Abstract
Background
Axillary web syndrome (AWS) can develop following breast cancer surgery and presents as a tight band of tissue in the axilla with shoulder abduction.
Objective
The objectives were to determine the prevalence and natural history of AWS and the association between AWS and function, range of motion, pain, lymphedema, and body mass index (BMI).
Design
This study was a longitudinal prospective cohort study utilizing a repeated measures design.
Methods
Axillary web syndrome, function, shoulder range of motion, pain, and lymphedema (using circumference, bioimpedance spectroscopy, tissue dielectric constant) were assessed in women at 2, 4, and 12 weeks and 18 months following breast cancer surgery. Prevalence of AWS and the association with the measured outcomes were analyzed.
Results
Thirty-six women agreed to participate in the study. The cumulative prevalence of AWS was 50% (18/36) at 18 months following breast cancer surgery. AWS was identified as a risk factor for reduced function. Women with AWS had statistically reduced range of motion, lower BMI, and higher number of lymph nodes removed compared to the non-AWS group. Forty-one percent (13/32) of women had AWS at 18 months. AWS reoccurred in 6 women following resolution, and a new case developed beyond the early postoperative period. The overall prevalence of physical impairments ranged from 66% to 97% within the first 18 months following surgery regardless of AWS.
Limitations
Limitations include a small sample size and potential treatment effect.
Conclusion
AWS occurs in approximately 50% of women following breast cancer surgery. It can persist for 18 months and potentially longer, develop beyond the early postoperative time period, and reoccur after resolution. Clinicians need to be aware of the chronicity of AWS and its association with reduced range of motion and function.
Treatment for breast cancer can lead to long-term complications such as reduced function, reduced range of motion (ROM), pain, and lymphedema.1–3 Axillary web syndrome (AWS), or cording, is another complication that often develops within weeks following breast cancer surgery and may present as one or more visible or palpable tight cords of tissue in the axilla (Fig. 1). The cords can also extend down to the medial aspect of the upper extremity as far as the base of the thumb and may also extend down along the lateral chest wall.4–6 The incidence of AWS ranges from 6% to 86% depending on the length and frequency of follow-up, the thoroughness of the postoperative physical exam of the axilla, and the extent of surgery.4–8 Women with more invasive surgery, such as more extensive axillary lymph node dissection (ALND) or contralateral prophylactic mastectomy (CPM), have a higher incidence of AWS.6 Women who developed AWS had statistically lower BMI or slimmer body for reasons unknown.5–8
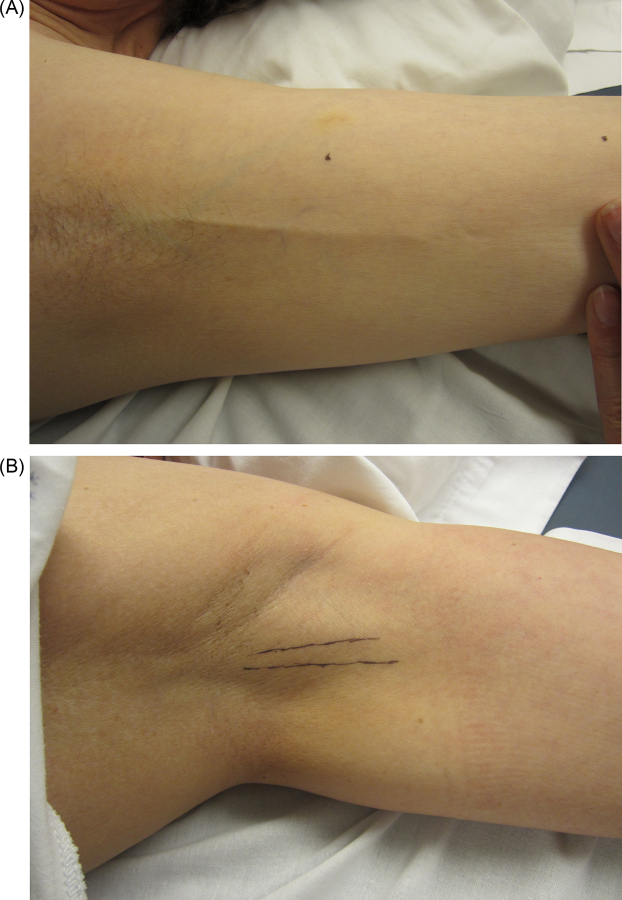
Figure 1.
Axillary web syndrome of the left axilla in two women 18 months following breast cancer surgery. (a) Visible cord indicated by arrow. (b) Palpable cords with lines drawn on the skin marking the location of the cords.
The most common accepted mechanism underlying the development of AWS involves iatrogenic damage to the lymphatic and/or venous system, resulting in local stasis, hypercoagulation, and inflammation of the involved superficial vein or lymphatic vessel.4,5 It has been suggested that AWS is a variant of Mondor disease, which is thrombophlebitis of a subcutaneous vein.4 Recent imaging studies on AWS disproved the thrombophlebitis hypothesis.9,10 Although the etiology and pathophysiology of AWS remains unknown, there is a growing body of evidence that appears to support the hypothesis that AWS results from a local pathophysiological injury to the lymphatic system.9–11
AWS was once thought to be a self-limiting condition, which resolved spontaneously by 3 months after surgery.4,5 We disproved this concept in a previous investigation which demonstrated that AWS persisted at 12 weeks following breast cancer surgery in 59% (10/17) of women who developed AWS.6 This study also demonstrated that early postoperative decrease in shoulder ROM was significantly worse in individuals with AWS, and that women with lower BMI were at higher risk of AWS.6 In the current study, we extended the follow-up to 18 months to determine the “medium to long” –term prevalence and natural history of AWS, and the association of AWS with ipsilateral extremity function, ROM, pain, and lymphedema. We hypothesized new cases of AWS would develop after 12 weeks, and AWS would persist in women beyond one year following breast cancer surgery. In addition, we hypothesized AWS would be a risk factor for reduced function, reduced ROM, pain, and lymphedema, and women with AWS would have a lower BMI than women without AWS.
