Abstract
Post-translational prenylation involves incorporation of 15-(farnesyl) or 20-(geranylgeranyl) carbon derivatives of mevalonic acid into highly conserved C-terminal cysteines of proteins. The farnesyl transferase (FTase) and the geranylgeranyl transferase (GGTase) mediate incorporation of farnesyl and geranylgeranyl groups, respectively. At least 300 proteins are prenylated in the human genome; the majority of these are implicated in cellular processes including growth, differentiation, cytoskeletal function and vesicle trafficking. From a functional standpoint, isoprenylation is requisite for targeting of modified proteins to relevant cellular compartments for regulation of effector proteins. Pharmacological and molecular biological studies have provided compelling evidence for key roles of this signaling pathway in physiological insulin secretion in normal rodent and human islets. Recent evidence indicates that inhibition of prenylation results in mislocalization of unprenylated proteins, and surprisingly, they remain in active (GTP-bound) conformation. Sustained activation of G proteins has been reported in mice lacking GGTase, suggesting alternate mechanisms for the activation of non-prenylated G proteins. These findings further raise an interesting question if mislocalized, non-prenylated and functionally active G proteins cause cellular pathology since aberrant protein prenylation has been implicated in the onset of cardiovascular disease and diabetes. Herein, we overview the existing evidence to implicate prenylation in islet function and potential defects in this signaling pathways in the diabetic β-cell. We will also identify critical knowledge gaps that need to be addressed for the development of therapeutics to halt defects in these signaling steps in β cells in models of impaired insulin secretion, metabolic stress and diabetes.
Keywords: Prenylation, G proteins, Islet β-cell, Insulin secretion, Diabetes
1. Introduction
It is well established that glucose-stimulated insulin secretion (GSIS) is mediated largely via the generation of soluble second messengers including cyclic nucleotides, adenine nucleotides, and hydrolytic products of phospholipases (A2, C and D). However, the precise molecular and cellular mechanisms underlying GSIS remain only partially understood [1–4]. It is widely accepted that, following its entry into the β-cell (facilitated by the glucose-transporter protein, Glut-2), glucose is metabolized via glycolytic and tricarboxylate cycles leading to an increase in intracellular ATP/ADP ratio, which, in turn, results in the closure of plasma membrane-associated ATP-sensitive K+ channels culminating in depolarization of the membrane. This facilitates the influx of extracellular calcium via the voltage-gated calcium channels. Increase in intracellular calcium has been shown to be critical for the transport of insulin-laden secretory granules to the plasma membrane for fusion and exocytotic secretion of insulin into the circulation [1–4].
In addition to the regulation of GSIS by adenine nucleotides, current evidence implicates guanine nucleotides (e.g., GTP) in physiological insulin secretion. For example, using selective inhibitors of the GTP biosynthetic pathway (e.g., mycophenolic acid), Metz and associates have demonstrated a permissive role for GTP in GSIS [5]. Although, the precise mechanisms underlying GTP-mediated GSIS remain elusive, experimental evidence from several laboratories indicates that they might involve activation of one (or more) GTP binding proteins (G proteins; Refs. [6,7] for reviews). Along these lines, two major groups of G proteins have been identified in pancreatic islet β-cells. The first group consists of heterotrimeric G proteins comprised of αβγ subunits; this class of Gproteins plays essential roles in the coupling of various receptors to their intracellular effectors, including adenylate cyclases, phosphodiesterases and phospholipases. The second group of G proteins is comprised of small molecular weight, monomeric G proteins, which are involved in protein sorting and trafficking to subcellular compartments. They have also been implicated in transport of secretory vesicles to the docking sites on the plasma membrane for fusion and release of their cargo into circulation [6,7].
2. Post-translational modification of G-proteins
A large body of evidence indicates that the majority of small G proteins (e.g., Cdc42, Rac1, Rap1 and Ras), γ-subunits of trimeric G proteins, and nuclear lamins (lamin A and B) undergo a series of post-translational modifications at their C-terminal cysteine residues (referred to as the CAAX motif; Fig. 1A). Such modification steps control trafficking of modified proteins to appropriate cellular compartments (plasma membrane, insulin granules, mitochondria, nucleus) for optimal interaction with their respective effector proteins and regulation of cellular function [7–12]. The first of a four-step modification sequence (Fig. 1A) includes incorporation of mevalonic acid (MVA)-derived 15-carbon (farnesyl) or 20-carbon (geranylgeranyl) isoprenoid (prenyl) moiety onto the C-terminal cysteine residue of the candidate G protein. These reactions are mediated by either farnesyltransferase (FTase) or geranylgeranyltransferase (GGTase; collectively referred to as prenyl transferases; see below for additional details). The second committed step following prenylation is the proteolytic removal of the C-terminal amino acids (-AAX) after the prenylated cysteine; this is catalyzed by the ras converting enzyme 1 (Rce1), a protease of microsomal origin thus exposing the carboxylate anion of the prenylated cysteine. This is further subjected to a methylation step catalyzed by isoprenyl cysteine methyl transferases (ICMT) in the presence of S-adenosylmethione (SAM), serving as the methyl donor (Fig. 1A). Not shown in Fig. 1A is an additional modification step involving incorporation of a long-chain fatty acid, typically palmitate, at a cysteine residue, which is upstream to the isoprenylated cysteine. This palmitoylation step, which is catalyzed by the palmitoyl transferase, is thought to render the modified G proteins more hydrophobic and enable them to associate with relevant membrane compartments for their interaction with respective effector proteins [13,14]. Since the isoprenylation of Gproteins occurs shortly after their synthesis, and the half-lives of prenylated proteins are relatively long, this is not likely to be an acute regulatory step; however, in many cases, prenylation is necessary to allow candidate G proteins to intercalate into the relevant membranes. In contrast to prenylation steps, the methylation and palmitoylation steps are subject to acute regulation at the level of the “on” steps (addition of methyl or palmitoyl groups) as well as the “off” steps (removal of methyl or palmitoyl groups). The addition and removal of methyl groups are mediated by ICMT and isoprenyl cysteine methylesterase, respectively. Likewise, addition and deletion of palmitoyl groups are facilitated by palmitoyl transferase and esterases, respectively. The reader is referred to recent reviews that highlight the requisite nature and roles of post-translational carboxylmethylation and palmitoylation of small G protein as they relate to GSIS [7,13].
Fig. 1.
