ABSTRACT
Background
Due to insufficient evidence, extremely preterm infants (≤28 wk of gestation) rarely receive early progressive feeding (small increments of feeding volumes between 1 and 4 d after birth). We hypothesized that early progressive feeding increases the number of full enteral feeding days in the first month after birth.
Objective
The aim of this study was to determine the feasibility and efficacy of early progressive feeding in extremely preterm infants.
Design
In this single-center randomized trial, extremely preterm infants born between September 2016 and June 2017 were randomly assigned to receive either early progressive feeding without trophic feeding (early feeding group) or delayed progressive feeding after a 4-d course of trophic feeding (delayed feeding group). Treatment allocation occurred before or on feeding day 1. The primary outcome was the number of full enteral feeding days in the first month after birth. Secondary outcomes were death, necrotizing enterocolitis (NEC), culture-proven sepsis, growth percentiles at 36 wk postmenstrual age, use of parenteral nutrition, and need for central venous access.
Results
Sixty infants were included (median gestational age: 26 wk; mean ± SD birth weight: 832 ± 253 g). The primary outcome differed between groups (median difference favoring the early feeding group: +2 d; 95% CI: 0, 3 d; P = 0.02). Early progressive feeding reduced the use of parenteral nutrition (4 compared with 8 d; P ≤ 0.01) and the need for central venous access (9 compared with 13 d; P ≤ 0.01). The outcome of culture-proven sepsis (10% compared with 27%; P = 0.18), restricted growth (weight, length, and head circumference <10th percentile) at 36 wk postmenstrual age (25% compared with 50%; P = 0.07), and the composite outcome of NEC or death (27% compared with 20%; P = 0.74) did not differ between groups.
Conclusion
Early progressive feeding increases the number of full enteral feeding days in extremely preterm infants. This trial was registered at www.clinicaltrials.gov as NCT02915549.
Keywords: minimal enteral nutrition, parenteral nutrition, necrotizing enterocolitis, central venous access, late-onset sepsis, postnatal growth restriction, premature infants
INTRODUCTION
In many hospitals that provide neonatal care for extremely preterm infants (≤28 wk of gestation), the transition from parenteral to enteral nutrition often begins with minimal enteral feeding (MEF) or trophic feeding (≤24 mL ⋅ kg−1 ⋅ d−1), then changes to progressive feeding (daily increments of feeding volumes usually by 20–24 mL ⋅ kg−1 ⋅ d−1), and concludes with full enteral feeding (≥120 mL ⋅ kg−1 ⋅ d−1) (1–4). By initiating a 3- to 5-d course of MEF within the first 96 h after birth (1), most clinicians assume that prevention of gastrointestinal atrophy will reduce the risk of feeding intolerance and necrotizing enterocolitis (NEC) in extremely preterm infants (5–7).
A meta-analysis of 9 randomized trials that compared early with delayed progressive feeding in predominantly moderate-preterm infants (29–32 wk of gestation) (8) and a retrospective study that compared short with extended periods of trophic feeding in extremely preterm infants (1) provide clinicians with evidence that early progressive feeding (small increments of feeding volumes between 1 and 4 d after birth) reduces the time to establish full enteral feeding without increasing the risk of NEC. However, many clinicians considered this evidence insufficient to standardize the practice of early progressive feeding in extremely preterm infants. Feasibility, safety, and efficacy are the main concerns (9, 10).
Because retrospective studies introduce selection bias mediated by severity of illness (1, 11) and randomized trials often exclude extremely preterm infants (10, 12), this randomized trial assessed the feasibility and efficacy of early progressive feeding in extremely preterm infants. We hypothesized that in extremely preterm infants receiving human milk, progressive feeding without MEF compared with delayed progressive feeding after a 4-d course of MEF would result in an increased number of full enteral feeding days in the first month after birth.
METHODS
In this parallel-group randomized controlled trial, participants were randomly assigned in a 1:1 allocation ratio to receive either early progressive feeding without MEF (early feeding group) or delayed progressive feeding after a 4-d course of MEF (delayed feeding group). Extremely preterm infants with gestational ages between 22 and 28 wk of gestation admitted to the neonatal unit at the University of Alabama at Birmingham Hospital were included. Infants born small for gestational age (SGA) with a birth weight below the fifth percentile were excluded. Infants with a terminal illness in whom decisions to withhold or limit life support were made and infants with major congenital or chromosomal anomalies were also excluded.