Methods
Design
This prospective repeated measures cohort study tracked women for 18 months (±6 months) following breast cancer surgery with visits at 2, 4, and 12 weeks and 18 months after surgery. Figure 2 displays the study flowchart. The University of Minnesota Internal Review Board approved the study, including the additional follow-up visit. Eligible women undergoing breast cancer surgery were initially recruited from the University of Minnesota Breast Center between September 2011 and April 2012 by the primary investigator (LAK). The primary investigator (PI) was directly located in the clinic and allowed to recruit eligible participants at the time of their preoperative or postoperative visit with the breast surgeon. Recruitment was complete once the powered sample size of 36 women was attained. Participants provided written consent at the first postoperative study visit. For the 18-month follow-up visit, the women were contacted by phone and asked permission to participate in an additional follow-up visit. The women provided written re-consent at the 18-month visit.
Figure 2.
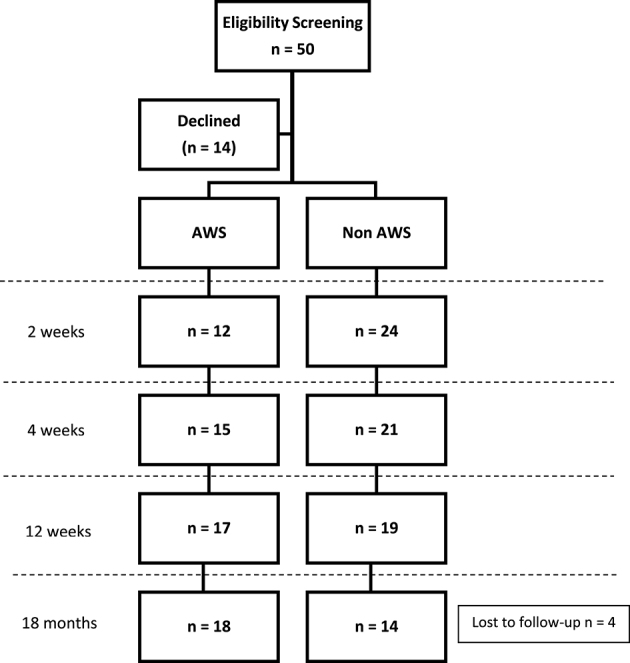
Flow diagram of the longitudinal study of axillary web syndrome (AWS) with the 18-month period prevalence.
Participants
Inclusion criteria consisted of women having undergone early stage surgical breast cancer treatment (lumpectomy or mastectomy) with a minimum removal of one axillary lymph node by sentinel node biopsy. Women with prophylactic contralateral mastectomy were allowed to participate. Women with previous surgical treatment for breast cancer or synchronous bilateral breast cancer, or a previous history of shoulder surgery, shoulder dysfunction, or history of upper extremity deep vein thrombosis, were excluded. Eligible consecutive participants undergoing surgery at the University of Minnesota Breast Center during the time of recruitment were invited to participate in the study.
Clinical Outcome Measures
This study incorporated examination of function, shoulder ROM, pain, and lymphedema at each study visit. The primary investigator, a physical therapist and certified lymphedema therapist with over 15 years of experience working with women with breast cancer, performed all of the examinations for this study.
ROM
Bilateral upper extremity active ROM for shoulder flexion and abduction were assessed in the standing position using a standard goniometric method.12 Bilateral passive shoulder flexion and abduction ROM was taken in the supine position. Special attention was given to maintain full elbow extension during the movements to prevent individuals from flexing the elbow, which could potentially take tension off the cords, if present. ROM difference of 10 or more degrees between the ipsilateral and contralateral extremity in either shoulder flexion or abduction indicated reduced ROM based on previous research.1,13
Lymphedema
Lymphedema assessment involved taking upper extremity circumference, bioimpedance spectroscopy (BIS), and trunk tissue dielectric constant measurements. Circumferential measurements were taken bilaterally at 8 cm increments starting at the ulnar styloid using a nonstretch, flexible tape measure with a tension gauge. The affected side was compared to the non-affected side by calculating volume difference. Volume was calculated using the truncated cone formula V = h/12π (C12 + C1C2 + C22).14 BIS (Dex U400, Impedimed, San Diego, California) utilized electrical tissue resistance to determine the amount of extracellular fluid volume in the upper extremities. The BIS device displayed the measurement as a lymphedema index (L-Dex) score, with normal values ranging from –10 to 10. The following criteria were indicative of upper extremity lymphedema: more than 10% difference in volume, >200 mL difference in volume, >2cm difference at any site on the upper extremity using circumferential measurements,15,16 or an L-Dex value over 10.17 Trunk local tissue water (LTW) was taken using the MoisturemeterD (Delfin Technologies) at a location 8 cm below the axillary fold bilaterally. The MoisturemeterD measures the amount of water in the tissue based on the tissue electric properties and displays the results as a number called the tissue dielectric constant (TDC). TDC has been described in previous literature.6,18 A TDC ratio was calculated using the formula TDC affected/TDC unaffected. A TDC ratio >1.2 and/or subjective confirmation by visual inspection was indicative of trunk lymphedema.19–21 “Lymphedema impairment” was diagnosed if individuals met one of the above criteria for upper extremity or the trunk lymphedema criteria.
