Abstract
Background and Purpose
Much of the opioid epidemic arose from abuse of prescription opioid drugs. This study sought to determine if the combination of a cannabinoid with an opioid could produce additive or synergistic effects on pain, allowing reduction in the opioid dose needed for maximal analgesia.
Experimental Approach
Pain was assayed using the formalin test in mice and the carrageenan assay in rats. Morphine and two synthetic cannabinoids were tested: WIN55,212‐2 (WIN), which binds to both CB1 and CB2 receptors, and possibly TRPV1 channels; and GP1a, which has activity at CB2 receptors and is reported to inhibit fatty acid amide hydrolase, thus raising levels of endogenous cannabinoids.
Key Results
Morphine in combination with WIN in the formalin test gave synergistic analgesia. Studies with selective antagonists showed that WIN was acting through CB1 receptors. Morphine in combination with GP1a in the formalin test was sub‐additive. In the carrageenan test, WIN had no added effect when combined with morphine, but GP1a with morphine showed enhanced analgesia. Both WIN and Gp1a used alone had analgesic activity in the formalin pain test, but not in the carrageenan pain test.
Conclusions and Implications
The ability of a cannabinoid to produce an additive or synergistic effect on analgesia when combined with morphine varies with the pain assay and may be mediated by CB1 or CB2 receptors. These results hold the promise of using cannabinoids to reduce the dose of opioids for analgesia in certain pain conditions.
1. INTRODUCTION
The United States is currently in the midst of an opioid epidemic that has been declared a “public health emergency” by the Department of Health and Human Services (https://www.hhs.gov/about/news/2017/10/26/hhs‐acting‐secretary‐declares‐public‐health‐emergency‐address‐national‐opioid‐crisis.html). The United Kingdom has also seen a significant rise in prescription opioid abuse (Giraudon, Lowitz, Dargan, Wood, & Dart, 2013) (https://www.nytimes.com/2018/02/04/world/europe/uk‐fentanyl‐opioid‐addiction.html). The National Survey on Drug Use and Health carried out by the Substance Abuse and Mental Health Services Administration in 2016 found that 11.5 million Americans misused prescription opioids and 62.3% gave as the reason for their use, relief of pain (Ahrnsbrak, Bose, Hedden, Lipari, & Park‐Lee, 2017). It is estimated that 30% of Americans suffer from acute or chronic pain (Volkow & McLellan, 2016; Volkow, McLellan, Cotto, Karithanom, & Weiss, 2011), leading to the use of prescribed or illicit opioids for pain relief. One potential mitigating strategy for decreasing opioid use is to employ combination therapies, with the objective of producing equal analgesia using a lower dose of opioid. In animal models, cannabinoids have demonstrated analgesic activity (Pertwee, 2001; Walker & Hohmann, 2005) as well as anti‐inflammatory effects (Eisenstein & Meissler, 2015; Klein, 2005).
There are complexities to the cannabinoid system, as two cannabinoid receptors, CB1 and CB2, have been identified (Matsuda, Lolait, Brownstein, Young, & Bonner, 1990; Munro, Thomas, & Abu‐Shaar, 1993). CB1 receptors are highly expressed on neurons in the CNS (Herkenham et al., 1991) and to a lesser extent on cells of the immune system (Daaka, Friedman, & Klein, 1996; Galiegue et al., 1995). CB2 receptors are primarily expressed on cells of the immune system (Carlisle, Marciano‐Cabral, Staab, Ludwick, & Cabral, 2002; Daaka et al., 1996; Galiegue et al., 1995), as well as on activated microglia (Carlisle et al., 2002). CB2 receptors have been detected on neurons in the CNS, but levels of expression of this receptor are low compared to those of CB1 receptors (Gong et al., 2006; Van Sickle et al., 2005).
Both endogenous cannabinoid system ligands and exogenous cannabinoid receptor ligands have been shown to mitigate pain (Woodhams, Chapman, Finn, Hohmann, & Neugebauer, 2017). Δ9‐Tetrahydrocannabinol (Δ9‐THC) has activity at both CB1 and CB2 receptors and exerts analgesic activity through both receptors (Agarwal et al., 2007; Craft, Kandasamy, & Davis, 2013; Elikottil, Gupta, & Gupta, 2009; Thapa et al., 2018). CB2 receptors have also been shown to modulate pain (Brownjohn & Ashton, 2012; Deng et al., 2015; Guindon & Hohmann, 2008; Gutierrez, Crystal, Zvonok, Makriyannis, & Hohmann, 2011; Kinsey et al., 2011). Activation of these receptors decreased sciatic nerve injury pain in CB2 receptor knockout mice, as well as in CB2 receptor‐overexpressing transgenic mice (Racz et al., 2008). A synthetic CB2 receptor‐selective agonist was shown to have analgesic activity in a model of chemotherapy‐induced neuropathic pain in mice, which correlated with a reduction in mRNA for selected pro‐inflammatory cytokines and chemokines (Deng et al., 2015). Further, a synergistic combination between morphine and a selective CB2 receptor agonist, JWH015, was also shown in rodent models of post‐surgery and neuropathic pain (Grenald et al., 2017).CB1 and CB2 receptors were reported to participate in synergistic combinations of cannabinoids with morphine in a mouse model of cancer pain (Khasabova et al., 2011). Activation of the CB2 receptors on cells of the immune system results mainly in immunosuppression (Eisenstein & Meissler, 2015; Klein, 2005). Use of a CB2 receptor‐selective agonist would, therefore, be predicted to reduce inflammation, which plays a major role in many types of pain.
There are several preclinical reports showing that combinations of cannabinoids and opioids have additive or synergistic analgesic effects (Cichewicz, 2004; Nielsen et al., 2017; Welch, 2009). Most of these studies did not provide information on which cannabinoid receptor was mediating the opioid‐sparing effect. The hypothesis being tested in the present studies is that a combination of a cannabinoid with a sub‐analgesic dose of an opioid can achieve a level of pain relief observed with an optimal dose of the opioid alone. Such combinations, which would permit use of opioids at lower doses, could have the added advantage of reducing unwanted adverse effects of opioids, such as nausea, vomiting, constipation, sedation, respiratory depression, and pruritus, as well as potential development of tolerance and dependence. The present project was undertaken to investigate the feasibility of using morphine in combination with two different cannabinoids, one of which (WIN55,212‐2 [WIN]) binds to both CB1 and CB2 receptors, and the other ,GP1a, which has CB2 receptor agonist activity in in vivo studies (Franklin & Carrasco, 2013; Kong, Li, Tuma, & Ganea, 2014). We also used the cannabinoid receptor antagonists SR141716A (rimonabant) for CB1 receptors and SR144528 for CB2 receptors to understand which cannabinoid receptor mediates additive or synergistic analgesic effects with morphine. We chose WIN because there is a history of its use in animals for pain studies (Martin et al., 1999). WIN has been reported to have activity at the CB1 and CB2 receptors and SR141716A (Lowin, Pongratz, & Straub, 2016). In the present study, a TRPV1 channel antagonist, SB366791, was also studied using the cannabinoid and opioid combination to investigate the possible involvement of TRPV1 channels. Kong et al. (2014) reported that GP1a treatment decreased demyelination and axonal loss and reduced clinical scores and facilitated recovery in experimental autoimmune encephalomyelitis in mice. Analgesic effects of the compounds alone and in combination with morphine were tested in two different pain assays. Formalin‐induced nociception was used in mice as a tonic/chronic pain model (Murray, Porreca, & Cowan, 1988), and carrageenan‐induced inflammation was used in rats as a moddel of inflammatory pain (Kocher, Anton, Reeh, & Handwerker, 1987).
What is already known
Combinations of some cannabinoids plus morphine give enhanced analgesia over either drug alone.
Cannabinoids alone can mediate analgesia through CB1 or CB2 receptors .
What this study adds
Additive or synergistic analgesic effects of cannabinoids, when combined with morphine, vary with the pain assay.
CB1 or CB2 receptors can mediate additive/synergistic analgesic effects of cannabinoids combined with morphine.
What is the clinical significance
Combinations of cannabinoids with opioids may be an effective way of reducing opioid doses.
2. METHODS
2.1. Animals
All animal care and experimental procedures were carried out under protocols approved by the University IACUC. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology. Outbred Swiss‐Webster male mice (20–25 g) and outbred male Sprague–Dawley rats (120–150 g) were purchased from Taconic Biosciences (Albany, NY). Animals were housed in the Central Animal Facility of the Medical School in ventilated micro‐isolator cages under a schedule of 12 hr of light and 12 hr of darkness. They had access to food and water ad libitum. In all experiments, there were 6–10 animals per group, as designated in the individual figures.