This trial was approved by the University of Alabama at Birmingham Institutional Review Board (clinicaltrials.gov: NCT02915549). Written informed consent was obtained during the first 48 h after birth to allow treatment allocation before or on feeding day 1, usually between 48 and 96 h after birth. Participants were randomly assigned to one of the study groups following computer-generated random-block sequences and with the use of sequentially numbered, opaque, sealed envelopes, which were opened in sequential order only after informed consent was obtained. Twin infants were randomly assigned individually. The intervention was not masked.
Enteral nutrition was administered as an intermittent bolus gavage every 3 h. Infants in the early feeding group received 20–24 mL enteral nutrition ⋅ kg−1 ⋅ d−1 on feeding day 1. On feeding day 2, early progressive feeding began with daily increments of 24–25 mL ⋅ kg−1 ⋅ d−1 and continued until full enteral feeding was established (≥120 mL ⋅ kg−1 ⋅ d−1). Infants in the delayed feeding group received 20–24 mL enteral nutrition ⋅ kg−1 ⋅ d−1 from feeding day 1 to feeding day 4. On feeding day 5, progressive feeding began with daily increments of 24–25 mL ⋅ kg−1 ⋅ d−1 until full enteral feeding was established. Unfortified donor human milk was offered as an alternative to mother's own milk until full enteral feeding was established. Subsequently, infant formula was offered as an alternative to human milk if the mother was no longer able to supply her own milk.
Although a birth weight–based feeding protocol was created for each study participant to standardize daily rates of progressive feeding (24–25 mL ⋅ kg−1 ⋅ d−1) and verify compliance, deviations from the feeding protocol were allowed. Enteral feeding discontinued for <5 d due to feeding intolerance or clinical deterioration was resumed at the clinician's discretion with the feeding volume defined in the birth-weight–based feeding protocol (preferred approach), with a feeding volume previously tolerated, or with feeding volumes that did not meet any of those criteria. If enteral feeding was discontinued for ≥5 d, infants in the early feeding group received a feeding volume of 20–24 mL ⋅ kg−1 ⋅ d−1 on day 1 of re-initiation of enteral feeding before receiving progressive feeding, and infants in the delayed feeding group received a feeding volume of 20–24 mL ⋅ kg−1 ⋅ d−1 for 4 d before receiving progressive feeding. Re-initiation of enteral feeding after the diagnosis of NEC or spontaneous intestinal perforation (SIP) was not regulated by the study protocol.
The primary efficacy endpoint of the trial was the number of full enteral feeding days in the first month after birth. Secondary efficacy endpoints of the trial were time to establish full enteral feeding, use of parenteral nutrition (PN) in days, use of central venous access in days, culture-proven sepsis, growth percentiles at 36 wk postmenstrual age or time of hospital discharge (whichever occurred first), and duration of hospital stay in days.
The primary safety endpoints of the trial were death, NEC stage 2 or 3, or SIP. Due to insufficient power to detect significant group differences in these primary safety endpoints (∼25%), a data safety and monitoring committee reviewed individual patient data at 50% enrollment to exclude the possibility of a temporal association between the intervention under investigation and the primary safety endpoints of the trial.
A sample size of 48 patients achieved 80% power to detect a 5-d difference in the number of full enteral feeding days in the first month after birth with an SD of 6 d under the 0.05 significance level. However, anticipating that ∼20% of study participants would not be able to complete the intervention as assigned due to acute complications (3), 6 patients were added to each group and the sample size was increased to 60.
All of the continuous endpoints were summarized as means ± SDs or as medians and IQRs. The categorical endpoints were summarized as frequencies and proportions. Group differences were evaluated by using the Wilcoxon test for continuous variables and chi-square test or Fisher's exact test for categorical variables. The effect size for the primary efficacy endpoint was expressed as the median difference with 95% CIs. Differences in mean values were reported for other continuous endpoints. RRs with 95% CIs were reported for categorical endpoints.
We also performed a prespecified time-to-full-enteral-feeding analysis by using the Kaplan-Meier method and the log-rank test. For this analysis, infants who died or developed NEC before postnatal day 28 were censored.