Pain
The visual analog pain scale (VAS) provided quantifiable, reliable measurements of pain ranging from 0 to 100, with 0 reflecting no pain and 100 indicating worst pain possible.22 A VAS score of 3 or more indicated a pain induced “physical impairment,” based on previous research which indicated that pain scores of less than 3 have little effect on quality of life.23,24
Function
The Disabilities of the Arm, Shoulder, and Hand (DASH) self-report questionnaire measured upper extremity functional disability and has been validated in individuals with breast cancer.25 The questionnaire consists of 30 questions ranked on a scale from 1 (no difficulty) to 5 (unable to perform activity). The DASH scores are then calculated using the formula [(Sum of n responses)–1]/n*24 = DASH disability/symptom score. The score ranges from 0 to 100, with 0 indicating no disability/symptoms and higher scores reflecting an increase in disability/symptoms. A score of more than 10.1 indicated reduced function. This is based on a comparison to the general population DASH score.26 This is a conservative cutoff, considering that healthy women of approximately the same age as women in our study have a median DASH score of 1.627 and women with breast cancer have a median DASH score of 7.28
AWS assessment
Assessment for AWS was performed during each study visit by the primary investigator of the study. Women were determined to have AWS if there was a visible or palpable cord of tissue in the axilla, upper extremity, or trunk region during maximal shoulder abduction at any of their scheduled visits. Women were assessed for AWS at the end of their scheduled visit after the outcome measures were taken to reduce bias.
This was a nonintervention study, although written and verbal lymphedema education consisting of lymphedema precautions, signs and symptoms, and description of treatment was provided by the PI at 12 weeks following surgery. Participants followed the normal plan of care as determined by their health care providers and were referred for rehabilitation intervention only if the attending health care team initiated a referral.
Data Analysis
Participant characteristics were analyzed using Chi Squared test for categorical variables and a two-sample t-test for continuous variables. For group analysis, women were categorized into the AWS group if the participant had AWS at any of the scheduled visits regardless of what visit the participant had AWS. A repeated measures analysis of variance (ANOVA) compared differences between groups (AWS/Non-AWS) on the clinical outcome measures (shoulder ROM, function, pain, lymphedema, anxiety, depression) at each time point (2, 4, 12 weeks and 18 months) using a significance level of .05. If there was an interaction effect, a Tukey-Kramer adjustment was utilized to determine a visit effect per group or a group effect per visit. Women lost to follow-up were included in the analysis. Descriptive statistics were used to calculate the prevalence of AWS and physical impairments (functional impairment, reduced ROM, pain, and lymphedema) at each time point. The number of women who received rehabilitation was descriptively compared to the number of women identified with a physical impairment. Univariate logistic regression determined if AWS was a risk factor for functional impairments, ROM impairments, pain, and lymphedema at 18 months using a more lenient P value of .10, so as not to exclude a potentially important variable considering the low sample size. A calculated sample size of 36 was based on finding a difference of 20 in DASH scores between groups, which provided .95 power at a .5 significance level. Women lost to follow-up at 18 months were included in the participant characteristic analysis, and complete case analysis was used for the remaining analysis. A sample size of 32 (18 in the AWS group and 14 in the non-AWS group) provided 80% power at the .05 level of significance (2-sided) to detect a difference of 8.9 in the DASH score with a SD of 8.5. NCSS statistical program version 9.0 (NCSS, Kaysville, Utah) was used for statistical analysis.
Role of the Funding Source
This project was supported in part by the University of Minnesota Foundation's Doctoral Dissertation Fellowship (ref. no. 4072-9201-11) and the National Cancer Institute (ref. no. P30 CA77598), utilizing the Masonic Cancer Center and University of Minnesota share resources. The funders played no role in conducting this study.
Results
Fifty women were eligible for the study, with 14 declining participation because they received follow-up treatment at an outside facility. The target sample size of 36 women were successfully recruited and consented to participate. There was 100% participation for the first 3 visits of the study (2, 4, and 12 weeks). At 18 months, 4 individuals dropped out secondary to 1 relocating, 1 death, and 2 who did not return a phone call when contacted. This was an 11% dropout, resulting in 32 participants at 18 months. Prevalence of AWS is displayed in Figure 2. Demographic characteristics are presented in Table 1. Women with AWS had statistically lower BMI and a higher number of lymph nodes removed.
Table 1.
Participant Characteristics of the Study Cohorta at 12 Weeks Postsurgery.
| Characteristic | AWS group n = 18 (50%) | Non-AWS group n = 18 (50%) | Total n = 36 (100%) | Pb |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Breast surgery | .08 | |||
| Lumpectomy | 7 (39) | 11(61) | 18 (50) | |
| Mastectomy | 5 (45) | 6 (33) | 11 (31) | |
| Contralateral prophylactic mastectomy | 6 (33) | 1 (6) | 7 (19) | |
| Axillary surgery | .22 | |||
| SNB | 12 (67) | 16 (89) | 28 (78) | |
| ALND | 6 (33) | 2 (11) | 8 (22) | |
| Radiation | 1.00 | |||
| Yes | 11 (61) | 11 (61) | 22 (61) | |
| No | 7 (39) | 7 (39) | 14 (39) | |
| Chemotherapy | .74 | |||
| Yes | 9 (50) | 7 (39) | 16 (44) | |
| No | 9 (50) | 11 (61) | 20 (55) | |
| Reconstruction | .69 | |||
| Yes | 5 (28) | 3 (17) | 8 (22) | |
| No | 13 (72) | 15 (83) | 28 (78) | |
| Age at diagnosis (y) (SD) | 54 (10) | 59 (9) | 56 | .11 |
| Range | 35–73 | 40–69 | 35–73 | |
| BMI (kg/m2) (SD) | 25.1 (3.8) | 28.8 (7.3) | 27 | .05c |
| Range | 20–34 | 18–45 | 18–45 | |
| No. of LN removed (SD) | 8 (9) | 3 (4) | 5 | .04c |
| Range | 1–32 | 1–14 | 1–32 |
aValues are numbers (percentages) unless otherwise indicated. ALND = axillary lymph node dissection, BMI = body mass index, LN = lymph node, SNB = sentinel node biopsy.
bChi2 and t-test P values.
cSignificance level: P ≤ .05.
