistic regression model to find significant predictors. A two-sided p-value < 0.05 in the multivariable model indicated independent statistical significance.
We performed several a priori–defined stratified analyses, such as analyses by type of IBD, and by varying the cut-offs to define high-dose compared with low-dose MTX use. In addition, we examined the effect of oral compared with subcutaneous administration. The study was approved by the Institutional Review Board of Partners Healthcare.
3. Results
3.1. Study cohort
Our study cohort included 222 patients [163 CD, 59 UC] receiving MTX and anti-TNF therapy at the same time. The mean age of included patients was 37 years, and 47% of the cohort were women. Fewer than half the patients were taking oral steroids at the time of initiating MTX [49%]. Over three-quarters [76%] of patients had a prior history of biologic therapy before the index anti-TNF agent. The median baseline CRP and mean albumin were 5.8 mg/L and 4.1 g/dL, respectively. In three-quarters of patients [79%], MTX was initiated at the same time or within 90 days of anti-TNF initiation, and the remaining 21% had continuation of their previously initiated MTX. A total of 15, 29, 19, 50, 1, 13, and 95 patients were receiving a concomitant MTX dosage of 7.5 mg, 10 mg, 12.5 mg, 15 mg, 17.5 mg, 20 mg, and 25 mg, respectively.
Table 1 compares the characteristics of low-dose [≤12.5 mg weekly] and high-dose [>12.5 mg weekly] MTX users. We found no difference in age, gender, IBD type, disease location and behavior, disease duration, prior IBD treatments, serum albumin, or CRP between the two groups. Nearly three-quarters of patients in both groups had CD, with no differences in disease behaviour or extent. Patients in the high-dose MTX group were more likely to be former or current smokers [p = 0.021] than the low-dose MTX users. Two-thirds [65%] of patients in the high-dose MTX group and half [54%] in the low-dose MTX group initiated MTX in conjunction with or within 90 days of anti-TNF therapy initiation. The most common anti-TNF agent in both groups was infliximab [41% high-dose MTX, 44% low-dose MTX]. A similar proportion in both groups had previous loss of response to at least one biologic agent [p = 0.33].
Table 1.
Baseline characteristics of the study cohort
| Characteristic | Low dose ≤12.5 mg [n = 63] | High dose >12.5 mg [n = 159] | p-value |
|---|---|---|---|
| Female, n [%] | 29 [46.0] | 76 [47.8] | 0.812 |
| Age, mean [SD] | 35.0 ± 14.0 | 38.1 ± 13.9 | 0.125 |
| IBD type | 0.802 | ||
| Crohn’s disease, n [%] | 47 [74.6] | 116 [73.0] | |
| Ulcerative colitis, n [%] | 16 [25.4] | 43 [27.0] | |
| Disease duration, mean [SD] | 10.7 ± 9.6 | 10.4 ± 9.4 | 0.802 |
| Smoking | 0.021 | ||
| Never, n [%] | 53 [86.9] | 106 [68.4] | |