All of the efficacy and safety endpoints of the trial were analyzed with the intention-to-treat principle by using SAS 9.4 (SAS Institute).
RESULTS
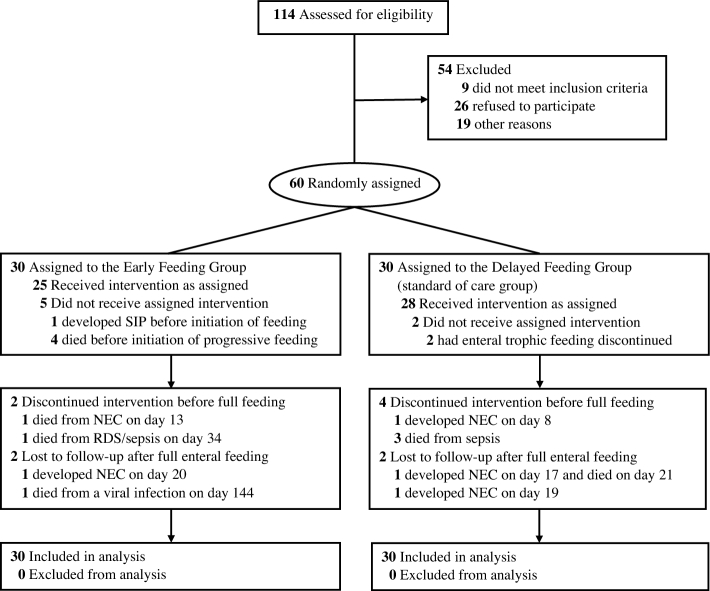
Of 114 eligible extremely preterm infants admitted between September 2016 and June 2017, 60 extremely preterm infants with gestational ages of 22 wk, 0 d, through 28 wk, 6 d, were randomly assigned to receive either early progressive feeding without MEF or delayed progressive feeding after a 4-d course of MEF. Four infants in the early feeding group died of respiratory distress syndrome before initiation of progressive feeding. Three infants in the delayed feeding group died of sepsis before establishment of full enteral feeding (Figure 1).
FIGURE 1.
Study enrollment, randomization, and outcomes. NEC, necrotizing enterocolitis; RDS, respiratory distress syndrome; SIP, spontaneous intestinal perforation.
Baseline characteristics of the study participants are shown in Table 1. Mean ± SD birth weight was 832 ± 253 g, and the median gestational age was 26 wk (IQR: 24–28 wk). More than one-half of infants were of non-Hispanic black race/ethnicity. All of the study participants received either mother's own milk or unfortified donor human milk during the first 2 wk after birth. Subsequently, approximately one-third of the study participants received formula.
TABLE 1.
Baseline characteristics
| Early feeding group (n = 30) | Delayed feeding group (n = 30) | |
|---|---|---|
| Demographic characteristics | ||
| Birth weight, g | 873 ± 2691 | 793 ± 234 |
| Gestational age, wk | 26 (24–28)2 | 26 (24–27) |
| Weight-for-age z score3 | −0.10 ± 0.86 | −0.17 ± 0.82 |
| Male, n (%) | 13 (43) | 9/30 (30) |
| Black race, n (%) | 15 (50) | 20 (67) |
| Exposure to a full course (2 doses) of antenatal steroids, n (%) | 22 (73) | 17 (57) |
| Apgar score at 5 min | 6 (4–7) | 6 (4–7) |
| Initiation of enteral feeding, d | 3 (1–3) | 3 (1–3) |
| Human milk–based diet with the use of mother's own milk (>80%) in the first 28 d after birth, n (%) | ||
| Week 1 | 13 (43) | 16 (53) |
| Week 2 | 13 (43) | 10 (33) |
| Week 3 | 9 (30) | 11 (37) |
| Week 4 | 8 (27) | 10 (33) |
| Formula-based diet (>80%) in the first 28 d after birth, n (%) | ||
| Week 3 | 11 (37) | 7 (23) |
| Week 4 | 12 (40) | 12 (40) |
1Mean ± SD (all such values).
2Median; 25th–75th percentile in parentheses (all such values).
3 z Scores were estimated by using the Fenton 2013 growth curves.