2.2. Formalin pain assay
In this assay, the analgesic effects of morphine alone, a cannabinoid alone, or a combination of morphine plus a cannabinoid were tested (n = 7–8). Five minutes before formalin injection (t = −5), animals were placed into a transparent chamber connected to an isoflurane vaporizer and briefly anaesthetized. At t = 0, morphine or saline vehicle, or a cannabinoid (WIN or Gp1a), or 5% DMSO alone, was injected subcutaneously into the dorsal flank area of mice. In combination experiments, opposite sides of the dorsal flank were used. Immediately after drug or vehicle injections, the dorsal side of the left hind paw was injected subcutaneously with 20 μl of a 5% formalin solution diluted in saline. In experiments in which an antagonist was used, it was given intraperitoneally 30 min before agonists and formalin. After receiving the formalin injection, animals were placed into a large glass jar where their actions could be monitored. Formalin injection causes intense licking of the injected paw in two phases. The late phase of licking, between 20 and 35 min after administration of formalin, represents a combination of peripheral inflammatory‐ and spinal‐mediated pain (Tjolsen, Berge, Hunskaar, Rosland, & Hole, 1992), and thus, total licking time between 20 and 35 min was chosen for measurement of the response. Licking was scored for each animal as the number of seconds of licking that occurred during that 15‐min period (20 to 35 min after formalin injection). Animals were randomly assigned to groups, and experiments were carried out with the observer/recorder of the behaviour blinded to the treatment given to the animal.
2.3. Carrageenan pain assay
The carrageen test has two parameters, pain and swelling (oedema) of rat paws following injection of the irritant, carrageenan. To carry out the test, unrestrained rats were placed inside a clear plastic chamber (10 cm wide × 21 cm deep × 13 cm high) with a glass floor (32°C) that is part of the Hargreaves' Plantar Test Apparatus (Model 400, IITC Life Science, Woodland Hills, CA). After 60 min of habituation, all animals were exposed on the plantar surface of the left hind paw to a beam of radiant heat (intensity = 45) through the glass floor. They were tested three times, with 5‐min intervals between each stimulus. The latency (seconds) to paw withdrawal was used as the antinociceptive index and was automatically scored by the apparatus. Oedema was measured using a digital plethysmometer. To carry out the pain assay, rats were injected intraperitoneally with the desired cannabinoid, WIN or GP1a, or the vehicle (10% DMSO), 15 min before the carrageenan injection (t = −15). At t = 0, rats were injected into the plantar side of the left hind paw with 0.1 ml of a 2% carrageenan solution (FMC, Philadelphia, PA), freshly prepared in saline. All animals were tested by exposure to the heat beam three times at 5‐min intervals to establish the baseline pain value 180 min after carrageenan injection. Fifteen minutes after the post‐carrageenan baseline, the animals received a second injection of cannabinoid or vehicle. At t = +210 min (15 min after second cannabinoid or vehicle injection), they received an injection of morphine (3.0 mg·kg−1) or saline subcutaneously into the dorsal flank area. Latency of paw withdrawal to radiant heat and oedema was measured at 240 min (30 min after morphine or saline injection) and expressed as a percentage change from post‐carrageenan baseline. The per cent of maximal possible analgesia (%MPA) for each animal at each time was calculated using the following formula: %MPA = [(test latency at t = +240 min − baseline latency at t = +180 min)/(22 s − baseline latency at t = +180 min)] × 100. A cut‐off limit of 22 s was set to avoid damage to the paw. To measure oedema induced by the carrageenan and the effect of cannabinoids, morphine, or the combination of the two compounds, swelling of the left paw was measured at t = 0 (just after carrageenan injection) 180 and 240 min post‐carrageenan administration. To quantitate oedema, the following formula was used: Volume difference of oedema (ml) = Volume at t = +240 min − Volume at t = +180 min. Experiments were carried out with the observer/recorder of the behaviour blinded to the treatment given to the animal. The timeline for the carrageenan experiments is shown below:

2.4. mRNA levels of immune mediators
For determining mRNA levels, the draining popliteal lymph nodes from two animals in each group, as well as from two animals that received no carrageenan or drug (to obtain baseline values), were collected at t = 35 min from mice in the formalin test and at t = 4 hr from rats in the carrageenan test. The lymph nodes were then extracted by the RNeasy® Microarray Tissue Mini Kit (Qiagen, Gaithersburg, MD). These RNAs were used to generate cDNA using the RT2 First Strand Kit (Qiagen). The cDNAs from the two duplicate animals were pooled and assayed using the RT2 Profiler® PCR Arrays for Rat or Mouse Inflammatory Cytokines and Receptors (Qiagen), with the RT2 SYBR® Green ROX® qPCR Mastermix (Qiagen). The rat and mouse arrays have probes for mRNA for 84 immune mediators. The PCR arrays were run on an ABI StepOne Plus® qPCR thermocycler (Applied Biosystems, Foster City, CA), using the cycling conditions given in the protocol supplied with the RT2 Array. Data were processed by the online GeneGlobe Data Analysis Center (Qiagen). Results are presented as heat maps showing mRNA for cytokines and chemokines in the array of the treated groups compared with mRNA level expression in the baseline, untreated control group.
2.5. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology. For dose–response curves where multiple doses were tested, a one‐way ANOVA was performed followed by Sidak's multiple comparison test when comparing drug‐treated animals to a control group. Sidak's test was used for comparisons between groups treated with different drugs or different drug dosages. P < .05 was considered to be a statistically significant difference.
Isobolographic analysis was used to determine if there were additive or synergistic effects when morphine was combined with a cannabinoid, where both drugs demonstrated analgesic activity. The ED50 values of the individual drugs were plotted on the x and y axes and connected by an intersecting line. The intersecting line represents points along the line that shows additivity. A point above the line represents sub‐additivity, and a point below the line represents synergy. The new ED50 value of the combinations can be plotted to determine if the combinations are additive, sub‐additive, or synergistic.
In the case where one drug in the combination was effective (morphine), and the other drug was ineffective or only slightly effective, precluding calculation of its ED50, the dose equivalence method (Tallarida, 2006) was used to determine if the combinations were different from either drug alone. In this method, the value for the second drug was converted to the equally effective dose of morphine, and expected effects were calculated. To evaluate if the two drugs interact, these expected effects were compared to the effects observed. If the observed effects were below what was expected, the interaction was classified as sub‐additive; if the effects were equal to the expected effect, the interaction was classified as additive; and if the observed effects were greater than what was expected, the interaction was classified as synergistic.
2.6. Materials
Cannabinoids and their antagonists were purchased from Tocris (Minneapolis, MN). WIN was dissolved in 5% DMSO when used in mice and in 10% DMSO when used in rats. GP1a was dissolved in 5% DMSO. The cannabinoid antagonists, SR141716A (CB1), SR144528 (CB2), and the TRPV1 channel antagonist, SB366791, were dissolved in 5% DMSO. Morphine sulfate (doses expressed as the salt) was obtained from NIDA and dissolved in saline.
2.7. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos et al., 2017; Alexander, Fabbro et al., 2017; Alexander, Striessnig et al., 2017).
3. RESULTS
3.1. Analgesia induced by WIN alone, morphine alone, or a combination treatment in the formalin assay
Initial experiments were undertaken to assess the analgesic activity of morphine (0.5–10 mg·kg−1, s.c.) alone, WIN (0.5–5 mg·kg−1, s.c.) alone, or the two drugs (morphine:WIN, 1:1 ratio, 0.1–1 mg·kg−1, s.c.) in combination. Figure 1a shows that morphine significantly decreases formalin‐induced licking in a dose‐related manner compared to vehicle. Figure 1b shows that WIN also significantly decreases licking time in a dose‐related manner, compared to the vehicle group. Thus, both the opioid and the cannabinoid gave strong, dose‐related antinociceptive effects in the formalin test. Combination experiments were then carried out using these two compounds. The doses used in the combination studies were derived from the data gleaned from the individual dose–response curves, with attention to the range within which an increase or decrease in analgesic effect could be observed. Figure 2a shows that the licking time in response to formalin injected into the paw significantly decreased in a dose‐related manner in the animals given the combination of morphine and WIN. At the 0.5 and the 1.0 mg·kg−1 doses, morphine alone and WIN alone produced significantly reduced licking in response to the formalin injection, which was even further reduced when the drugs were combined at those doses. Using isobolographic analysis, the drug interactions were found to be highly synergistic. The results demonstrated the predicted increase in antinociceptive effect of the combination over each individual drug alone (Figure 2b).
Figure 1.