| Former, n [%] | 5 [8.2] | 32 [20.7] | |
| Current, n [%] | 3 [4.9] | 17 [11.0] | |
| Disease location | 0.894 | ||
| CD ileitis, n [%] | 6 [9.5] | 12 [7.8] | |
| CD ileocolitis, n [%] | 27 [42.9] | 68 [44.4] | |
| CD colitis, n [%] | 15 [23.8] | 32 [20.9] | |
| UC proctitis, n [%] | 2 [3.2] | 2 [1.3] | |
| UC left-sided colitis, n [%] | 3 [4.8] | 8 [5.2] | |
| UC pancolitis, n [%] | 10 [15.9] | 31 [20.3] | |
| Perianal disease | 14 [22.2] | 49 [31.4] | 0.174 |
| Stricturing | 14 [22.2] | 35 [22.4] | 0.973 |
| Penetrating | 21 [33.3] | 59 [37.8] | 0.532 |
| Stoma | 2 [3.2] | 13 [8.4] | 0.168 |
| Pouch | 8 [12.7] | 5 [3.2] | 0.007 |
| Past history | |||
| Prior anti-TNF, n [%] | 45 [71.4] | 123 [77.4] | 0.353 |
| Prior surgery, n [%] | 16 [25.4] | 48 [30.8] | 0.429 |
| CRP mg/L, mean [SD] | 14.1 ± 27.8 | 19.8 ± 28.6 | 0.209 |
| Albumin g/dL, mean [SD] | 4.2 ± 0.5 | 4.0 ± 0.5 | 0.116 |
| Started MTX within 90 days of anti-TNF, n [%] | 34 [54.0] | 104 [65.4] | 0.113 |
| Route of MTX administration n [%] | <0.001 | ||
| Oral administration | 61 [96.8] | 62 [39.0] | |
| Injection | 2 [3.2] | 97 [61.0] | |
| Anti-TNF agent | 0.412 | ||
| Infliximab, n [%] | 24 [38.1] | 60 [37.7] | |
| Adalimumab, n [%] | 28 [44.4] | 65 [40.9] | |
| Certolizumab, n [%] | 6 [9.5] | 27 [17.0] | |
| Simponi, n [%] | 5 [7.9] | 7 [4.4] |
3.2. Efficacy
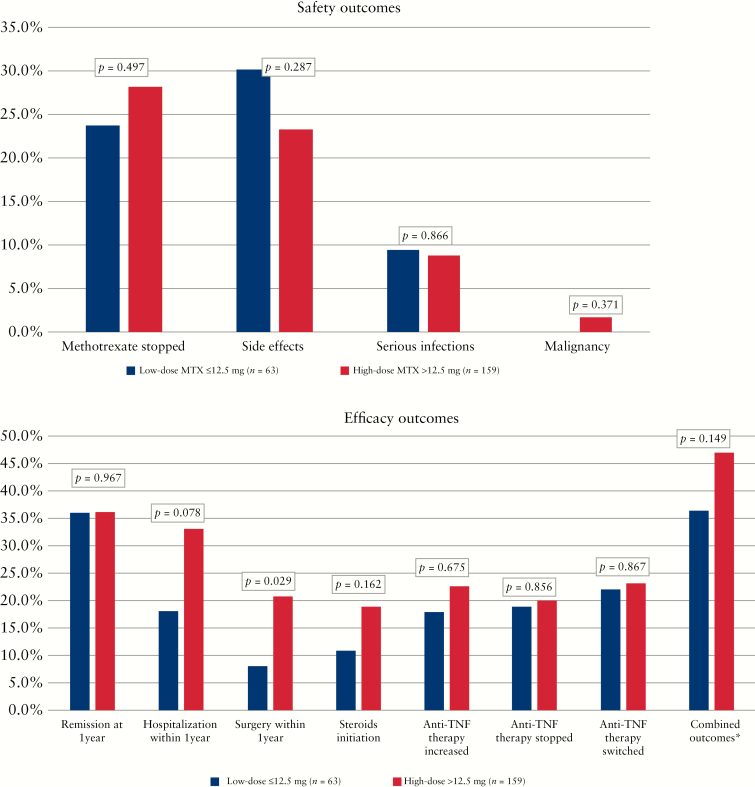
The primary composite outcome [IBD-related hospitalization or surgery, biologic change, or steroid initiation] was noted in 75 patients [47%] in the high-dose MTX group, compared with 23 patients [37%] in the low-dose MTX group [p = 0.15]. After adjusting for relevant confounders [gender, age, IBD type, disease duration, smoking status, prior failed anti-TNF therapy and surgical treatment, and CRP at baseline], there was no significant difference in primary outcome between the two dose groups of MTX (odds ratio [OR] 0.49, 95% confidence interval [CI] 0.23–1.04, p = 0.063) [Table 2]. For the individual outcomes, there was a numerically higher rate of surgery and hospitalization in the high-dose compared with low-dose groups [Figure 1], but these differences did not remain significant on multivariable analysis [p = 0.19 and p = 0.06, respectively] [Table 2]. Stratifying the analysis by type of IBD, neither CD nor UC had significant impact on the outcomes [Supplemental Table 1]. A similar proportion of patients in the low-dose [39%] and high-dose [36%] groups achieved clinical remission at 1 year [p = 0.71], with no difference on adjusting for potential confounders [OR 1.02, 95% CI 0.47–2.22, p = 0.97]. A similar proportion of patients in both groups required an increase in anti-TNF dose [23% high dose vs 17% low dose] or ceased the index anti-TNF therapy [25% high dose vs 15% low dose, p = 0.069].