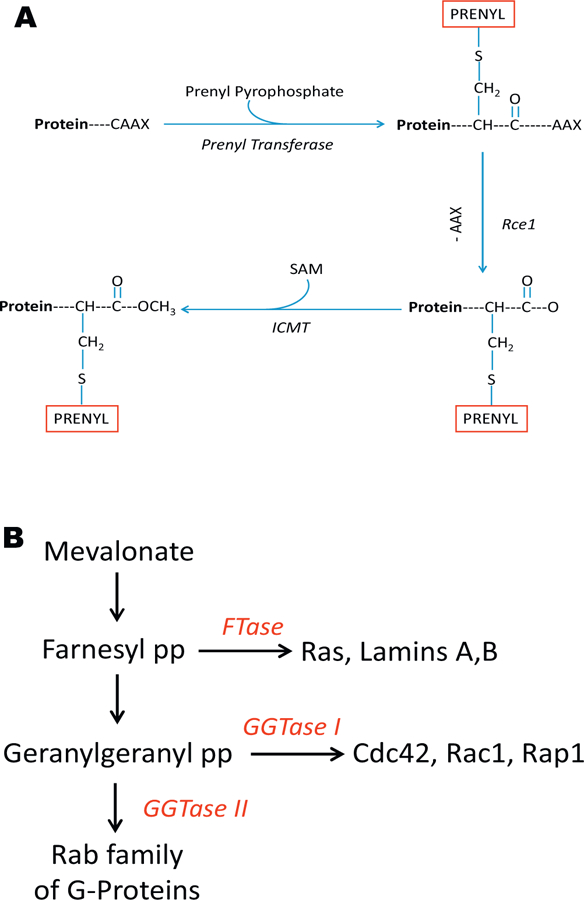
(A) Schematic representation of post-translational prenylation of G-proteins. The first of the four-step modifications is incorporation of either a 15 (farnesyl)- or a 20 (geranylgeranyl)-carbon derivative of MVA into the COOH-terminal cysteine (CAAX motif) via a thioether linkage. This reaction is catalyzed by either the FTase and GGTase, respectively. Collectively, they are referred to as prenyl transferase. After this, the three amino acids after the prenylated cysteine (AAX) are removed by a protease (Rce1) of microsomal origin, thereby exposing the carboxylate anion. This site is then methylated by a carboxyl methyl transferase (ICMT), which transfers a methyl group onto the carboxylate group using S-adenosyl methionine (SAM) as the methyl donor. We have shown that the carboxyl methylation of specific G proteins (e.g., Cdc42) increases their hydrophobicity and translocation to the membrane fraction. In addition to these, certain G proteins (e.g., H-Ras) have also been shown to undergo palmitoylation at a cysteine residue, which is upstream to the prenylated cysteine. It is thought that palmitoylation provides a “firm” anchoring for the modified protein into the cell membrane for optimal interaction with its respective effector proteins. (B) Schematic representation of protein prenylation pathway. MVA is the precursor for the biosynthesis of Fpp and GGpp. FTase farnesylates nuclear lamins (A and B), Ras and g-subunits of trimeric G proteins. GGTase I geranylgeranylates G proteins, such as Cdc42, Rac1 and Rap1. GGTase II facilitates prenylation of Rab subfamily of G proteins. Recent studies have characterized these enzymes in the islet b-cell in relation to their roles in various cellular events including vesicular transport, cytoskeletal remodeling, proliferation and insulin secretion (see text for additional details).
3. Protein prenyl transferases, subunit composition and substrate specificity
As stated above, protein prenylation involves incorporation of either farnesyl pyrophosphate (Fpp) by FTase or geranylgeranyl pyrophosphate (GGpp) by GGTase (Fig. 1B). At least three distinct prenyl transferases have been described in the islet β-cell. The FTase and GGTase-I are referred to as CAAX prenyl transferases because their substrate proteins share a conserved CAAX motif at the C-terminal region (as above). The GGTase-II (also termed as Rabgeranyl geranyl transferase; RGGT) prenylates Rab Gproteins at a different motif (CXC or CC motif) and hence is termed as non-CAAX prenyltransferase. FTase, GGTase-I and GGTase-II are heterodimeric consisting of α-and β-subunits. The FTase and GGTase-I share a common α-subunit (FTase/GGTase-α), but distinct β-subunits. The α-subunit is the regulatory subunit whereas the β-subunit confers substrate specificity. Examples of farnesylated proteins include Ras, lamins A/B and certain γsubunits of trimeric Gproteins. Cdc42, Rac1 and Rap1 are prenylated by GGTase-I, whereas Rab Gproteins (Rab3A, Rab27A) undergo prenylation mediated by GGTase-II (Fig. 1B; Refs. [7–12]). Recent studies have demonstrated expression and subcellular distribution of subunits of FTase, GGTase-I and GGTase-II in clonal β-cells, rodent islets and human islets [7,15–18].
4. Summary of evidence to implicate protein prenylation is essential for islet function including GSIS
4.1. Studies of pharmacological inhibitors
Original studies from the laboratories of Metz et al. [19] and Li et al. [20] utilized inhibitors of cholesterol biosynthesis (e.g., lovastatin) to examine roles of protein prenylation in insulin secretion. Statins inhibit the synthesis of MVA, a precursor for the biosynthesis of Fpp and GGpp. These studies in clonal β-cells and normal rodent islets have demonstrated significant inhibition of nutrient-induced insulin secretion by lovastatin [19,20]. Coprovision of MVA reversed the inhibitory effect of statins on GSIS. Furthermore, these studies have also demonstrated significant alterations in the subcellular distribution of G proteins following inhibition of prenylation with statins. Based on these investigations, it was concluded that inhibition of protein prenylation in β-cells results in selective accumulation of unprenylated G proteins in the soluble compartment, possibly interfering with the interaction of these proteins with their respective effector proteins, which may be required for nutrient-induced insulin secretion [19]. Further studies along these lines utilized more generic inhibitors of protein isoprenylation (e.g., limonene, perillic acid) were not very conclusive because of their nonspecific and cytotoxic effects on islet function [19]. More recent studies utilized selective inhibitors of FTase and GGTases including allyl- or vinyl-farnesols or geranylgeraniols, FTI-277, GGTI-2147 to inhibit islet endogenous FTase and GGTases (Table 1; see Fig. 2 for chemical structures of these inhibitors). Data from these studies provided compelling evidence to implicate that protein prenylation is essential for GSIS to occur.
Table 1.