The median number of full enteral feeding days in the first 28 d after birth was 19 d (IQR: 0–20 d) in the early feeding group and 17 d (IQR: 13–18 d) in the delayed feeding group (median difference favoring the early feeding group: +2 d; 95% CI 0, 3 d; P = 0.02) (Table 2). In the time-to-event analysis, the median time to establish full enteral feeding was 9 d in the early feeding group and 12 d in the delayed feeding group (P = 0.01) (Figure 2).
TABLE 2.
Feeding and safety outcomes1
| Early feeding | Delayed feeding | ||
|---|---|---|---|
| Outcomes | group (n = 30) | group (n = 30) | P |
| Full enteral feeding in the first 28 d after birth, d | 19 (0–20)2 | 17 (13–18) | 0.023 |
| Time to full enteral feeding, d | 10 ± 34 | 12 ± 2 | 0.00035 |
| Duration of parenteral nutrition, d | 4 ± 6 | 8 ± 6 | 0.00055 |
| Duration of central venous access, d | 9 ± 7 | 13 ± 6 | 0.00015 |
| Culture-proven sepsis, n (%) | 3 (10) | 8 (27) | 0.186 |
| Duration of mechanical ventilation, d | 8 ± 9 | 10 ± 12 | 0.615 |
| Supplemental oxygen at 36 wk,7n (%) | 12 (50) | 13 (50) | 1.006 |
| Weight <10th percentile at 36 wk,7,8n (%) | 12 (50) | 16 (62) | 0.416 |
| Length <10th percentile at 36 wk,7,8n (%) | 13 (54) | 18 (69) | 0.276 |
| Head circumference <10th percentile at 36 wk,7,8n (%) | 9 (38) | 16 (62) | 0.096 |
| Restricted growth at 36 wk (weight, length, and head circumference <10th percentile),7,8n (%) | 6 (25) | 13 (50) | 0.076 |
| NEC, n (%) | 2 (7) | 3 (10) | 1.006 |
| Mortality before postnatal day 28, n (%) | 5 (16) | 3 (10) | 0.706 |
| Mortality, n (%) | 7 (23) | 4 (12) | 0.376 |
| NEC or death, n (%) | 8 (27) | 6 (20) | 0.566 |
| Age at the time of hospital discharge, d | 74 (53–92) | 80 (66–92) | 0.205 |
1NEC, necrotizing enterocolitis.
2Median; 25th–75th percentile in parentheses (all such values).
3Derived by using Wilcoxon's rank-sum test. Normal distribution could not be assumed because the early feeding group had 2 distinctive peaks in the distribution of the outcome (bimodal distribution).
4Mean ± SD (all such values).
5Derived by using t test for independent samples assuming equal variances.
6Derived by using chi-square test or Fisher's exact test if some cells have an expected count of ≤5.
7Only participants with outcome data at 36 wk (n = 50): 24 in the early feeding group and 26 in the delayed progressive group.
8Percentiles were estimated by using the Fenton 2013 growth curves.
FIGURE 2.
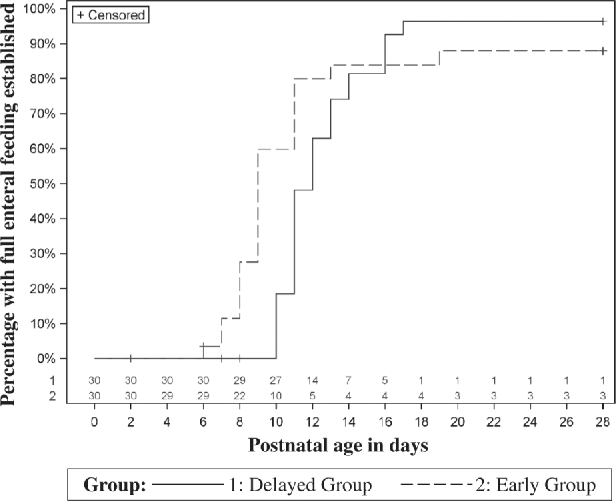
Percentage of infants with full enteral feeding established according to the intervention group. In this time-to-event analysis, the observation period began at birth and continued until postnatal day 28. The number of infants eligible for or “at risk” of developing the outcome of interest (i.e., full enteral feeding) changed over time (numbers at the bottom of the graph). Infants removed from the study as a consequence of serious adverse events (i.e., necrotizing enterocolitis, spontaneous intestinal perforation, or death) were censored (not counted in the denominator used to report the percentage of infants with full enteral feeding established). Infants unable to achieve full enteral feeding by postnatal day 28 were also censored.