Table 2.
Multivariable analysis of impact of high-dose [>12.5 mg] or low-dose MTX [≤12.5 mg] on efficacy and safety outcomes in patients with inflammatory bowel diseases
| OR† | 95% CI | p-value | |
|---|---|---|---|
| Side effects | 1.59 | 0.77–3.31 | 0.21 |
| Serious infections | 1.19 | 0.41–3.45 | 0.75 |
| Remission at 1 year | 1.02 | 0.47–2.22 | 0.97 |
| Hospitalization within 1 year | 0.42 | 0.17–1.04 | 0.06 |
| Surgery within 1 year | 0.46 | 0.14–1.47 | 0.19 |
| Combined outcomes‡ | 0.49 | 0.23–1.04 | 0.06 |
†High-dose compared with low-dose MTX [reference].
‡Composite outcome included switch of biologic therapy, hospitalization within 1 year, surgery within 1 year, and steroid therapy initiated in the first year
Figure 1.
Comparison of safety [a] and efficacy [b] outcomes between low-dose MTX and high-dose MTX combination therapy.
A subgroup analysis was done for those patients who started MTX and anti-TNF therapy at the same time or added MTX within 90 days after anti-TNF therapy started [n = 140]. Similar to our prior results, there were no differences in efficacy outcomes between high-dose MTX [>12.5 mg] and low-dose MTX [≤12.5 mg] while on anti-TNF therapy. Including the variable for year of anti-TNF initiation in the multivariable model did not change the efficacy and safety outcomes, suggesting lack of impact of secular changes in IBD practice]. Additionally, we repeated the multivariable analysis separately for oral and subcutaneous administration of MTX and found no difference in efficacy or safety outcomes between low-dose and high-dose MTX [Supplemental Table 2].
3.3. Safety
Of the 222 patients in our cohort who were on combination therapy, one-quarter [25.2%] of the patients reported an adverse event. Nausea [n = 16] was the most common, followed by elevated liver enzymes [n = 13]. Other side effects included infections, anti-TNF–induced lupus, rash, joint pain, headache, psoriasis, bronchitis, and fatigue. MTX dosage was not associated with likelihood of side effects [high dose 23.3% vs low dose 30.2%, p = 0.28]. A total of 20 [9%] serious infections were reported during the first year of combination therapy, but there was no significant difference between high-dose versus low-dose MTX [8.8% vs 9.5%, p = 0.87]. Reported infections were Herpes zoster, Clostridium difficile, pneumonia, and herpes simplex virus infection. Discontinuation of MTX therapy occurred in approximately one-quarter [23.8%] of the patients receiving low-dose MTX and 28.3% of the patients receiving high-dose MTX [p = 0.50]. Two cases developed a malignancy [thyroid carcinoma and hepatocellular carcinoma] during the first year of combination therapy, both of whom were in the high-dose MTX group. On multivariable analysis, adjusting for gender, age, IBD type, disease duration, smoking status, CRP at baseline, MTX dosage, prior anti-TNF therapy, prior surgery, and prior MTX failure, we found no significant associations with low-dose MTX or any side effect [OR 1.59, 95% CI 0.77–3.31, p = 0.21] or development of serious infections [OR 1.19, 95% CI 0.42–3.45, p = 0.76].