Pharmacological inhibitors used to study roles of protein prenylation in islet function.
| Inhibitors used | Target proteins | Consequence | References |
|---|---|---|---|
| Lovastatin, Simvastatin | HMG-CoA reductase | Inhibition of GSIS | [19,20,22] |
| Limonene | FTase | Inhibition of GSIS | [19] |
| Manumycin | FTase | Inhibition of GSIS | [22] |
| Perillic acid | FTase | Inhibition of GSIS | [19] |
| Allyl and vinyl farnesols | FTase | Inhibition of GSIS | [21] |
| Ally and vinyl geraniols | GGTase | Inhibition of GSIS | [21] |
| FTI-277 | FTI | Inhibition of GSIS | [16] |
| GGTI-2147; GGTI-2368 | GGTase | Inhibition of GSIS | [15] |
| Zaragozic acid A | Squalene synthetase | Inhibition of GSIS | [22] |
Fig. 2.
Structures of various inhibitors of prenyltransferases employed to examine the roles of this pathway in glucose-stimulated insulin secretion. Structures of some generic and specific inhibitors of FTase/GGTase and sqaulenesynthetase used to study roles of these pathways in physiological insulin secretion (see Table 1 for their target proteins and functional effects on GSIS).
Recent investigations by Zuniga-Hertz and associates have suggested additional roles for events in cellular cholesterol metabolic pathway in GSIS from INS-1E cells and mouse islets [22]. They observed a significant reduction in GSIS by simvastatin under acute conditions (2 h exposure). Statin-mediated inhibition of GSIS was reversed by exogenous GGpp suggesting that statin treatment results in depletion of endogenous pools of GGpp necessary for prenylation and GSIS. They also noted no significant alterations in membrane cholesterol content under these conditions. However, long-term exposure of these cells to simvastatin (24 h) significantly attenuated total cellular content of cholesterol and GSIS. In addition, the membrane fluidity is also significantly increased under these conditions. Interestingly, however, zaragozic acid A, a specific inhibitor of sqaulene synthetase (Table 1 and Fig. 2), a signaling step downstream to Fpp biosynthesis, inhibited cholesterol biosynthesis as well as GSIS. Based on these findings the authors concluded that protein prenylation and cholesterol biosynthetic pathways represent dual signaling mechanisms involved in GSIS [22]. Together, these findings provided additional clues with regard to regulatory roles of MVA-derived intermediates in GSIS. However, it should be kept in mind that pharmacological inhibition of squalene synthetase also results in significant accumulation of Fpp [23,24], which, in turn, could contribute to the observed inhibitory effects on GSIS. Additional studies are needed to further assess this working model.
4.2. Studies utilizing molecular biological approaches
The aforestated data from pharmacological approaches suggesting novel regulatory roles of protein prenylation in GSIS were further confirmed by molecular biological approaches. For example, Veluthakal et al. [15] have demonstrated that overexpression of an inactive mutant of FTase/GGTase-a markedly attenuated glucose- but not KCl-induced insulin secretion from pancreatic β-cells. In addition, siRNA-mediated knockdown of FTase-b subunit significantly suppressed glucose- and mitochondrial fuel-induced insulin release in INS 832/13 cells [16,25]. Further- more, published evidence suggests that siRNA-mediated knock-down of α- or β-subunits of GGTase-II and Rab escort protein (REP1) markedly attenuates GSIS in INS 832/13 cells [17]. These findings provided the first evidence in support of key roles for GGTase-II and its regulatory proteins in GSIS. Together, these observations further affirm roles of FTase/GGTase in nutrient-induced insulin release, and the data from pharmacological and molecular biological approaches further validate our original hypothesis that protein prenylation represents a key signaling step in the cascade of events leading to GSIS.
5. How does glucose regulate protein prenylation to promote GSIS?
Emerging data appear to provide support for, at least, three potential mechanisms whereby stimulatory concentrations of glucose regulate protein prenylation culminating in GSIS.
First, key observations from Lorenz and associates in INS-1 832/13 cells suggested significant increase in Fpp levels rapidly following glucose stimulation in a time course that mirrors first phase of GSIS [26]. Such an increase in Fpp, a substrate for FTase, would be ideal for prenylation of key G proteins necessary for GSIS to occur. These studies, however, did not quantify the levels of GGpp under these conditions.
Second, it is likely that stimulatory concentrations of glucose increase the catalytic activation of FTase/GGTase activities under conditions favorable for GSIS. In an attempt to address this question, Goalstone and associates quantified the levels of expression and catalytic activity of FTase and GGTase-I in INS-1 832/13 cells and normal rodent islets exposed to stimulatory glucose concentrations [18]. Their findings suggested that an insulinotropic concentration of glucose (20 mM) markedly stimulated the expression of the FTase/GGTase-a, but not the β-subunits of FTase or GGTase-1 INS 832/13 cells and rat islets. Furthermore, they also observed that glucose significantly stimulated (2.5- to 4.0-fold over basal) the activities of FTase and GGTase-1 in both cell types [18].
A third potential possibility would be acute regulation of FTase/GGTase-α activation via phosphorylation–dephosphorylation mechanisms. Published evidence, albeit not in the islet β-cell, suggests that FTase/GGTase-a undergoes phosphorylation, which, in turn, results in the functional activation of the enzyme. Studies by Goalstone and associates demonstrated that insulin stimulates phosphorylation and activation of the FTase/GGTase thereby increasing the abundance of Ras (a farnesylated protein) and RhoA (a geranylated protein) necessary for its mitogenic effects [27]. They also reported that substitution of alanine for serine residues of FTase/GGTase-a creates a dominant negative mutant for FTase/GGTase, and overexpression of this mutant markedly attenuated the ability of insulin to increase the activities of FTase/GGTases and the abundance of prenylated Ras and Rho [27]. Along these lines, studies by Veluthakal and associates have shown that forced expression of dominant negative FTase/GGTase-a resulted in significant inhibition of glucose-, but not KCl-mediated insulin secretion from pancreatic β-cells, suggesting a potential possibility that phosphorylation of critical serine residues on FTase/GGTase-α may be necessary for GSIS to occur [15]. Together, findings from above described studies provide evidence to suggest that GSIS involves activation of the endogenous islet prenyltransferases by glucose. The above data provide a working model and basis for future investigations in this field including understanding of the regulatory effects of glucose on expression and activity of GGTase-II in pancreatic β-cells.