The period prevalence of AWS was 33%, 42%, 47%, and 50% at 2, 4, and 12 weeks and 18 months, respectively. Thirteen women (41%) had AWS at 18 months. A new case of AWS was discovered at 18 months that was not present at previous visits. AWS returned in 6 women at 18 months following no identifiable signs of AWS at 12 weeks. Six individuals had AWS present at all 4 visits. One woman with AWS that resolved by 12 weeks reported that she experienced recurrence of AWS cords on 2 separate occasions approximately 1 year postoperatively (confirmed by photos taken by the individual). She did not have signs of AWS at 18 months. The 18-month prevalence of AWS in women with a lumpectomy was 38%, mastectomy 45%, and bilateral mastectomy 86%. Women with sentinel node biopsy (SNB) had a 43% prevalence, and those with axillary lymph node dissection (ALND) had a 75% prevalence. Visible cords were detected in 48% of women. Fifty-two percent of the women had cords that were palpable but not visible, requiring careful palpation of the axilla in the abducted position for detection (Fig. 1).
Clinical Outcome Measures
Table 2 displays the comparison of function, ROM, pain, and lymphedema between the AWS and non-AWS groups at each visit.
Table 2.
Comparison of Function, Range of Motion (ROM), Pain, and Lymphedema Between Groups at Each Visit.
| Variable | AWS mean (95% CI) | Non-AWS mean (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| Time | Time | |||||||
| 2 Weeks | 4 Weeks | 12 Weeks | 18 Months | 2 Weeks | 4 Weeks | 12 Weeks | 18 Months | |
| Function (DASH) | 29.2 (25.0 to 333.5) | 19.8 (15.6 to 24,1) | 8.3 (4.1 to 12.6) | 9.8 (5.5 to 14.1) | 24.1 (19.9 to 28.4) | 13.0 (8.6 to 17.4) | 12.8 (8.5 to 17.0) | 5.4 (0.4 to 10.4) |
| Shoulder ROM (°) | ||||||||
| Abduction PROMa | 105b (95 to 114) | 127b (117 to 137) | 155 (145 to 165) | 153 (143 to 163) | 139b (129 to 148) | 154b (145 to 164) | 163 (154 to 173) | 160 (149 to 171) |
| Flexion PROM | 138 (132 to 144) | 151 (145 to 157) | 164 (158 to 170) | 166 (160 to 172) | 151 (145 to 157) | 159 (153 to 165) | 165 (159 to 171) | 165 (158 to 172) |
| Abduction AROMa | 108b (100 to 117) | 128b (120 to 137) | 155 (147 to 164) | 151 (143 to 160) | 135b (126 to 143) | 151b (142 to 159) | 157 (149 to 166) | 152 (142 to 162) |
| Flexion AROMa | 125c (119 to 131) | 138 (132 to 144) | 150 (144 to 156) | 154 (148 to 160) | 143 c (137 to 149) | 152 (146 to 158) | 155 (149 to 161) | 150 (143 to 157) |
| Pain on VAS (mm) | 35.0 (27.1 to 42.9) | 25.4 (17.5 to 33.3) | 14.7 (6.8 to 22.6) | 19.7 (11.8 to 27.6) | 30.7 (22.8 to 38.6) | 19.2 (11.0 to 27.3) | 19.3 (10.9 to 27.6) | 14.6 (5.6 to 23.5) |
| Lymphedema | ||||||||
| Volume difference (%) | 0.32 (–1.1 to 1.8) | 0.28 (–1.2 to 1.7) | 0.62 (–0.8 to 2.1) | 0.01 (–1.4 to 1.5) | 1.51 (0.1 to 3.0) | 0.67 (–0.8 to 2.1) | 0.74 (–0.7 to 2.2) | –0.05 (–1.7 to 1.6) |
| Volume difference (mL) | 7.3 (–23.1 to 37.7) | 3.7 (–26.7 to 34.2) | 10.8 (–19.6 to 41.3) | –0.7 (–31.2 to 29.7) | 33.3 (2.9 to 63.7) | 13.5 (–16.9 to 43.9) | 14.9 (–15.6 to 45.3) | 6.0 (–28.5 to 40.5) |
| BIS (L-Dex value) | 2.34 (0.9 to 3.8) | 0.02 (–1.4 to 1.4) | 0.23 (–1.2 to 1.6) | 0.71 (–0.7 to 2.1) | 0.40 (–1.0 to 1.8) | –0.72 (–2.1 to 0.7) | 0.11 (–1.3 to 1.6) | –1.15 (–2.8 to 0.5) |
| Lateral chest wall TDC | 1.3 (1.2 to 1.4) | 1.2 (1.1 to 1.4) | 1.3 (1.2 to 1.4) | 1.1 (1.0 to 1.2) | 1.3 (1.2 to 1.5) | 1.1 (1.0 to 1.2) | 1.3 (1.1 to 1.4) | 1.1 (1.0 to 1.3) |
aStatistical comparisons made between groups at each visit. Differences at P < .05 indicated by superscript letters. AROM = active range of motion, BIS = bioimpedance spectroscopy, DASH = Disabilities of the Arm, Shoulder, and Hand, mL = milliliters, PROM = passive range of motion, TDC = tissue dielectric constant, VAS = visual analog scale.
bDifferent at 2 and 4 weeks.
cDifferent at 2 weeks.