The use of PN (4 compared with 8 d; P = 0.0005) and the use of central venous access (9 compared with 13 d; P = 0.0001) were also significantly lower in the early feeding group. The differences in culture-proven sepsis and growth outcomes at 36 wk postmenstrual age did not reach significance, but the risk of culture-proven sepsis (RR: 0.38; 95% CI: 0.11, 1.28; P = 0.12) and the risk of restricted growth (weight, length, and head circumference <10th percentile) at 36 wk of postmenstrual age (RR: 0.50; 95% CI: 0.23, 1.10; P = 0.07) tended to be lower in the early feeding group. The risk of NEC (RR: 0.67; 95% CI: 0.12, 3.71; P = 0.68), death (RR: 1.98; 95% CI: 0.64, 6.11; P = 0.25), and the combined outcome of NEC or death (RR: 1.33; 95% CI: 0.53, 3.38; P = 0.56) did not differ between groups (Table 2).
DISCUSSION
In this single-center randomized trial, we compared early progressive feeding without MEF with delayed progressive feeding after a 4-d course of MEF. We showed that early progressive feeding increases the total number of full enteral feeding days, reduces the use of PN, and reduces the need for central venous access in extremely preterm infants. We also found that early progressive feeding, compared with delayed progressive feeding after a 4-d course of MEF, reduces the use of PN without increasing the risk of postnatal growth restriction at 36 wk of postmenstrual age. To our knowledge, this is the first trial of early progressive feeding that includes only extremely preterm infants.
This trial confirms the results of our retrospective study that compared short with extended periods of trophic feeding in 192 extremely preterm infants (1). After adjustment for birth weight, gestational age, SGA status, race, sex, type of enteral nutrition, and day of initiation of trophic feeding, we previously concluded that a short period of trophic feeding is associated with early establishment of full enteral feeding (1). Our results also corroborate the results of a meta-analysis that included >1000 moderately preterm infants randomly assigned to receive early progressive feeding, in which early progressive feeding reduced the time to establish full enteral feeding (8). The largest randomized trial included in the meta-analysis also concluded that early progressive feeding reduces the duration of PN and the risk of poor growth at the time of hospital discharge (9). We could not detect a significant reduction in poor growth or culture-proven sepsis with early progressive feeding, but large observational studies show that more aggressive enteral nutrition and less PN are associated with a lower risk of sepsis (9, 13–15).
Our results contradict the results of a randomized trial that favored MEF over progressive feeding in predominantly formula-fed infants with limited exposure to antenatal steroids (6, 16). The trial that compared MEF with progressive feeding listed several contraindications to initiate enteral feeding and delayed initiation of enteral feeding for ∼10 d in all study participants (6). Because antenatal steroids increase survival and reduce short-term complications in preterm infants (17), early initiation of enteral feeding is recommended in the current era of antenatal steroid use. Moreover, the presence of umbilical catheters or the infusion of pressor agents are no longer listed as contraindications to initiate enteral feeding, particularly if human milk is available (18). This important difference between trials suggests that, when human milk is not available, early progressive feeding may not be suitable for extremely preterm infants who develop intestinal atrophy due to delayed initiation of enteral feeding. The results of a subgroup analysis of growth-restricted extremely preterm infants randomly assigned to receive early progressive feeding also suggest that early progressive feeding might be less effective in extremely preterm infants born SGA (10).
Although we adequately powered this trial to test the effect of early progressive feeding on the outcome “full enteral feeding days in the first 28 d after birth,” a surrogate outcome measurable in all study participants that quantifies the negative effects of SIP, NEC, or death on enteral feeding and combines efficacy and safety endpoints of the trial in a continuous scale, the power of this trial was insufficient to determine the effect of early progressive feeding on the outcome of NEC. Unlike the interim analysis of a large randomized trial of trophic feeding compared with delayed progressive feeding (6, 16), neither the interim nor the final analysis of this trial identified a temporal association between progressive feeding and NEC. All but one of the NEC cases were attributed to severity of critical illness, and the overall risk of NEC reported in this trial was comparable to the baseline risk of NEC observed in extremely preterm infants admitted to our unit (8% compared with 10%, respectively).