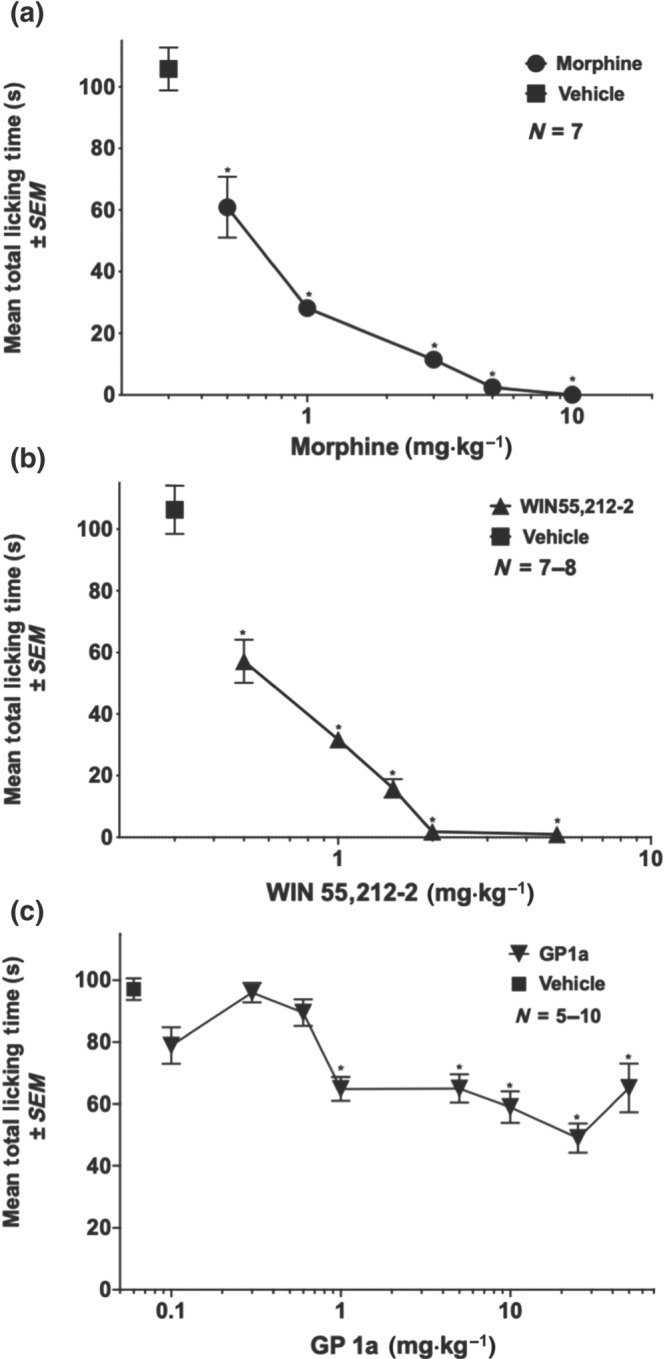
Dose–response curves for morphine, WIN, and GP1a on formalin‐induced nociception in mice. (a) Morphine, (b) WIN, or (c) GP1a were given subcutaneously immediately before injection of 20 μl of 5% formalin into the dorsal side of the left hind paw. Formalin control mice received saline subcutaneously (vehicle for morphine) or 5% DMSO (vehicle for WIN and GP1a). Total licking time was scored between 20 and 35 min post‐formalin injection. Each point represents the mean ± SEM paw licking time (seconds). *P < .05, significantly different from control; one‐way ANOVA followed by Sidak's multiple comparisons test
Figure 2.
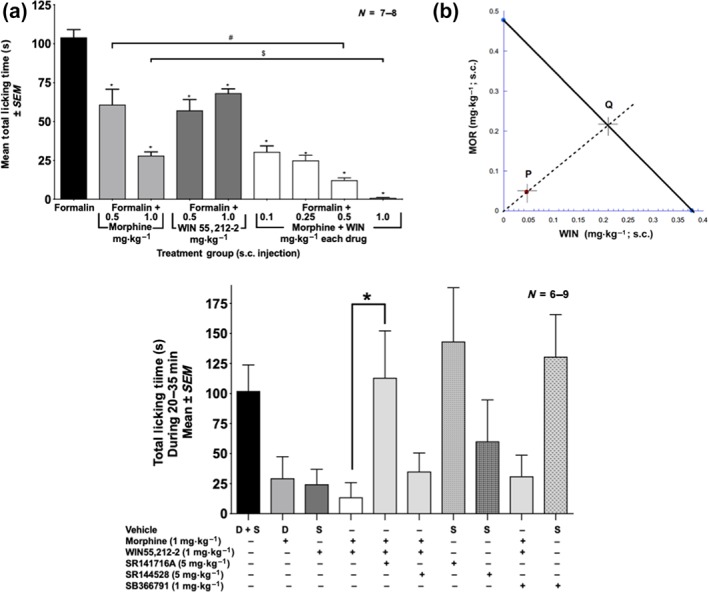
Effect of morphine alone, WIN alone, and the combination of WIN plus morphine on formalin‐induced nociception in mice. Mice received a formalin injection into the paw and either subcutaneously morphine, WIN, or combinations of morphine plus WIN. Formalin control mice received subcutaneously saline (vehicle for morphine) or 5% DMSO (vehicle for WIN) or a saline injection and a 5% DMSO injection. Values for all three vehicle controls were averaged. (a) Data are the mean paw licking time (seconds) ± SEM of treated mice. *P<.05, significantly different from formalin alone; #P<.05, significantly different as indicated; $ P<.05, significantly different as indicated; one‐way ANOVA with Sidak's multiple comparisons test. (b) Isobolographic analysis to determine synergism between morphine and WIN. The 1:1 ratio of WIN:morphine produced the half maximal effect shown here as point P. The expected additive point is shown as Q. (c) Effect of antagonists (SR141716A [CB1 receptor antagonist], SR144528 [CB2 receptor antagonist], and SB366791 [TRPV1 channel antagonist]) on licking time produced by the morphine plus WIN combination. D, DMSO; S, saline. *P < .05, significantly different as indicated; one‐way ANOVA followed by Sidak's multiple comparison test between the groups
To examine the receptor through which WIN (1 mg·kg−1) in combination with morphine (1 mg·kg−1) produced enhanced analgesia in the formalin test, antagonists for CB1 or CB2 receptors, and TRPV1 channels were used. SR141716A (CB1 receptors; 5 mg·kg−1, s.c.), SR144528 (CB2 receptors; 5 mg·kg−1, s.c.), and SB366791 (TRPV1 channels, 1 mg·kg−1, s.c.) were administered 20 min before the test compounds and formalin administration. Figure 2c shows that the synergistic activity of WIN and morphine in the formalin test is through CB1 receptors, as the CB1 receptor antagonist (SR141716A) returned licking time to that seen with vehicle alone. Neither the CB2 receptor antagonist nor the TRPV1 channel antagonist had an effect on the analgesic activity of the WIN plus morphine combination. Also, none of the three antagonists had any effect on licking by themselves (Figure 2c).
3.2. Analgesia induced by GP1a alone, morphine alone, or a combination treatment in the formalin assay
Figure 1c shows the dose–response effect for GP1a alone in the formalin assay. Gp1a significantly reduced (around 30–50%) licking time at doses ranging between 1.0 and 50.0 mg·kg−1 in a non‐dose‐related manner. The next experiments tested the effect of the combination of GP1a with morphine, using the morphine dose–response curve shown in Figure 1a as a reference. Figure 3a–c shows the effect of GP1a alone (1.0, 5.0, or 10.0 mg·kg−1) or a combination of these three different doses of GP1a with two different doses (0.5 and 1 mg·kg−1) of morphine. Using dose equivalence analysis, as explained in Section 2.5, the combinations of GP1a and morphine were sub‐additive.
Figure 3.