Function
There was an interaction (F = 2.63, P = .05) effect between the AWS and non-AWS group related to DASH scores. Both groups had a decrease in DASH scores (indicating an improvement in function) from 2 weeks to 12 weeks, but at 18 months the AWS group had an increase in DASH scores while the non-AWS group had a continued decrease in DASH scores.
ROM
There was an interaction effect between the AWS and non-AWS groups related to ROM measurements. The AWS group had statistically impaired abduction AROM (F = 4.5, P = .005) and PROM (F = 3.6, P = .02) at 2 and 4 weeks but not at subsequent visits (Tab. 2).
There was an interaction effect between the AWS and non-AWS groups over time related to shoulder flexion AROM (F = 4.5, P = .006). Shoulder flexion AROM was statistically lower in the AWS group compared to the non-AWS group at 2 weeks (P < .05) but not subsequent visits (Tab. 2). There was no interaction effect between the AWS and non-AWS group related to shoulder flexion PROM (F = 2.37, P = .07). No significant shoulder flexion PROM (F = 1.5, P = 0.23) group differences were present between the AWS (PROM = 155, SE = 3.0) and non-AWS (PROM = 160, SE = 3.1) groups collapsed across visits.
Pain
There was no interaction effect between the AWS and non-AWS group related to pain with movement (F = 0.71, P = .55), nor was there a statistical difference between the AWS group (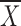 = 24, SE = 3.7) and the non-AWS group (
= 24, SE = 3.7) and the non-AWS group (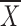 = 21, SE = 3.9) collapsed across visits (F = 0.27, P = .61).
= 21, SE = 3.9) collapsed across visits (F = 0.27, P = .61).
Lymphedema measures
There were no statistical group differences between the AWS and non-AWS groups related to upper extremity percent volume difference (F = .07, P = .79), mL difference (F = 0.15, P = .70), and L-Dex values (F = 0.58, P = .45) between the AWS group (Volume mean = 0.31%, SE = 1.0, mL difference mean = 5.3, SE = 21.0, L-Dex mean = 0.82, SE = 1.1) and non-AWS group (Volume mean = 0.72%, SE = 1.1, mL difference mean = 16.9, SE = 21.6, L-Dex mean = –0.34, SE = 1.1) collapsed across time. There was no interaction effect between the AWS and non-AWS group related to percent volume difference (F = 0.3, P = .85), mL difference (F = 0.20, P = .90), or L-Dex values (F = 0.73, P = .54). Lateral chest wall TDC had no interaction (F = 0.85, SE = 0.47) or statistical group difference (F = .08, P = .78) between the AWS (mean = 1.2, SE = 0.05) and non-AWS groups (mean = 1.2, SE = 0.05).
Physical impairments
The overall prevalence of physical impairments is displayed in Table 3, demonstrating a range from 66 to 97% dependent on the visit. Univariate analysis for association of AWS with impairments is presented in Table 4. AWS was identified as a risk factor for functional impairment (P = .05).
Table 3.
Incidence of Physical Impairments at Each Visit.
| 2 Weeks | 4 Weeks | 12 Weeks | 18 Months | |||||
|---|---|---|---|---|---|---|---|---|
| Reduced function | 26 | 72% | 15 | 47% | 9 | 25% | 10 | 31% |
| Reduced range of motion | 32 | 89% | 24 | 67% | 19 | 53% | 16 | 50% |
| Reduced abduction | 28 | 78% | 22 | 61% | 13 | 36% | 15 | 47% |
| Reduced flexion | 25 | 70% | 17 | 47% | 9 | 25% | 5 | 16% |
| Pain | 16 | 44% | 12 | 33% | 8 | 22% | 9 | 28% |
| Lymphedema | 24 | 67% | 15 | 42% | 18 | 50% | 11 | 34% |
| Upper extremity | 4 | 11% | 3 | 8% | 5 | 14% | 6 | 19% |
| Lateral chest wall | 23 | 64% | 14 | 39% | 15 | 42% | 7 | 22% |
| One or more physical impairments | 35 | 97% | 29 | 81% | 29 | 81% | 21 | 66% |
Table 4.
Univariate Analysis of Axillary Web Syndrome as a Risk Factor for Physical Impairments.
| Odds Ratio | Lower 95% CIa | Upper 95% CI | Wald Probability | |
|---|---|---|---|---|
| Reduced function | 9.60 | 0.02 | 4.50 | .05b |
| Reduced range of motion | 2.83 | 0.67 | 12.02 | .16 |
| Pain | 1.83 | 0.37 | 9.17 | .46 |
| Lymphedema | 2.93 | 0.60 | 14.23 | .18 |
aSignificance level: P ≤ .10.
Rehabilitation
A total of 13 individuals reported receiving rehabilitation within the first 18 months. Twelve of these women described receiving lymphedema treatment and exercise, and 1 described receiving ROM exercises. Three women reported rehabilitation early during their recovery (within the first 12 weeks) without further follow-up.