6. Some examples of prenylation-dependent signaling events in the islet β-cell
6.1. Protein prenylation in acute regulation of generation of reactive oxygen species (ROS)
Experimental evidence in multiple cell types suggests that small G proteins play key regulatory roles in cytoskeletal remodeling, a requisite signaling step for vesicular fusion with the plasma membrane [6,7]. Reactive oxygen species (ROS) generated via activation of NADPH-oxidase has been shown to promote cytoskeletal remodeling [28]. In the context of islet β-cell and GSIS, recent evidence from the Carpinelli’s laboratory suggested that an acute increase in the generation of phagocyte-like NADPH-oxidase (Nox2)-mediated ROS may be necessary for GSIS [29]. Using rodent islets and INS-1 832/13 cells, we further tested the hypothesis that activation of specific G proteins is necessary for nutrient-mediated intracellular generation of ROS [30]. We observed significant increase in ROS generation in β-cells following exposure to stimulatory glucose or a mixture of mitochondrial fuels (mono-methylsuccinate plus α-ketoisocaproic acid). Selective inhibitors of Nox2 (apocynin or diphenyleneiodonium chloride) or siRNA-p47phox, one of the subunits of Nox2, markedly suppressed nutrient-induced ROS generation suggesting that Nox2 mediates nutrient-induced ROS generation in pancreatic β-cells. Moreover, FTI-277 or GGTI-2147, known inhibitors of protein prenylation (Table 1) significantly suppressed nutrient-induced ROS generation, suggesting that activation of one (or more) prenylated small G proteins and/or γ-subunits of trimeric G proteins is involved in this signaling axis. In addition, depletion of endogenous GTP levels with mycophenolic acid significantly reduced glucose-induced Rac1 activation and ROS generation in these cells. Treatment of INS 832/13 cells or rat islets with pertussis toxin (Ptx), which ADP ribosylates and blocks inhibitory class of trimeric G proteins (i.e., Gi or Go), markedly attenuated glucose-induced ROS generation in these cells, implicating activation of a Ptx-sensitive G protein in this signaling cascade. Based on these data we concluded that a prenylated Ptx-sensitive signaling step couples Rac1 activation in the signaling steps necessary for glucose-mediated generation of ROS in the pancreatic β-cells [30]. In addition to ROS generation several intracellular signaling events involved in GSIS appear to be sensitive to protein prenylation (see below).
6.2. Protein farnesylation is essential for cytoskeletal remodeling
To further evaluate regulatory roles for prenylation in GSIS, we studied the signaling steps involved in Raf-1/extracellular signal-related kinase (ERK1/2) signaling pathway, which has been implicated in cytoskeletal remodeling, in glucose-induced Rac1 activation and insulin secretion in the pancreatic β-cell. We demonstrated that specific inhibitors of FTase (FTI-277 or FTI-2628) or siRNA-FTase-b resulted in a significant inhibition of glucose-stimulated ERK1/2 and Rac1 activation and insulin secretion [16]. Pharmacologic inhibition of Raf-1 kinase using GW-5074 markedly reduced the stimulatory effects of glucose on ERK1/2 phosphorylation, Rac1 activation, and insulin secretion, suggesting that Raf-1 kinase activation may be upstream to activation of ERK1/2 and Rac1 leading to GSIS. Lastly, siRNA-ERK1/2 markedly attenuated glucose-induced Rac1 activation and insulin secretion. Based on these findings we proposed that protein farnesylation is critical for glucose-mediated regulation of the Raf-ERK-Rac1 signaling pathway to facilitate cytoskeletal reorganization and exocytotic secretion of insulin [16].
6.3. A farnesylated G protein regulates cell survival signaling pathways in pancreatic β-cells
Recently, we investigated putative roles of protein prenylation, specifically farnesylation in the regulation of insulin-like growth factor (IGF)-induced protein kinase-B/Akt phosphorylation in INS 1–832/13 cells and normal rodent islets [31]. Our findings indicated that either pharmacological inhibition of FTase (FTI-277 or FTI-2628) or transfection of siRNA-FTase-b subunit markedly augmented Akt activation under basal as well as IGF-1-stimulated conditions. Furthermore, we also noted that the relative abundance of phosphorylated FoxO1 and Bad was increased implicating inactivation of critical components of the cell death machinery. FTI-induced Akt activation was also suppressed by the PI3-kinase inhibitor, LY294002. Interestingly, exposure of pancreatic β-cells to Ptx significantly potentiated Akt activation suggesting involvement of a Ptx-sensitive G protein in this signaling axis. In addition, prostaglandin E2, a known agonist of inhibitory G proteins, significantly attenuated FTI-induced Akt phosphorylation. Taken together, our observations suggested expression of a farnesylated G-protein in pancreatic β-cells (named protein kinase B inhibitor; Probin; Ref. [31]), which appears to suppress Akt activation and subsequent cell survival signaling steps. Further charcterization of this protein in underway in our laboratory.
Based on the evidence we reviewed above, we propose a model (Fig. 3) which implicates protein prenylation in physiologicalinsulin secretion from the pancreatic β-cell. Glucose metabolism leads to increases in the levels of soluble second messenger molecules including GTP [5] and intermediates of isoprenoid pathway (Fpp; 26). Published evidence also suggests increases in the catalytic activity of FTase/GGTase in a glucose-stimulated β-cell [18]. These signaling steps result in increased prenylation of key G proteins culminating in their targeting to appropriate cellular compartment (plasma membrane and secretory granule) for optimal interaction and regulation of their respective effector proteins (Raf-1, ERK1/2, Nox2) to promote conditions (e.g., ROS generation, cytoskeletal remodeling) conducive for secretory granule fusion with the plasma membrane leading to exocytotic secretion of insulin [32–34].
Fig. 3.