Our findings are consistent with the results of other randomized trials that compared early with delayed progressive feeding in moderate-preterm infants (8) and growth-restricted extremely preterm infants (10). In addition, a recently updated meta-analysis in >500 extremely preterm infants randomly assigned to either rapid (30 and 35 mL ⋅ kg−1 ⋅ d−1) or slow progressive (15–20 mL ⋅ kg−1 ⋅ d−1) feeding did not suggest that slow progressive feeding reduces the risk of NEC (19). Our trial used a rapid progressive feeding rate by definition (>24 mL ⋅ kg−1 ⋅ d−1) (19), but our progressive feeding rates were lower than the average rates reported in randomized trials of rapid progressive feeding.
If equipoise can be maintained to show that the unknown risk of NEC outweighs the proven benefits of early progressive feeding on full enteral feeding, PN use, and need for central access, a larger multicenter trial powered to detect differences in the outcome of NEC could add external validity to our results. The average time to establish full enteral feeding in this trial was shorter than the average time reported in other trials that included extremely preterm infants (20). A larger neonatal trial might not reduce uncertainty as anticipated (21), but it could show that early progressive feeding reduces the risk of postnatal growth restriction and culture-proven sepsis.
Because enteral feeding is often initiated earlier and advanced more rapidly in less critically ill preterm infants (11), randomization of high-risk extremely preterm infants is one of the most important strengths of this trial. Severe respiratory distress syndrome, sepsis, and hypotension were not listed as exclusion criteria for this trial. Progressive feeding was initiated according to treatment allocation and following an individualized birth-weight–based feeding protocol in all study participants. Variability in progressive feeding practices was minimized through daily measurements of compliance to avoid differential noncompliance, which often introduces bias; however, the 4-d delay in the progression of feeding was not reflected in the final difference between groups, likely because the progression of feeding after feeding day 1 in the early feeding group had the compliance challenges of any new intervention.
In summary, this trial shows that early progressive feeding is not only feasible in critically ill, extremely preterm infants but also effective in increasing the number of full enteral feeding days, reducing the use of PN, and reducing the use of central venous access. It remains uncertain whether early progressive feeding increases the risk of NEC in extremely preterm infants born at the limits of viability, but larger studies of feeding practices aimed at promoting early progression of feeding could reduce the risk of postnatal growth restriction and culture-proven sepsis in all extremely preterm infants.
Acknowledgements
The authors’ responsibilities were as follows—AAS: conceptualized and designed the study, carried out the initial analysis, and drafted the initial manuscript; PL: performed the randomization and carried out the statistical analysis; KP: designed the data collection instruments, monitored patient enrollment and compliance, and collected data; CVL, CRM, and WAC: helped design the study and critically reviewed the manuscript; and all authors: read and approved the final manuscript. The authors had no conflicts of interest relevant to this article to disclose.
Notes
Supported in part by a research award from the Gerber Foundation. WAC and PL are also supported by the NIH (grants U10 HD34216 and UL1TR001417, respectively).
Abbreviations used: MEF, minimal enteral feeding; NEC, necrotizing enterocolitis; PN, parenteral nutrition; SGA, small for gestational age; SIP, spontaneous intestinal perforation.