Discussion
Our results indicate that AWS is a common condition, with a prevalence of 50% at 18 months following breast cancer surgery. Cording can develop beyond the early postoperative time period, persist for up to 18 months, and reoccur following resolution. Our data also demonstrated that women with AWS have reduced ROM, lower BMI, and a higher number of lymph nodes removed, and AWS was identified as a risk factor for long-term reduced function. A wide variety of physical impairments such as AWS, reduced ROM, pain, lymphedema, and decreased function exist following breast cancer treatment, but few women are receiving rehabilitation assessment and treatment that could reduce the burden of the impairment.
The cumulative prevalence of AWS of 50% within the first 18 months in our study is higher than the previous prevalence of 36.2% at 24 months determined by self-report in previous literature.11 Participants in our study were assessed regularly by an experienced physical therapist providing an expert assessment compared to self-report.11 More than 50% of the cords in our study were not visible, requiring careful palpation for detection. This indicates the complex nature of concealed cords, which may be missed in studies that rely on self-reporting. Lacomba et al reported a similar AWS prevalence of 48.3% compared to our 50%, but had fewer cases of persistent AWS at 3 months (2 of 56) compared to our study (10 of 36).8 Lacomba et al provided a specific physical therapy protocol consisting of manual techniques and exercise, which may explain the lower number of women with residual AWS at 3 months compared to our study.8 This suggests that a physical therapist intervention may shorten the course of AWS.8 In our study examining the natural history of AWS without planned intervention, AWS persisted, reoccurred, and developed beyond 12 weeks following breast surgery, demonstrating that AWS is not self-limited as previously described.4,5 It is possible our prevalence may be an underestimate, considering that 4 women were lost to follow-up that may have had AWS, and the possibility that AWS occurred and resolved between visits in women in the non-AWS group. Health care providers should provide thorough AWS assessments at any time point following breast cancer surgery, considering the complex and dynamic nature of AWS.
This is one of the first studies to provide long-term follow-up in women with AWS and the only study to look at the long-term natural history. Our results demonstrate that AWS is associated with reduced shoulder ROM and an increased risk for long-term reduced function. One previous study that examined the association between AWS and function found similar results that identified AWS as a risk factor for reduced function.11 In that study, early ROM was lower in women with AWS, and there was a trend toward continued lower ROM at extended follow-up. Multiple other studies have reported reduced ROM in AWS.4,5,7 We did not find an association between AWS and lymphedema, which disagrees with findings from a similar study.11 Utilizing multiple methods for lymphedema diagnosis including trunk measurements brought strength to our study, but a treatment effect may have been present since lymphedema education was provided at 12 weeks. Providing lymphedema education may have influenced women to receive an intervention for lymphedema. A larger sample size may be needed to provide evidence as to whether or not there is an association between AWS and other physical impairments such as lymphedema. Considering that AWS appears in our study to be a more chronic condition than expected, with an increased risk of functional impairment, future research with a longer follow-up and larger sample size should focus on the long-term association between AWS and physical impairments. Four women were lost to follow-up at 18 months, which reduced power in the study. A larger number of patients from a more widespread patient population would be helpful to clearly define the relationship over time between breast cancer morbidity and effective treatment strategies. The women that participated in this study primarily represented women that received follow-up cancer care at an academic metropolitan based hospital; therefore, generalizability is limited.
Previous literature supports our BMI results and proposed hypothesis that AWS is associated with a lower BMI.4–8 The reason for this is unknown, but it has been suggested that AWS may go undetected because it is hidden by excess adipose tissue present in women with a higher BMI.5 It has also been suggested that AWS may be related to scar formation, and the thick layer of subcutaneous adipose tissue in obese individuals may prevent adhesions from developing.5 We have proposed a different theory that remains a hypothesis.29 We suggest that the pathophysiology of AWS is part of the body's natural response to lymphatic injury and may be suppressed in women with a higher BMI. When the lymphatic system has been injured, the body responds with a cascade of events, including inflammation, leakage of lymphatic fluid into the interstitial space, and coagulation. In order to remove lymphatic fluid and reestablish lymphatic flow, the lymphatic vessels attempt to recanalize and reconnect by forming lymphatic connections to existing lymph vessels (ie, lymphangiogenesis). The newly forming lymphatic vessels may become adhered to the underlying tissue during the attempt to make a lymphatic connection. The cords may be caused by tethering of the lymphatic vessel to the underlying tissue, which becomes taut when tension is put on the adhered lymphatic vessel during shoulder abduction. The resolution of the cords could be explained by successful connection of lymph vessels, reestablishing lymphatic flow. The non-resolution of cords may be an unsuccessful lymphatic connection, leaving the vessel adhered to the underlying tissue. This lymphatic response may not occur in women with higher BMI, therefore reducing the risk of AWS development. Obesity predisposes individuals to poor wound healing,30,31 therefore excess adipose tissue may suppress the lymphatic system's normal healing response following injury. This theory, which remains a hypothesis, provides a novel concept that helps explain why subjects with high BMI are at higher risk for lymphedema development but at lower risk for AWS.29 Further research is needed to support this theory and other theories to better understand the association between AWS and low BMI.