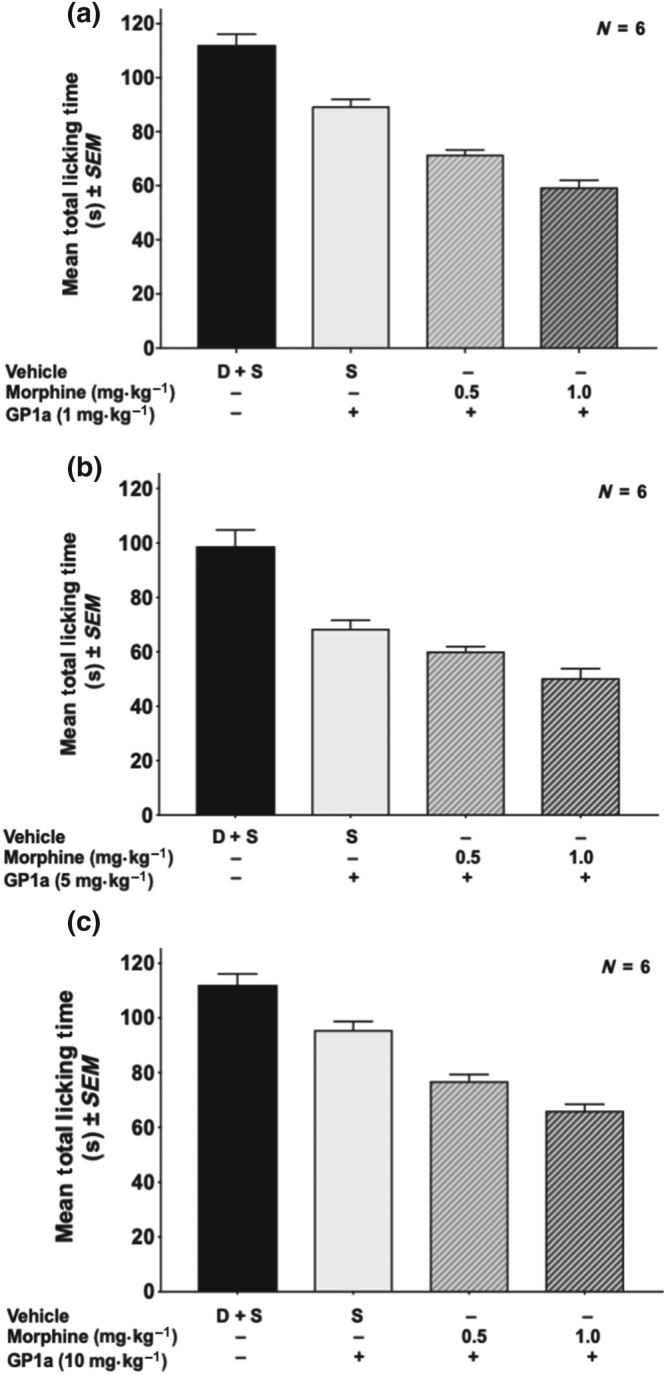
Effect of different doses of GP1a (1, 5, or 10 mg·kg−1) in combination with different doses of morphine in the formalin test. (a) GP1a = 1 mg·kg−1. (b) GP1a = 5 mg·kg−1. (c) GP1a = 10 mg·kg−1. D + S, DMSO plus saline. Dose equivalence analysis determined that the effect of combinations on analgesia was sub‐additive
3.3. Analgesia in the carrageenan assay induced by the cannabinoids, WIN or GP1a each alone, morphine alone, or combination treatments
Figure 4a shows the analgesic dose–response for morphine (0.5–10 mg·kg−1, s.c.), and Figure 4b,c presents the respective dose–responses for WIN (1–5 mg·kg−1, s.c.) and GP1a (0.5–10 mg·kg−1, s.c.) in the carrageenan test. Morphine produced significant analgesia in a dose‐dependent manner but had no effect on oedema (Figure S1A). Neither WIN nor GP1a had significant analgesic activity in this assay (Figure 4b,c) and was also without effect on paw oedema (Figure S1B,C). The animals that received only vehicle (10% DMSO) in Figure 4a–c appeared to show hyperalgesia, but it was not statistically significant due to the large standard errors. Also, no trend towards an increase in pain with vehicle was found in a subsequent experiment (see Figure 5a,b). Combination experiments of morphine with a cannabinoid were carried out using the carrageenan model. As shown in Figure 5a, WIN (1–5 mg·kg−1) did not enhance the analgesia induced by a suboptimal dose of morphine (3.0 mg·kg−1) in this test, nor did the WIN–morphine combination result in a reduction in oedema (Figure S1C). Thus, in contrast to the formalin assay in mice, in the carrageenan assay in rats, WIN showed no additive or synergistic effect with morphine. Figure 5b presents data on the combination of morphine at a suboptimal dose (3.0 mg·kg−1) given with GP1a (5.0 mg·kg−1). An increased analgesic effect was observed using this combination of GP1a plus morphine. No drug interactions were seen in reducing swelling of the paw (Figure S1D). Thus, GP1a showed an increased analgesic response with morphine in the carrageenan test, but in the formalin test, this drug combination was no better than either compound alone.
Figure 4.
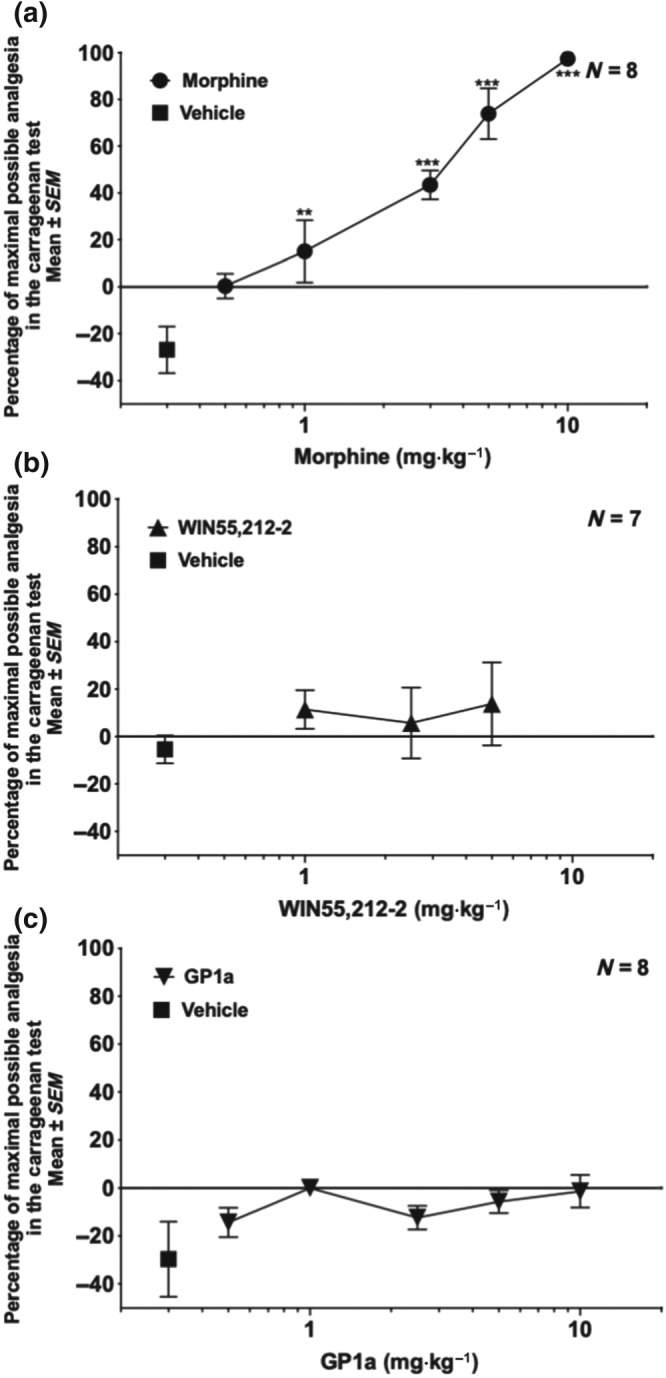
Dose–response curves for morphine, WIN, and GP1a in the rat carrageenan test. Rats received a carrageenan injection into the paw. WIN or GP1a was injected intraperitoneally 15 min before and 195 min after the carrageenan injection. Morphine was injected at 210 min post‐carrageenan subcutaneously into the dorsal flank. Analgesia was assessed 30 min after morphine injection using the Hargreaves' apparatus with a radiant heat beam. (a) Morphine provided analgesia at all but the lowest dose. *P < .05, significantly different from vehicle; one‐way ANOVA followed by Sidak's multiple comparison test. (b) WIN: percentage of maximal analgesia. (c) GP1a: percentage of maximal analgesia. (b, c) Not significant by one‐way ANOVA compared to vehicle
Figure 5.