3.4. Sensitivity analyses
We repeated the analysis stratifying MTX users into low-dose and high-dose groups at a cut-off of 15 mg instead of 12.5 mg, which resulted in 113 ‘low-dose’ and 109 ‘high-dose’ users. As in our primary analysis, there was no statistically significant difference in composite outcomes between the two groups [Supplemental Table 3]. Separately, we also observed no difference in hospitalization, surgery, or steroid initiation within 1 year between the low-dose and high-dose MTX groups. On multivariable analysis, the differences remained statistically insignificant for each of the outcomes. For the individual outcomes, there was a higher rate of surgery and hospitalization in the high-dose group compared with the low-dose group, but these differences did not remain significant on multivariable analysis [p = 0.061 and p = 0.189, respectively]. No differences between the high-dose and low-dose groups were found in remission rates, changes in anti-TNF therapy, side effects, infections, and laboratory values at 1 year. Additionally, we repeated the analysis stratifying the MTX dose at a cut-off of 10 mg, which resulted in 44 low-dose and 178 high-dose users. As in the primary analysis cut-off at 12.5 mg, there was no difference between the low-dose and high-dose groups for the efficacy or safety outcomes. However, there were too few patients in the low-dose group for robust interpretation of these results.
4. Discussion
Methotrexate is a widely used immunomodulator for the treatment of IBD. It is known to be beneficial in combination with anti-TNF therapy, particularly through reducing immunogenicity and increasing serum anti-TNF levels.11 However, there is little literature available regarding the optimal dosing of MTX when used in combination with anti-TNF therapy. In this large retrospective cohort study, we found no difference in safety or efficacy outcomes between low-dose MTX [≤15 mg/week] and high-dose MTX [>15 mg/week] during combination therapy with an anti-TNF biologic.
The initial evidence supporting efficacy of MTX in IBD was from the trial by Feagan and colleagues, randomizing patients with CD to 25 mg intramuscular MTX once a week or placebo. They demonstrated a statistically significant benefit in both induction7 and maintenance of remission when compared with placebo [65% MTX vs 39% placebo, p = 0.04].12 However, studies examining the efficacy of monotherapy with MTX for UC suggest no benefit. An initial study of MTX at 12.5 mg oral weekly was ineffective in UC.9 Recent larger randomized controlled trials of parenteral 25 mg weekly MTX found no statistically significant benefit of MTX monotherapy in UC. In the trial by Carbonnel et al., 25 mg parenteral MTX was not superior to placebo for induction of steroid-free remission in UC [31.7% MTX vs 19.6% placebo, p = 0.15],8 which was confirmed in the MERIT-UC trial by Herfarth et al.13 The only randomized trial examining the efficacy of MTX when used in combination with an anti-TNF was the COMMIT trial, which demonstrated that although there was no clinical benefit at 1 year in patients on combination infliximab-MTX when compared with infliximab alone, patients receiving MTX less frequently had antibodies to infliximab and had higher serum infliximab levels.14
One of the first pieces of evidence suggesting that a lower-than-therapeutic dose of the immunomodulator is sufficient when used in combination was from a single-centre cross-sectional study by Yarur et al.6 In a small cohort of 72 patients, a cut-off of 6-TGN levels of 125 pmol/8 × 108 RBCs [compared with the threshold of >230 pmol for therapeutic dosing] predicted higher IFX levels and lower rates of anti-drug antibody formation. Patients with 6-TGN levels below this threshold were more likely to have anti-drug antibodies. Only a few studies have examined optimal MTX dosing. In rheumatoid arthritis [RA], a large randomized, double-blind study of MTX in combination with ADA examined four different doses of MTX [2.5 mg, 5 mg, 10 mg, and 20 mg] in biologic-naïve patients. They found a similar benefit–risk profile for 10 and 20 mg/week of MTX, with increased ADA trough levels (6.5 [4.4] and 6.9 [3.4], respectively).10 These results suggest that a dose of 10 mg MTX/week may be used in conjunction with anti-TNF therapies in RA patients. Similar results came from a post hoc analysis of two pooled trials with combination