REFERENCES
- 1. Salas AA, Kabani N, Travers CP, Phillips V, Ambalavanan N, Carlo WA. Short versus extended duration of trophic feeding to reduce time to achieve full enteral feeding in extremely preterm infants: an observational study. Neonatology 2017;112(3):211–6. [DOI] [PubMed] [Google Scholar]
- 2. Klingenberg C, Embleton ND, Jacobs SE, O'Connell LA, Kuschel CA. Enteral feeding practices in very preterm infants: an international survey. Arch Dis Child Fetal Neonatal Ed 2012;97(1):F56–61. [DOI] [PubMed] [Google Scholar]
- 3. Salas AA, Cuna A, Bhat R, McGwin G Jr., Carlo WA, Ambalavanan N. A randomised trial of re-feeding gastric residuals in preterm infants. Arch Dis Child Fetal Neonatal Ed 2015;100(3):F224–8. [DOI] [PubMed] [Google Scholar]
- 4. Hans DM, Pylipow M, Long JD, Thureen PJ, Georgieff MK. Nutritional practices in the neonatal intensive care unit: analysis of a 2006 neonatal nutrition survey. Pediatrics 2009;123(1):51–7. [DOI] [PubMed] [Google Scholar]
- 5. Henderson G, Craig S, Brocklehurst P, McGuire W. Enteral feeding regimens and necrotising enterocolitis in preterm infants: a multicentre case-control study. Arch Dis Child Fetal Neonatal Ed 2009;94(2):F120–3. [DOI] [PubMed] [Google Scholar]
- 6. Berseth CL, Bisquera JA, Paje VU. Prolonging small feeding volumes early in life decreases the incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2003;111(3):529–34. [DOI] [PubMed] [Google Scholar]
- 7. Jasani B, Patole S. Standardized feeding regimen for reducing necrotizing enterocolitis in preterm infants: an updated systematic review. J Perinatol 2017;37(7):827–33. [DOI] [PubMed] [Google Scholar]
- 8. Morgan J, Young L, McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev 2014;12:CD001970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leaf A, Dorling J, Kempley S, McCormick K, Mannix P, Linsell L, Juszczak E, Brocklehurst P; Abnormal Doppler Enteral Prescription Trial Collaborative Group Early or delayed enteral feeding for preterm growth-restricted infants: a randomized trial. Pediatrics 2012;129(5):e1260–8. [DOI] [PubMed] [Google Scholar]
- 10. Kempley S, Gupta N, Linsell L, Dorling J, McCormick K, Mannix P, Juszczak E, Brocklehurst P, Leaf A. Feeding infants below 29 weeks' gestation with abnormal antenatal Doppler: analysis from a randomised trial. Arch Dis Child Fetal Neonatal Ed 2014;99(1):F6–F11. [DOI] [PubMed] [Google Scholar]
- 11. Ehrenkranz RA, Das A, Wrage LA, Poindexter BB, Higgins RD, Stoll BJ, Oh W; Eunice Kennedy Shriver National Institute of Child Health ; Human Development Neonatal Research Network Early nutrition mediates the influence of severity of illness on extremely LBW infants. Pediatr Res 2011;69(6):522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arnon S, Sulam D, Konikoff F, Regev RH, Litmanovitz I, Naftali T. Very early feeding in stable small for gestational age preterm infants: a randomized clinical trial. J Pediatr (Rio J) 2013;89(4):388–93. [DOI] [PubMed] [Google Scholar]
- 13. Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, Higgins RD, National Institute of Child Health;. Human Development Neonatal Research Network Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 2004;292(19):2357–65. [DOI] [PubMed] [Google Scholar]
- 14. Flidel-Rimon O, Friedman S, Lev E, Juster-Reicher A, Amitay M, Shinwell ES. Early enteral feeding and nosocomial sepsis in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 2004;89(4):F289–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hay WW. Optimizing nutrition of the preterm infant. Zhongguo Dang Dai Er Ke Za Zhi 2017;19(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Engle WD, Lair CS. Early feeding of premature infants questioned. Pediatrics 2004;113(4):931–2. [DOI] [PubMed] [Google Scholar]
- 17. Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, Laptook AR, Sanchez PJ, Van Meurs KP, Wyckoff M, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 2015;314(10):1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev 2014;4:CD002971. [DOI] [PubMed] [Google Scholar]
- 19. Oddie SJ, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev 2017;8:CD001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shah SD, Dereddy N, Jones TL, Dhanireddy R, Talati AJ. Early versus delayed human milk fortification in very low birth weight infants—a randomized controlled trial. J Pediatr 2016;174:126–31, e1. [DOI] [PubMed] [Google Scholar]
- 21. Hay SC, Kirpalani H, Viner C, Soll R, Dukhovny D, Mao WY, Profit J, DeMauro SB, Zupancic JAF. Do trials reduce uncertainty? Assessing impact through cumulative meta-analysis of neonatal RCTs. J Perinatol 2017;37(11):1215–9. [DOI] [PubMed] [Google Scholar]