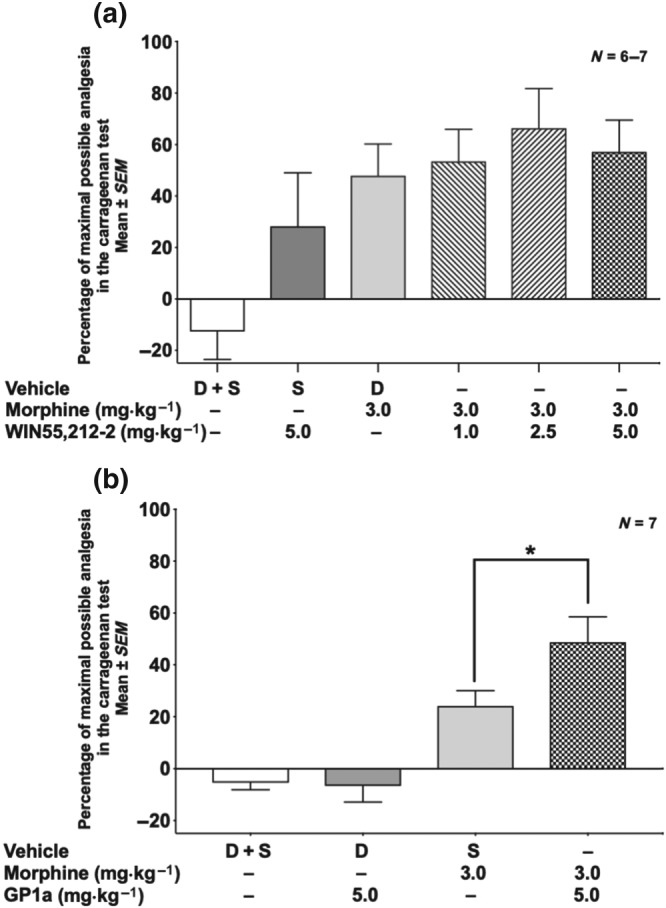
Effect of morphine, WIN, or GP1a alone, or in combination with morphine, in the rat carrageenan test. (a) Morphine plus WIN: percentage of maximal analgesia. Effect of the combination was not significant by one‐way ANOVA compared to morphine alone. (b) Morphine plus GP1a. *P < .05; significantly different as indicated; one‐way ANOVA with Sidak's multiple comparison test. D, DMSO; S, saline
3.4. Immune mediators, receptors, and other molecules
Preliminary data were obtained on the effect of opioid and cannabinoid treatment on production of inflammatory mediators using mRNA arrays. Data are displayed as heat maps. Figure 6a shows the results for the formalin test in mice, using pooled mRNA extracts of popliteal lymph nodes of two mice in each group. Animals in every group received a formalin injection in addition to the treatments noted at the bottom of each column. The left‐hand column represents the levels of mediators induced by the formalin injection alone plus saline and DMSO vehicles. Qualitatively, one can observe that many of the analytes were elevated (darker red colour). Morphine or WIN given alone each had a moderate suppressive effect on mediator expression. The morphine plus WIN combination dampened the chemokine/cytokine mRNA profile, as seen by the increase in the number of green bars between formalin alone (two bars) and the combination treatment (seven bars). The CB2 receptor antagonist, but not the CB1 receptor antagonist, returned the mediator intensity closer to that observed with formalin alone. Interestingly, the TRPV1 channel antagonist given with morphine and WIN resulted in highly depressed levels of mRNA for many mediators when compared to morphine plus WIN, even though it did not inhibit the analgesia induced by this combination. Figure 6b shows a similar analysis for the combination of morphine plus the CB2 receptor‐selective agonist, GP1a, on the average mRNA levels of tissue extracted from two individual rats in each treatment group. Carrageenan plus vehicles (left‐most column) induced a broad inflammatory response. The combination of morphine plus Gp1a in the carrageenan test was more suppressive than either morphine alone or GP1a alone.
Figure 6.
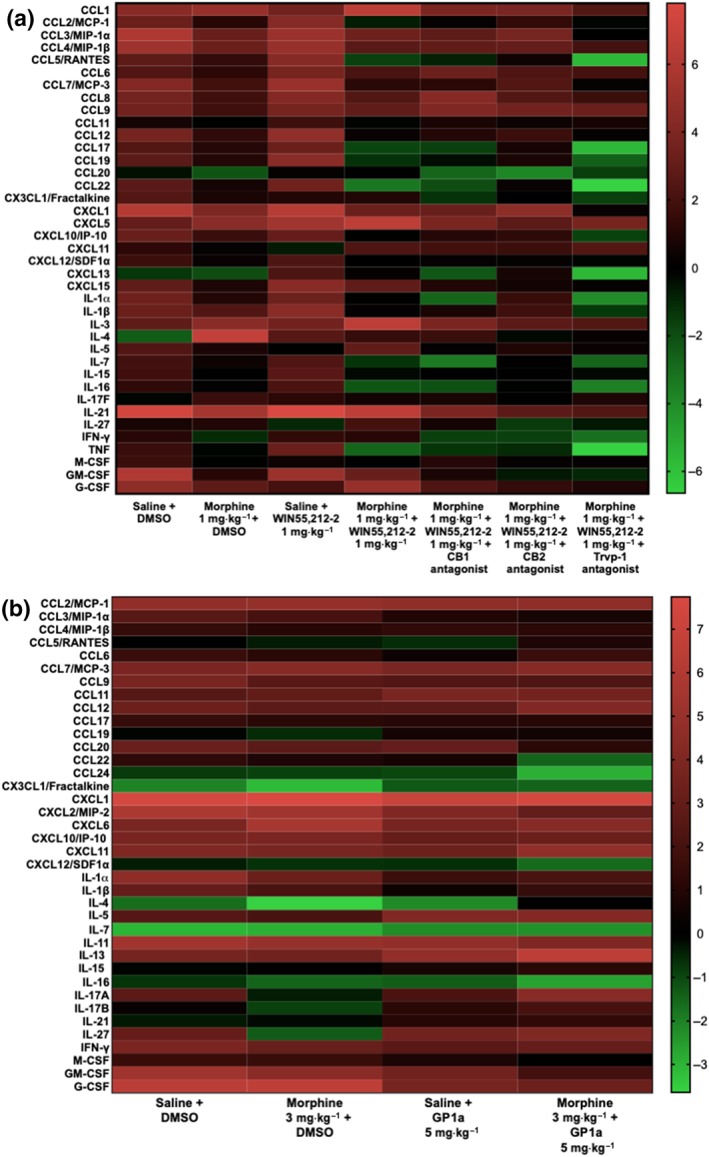
Change in mRNA expression in popliteal lymph nodes of mice receiving formalin or rats receiving carrageenan in the foot pad, with and without morphine or cannabinoid treatments. Heat maps show the changes in mRNA expression for panels of chemokines and cytokines, with or without treatments, compared with untreated, baseline popliteal lymph nodes. The scale is shown to the right of the graphs. Red indicates up‐regulation compared to baseline. Black represents no change. Green indicates down‐regulation of mediators. (a) Samples of tissue from mice injected with formalin into the foot pad and given different treatments. Each bar represents relative amounts of mRNA in a pooled extract of two mice per group. (b) Samples of tissue from rats injected with carrageenan into the foot pad and given different treatments. Each bar represents relative amounts of mRNA averaged from two arrays performed on samples from two different rats in each group
4. DISCUSSION AND CONCLUSIONS
Two different synthetic cannabinoid agonists, WIN and GP1a, were tested in combination with morphine in two different pain tests, the formalin assay and the carrageenan assay. Morphine, as expected, gave strong dose‐dependent analgesia in both tests (Figures 1a and 4a). WIN produced analgesia in the formalin test (Figure 1b) and when combined with morphine had a synergistic interaction in reducing pain (Figure 2a,b). Use of receptor‐selective antagonists, SR141716 (CB1), SR144528 (CB2), and SB366791 (TRPV1), showed that the synergistic interaction on the analgesic activity produced by WIN was mediated through CB1 receptors (Figure 2c). The other cannabinoid, GP1a, produced statistically significant analgesia in the formalin test at doses ranging between 1.0 and 50.0 mg·kg−1, without a clear dose–response effect in that range (Figure 1c). Combinations of three different doses of GP1a with two different doses of morphine did not result in additive or synergistic interactions in this assay (Figure 3a–c). In contrast, in the carrageenan assay, neither WIN nor GP1a produced analgesia when given alone over a range of doses (Figure 4b,c). Unlike the results with formalin, in the carrageenan test, WIN did not give statistically significant interactive effects when combined with morphine (Figure 5a), while the combination of GP1a and morphine resulted in a greater analgesic effect than with morphine alone at one dose combination (Figure 5b). Thus, the results for the combinations of the cannabinoids with morphine yielded opposite results in the two tests. These results are summarized in Table 1. Similarly, previous report from our laboratory (Inan et al., 2018) showed that combining sub‐analgesic doses of morphine with chemokine receptor antagonists could provide maximal analgesia in a rat model of incisional pain.
Table 1.
Summary of cannabinoid and opioid interactions in two pain assays
| Pain Assay | WIN alone | WIN + morphine | GP1a alone | GP1a + morphine |
|---|---|---|---|---|
| Formalin test | +a |
+ Synergyd |
+ |
−b
Sub‐additivec |
| Carrageenan test | − | − | − |
+ Enhanced analgesia |
+ means that it produced analgesia.
− means that there was no analgesia.
“Sub‐additive” means that the combination was not as efficacious as would be predicted from the sum of the individual drugs alone.
“Synergy” means that the effect of the combined drugs was greater than an additive effect of the two drugs. “Synergy” can also be called “a super additive effect.”