of etanercept and MTX therapy.15 Patients were stratified by MTX dosage at 24 months [low dose <10 mg/week, medium dose 10–17.5 mg/week, and high dose >17.5 mg/week], and no differences between the study groups were found for response to therapy, remission scores, or quality of life. In contrast are the results of Becciolini et al. that indicated better clinical response at 6 and 12 months and clinical outcome at 12 months in patients treated with high-dose MTX [>10 mg/week], rather than low-dose MTX, concomitant with etanercept.16 Interesting results regarding MTX reduction upon adding a TNF-inhibitor came from a large RA registry that followed patients on MTX combination therapy for 24 months. They found that 56% maintained their MTX dosage, 34% underwent tapering of MTX, and 10% stopped MTX after adding a TNF inhibitor for the first time, and they found no differences in disease activity scores for the three groups.17
Colman et al. compared the efficacy of higher- [15–25 mg] and lower- [<12.5 mg] dose MTX combined with anti-TNF agents in IBD patients in remission.18 Nearly three-quarters [71%] received high-dose MTX, and it was given orally in 75% of patients. They found that patients receiving high-dose MTX combination therapy were more likely to maintain remission and that they had less frequent relapses [p < 0.01]. There was no difference in adverse events or discontinuation. However, this study was restricted to those who were already in clinical remission and thus therapy responsive. In our study, examining the efficacy of MTX when used in combination for induction, we did not find a difference between >12.5 mg and ≤12.5 mg weekly MTX dose, in contrast to the Colman et al. data, and we found similar efficacy when stratifying at 15 mg/week and 10 mg/week. Thus, a lower dose of MTX may be sufficient to confer benefit when used in combination, as noted for thiopurine therapy. We also found no difference in safety, including infections, hepatotoxicity, and gastrointestinal intolerance between the two groups.
There were several limitations to this study. First, it was a retrospective analysis that was completed in a tertiary referral centre, which may have been biased towards more severe disease when compared with a population-based cohort. Second, since the data spanned a period where therapeutic drug monitoring was not frequent, we did not have information on sufficient drug trough level or antibodies to compare the two groups. Third, assessment of clinical remission and endoscopic evaluation was based upon global physician impression, and validated disease indices were not routinely used. We also used the highest dose of MTX during the first year of therapy, which may have biased against the high-dose MTX group. We also included patients who could have been on MTX for non-IBD indications. While one expects the pharmacokinetic and immunologic impact on patients to be independent of indication for use, it is possible that the low-dose group may have included more patients on it for a non-IBD indication, thereby contributing to the non-statistically significant trend towards better IBD outcomes in this group. Finally, the study period spanned a decade. Although, the reasons for initiation of MTX combination therapy have changed over recent years, we did not observe a change in our results when we included the year of anti-TNF initiation in our multivariable analysis, suggesting our findings do not merely represent secular trends.
In conclusion, we demonstrated that both low-dose [≤12.5 mg weekly] and high-dose MTX [>12.5 mg weekly] combination therapy were equally effective and well tolerated in patients with IBD. There is an important need for prospective trials comparing low-dose and high-dose MTX when used in combination with biologic therapy to determine optimal dosing.
Funding
ANA is supported in part by grants from the National Institutes of Health [R03 DK112909], the Chleck Family Foundation, and the Crohn’s and Colitis Foundation.
Conflict of Interest
ANA has served on advisory boards for Abbvie, Takeda, and Merck.
Author Contributions
NZB – study design, data extraction and statistical analysis, drafting of the manuscript, approval of the final manuscript. JL, JGG, FPC, HK – approval of the final manuscript. ANA – study design and supervision, statistical analysis, drafting of the manuscript, approval of the final manuscript.