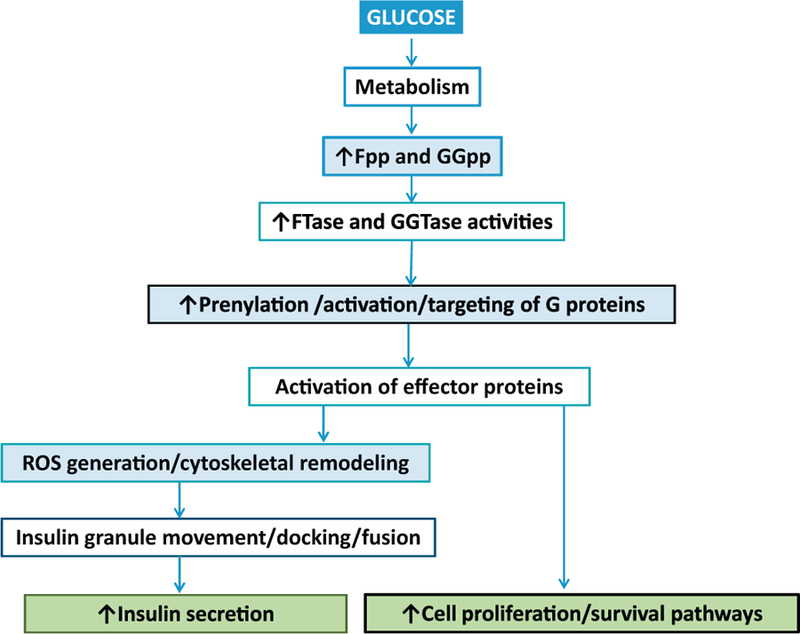
Our proposed model depicting roles of protein prenylation in islet β-cell function glucose metabolism leads to increase in intracellular levels of second messenger molecules such as GTP [5] and intermediates of cholesterol biosynthetic pathway, including Fpp [26]. It is not clear if GGpp levels are also increased under these conditions. Fpp and GGpp are the substrates for prenyltransferases (FTase, GGTase-I and GGTase-II; Fig. 1A and B), which prenylate and activate a variety of signaling G proteins, which are critical for islet β-cell function. Prenylation is also necessary for targeting of these proteins to appropriate cellular compartments [7] for optimal interaction of prenylated proteins with their respective effector proteins leading to regulation of intracellular pathways (e.g., ROS generation and cytoskeletal remodeling), which are requisite for translocation of insulin laden secretory granules from distal sites to the plasma membrane for docking and fusion to result in exocytotic secretion of insulin. We also propose that prenylation of specific Gproteins (small molecular mass as well as the g-subunits of trimeric G proteins) is necessary for coupling of signaling pathways leading to increased cell proliferation and activation of survival pathways in the pancreatic β-cell.
7. Protein prenylation in models of human disease: potential mechanisms underlying the islet dysfunction in models of impaired insulin secretion, metabolic stress and diabetes
Recent estimates indicate at least 300 prenylated proteins in the human genome; the majority of which are implicated in a variety of cellular processes including cell growth, differentiation, cytoskeletal function and vesicle trafficking [35]. It is becoming increasingly clear that alterations in prenylation of cellular proteins (Ras, nuclear lamins) are involved in multiple human diseases including cancer, neurodegenerative disorders, retinitis pigmentosa and premature ageing syndromes. FTIs are being actively investigated as therapeutics for the treatment of cancer and progeria [35,36]. Recently Khan et al. have reported hyper-activation of macrophages and associated induction of erosive arthritis in mice lacking GGTase-1, thus raising potential for regulatory roles of geranylgeranylation in inflammatory cell signaling [37]. Interestingly, significantly elevated levels of active (GTP-bound) Rac1, Cdc42 and RhoA were seen in GGTase-deficient cells suggesting potential alternate mechanisms for the activation of these unprenylated G proteins under pathological conditions [37]. Studies by Dunford and associates have suggested that inhibition of protein prenylation by nitrogen-containing bisphosphonates caused paradoxical and sustained activation of Rac, Cdc42 and Rho G-proteins in J774 macrophages [38]. Available evidence also suggests significant alterations in subcellular distribution and targeting of G proteins under conditions in which their prenylation is suppressed via mutation of prenylatable cysteine in the CAAX motif [37,39,40]. These observations clearly show prenylation-independent activation of G proteins in “inappropriate” cellular compartments, thus raising an important question if such a signaling step leads to cellular pathology.
Compatible with above observations are our findings in the islet β-cell to indicate increased abundance of GTP-bound (active) Rac1 in β-cell models of glucolipotoxicity, ER stress and diabetes [41]. We also observed hyperactivation of Rac1 in human islets exposed to glucotoxic conditions and in islets derived from the ZDF rat [42]. These findings have led us to propose that Rac1 plays both positive and negative modulatory roles in the regulation of β-cell function in the sense that while it is critical for GSIS, Rac1 also exerts damaging roles under pathological conditions by inducing Nox2 activity to create excessive oxidative stress, mitochondrial damage and cell demise [41,42]. In support of our findings implicating sustained activation of Rac1 in islet β-cell in in vitro and in vivo models of glucolipotoxicity and T2DM are observations by Zhou and associates demonstrating significant increase in the expression and activation of Rac1 in pancreatic tissue from ob/obmice, a model for obesity; these findings were replicated in insulin-secreting NIT-1b cells under hyper glycemic and hyper lipidemic conditions in vitro [43]. Recently, Sun et al. [44] implicated Rac1 as key signaling protein to link obesity and NADPH oxidase derived oxidative stress in Chinese overweight adolescents. Sustainedactivation of Rac1–Nox2 signaling pathway has been reported in multiple cell types under various pathological conditions including cardiovascular diseases and diabetes [41,45,46].
In the context of above discussion, one important question that needs to be addressed is whether Gprotein (Rac1) activation under various pathological conditions is independent of prenylation and membrane association. In other words, do these G proteins get activated in inappropriate cellular compartments (e.g., cytosol) and promote cellular dysfunction? Along these lines, recent studies by Reddy et al. [47] provide support for the formulation that G proteins, such as Rac1, may not require prenylation and plasma membrane localization to promote cell regulation. Briefly, these investigators generated a non-prenylatable emerald green fluorescent protein-Rac1 fusion protein (EmGFP-C189A-Rac1) to determine potential regulation of neurite growth by non-prenylatable Rac1. They observed that expression of EmGFP-C189A-Rac1 induced cell clustering while increasing neurite initiation. More importantly, the non-prenylatable Rac1 remained associated with the cytosolic compartment in its GTP-bound (active) conformation. Based on these observations these investigators proposed that activation of Rac1 may not require prenylation-dependent plasma membrane association and that it could contribute to differential activation of cytosolic signaling pathways for regulation of cell function [47]. Together, it is likely that the sustained (constitutive) activation of specific G proteins under various pathological conditions (discussed above) might occur in the absence of requisite prenylation and membrane association. Additional studies are needed to further assess the cellular mechanisms underlying G protein mediated signaling pathways or mechanisms responsible for aberrant prenylation of G proteins leading to pathology of diseases including T2DM and associated complications. Published evidence along these lines discussed below.