The prevalence of one or more physical impairments was 66%–97% at 1 or more visits. The impairment estimates are likely low, considering that the study did not include other possible impairments such as fatigue, reduced strength, endurance, cardiovascular status, or balance issues. Only 36% of the women received rehabilitation treatment at least once during the first 18 months following surgery, with 92% of those having received treatment for lymphedema. The prevalence of physical impairment and rehabilitation intervention was not the primary focus of the study, but the results demonstrate the need for better management of physical impairment, considering that the number of impairments far exceeded the number of women who received treatment. During this study, patients followed the normal plan of rehabilitation care as followed by their providers, which did not include routine rehabilitation assessment or intervention. The only exception to normal postoperative treatments was that lymphedema education was provided at the 12-week visit by the PI of the study. Providing lymphedema education likely affected the long-term results, which is a limitation to the study, considering that the education provided would have led the patient to seek treatment for lymphedema. Many studies and clinicians focus on lymphedema as the primary dysfunction following breast cancer surgery, when reduced ROM, reduced function, and pain appear to have a similar or higher prevalence. Physical therapists are trained to identify and treat physical impairments. Providing standard, comprehensive physical therapy assessments and effective treatment strategies may reduce the risk of chronic impairments following breast cancer treatment. Growing evidence demonstrates that physical therapist intervention can reduce cost and improve quality of life in breast cancer survivors.32–34 Future studies are needed to determine the effectiveness of periodic assessment and treatment strategies in reducing upper extremity impairment following breast cancer treatment. Considering AWS is associated with impairments and can develop beyond the early postoperative time period, a thorough assessment for AWS should be standardly performed at any time following surgery and not just in the early postoperative time period.
AWS occurs in up to 50% of women following breast cancer surgery, may persist for at least 18 months, may develop beyond the early postoperative time period, and may reoccur after resolution. Clinicians and patients need to be educated about both the persistent and transient nature of AWS, the known risk factors for AWS, and the association between AWS and reduced ROM and function. Physical therapist interventions may be beneficial in the prevention of AWS and other physical impairments, but optimal intervention has yet to be defined. Effective assessment and treatment strategies are needed to improve the physical health of breast cancer survivors with or without AWS.
Author Contributions and Acknowledgments
Concept/idea/research design: L.A. Koehler, D.W. Hunter, A.H. Blaes, T.C. Haddad
Writing: L.A. Koehler, D.W. Hunter, A.H. Blaes, T.C. Haddad
Data collection: L.A. Koehler
Data analysis: L.A. Koehler, D.W. Hunter, T.C. Haddad
Project management: L.A. Koehler
Fund procurement: L.A. Koehler, T.C. Haddad
Providing participants: L.A. Koehler, A.H. Blaes, T.C. Haddad
Providing facilities/equipment: L.A. Koehler
Providing institutional liaisons: L.A. Koehler, A.H. Blaes, T.C. Haddad
Clerical/secretarial support: L.A. Koehler
Consultation (including review of manuscript before submitting): L.A. Koehler, D.W. Hunter, A.H. Blaes, T.C. Haddad.
The authors thank Mackenzie Madsen for her volunteer time in the lab and give a very special thanks to the generous patients who participated in the study.
Ethics Approval
The University of Minnesota Internal Review Board approved the study, including the additional follow-up visit. Participants provided written consent at the first postoperative study visit. For the 18-month follow-up visit, the women were contacted by phone and asked permission to participate in an additional follow-up visit. The women provided written re-consent at the 18-month visit.
Funding
This project was supported in part by the University of Minnesota Foundation's Doctoral Dissertation Fellowship (ref. no. 4072-9201-11) and the National Cancer Institute (ref. no. P30 CA77598), utilizing the Masonic Cancer Center and University of Minnesota share resources and, as part of the Women's Health Scholar, the Powell Center Fund for Women's Health Advancement endowment at the University of Minnesota administrated by the University Minnesota Women's Health Research Program.
Disclosures
The authors completed the ICJME Form for Disclosure of Potential Conflicts of Interest. They reported no conflicts of interest.
References
- 1. Verbelen H, Gebruers N, Eeckhout FM, Verlinden K, Tjalma W. Shoulder and arm morbidity in sentinel node-negative breast cancer patients: A systematic review. Breast Cancer Res Treat. 2014;144:21–31. [DOI] [PubMed] [Google Scholar]
- 2. Hayes SC, Rye S, Battistutta D, DiSipio T, Newman B. Upper-body morbidity following breast cancer treatment is common, may persist longer-term and adversely influences quality of life. Health Qual Life Outcomes. 2010;8:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hickey OT, Burke SM, Hafeez P, Mudrakouski AL, Hayes ID, Shorten GD. Severity of acute pain after breast surgery is associated with the likelihood of subsequently developing persistent pain. Clin J Pain. 2010;26:556–560. [DOI] [PubMed] [Google Scholar]
- 4. Moskovitz AH, Anderson BO, Yeung RS, Byrd DR, Lawton TJ, Moe RE. Axillary web syndrome after axillary dissection. Am J Surg. 2001;181:434–439. [DOI] [PubMed] [Google Scholar]