Supplementary Material
References
- 1. Colombel JF, Sandborn WJ, Reinisch W, et al. ; SONIC Study Group Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95. [DOI] [PubMed] [Google Scholar]
- 2. Panaccione R, Ghosh S, Middleton S, et al. . Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014;146:392–400.e3. [DOI] [PubMed] [Google Scholar]
- 3. Sokol H, Seksik P, Carrat F, et al. . Usefulness of co-treatment with immunomodulators in patients with inflammatory bowel disease treated with scheduled infliximab maintenance therapy. Gut 2010;59:1363–8. [DOI] [PubMed] [Google Scholar]
- 4. Ungar B, Chowers Y, Yavzori M, et al. ; ABIRISK consortium The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut 2014;63:1258–64. [DOI] [PubMed] [Google Scholar]
- 5. Reenaers C, Louis E, Belaiche J, Seidel L, Keshav S, Travis S. Does co-treatment with immunosuppressors improve outcome in patients with Crohn’s disease treated with adalimumab? Aliment Pharmacol Ther 2012;36:1040–8. [DOI] [PubMed] [Google Scholar]
- 6. Yarur AJ, Kubiliun MJ, Czul F, et al. . Concentrations of 6-thioguanine nucleotide correlate with trough levels of infliximab in patients with inflammatory bowel disease on combination therapy. Clin Gastroenterol Hepatol 2015;13:1118–24.e3. [DOI] [PubMed] [Google Scholar]
- 7. Feagan BG, Rochon J, Fedorak RN, et al. . Methotrexate for the treatment of Crohn’s disease. The North American Crohn’s Study Group Investigators. N Engl J Med 1995;332:292–7. [DOI] [PubMed] [Google Scholar]
- 8. Carbonnel F, Colombel JF, Filippi J, et al. ; European Crohn’s and Colitis Organisation; Groupe d’Étude Thérapeutique des Affections Inflammatoires Digestives Methotrexate is not superior to placebo for inducing steroid-free remission, but induces steroid-free clinical remission in a larger proportion of patients with ulcerative colitis. Gastroenterology 2016;150:380–8.e4. [DOI] [PubMed] [Google Scholar]
- 9. Oren R, Arber N, Odes S, et al. . Methotrexate in chronic active ulcerative colitis: a double-blind, randomized, Israeli multicenter trial. Gastroenterology 1996;110:1416–21. [DOI] [PubMed] [Google Scholar]
- 10. Burmester GR, Kivitz AJ, Kupper H, et al. . Efficacy and safety of ascending methotrexate dose in combination with adalimumab: the randomised CONCERTO trial. Ann Rheum Dis 2015;74:1037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vermeire S, Noman M, Van Assche G, Baert F, D’Haens G, Rutgeerts P. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut 2007;56:1226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feagan BG, Fedorak RN, Irvine EJ, et al. . A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group Investigators. N Engl J Med 2000;342:1627–32. [DOI] [PubMed] [Google Scholar]
- 13. Herfarth H, Barnes EL, Valentine JF, et al. ; Clinical Research Alliance of the Crohn’s and Colitis Foundation Methotrexate is not superior to placebo in maintaining steroid-free response or remission in ulcerative colitis. Gastroenterology 2018;155:1098–08.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feagan BG, McDonald JW, Panaccione R, et al. . Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn’s disease. Gastroenterology 2014;146:681–8.e1. [DOI] [PubMed] [Google Scholar]
- 15. Gallo G, Brock F, Kerkmann U, Kola B, Huizinga TW. Efficacy of etanercept in combination with methotrexate in moderate-to-severe rheumatoid arthritis is not dependent on methotrexate dosage. RMD Open 2016;2:e000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Becciolini A, Biggioggero M, Favalli EG. The role of methotrexate as combination therapy with etanercept in rheumatoid arthritis: retrospective analysis of a local registry. J Int Med Res 2016;44:113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Manders SH, van de Laar MA, Rongen-van Dartel SA, et al. . Tapering and discontinuation of methotrexate in patients with RA treated with TNF inhibitors: data from the DREAM registry. RMD Open 2015;1:e000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Colman RJ, Rubin DT. Optimal doses of methotrexate combined with anti-TNF therapy to maintain clinical remission in inflammatory bowel disease. J Crohns Colitis 2015;9:312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.