Much of the literature on interactions between opioids and cannabinoids relating to analgesia, in both preclinical models and clinical studies, has involved Δ9‐THC. In rhesus monkeys, the dose–response curves for morphine and other opioids (fentanyl, etorphine, and buprenorphine, but not nalbuphine) in the warm water tail‐flick test were significantly shifted leftward by Δ9‐THC and also by the synthetic cannabinoid, CP55,940 (Maguire & France, 2014). CP55,940, like Δ9‐THC and WIN, is active at both CB1 and CB2 receptors. Synergy was also found between Δ9‐THC and morphine administered intrathecally, intracerebroventricularly, subcutaneously, or orally in inducing analgesia measured using the tail‐flick test in mice (Smith, Cichewicz, Martin, & Welch, 1998; Welch & Stevens, 1992; Welch, Thomas, & Patrick, 1995). Antagonist studies showed that Δ9‐THC was acting via CB1 receptors (Smith et al., 1998). Experiments using genetic deletion of the peripheral CB1 receptors in mice showed that the analgesia mediated by WIN in an assay of neuropathic pain was mainly through these receptors (Agarwal et al., 2007). There is also a report that Δ9‐THC used in combination with morphine in arthritic rats has synergistic analgesic interactions that were mediated via CB2 receptors (Cox, Haller, & Welch, 2007a; Cox, Haller, & Welch, 2007b). The experiments carried out in the present studies using WIN, with and without antagonists selective for CB1 and CB2 receptors, show that in the mouse formalin test, WIN was active via CB1 receptors. The results reveal very strong synergy of WIN with non‐analgesic doses of morphine (0.1–1 mg·kg−1, Figure 2).
There is currently marked interest in the analgesic activity of Δ9‐THC, as marijuana has been legalized for medicinal purposes in 24 states and the District of Columbia, including approval for use for many conditions that have pain as a primary symptom. There are reports that availability of medical marijuana has decreased prescriptions for other FDA‐approved medications to treat pain (Bradford & Bradford, 2016). Δ9‐THC has the disadvantage of being psychoactive and potentially leading to tolerance and dependence. The non‐selective cannabinoid agonists such as WIN and CP55,940 also bind to CB1 receptors with the same potentially negative side effects. The development of selective CB2 receptor agonists raises the possibility of utilizing cannabinoids devoid of psychoactive activity as analgesics. In fact, CB2 receptor agonists given acutely or chronically have been reported to have analgesic activity against neuropathic pain in mice (Deng et al., 2015; Lin, Dhopeshwarkar, Huibregtse, Mackie, & Hohmann, 2018). Further, CB2 receptor knockout mice have enhanced nociception in a mouse model of sciatic nerve injury (Racz et al., 2008).
The current experiments show that GP1a has an increased analgesic effect with morphine in the carrageenan test in rats but did not enhance morphine analgesia in the formalin test in mice. GP1a was promoted as a selective CB2 receptor agonist and found active on these receptors in vivo (Franklin & Carrasco, 2013; Kong et al., 2014) until it was reported recently as an inverse agonist by Soethoudt et al. (2017). Also, in the same study, it was stated that GP1a showed partial inhibition (30–40%) of fatty acid amide hydrolase (FAAH), an enzyme that breaks down endogenous anandamide. Inhibition of this enzyme will result in an increase in endogenous anandamide levels. Recently, inhibitors of FAAH have been investigated as potential therapeutic targets for pain and CNS disorders (Ahn, Johnson, & Cravatt, 2009; Deutsch, 2016). Our results show that GP1a has analgesic activity on chronic/tonic pain by itself, as well as increasing morphine's effect on inflammatory pain. GP1a might have analgesic activity through enhancing endogenous anandamide levels by blocking FAAH. A recent study (Slivicki et al., 2018) shows synergistic effects of FAAH inhibitors on morphine‐induced analgesia against chemotherapy‐induced neuropathic pain in mice. Further, and critically, Slivicki et al. (2018) also reported that this synergy was not shown on morphine‐induced reduction of gastrointestinal transit. Yuill, Hale, Guindon, and Morgan (2017) noted an additive interaction between the CB2 receptor‐selective agonist, JWH‐133, and morphine in the mouse formalin test at a single dose ratio. If GP1a is working by increasing endogenous levels of anandamide, it is possible that its effect may not have been robust enough to produce synergism when combined with morphine in the formalin test but was sufficient to increase the analgesic effect at one dose in the carrageenan test. Lin et al. (2018) reported that a CB2 receptor agonist used chronically to treat paclitaxel‐induced neuropathy can block development of tolerance to the analgesic effects of morphine when it is given post‐cannabinoid treatment. In the present experiments, all of the drugs were given acutely, and the effect of drug combinations on analgesic activity was only evaluated in an acute time frame.
The present studies show that there are differing results for analgesia evoked by two cannabinoids, each acting on distinct receptors, and also by combinations of opioids and cannabinoids in different pain assays. These results point to the conclusion that there are divergent mechanisms involved in pain induction, transmission, or perception in the two assays. Formalin causes a biphasic response with a rapid onset of pain that lasts for 3–5 min, followed by a later phase starting 15–20 min after the formalin injection, which has an inflammatory component (Tjolsen et al., 1992). Injection of carrageenan into the foot pad results in pain and swelling that increases over a period of 4 hr, the endpoint when measurements were taken. Time‐course studies showed that pain persisted for at least 5 hr. Thus, the pain induced by carrageenan is long lasting and is accompanied by a clear inflammatory component, which is manifested as measurable, marked paw oedema. As GP1a and CB2 receptor‐selective agonists have been shown to have suppressive effects on the immune system (Eisenstein & Meissler, 2015), one would have predicted that this cannabinoid would have had efficacy in the carrageenan assay. In spite of a lack of activity by itself in the carrageenan assay, GP1a increased morphine's antinociceptive effect at one dose.
The immune assays were undertaken to determine if morphine alone, the cannabinoids alone, or the combination of the two classes of drugs would show suppressive effects on levels of inflammatory mediators that might correlate with reductions in pain. The results presented are preliminary as only a limited number of samples were tested. Results in Figure 6a represent the pooled mRNA extracted from two mice, and the results in Figure 6b represent the average of results from two arrays from two rats. From these preliminary data, it would appear that suboptimal analgesic levels of morphine alone in the formalin and carrageenan assays moderately suppressed mRNA for a broad panel of mediators induced by the corresponding painful insults, which is consonant with the literature showing that morphine is immunosuppressive (Eisenstein, Rahim, Feng, Thingalaya, & Meissler, 2006; Ninković & Roy, 2013). Overall, these preliminary data suggest that the combination treatment of morphine plus a cannabinoid was more effective in reducing inflammatory mediators compared to morphine alone or a cannabinoid alone in two different pain tests in two different rodent species, which was consonant with a greater analgesic effect of the combinations in these two assays. The assays would need to be repeated with more animals to reach a firm conclusion. The mechanisms underlying these observations will require further investigation. Welch (2009) proposed that additive or synergistic interactions between cannabinoid and opioid receptors might be due to release of endogenous opioids by cannabinoids, for which there is evidence, and possibly also through formation of opioid–cannabinoid receptor heterodimers (Rios, Gomes, & Devi, 2006).
In summary, the present studies demonstrate that synthetic cannabinoids, when combined with morphine, can exert synergistic or increased analgesic effects in rodents in two pain models. These results provide a rationale for the use of such drug combinations in the treatment of pain. In a small clinical study, Abrams, Couey, Shade, Kelly, and Benowitz (2011) reported that vaporized cannabis enhanced analgesia in chronic pain patients on sustained‐release morphine or oxycodone. Combinations of cannabinoids with opioids may be an effective way of reducing opioid doses, with the potential for reducing opioid‐induced side effects and subsequent opioid physical dependence.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
X.C., M.W.A., T.K.E., A.C., S.M.R., S.I., and E.B.G. contributed to the design of the experiments. X.C., S. I., and M.N.W. performed the in vivo experiments. J.J.M. performed the RT‐PCR arrays. R.J.T. and C.S.T. performed the data analysis. X.C., S.I., T.K.E., A.C., S.M.R., J.J.M., and E.B.G contributed to the writing of the manuscript. All authors reviewed the final draft of the manuscript.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, and Animal Experimentation, and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
Supporting information
Figure S1.
Left paw edema for morphine (panel A), WIN55,212‐2 (panel B) and GP1a (panel C). Differences in paw volumes between left paws prior to injection with carrageenan and post carrageenan, using vehicle or various doses of compounds. Differences not significant by one‐vehicle. D = DMSO.
Figure S2.
Left paw edema for morphine plus cannabinoid combinations in the rat carrageenan test. Panel A: Morphine plus WIN55,212‐2. Panel B: Morphine plus GP1a. Differenced not significant by one‐way ANOVA followed by Sidak's multiple comparison test compared to vehicle. D = DMSO; S = saline.
ACKNOWLEDGEMENTS
This work was supported by a CURE grant from the Pennsylvania Department of Health and by National Institute on Drug Abuse P30 Grant DA013429.