8. How do glucotoxic or diabetic conditions affect protein prenylation?
Unfortunately, limited information is available in this field, specifically in the functional status of protein prenyltransferases in the islet β-cell. Recent observations by Chen et al. [48] in vascular smooth muscle cells (VSMC) offer fresh insights into potential alterations in enzymes of the MVA pathway in streptozotocin-induced diabetic BALB/c mouse model. These investigators demonstrated a temporal relationship between the onset of atherogenesis (8–16 weeks following induction of diabetes) and the expression (mRNA and protein) levels of key enzymes of MVA pathway, including HMG CoA reductase, Fpp synthase, GGpp synthase, FTase and GGTase in VSMC. Expression levels of sqaulene synthase and phospho-HMG CoA reductase remained unchanged in these cells under diabetic conditions. The data accrued in diabetic animal models were replicated in VSMC incubated under glucotoxic conditions (22.2 mM glucose; 48 h; 48). These findings offer additional clues with regard to potential pathophysiological roles of this signaling pathway in the induction of atherosclerosis, specifically under glucotoxic/diabetic conditions. Future studies will determine the flux of metabolites in this pathway in addition to the prenylation status of specific G proteins and activation of downstream effector proteins.
9. Evidence of fate of protein prenyltransferases under various pathological conditions
Original studies by Kim et al. in 2001 [49] reported that FTase/GGTase-a was cleaved by caspase-3 during apoptosis in Rat-2/H-ras, W4 and Rat-1 cells treated with LB42708 (an FTI), anti-Fas antibody and etoposide, respectively. Caspase-3 mediatedproteolysis of FTase/GGTase-a was prevented by caspase-3 inhibitors. In addition, expression of D59A FTase/GGTase-a mutant significantly desensitized cells to etoposide-induced death. Based on these findings, the authors proposed that cleavage of FTase/GGTase-a by caspase contributes to the progression of apoptosis. We arrived at similar conclusions in pancreatic β-cells undergoing apoptosis following exposure to genotoxic agents, such as etoposide [50]. We were able to demonstrate that exposure of INS-1 832/13 cells or normal rat islets to etoposide leads to significant activation of caspase-3 and subsequent degradation of FTase/GGTase-a; this was prevented by Z-DEVD-FMK, a known inhibitor of caspase-3. The results showed that treatment of cell lysates with recombinant caspase-3 also caused FTase/GGTaseα-subunit degradation. Moreover, nifedipine, a calcium channel blocker, markedly attenuated etoposide-induced caspase-3 activation, FTase/GGTase α-subunit degradation in INS 832/13 cells and normal rat islets. Further, nifedipine significantly restored etopo-side-induced loss in metabolic cell viability in INS 832/13 cells. Based on these findings, we concluded that etoposide induces loss in cell viability by inducing mitochondrial dysfunction, caspase-3 activation and degradation of FTase/GGTase α-subunit. Together, these data provide additional insights into mechanisms underlying FTase/GGTase degradation and potentially dysfunction leading to cellular apoptosis. It is also noteworthy that our findings indicate regulatory roles for alterations in cytosolic calcium in etoposide [50] and glucose-induced effects on caspase 3 activation [51] in isolated β-cells as evidenced by sensitivity of this signaling step to calcium channel blockers. These findings are in agreement with observations by Wang et al. who reported cytoprotective effects, by nifedipine, against endoplasmic reticulum stress and apoptosis induced by glucotoxic conditions in pancreatic β-cells [52]. Therefore, it is likely that such conditions could also promote β-cell dysfunction via calcium-mediated mitochondrial dysregulation, caspase 3 activation and potentially degradation of key cellular proteins leading to loss in cell viability. This needs to be verified experimentally. Indeed, recent observations by Xu et al. have provided compelling evidence to suggest a marked reduction by verapamil, a calcium channel blocker, of increased expression of pro-apoptotic thioredoxin-interacting protein expression and restoration of normal β-cell survival and function in the BTBR ob/ob mice [53]. Together, findings from above studies support the hypothesis that increased influx and subsequent overload of mitochondrial calcium leads to dysregulation of cellular function in the islet β-cell. Thus, calcium channel blockers (verapamil and nifedipine) appear to exert cytoprotective effects in β-cells against noxious stimuli and improve β-cell survival and function.
10. Conclusions and future directions
Several lines of evidence appear to implicate novel regulatory roles for protein prenylation in physiological insulin secretion. It is becoming increasingly clear that early steps in glucose metabolism lead to generation of relevant signals (biosynthesis of GTP, Fpp and GGpp) leading to functional activation of various prenyl transferases (FTase, GGTase-I and GGTase-II) and their regulatory proteins/factors (REP-1) endogenous to the pancreatic β-cell. Timely activation of these proteins leads to prenylation and location/association of candidate Gproteins with relevant membranous compartments (plasma membrane, mitochondria, secretory granules) for optimal regulation of their downstream effector proteins and signaling pathways leading to physiological insulin secretion.
Data accrued in several recent studies involving pharmacological inhibitors of prenyl transferases clearly suggest that prenylation plays a requisite role in physiological insulin secretion. Furthermore, molecular biological (dominant negative mutants and siRNAs) observations affirm support to pharmacological data. It would be worthwhile to extend these studies to animal models in which the prenyl transferases and their regulatory proteins/factors are conditionally deleted in the pancreatic β-cell to further assess the roles of these signaling pathways in islet function including insulin secretion.
As stated above, it is noteworthy that, even though protein prenylation is not acutely regulable, it dictates the subsequent modification steps, such as carboxylmethylation (Fig. 1A) that are acutely regulated and could determine the functional status and subcellular location of a given G protein. Therefore, in the context of G protein prenylation status under pathological conditions, it is important to further understand the mechanisms underlying potential defects in the prenylation signaling steps in target cells/tissues under conditions of gluco-, lipo-, or glucolipotoxicity and diabetes. It is likely that specific G proteins might undergo prenylation-independent sustained activation in non-relevant cellular compartments under these conditions leading to cellular pathology. A deeper understanding of the functional consequences of aberrant prenylation of specific G proteins leading to alterations in their subcellular targeting and activation would prove beneficial in the development of novel therapeutics for the treatment and/or halting metabolic defects of the islet leading to impaired insulin secretion, loss of β-cell mass and the onset of diabetes. Along these lines, recent studies have identified T-cell lymphoma invasion and metastassis-1 (Tiam1) as one of the guanine nucleotide exchange factors for Rac1 in the islet β-cell and retina [54–56]. It might represent one of the factors that could mediate sustained activation of Rac1 in target tissues under the duress of glucolipotoxicity and diabetes [54–56]. It is our hope that these observations provide basis for future investigations in the development novel therapeutics to prevent/halt inappropriate activation of Gproteins in non-relevant cellular compartments. These possibilities remain to be verified in-depth, and they are being actively pursued in our laboratories currently.