- 5. Leidenius M, Leppanen E, Krogerus L, von Smitten K. Motion restriction and axillary web syndrome after sentinel node biopsy and axillary clearance in breast cancer. Am J Surg. 2003;185:127–130. [DOI] [PubMed] [Google Scholar]
- 6. Koehler LA, Blaes AH, Haddad TC, Hunter DW, Hirsch AT, Ludewig PM. Movement, function, pain, and postoperative edema in axillary web syndrome. Phys Ther. 2015;95:1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bergmann A, Mendes VV, de Almeida Dias R, do Amaral E, Silva B, da Costa Leite Ferreira MG, Fabro EA. Incidence and risk factors for axillary web syndrome after breast cancer surgery. Breast Cancer Res Treat. 2012;131:987–992. [DOI] [PubMed] [Google Scholar]
- 8. Torres Lacomba M, Mayoral Del Moral O, Coperias Zazo JL, Yuste Sanchez MJ, Ferrandez JC, Zapico Goni A. Axillary web syndrome after axillary dissection in breast cancer: A prospective study. Breast Cancer Res Treat. 2009;117:625–630. [DOI] [PubMed] [Google Scholar]
- 9. Koehler LA, Hunter DW, Haddad TC, Blaes AH, Hirsch AT, Ludewig PM. Characterizing axillary web syndrome: Ultrasonographic efficacy. Lymphology. 2014;47:156–163. [PMC free article] [PubMed] [Google Scholar]
- 10. Leduc O, Fumiere E, Banse S et al. . Identification and description of the axillary web syndrome (AWS) by clinical signs, MRI and US imaging. Lymphology. 2014;47:164–176. [PubMed] [Google Scholar]
- 11. O’Toole J, Miller CL, Specht MC et al. . Cording following treatment for breast cancer. Breast Cancer Res Treat. 2013;140:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Norkin C, White J. Measurement of Joint Motion: A Guide to Goniometry. 4th ed Philadelphia, PA: F.A. Davis Company; 2009. [Google Scholar]
- 13. Kuehn T, Klauss W, Darsow M et al. . Long-term morbidity following axillary dissection in breast cancer patients—clinical assessment, significance for life quality and the impact of demographic, oncologic and therapeutic factors. Breast Cancer Res Treat. 2000;64:275–286. [DOI] [PubMed] [Google Scholar]
- 14. Casley-Smith JR, Casley-Smith JR. Modern treatment of lymphoedema. I. Complex physical therapy: The first 200 Australian limbs. Australas J Dermatol. 1992;33:61–68. [DOI] [PubMed] [Google Scholar]
- 15. Armer JM, Stewart BR. Post-breast cancer lymphedema: Incidence increases from 12 to 30 to 60 months. Lymphology. 2010;43:118–127. [PMC free article] [PubMed] [Google Scholar]
- 16. Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol. 2005;3:208–217. [DOI] [PubMed] [Google Scholar]
- 17. Ward LC, Czerniec S, Kilbreath SL. Quantitative bioimpedance spectroscopy for the assessment of lymphoedema. Breast Cancer Res Treat. 2009;117:541–547. [DOI] [PubMed] [Google Scholar]
- 18. Mayrovitz HN, Davey S, Shapiro E. Suitability of single tissue dielectric constant measurements to assess local tissue water in normal and lymphedematous skin. Clin Physiol Funct Imaging. 2009;29:123–127. [DOI] [PubMed] [Google Scholar]
- 19. Mayrovitz HN. Assessing lymphedema by tissue indentation force and local tissue water. Lymphology. 2009;42:88–98. [PubMed] [Google Scholar]
- 20. Mayrovitz HN, Weingrad DN, Davey S. Local tissue water in at-risk and contralateral forearms of women with and without breast cancer treatment-related lymphedema. Lymphat Res Biol. 2009;7:153–158. [DOI] [PubMed] [Google Scholar]
- 21. Mayrovitz HN. Assessing local tissue edema in postmastectomy lymphedema. Lymphology. 2007;40:87–94. [PubMed] [Google Scholar]
- 22. Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56. [DOI] [PubMed] [Google Scholar]
- 23. Janz NK, Mujahid M, Chung LK et al. . Symptom experience and quality of life of women following breast cancer treatment. J Womens Health (Larchmt). 2007;16:1348–1361. [DOI] [PubMed] [Google Scholar]
- 24. Tengrup I, Tennvall-Nittby L, Christiansson I, Laurin M. Arm morbidity after breast-conserving therapy for breast cancer. Acta Oncol. 2000;39:393–397. [DOI] [PubMed] [Google Scholar]
- 25. Harrington S, Michener LA, Kendig T, Miale S, George SZ. Patient-reported upper extremity outcome measures used in breast cancer survivors: A systematic review. Arch Phys Med Rehabil. 2014;95:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: Normative values from the general population. J Bone Joint Surg Am. 2002;84-A:208–215. [DOI] [PubMed] [Google Scholar]
- 27. Harrington S, Padua D, Battaglini C et al. . Comparison of shoulder flexibility, strength, and function between breast cancer survivors and healthy participants. J Cancer Surviv. 2011;5:167–174. [DOI] [PubMed] [Google Scholar]
- 28. Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer: Incidence, risk factors, and effect on upper body function. J Clin Oncol. 2008;26:3536–3542. [DOI] [PubMed] [Google Scholar]
- 29. Koehler LA, Hunter DW. The axillary web syndrome and its lymphatic origin. Lymphology. 2016;49:185–191. [PubMed] [Google Scholar]
- 30. Bellon JM, Duran HJ. Biological factors involved in the genesis of incisional hernia. Cir Esp. 2008;83:3–7. [DOI] [PubMed] [Google Scholar]
- 31. de Jongh RT, Serne EH, IJzerman RG, de Vries G, Stehouwer CD. Impaired microvascular function in obesity: Implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation. 2004;109:2529–2535. [DOI] [PubMed] [Google Scholar]
- 32. Stout NL, Pfalzer LA, Springer B et al. . Breast cancer-related lymphedema: Comparing direct costs of a prospective surveillance model and a traditional model of care. Phys Ther. 2012;92:152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Soran A, Ozmen T, McGuire KP et al. . The importance of detection of subclinical lymphedema for the prevention of breast cancer-related clinical lymphedema after axillary lymph node dissection; A prospective observational study. Lymphat Res Biol. 2014;12:289–294. [DOI] [PubMed] [Google Scholar]
- 34. Pidlyskyj K, Roddam H, Rawlinson G, Selfe J. Exploring aspects of physiotherapy care valued by breast cancer patients. Physiotherapy. 2014;100:156–161. [DOI] [PubMed] [Google Scholar]