Chen X, Cowan A, Inan S, et al. Opioid‐sparing effects of cannabinoids on morphine analgesia: participation of CB1 and CB2 receptors. Br J Pharmacol. 2019;176:3378–3389. 10.1111/bph.14769
REFERENCES
- Abrams, D. I. , Couey, P. , Shade, S. B. , Kelly, M. E. , & Benowitz, N. L. (2011). Cannabinoid‐opioid interaction in chronic pain. Clinical Pharmacology & Therapeuticsc, 90, 844–851. 10.1038/clpt.2011.188 [DOI] [PubMed] [Google Scholar]
- Agarwal, N. , Pacher, P. , Tegeder, I. , Amaya, F. , Constantin, C. E. , Brenner, G. J. , … Kuner, R. (2007). Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nature Neuroscience, 10, 870–879. 10.1038/nn1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, K. , Johnson, D. S. , & Cravatt, B. F. (2009). Fatty acid amide hydrolase as a potential therapeutic target for the treatment of pain and CNS disorders. Expert Opinion on Drug Discovery, 4, 763–784. 10.1517/17460440903018857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrnsbrak, R. , Bose, J. , Hedden, S. L. , Lipari, R. N. , & Park‐Lee, E. (2017). Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health (NSDUH). Washington, D.C.: Substance Abuse and Mental Health Services Administration, DHHS. [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Striessnig, J. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. British Journal of Pharmacology, 174, S160–S194. 10.1111/bph.13884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford, A. C. , & Bradford, W. D. (2016). Medical marijuana laws reduce prescription medication use in Medicare Part D. Health Affairs, 35, 1230–1236. 10.1377/hlthaff.2015.1661 [DOI] [PubMed] [Google Scholar]
- Brownjohn, P. W. , & Ashton, J. C. (2012). Spinal cannabinoid CB2 receptors as a target for neuropathic pain: An investigation using chronic constriction injury. Neuroscience, 203, 180–193. 10.1016/j.neuroscience.2011.12.028 [DOI] [PubMed] [Google Scholar]
- Carlisle, S. J. , Marciano‐Cabral, F. , Staab, A. , Ludwick, C. , & Cabral, G. A. (2002). Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage‐like cells in relation to cell activation. International Immunopharmacology, 2, 69–82. 10.1016/S1567-5769(01)00147-3 [DOI] [PubMed] [Google Scholar]
- Cichewicz, D. L. (2004). Synergistic interactions between cannabinoid and opioid analgsics. Life Sciences, 74, 1317–1324. 10.1016/j.lfs.2003.09.038 [DOI] [PubMed] [Google Scholar]
- Cox, M. L. , Haller, V. L. , & Welch, S. P. (2007a). The antinociceptive effect of Δ9‐THC in the arthritic rat involves the CB2 cannabinoid receptor. European Journal of Pharmacology, 570, 50–56. 10.1016/j.ejphar.2007.05.024 [DOI] [PubMed] [Google Scholar]
- Cox, M. L. , Haller, V. L. , & Welch, S. P. (2007b). Synergy between Δ9‐tetrahydrocannabinol and morphine in the arthritic rat. European Journal of Pharmacology, 567, 125–130. 10.1016/j.ejphar.2007.04.010 [DOI] [PubMed] [Google Scholar]
- Craft, R. M. , Kandasamy, R. , & Davis, S. M. (2013). Sex differences in anti‐allodynic, anti‐hyperalgesic and anti‐edema effects of Δ9‐tetrahydrocannabinol in the rat. Pain, 154, 1709–1717. 10.1016/j.pain.2013.05.017 [DOI] [PubMed] [Google Scholar]
- Daaka, Y. , Friedman, H. , & Klein, T. W. (1996). Cannabinoid receptor proteins are increased in jurkat, human T‐cell line after mitogen activation. The Journal of Pharmacology and Experimental Therapeutics, 276, 776–783. [PubMed] [Google Scholar]
- Deng, L. , Guindon, J. , Cornett, B. L. , Makriyannis, A. , Mackie, K. , & Hohmann, A. G. (2015). Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1‐dependent withdrawal. Biological Psychiatry, 77, 475–487. 10.1016/j.biopsych.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch, D. G. (2016). A personel retrospective: Elevating anandamide (AEA) by targeting fatty acid amid hydrolase (FAAH) and the fatty acid binding proteins (FABPs). Frontiers in Pharmacology, 7, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein, T. K. , & Meissler, J. J. (2015). Effects of cannabinoids on T‐cell function and resistance to infection. Journal of Neuroimmune Pharmacology, 10, 204–216. 10.1007/s11481-015-9603-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein, T. K. , Rahim, R. T. , Feng, P. , Thingalaya, N. K. , & Meissler, J. J. (2006). Effects of opioid tolerance and withdrawal on the immune system. Journal of Neuroimmune Pharmacology, 1, 237–249. 10.1007/s11481-006-9019-1 [DOI] [PubMed] [Google Scholar]
- Elikottil, J. , Gupta, P. , & Gupta, K. (2009). The analgesic potential of cannabinoids. Journal of Opioid Management, 5, 341–357. [PMC free article] [PubMed] [Google Scholar]
- Franklin, J. M. , & Carrasco, G. A. (2013). Cannabinoid receptor agonists upregulate and enhance serotonin 2A (5‐HT2A) receptor activity via ERK 1/2 signaling. Synapse, 67, 145–159. 10.1002/syn.21626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiegue, S. , Mary, S. , Marchand, J. , Dussossoy, D. , Carriere, D. , Carayon, P. , … Casellas, P. (1995). Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. European Journal of Biochemistry, 232, 54–61. 10.1111/j.1432-1033.1995.tb20780.x [DOI] [PubMed] [Google Scholar]
- Giraudon, I. , Lowitz, K. , Dargan, P. I. , Wood, D. M. , & Dart, R. C. (2013). Prescription opiioid abuse in the U.K. British Journal of Clinical Pharmacology, 76, 823–824. 10.1111/bcp.12133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, J. P. , Onaivi, E. S. , Ishiguro, H. , Liu, Q. R. , Tagliaferro, P. A. , Brusco, A. , & Uhl, G. R. (2006). Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Research, 1071, 10–23. 10.1016/j.brainres.2005.11.035 [DOI] [PubMed] [Google Scholar]
- Grenald, S. A. , Young, M. A. , Wang, Y. , Ossipov, M. H. , Ibrahim, M. M. , Largent‐Milnes, T. M. , & Vanderah, T. W. (2017). Synergistic attenuation of chronic pain using mu opioid and cannabinoid receptor 2 agonists. Neuropharmacology, 116, 59–70. 10.1016/j.neuropharm.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon, J. , & Hohmann, A. G. (2008). Cannabinoid CB2 receptors: A therapeutic target for the treatment of inflammatory and neuropathic pain. British Journal of Pharmacology, 153, 319–334. 10.1038/sj.bjp.0707531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez, T. , Crystal, J. D. , Zvonok, A. M. , Makriyannis, A. , & Hohmann, A. G. (2011). Self‐medication of a cannabinoid CB2 agonist in an animal model of neuropathic pain. Pain, 152, 1976–1987. 10.1016/j.pain.2011.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham, M. , Lynn, A. B. , Johnson, M. R. , Melvin, L. S. , de Costa, B. R. , & Rice, K. C. (1991). Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. Journal of Neuroscience, 11, 563–583. 10.1523/JNEUROSCI.11-02-00563.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan, S. , Eisenstein, T. K. , Watson, M. N. , Doura, M. , Meissler, J. J. , Tallarida, C. S. , … Adler, M. W. (2018). Coadministration of chemokine receptor antagonists with morphine potentiates morphine's analgesic effect on incisional pain in rats. The Journal of Pharmacology and Experimental Therapeutics, 367, 433–441. 10.1124/jpet.118.252890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasabova, I. A. , Gielissen, J. , Chandiramani, A. , Harding‐Rose, C. , Odeh, D. A. , Simone, D. A. , & Seybold, V. S. (2011). CB1 and CB2 receptor agonists promote analgesia through synergy in a murine model of tumor pain. Behavioural Pharmacology, 22(5‐6), 607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey, S. G. , Mahadevan, A. , Zhao, B. , Sun, H. , Naidu, P. S. , Razdan, R. K. , … Lichtman, A. H. (2011). The CB2 cannabinoid receptor‐selective agonist O‐3223 reduces pain and inflammation without apparent cannabinoid behavior effects. Neuropharmacology, 60, 244–251. 10.1016/j.neuropharm.2010.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, T. W. (2005). Cannabinoid‐based drugs as anti‐inflammatory therapeutics. Nature Reviews Immunology, 5, 400–411. 10.1038/nri1602 [DOI] [PubMed] [Google Scholar]
- Kocher, L. , Anton, F. , Reeh, P. W. , & Handwerker, H. O. (1987). The effect of carrageenan‐induced inflammation on the sensitivity of unmyelinated skin nociceptors in the rats. Pain, 29, 363–373. 10.1016/0304-3959(87)90051-0 [DOI] [PubMed] [Google Scholar]