Acknowledgements
The work described in this commentary was supported by grants (to AK and RAK) from the National Institutes of Health, the Juvenile Diabetes Research Foundation and (to AK) the Department of Veterans Affairs. AK is the recipient of a Senior Research Career Scientist Award from the Department of Veterans Affairs. RK is also supported by an unrestricted grant to the Ophthalmology Department from Research to Prevent Blindness. We thank our colleagues and collaborators for their support and assistance in the accrual of experimental data, which are cited in this commentary. We also thank Ms. Syeda Khadija and Mr. Vaibhav Sidarala for assistance in preparing figures.
Abbreviations:
- ERK1/2
extracellular signal-related kinase 1/2
- Fpp
farnesyl pyrophosphate
- FTase
farnesyl transferase
- FTase/GGTase-α
common α-subunit of FTase and GGTase
- FTI
farnesyl transferase inhibitor
- GGpp
geranylgeranyl pyrophosphate
- GGTase-I
geranylgeranyl transferase-I
- GGTase-II
geranylgeranyl transferase-II
- GGTI
geranylgeranyl transferase inhibitor
- GSIS
glucose-stimulated insulin secretion
- GTP
guanosine triphosphate
- G proteins
GTP-binding proteins
- ICMT
isoprenylcysteine methyl transferase
- MVA
mevalonic acid
- Nox2
phagocyte-like NADPH oxidase
- Ptx
pertussis toxin
- Rac1
Ras-related C3 botulinum toxin substrate-1
- Rce1
Ras converting enzyme 1
- REP-1
Rab escort protein 1
- RGGT
RabGGTase (also referred to as GGTase-II)
- ROS
reactive oxygen species
- VSMC
vascular smooth muscle cells
- ZDF
rat Zucker diabetic fatty rat
References
- [1].Jitrapakdee S, Wutthisathapornchai A, Wallace JC, MacDonald MJ, Regulation of insulin secretion: role of mitochondrial signaling, Diabetologia 53 (2010) 1019–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Prentki M, Matschinsky FM, Madiraju SR, Metabolic signaling in fuel-induced insulin secretion, Cell Metab 18 (2013) 162–185. [DOI] [PubMed] [Google Scholar]
- [3].Berggren PO, Leibiger IB, Novel aspects on signal transduction in the pancreatic beta cell, Nutr. Metab. Cardiovasc. Dis 16 (Suppl. 1) (2006) S7–S10. [DOI] [PubMed] [Google Scholar]
- [4].Komatsu M, Takei M, Ishii H, Sato Y, Glucose-stimulated insulin secretion: a newer perspective, J. Diabetes Investig 4 (2013) 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Metz SA, Rabaglia ME, Pintar TJ, Selective inhibitors of GTP synthesis impede exocytotic insulin release from intact rat islets, J. Biol. Chem 267 (1992) 12517–12527. [PubMed] [Google Scholar]
- [6].Wang Z, Thurmond DC, Mechanisms of biphasic insulin-granule exocytosis-roles of the cytoskeleton, small GTPases and SNARE proteins, J. Cell Sci 122 (2009) 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kowluru A, Small G proteins in islet beta-cell function, Endocr. Rev 31 (2010) 52–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kowluru A, Regulatory roles for small G proteins in the pancreatic beta cell: lessons from models of impaired insulin secretion, Am. J. Physiol. Endocrinol. Metab 285 (2003) E669–E684. [DOI] [PubMed] [Google Scholar]
- [9].Kowluru A, Protein prenylation in glucose-induced insulin secretion from the pancreatic islet beta cell: a perspective, J. Cell. Mol. Med 12 (2008) 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Casey PJ, Seabra MC, Protein prenyl transferases, J. Biol. Chem 271 (1996) 5289–5292. [DOI] [PubMed] [Google Scholar]
- [11].Lane KT, Beese LS, Thematic review series: lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I, J. Lipid Res 47 (2006) 681–699. [DOI] [PubMed] [Google Scholar]
- [12].McTaggart SJ, Isoprenylated proteins, Cell. Mol. Life Sci 63 (2006) 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mohammed AM, Chen F, Kowluru A, The two faces of protein palmitoylation in islet beta-cell function: potential implications in the pathophysiology of islet metabolic dysregulation and diabetes, Recent Pat. Endocr. Metab. Immune Drug Discov 7 (2013) 203–212. [DOI] [PubMed] [Google Scholar]
- [14].Navarro-Lerida I, Sanchez-Perales S, Calvo M, Rentero C, Zheng Y, Enrich C, Del Pozo MS, A palmitoylation switch mechanism regulates Rac1 function and membrane organization, EMBO J 31 (2011) 534–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Veluthakal R, Kaur H, Goalstone M, Kowluru A, Dominant-negative alpha-subunit of farnesyl- and geranyltransferase inhibits glucose-stimulated, but not KCl-stimulated, insulin secretion in INS 832/13 cells, Diabetes 56 (2007) 204–210. [DOI] [PubMed] [Google Scholar]
- [16].Kowluru A, Veluthakal R, Rhodes CJ, Kamath V, Syed I, Koch BJ, Protein farnesylation-dependent Raf/extracellular signal-related kinase signaling links to cytoskeletal remodeling to facilitate glucose-induced insulin secretion in pancreatic beta-cells, Diabetes 59 (2010) 967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Arora D, SyedI I, Machhadieh B, McKenna C, Kowluru A, Rab-geranyl geranyl transferase regulates glucose-stimulated insulin secretion from pancreatic beta cells, Islets 4 (2012) 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Goalstone M, Kamath V, Kowluru A, Glucose activates prenyl transferases in pancreatic islet beta-cells, Biochem. Biophys. Res. Commun 391 (2010) 895–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Metz SA, Rabaglia ME, Stock JB, Kowluru A, Modulation of insulin secretion from normal rat islets by inhibitors of the post-translational modifications of GTP-binding proteins, Biochem. J 295 (1993) 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li G, Regazzi R, Roche E, Wollheim CB, Blockade of mevalonate production by lovastatin attenuates bombesin and vasopressin potentiation of nutrient-induced insulin secretion from HIT-T15 cells, Biochem. J 289 (1993) 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Amin R, Chen HQ, Tannous M, Gibbs R, Kowluru A, Inhibition of glucose-and calcium-induced insulin secretion from betaTC3 cells by novel inhibitors of protein isoprenylation, J. Pharmacol. Exp. Ther 303 (2002) 82–88. [DOI] [PubMed] [Google Scholar]