- Kong, W. , Li, H. , Tuma, R. F. , & Ganea, D. (2014). Selective CB2 receptor activation ameliorates EAE by reducing Th17 differentiation and immune cell accumulation in the CNS. Cellular Immunology, 287, 1–17. 10.1016/j.cellimm.2013.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, X. , Dhopeshwarkar, A. S. , Huibregtse, M. , Mackie, K. , & Hohmann, A. G. (2018). Slowly signalling G protein‐biased CB2 cannabinoid receptor agonist LY2828360 suppresses neuropathic pain with sustained efficacy and attenuates morphine tolerance and dependence. Molecular Pharmacology, 93, 49–62. 10.1124/mol.117.109355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowin, T. , Pongratz, G. , & Straub, R. H. (2016). The synthetic cannabinoid WIN55,212‐2 mesylate decreases the production of inflammatory mediators in rheumatoid arthritis synovial fibroblasts by activating CB2, TRPV1, TRPA1 and yet unidentified receptor targets. Journal of Inflammation, 13, 15 10.1186/s12950-016-0114-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire, D. R. , & France, C. P. (2014). Impact of efficacy at the μ‐opioid receptor on antinociceptive effects of combinations of μ‐opioid receptor agonists. The Journal of Pharmacology and Experimental Therapeutics, 351, 383–389. 10.1124/jpet.114.216648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, W. J. , Coffin, P. O. , Attias, E. , Balinsky, M. , Tsou, K. , & Walker, J. M. (1999). Anatomical basis for cannabinoid‐induced antinociception as revealed by intracerebral microinjections. Brain Research, 822, 237–242. 10.1016/S0006-8993(98)01368-7 [DOI] [PubMed] [Google Scholar]
- Matsuda, L. A. , Lolait, S. J. , Brownstein, M. J. , Young, A. C. , & Bonner, T. I. (1990). Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature, 346, 561–564. 10.1038/346561a0 [DOI] [PubMed] [Google Scholar]
- Munro, S. , Thomas, K. K. , & Abu‐Shaar, M. (1993). Molecular characterization of a peripheral receptor for cannabinoids. Nature, 365, 61–65. 10.1038/365061a0 [DOI] [PubMed] [Google Scholar]
- Murray, C. W. , Porreca, F. , & Cowan, A. (1988). Methodological refinements to the mouse paw formalin test. An animal model of tonic pain. Journal of Pharmacological Methods, 20, 175–186. [DOI] [PubMed] [Google Scholar]
- Nielsen, S. , Sabioni, P. , Trigo, J. M. , Ware, M. A. , Betz‐Stablein, B. D. , Murnion, B. , … le Foll, B. (2017). Opioid‐sparing effects of cannabinoids: A systematic review and meta‐analysis. Neuropsychopharmacology, 42, 1752–1765. 10.1038/npp.2017.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninković, J. , & Roy, S. (2013). Role of the mu‐opioid receptor in opioid modulation of immune function. Amino Acids, 45, 9–24. 10.1007/s00726-011-1163-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee, R. G. (2001). Cannabinoid receptors and pain. Progress in Neurobiology, 63, 569–611. 10.1016/S0301-0082(00)00031-9 [DOI] [PubMed] [Google Scholar]
- Racz, I. , Nadal, X. , Alferink, J. , Baños, J. E. , Rehnelt, J. , Martin, M. , … Maldonado, R. (2008). Crucial role of CB2 cannabinoid receptor in the regulation of central immune responses during neuropathic pain. The Journal of Neuroscience, 28, 12125–12135. 10.1523/JNEUROSCI.3400-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios, C. , Gomes, I. , & Devi, L. A. (2006). Opioid and CB1 cannabinoid receptor interactions: Reciprocal inhibition of receptor signaling and neuritogenesis. British Journal of Pharmacology, 148, 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slivicki, R. A. , Saberi, S. A. , Iyer, V. , Vemuri, K. , Makriyannis, A. , & Hohmann, A. G. (2018). Brain permeant and impermeant inhibitors of fatty‐acid amide hydrolase synergize with the opioid analgesic morphine to suppress chemotherapy‐induced neuropathic nociception without enhancing effects of morphine on gastrointestinal transit. The Journal of Pharmacology and Experimental Therapeutics, 367, 551–563. 10.1124/jpet.118.252288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, F. L. , Cichewicz, D. L. , Martin, Z. L. , & Welch, S. P. (1998). The enhancement of morphine antinocieption in mice by Δ9‐tetrahydrocannabinol. Pharmacology, Biochemistry, and Behavior, 60, 559–566. 10.1016/S0091-3057(98)00012-4 [DOI] [PubMed] [Google Scholar]
- Soethoudt, M. , Grether, U. , Fingerle, J. , Grim, T. W. , Fezza, F. , de Petrocellis, L. , … van der Stelt, M. (2017). Cannabinoid CB2 receptor ligand profiling reveals biased signaling and off‐target activity. Nature Communications, 8, 13958 10.1038/ncomms13958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida, R. J. (2006). An overview of drug combination analysis with isobolograms. The Journal of Pharmacology and Experimental Therapeutics, 319, 1–7. 10.1124/jpet.106.104117 [DOI] [PubMed] [Google Scholar]
- Thapa, D. , Cairns, E. A. , Szczesniac, A. M. , Toguri, J. T. , Caldwell, M. D. , & Kelly, M. E. M. (2018). The cannabinoids Δ8 THC, CBD, and HU‐308 act via distinct receptors to reduce corneal pain and inflammation. Cannabis and Cannabinoid Research, 3, 11–20. 10.1089/can.2017.0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjolsen, A. , Berge, O. , Hunskaar, S. , Rosland, J. H. , & Hole, K. (1992). The formalin test: An evaluation of the method. Pain, 51, 5–17. 10.1016/0304-3959(92)90003-T [DOI] [PubMed] [Google Scholar]
- Van Sickle, M. D. , Duncan, M. , Kingsley, P. J. , Mouihate, A. , Urbani, P. , Mackie, K. , … Sharkey, K. A. (2005). Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science, 3100, 329–332. [DOI] [PubMed] [Google Scholar]
- Volkow, N. D. , & McLellan, A. T. (2016). Opioid abuse in chronic pain—Misconceptions and mitigation strategies. NEJM, 374, 1253–1263. 10.1056/NEJMra1507771 [DOI] [PubMed] [Google Scholar]
- Volkow, N. D. , McLellan, A. T. , Cotto, J. H. , Karithanom, M. , & Weiss, S. R. (2011). Characteristics of opioid prescriptions in 2009. JAMA, 305, 1299–1301. 10.1001/jama.2011.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, J. M. , & Hohmann, A. G. (2005). Cannabinoid mechanisms of pain suppression. Handbook of Experimental Pharmacology, 168, 509–554. 10.1007/3-540-26573-2_17 [DOI] [PubMed] [Google Scholar]
- Welch, S. P. (2009). Interaction of the cannabinoid and opioid systems in the modulation of nociception. International Review of Psychiatry, 21, 143–151. 10.1080/09540260902782794 [DOI] [PubMed] [Google Scholar]
- Welch, S. P. , & Stevens, D. L. (1992). Antinociceptive activity of intrathecally administered cannabinoids alone, and in combination with morphine, in mice. Journal of Pharmacology and Experimental Therapeutics, 262, 10–18. [PubMed] [Google Scholar]
- Welch, S. P. , Thomas, C. , & Patrick, G. S. (1995). Modulation of cannabinoid‐induced antinociception after intracerebroventricular versus intrathecal administration to mice: Possible mechanisms for interaction with morphine. The Journal of Pharmacology and Experimental Therapeutics, 272, 310–321. [PubMed] [Google Scholar]
- Woodhams, S. G. , Chapman, V. , Finn, D. P. , Hohmann, A. G. , & Neugebauer, V. (2017). The cannabinoid system and pain. Neuropharmacology, 124, 105–120. 10.1016/j.neuropharm.2017.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuill, M. B. , Hale, D. E. , Guindon, J. , & Morgan, D. J. (2017). Anti‐nociceptive interactions between opioids and a cannabinoid receptor 2 agonist in inflammatory pain. Molecular Pain, 13, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Left paw edema for morphine (panel A), WIN55,212‐2 (panel B) and GP1a (panel C). Differences in paw volumes between left paws prior to injection with carrageenan and post carrageenan, using vehicle or various doses of compounds. Differences not significant by one‐vehicle. D = DMSO.
Figure S2.
Left paw edema for morphine plus cannabinoid combinations in the rat carrageenan test. Panel A: Morphine plus WIN55,212‐2. Panel B: Morphine plus GP1a. Differenced not significant by one‐way ANOVA followed by Sidak's multiple comparison test compared to vehicle. D = DMSO; S = saline.