- [22].Zúñiga-Hertz JP, Rebelato E, Kassan A, Khalifa AM, Ali SS, Patel HH, Abdulkader F, Distinct pathways of cholesterol biosynthesis impact on insulin secretion, J. Endocrinol 224 (2015) 75–84. [DOI] [PubMed] [Google Scholar]
- [23].Weivoda MM, Hohl RJ, Effects of farnesyl pyrophosphate accumulation on calvarial osteoblast differentiation, Endocrinology 152 (2011) 3113–3122. [DOI] [PubMed] [Google Scholar]
- [24].Henneman L, van Cruchten AG, Kulik W, Waterham HR, Inhibition of the isoprenoid biosynthesis pathway: detection of intermediates by UPLC-MS/MS, Biochim. Biophys. Acta 1811 (2011) 227–233. [DOI] [PubMed] [Google Scholar]
- [25].Matti A, Kyathanahalli C, Kowluru A, Protein farnesylation is requisite for mitochondrial fuel-induced insulin release: further evidence to link reactive oxygen species generation to insulin secretion in pancreatic beta-cells, Islets 4 (2012) 74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lorenz MA, El Azzouny MA, Kennedy RT, Burant CF, Metabolome response to glucose in the beta-cell line INS-1 832/13, J. Biol. Chem 288 (2013) 10923–10935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Goalstone M, Carel K, Leitner JW, Draznin B, Insulin stimulates the phosphorylation and activity of farnesyltransferase via the Ras-mitogen-activated protein kinase pathway, Endocrinology 138 (1997) 5119–5124. [DOI] [PubMed] [Google Scholar]
- [28].Stanley A, Thompson K, Hynes A, Brakebusch C, Quondamatteo F, NADPH oxidase complex-derived reactive oxygen species, the actin cytoskeleton, and Rho GTPases in cell migration, Antioxid. Redox Signal 20 (2014) 2026–2042. [DOI] [PubMed] [Google Scholar]
- [29].Morgan D, Rebelato E, Abdulkader F, Graciano MF, Oliveira-Emilio HR, Hirata AE, et al. , Association of NAD(P)H oxidase with glucose-induced insulin secretion by pancreatic beta-cells, Endocrinology 150 (2009) 2197–2201. [DOI] [PubMed] [Google Scholar]
- [30].Syed I, Kyathanahalli CN, Kowluru A, Phagocyte-like NADPH oxidase generates ROS in INS 832/13 cells and rat islets: role of protein prenylation, Am. J. Physiol. Regul. Integr. Comp. Physiol 300 (2011) R756–R762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kyathanahalli CN, Kowluru A, A farnesylated G-protein suppresses Akt phosphorylation in INS 832/13 cells and normal rat islets: regulation by pertussis toxin and PGE2, Biochem. Pharmacol 81 (2011) 1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kalwat MA, Yoder SM, Wang Z, Thurmond DC, A p21-activated kinase (PAK1) signaling cascade coordinately regulates F-actin remodeling and insulin granule exocytosis in pancreatic b cells, Biochem. Pharmacol 85 (2013) 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Thunduguru R, Chiu TT, Ramalingam L, Elmendorf JS, Klip A, Thurmond DC, Signaling of the p21-activated kinase (PAK1) coordinatesinsulin-stimulated actin remodelling and glucose uptake in skeletal muscle cells, Biochem. Pharmacol 92 (2014) 380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Asahara S, Shibutani Y, Truyama K, Inoue HY, Kawada Y, Etoh H, et al. , Ras-related C3 botulinum toxin substrate 1 (Rac1) regulates glucose-stimulated insulin secretion via modulation of F-actin, Diabetologia 56 (2013) 1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Novelli G, D’Apice MR, Protein farnesylation and disease, J. Inherit. Metab. Dis 35 (2012) 917–926. [DOI] [PubMed] [Google Scholar]
- [36].Resh MD, Targeting protein lipidation in disease, Trends Mol. Med 18 (2012) 206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Khan OM, Ibrahim MX, Jonsson IM, Karlsson C, Liu M, Sjogren AK, et al. , Geranyl geranyl transferase type I (GGTase-I) deficiency hyperactivates macrophages and induces erosive arthritis in mice, J. Clin. Investig 121 (2011) 628–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dunford JE, Rogers MJ, Ebetino FH, Phipps RJ, Coxon FP, Inhibition of protein prenylation by bisphosphonates causes sustained activation of Rac, Cdc42, and Rho GTPases, J. Bone Miner. Res 21 (2006) 684–694. [DOI] [PubMed] [Google Scholar]
- [39].Wong KW, Isberg RR, Yersinia psuedotuberculosis spatially controls activation and misregulation of host cell Rac1, PLoS Pathog 1 (2005) e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Michaelson D, Silletti J, Murphy G, D’Eusachio P, Rush M, Philips MR, Differential localization of Rho GTPases in live cells: regulation by hypervariable regions of RhoGDI binding, J. Cell Biol 152 (2001) 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kowluru A, Friendly, and not so friendly, roles of Rac1 in islet beta-cell function: lessons learnt from pharmacological and molecular biological approaches, Biochem. Pharmacol 81 (2011) 965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Syed I, Kyathanahalli CN, Jayaram B, Govind S, Rhodes CJ, Kowluru RA, et al. , Increased phagocyte-like NADPH oxidase and ROS generation in type 2 diabetic ZDF rat and human islets: role of Rac1-JNK1/2 signaling pathway in mitochondrial dysregulation in the diabetic islet, Diabetes 60 (2011) 2843–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhou S, Yu D, Ning S, Zhang H, Jiang L, He L, et al. , Augmented Rac1 expression and activity are associated with oxidative stress and decline of beta cell function in obesity, Cell. Physiol. Biochem 35 (2015) 2135–2148. [DOI] [PubMed] [Google Scholar]
- [44].Sun M, Huang X, Yan Y, Chen J, Wang Z, Xie M, et al. , Rac1 is a possible link between obesity and oxidative stress in chinese overweight adolescents, Obesity (Silver Spring) 20 (2012) 2233–2240. [DOI] [PubMed] [Google Scholar]
- [45].Kowluru A, Kowluru RA, Phagocyte-like NADPH oxidase [Nox2] in cellular dysfunction in models of glucolipotoxicity and diabetes, Biochem. Pharmacol 88 (2014) 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Elnakish MT, Hassanian HH, Janssen PM, Angelos MG, Khan M, Emerging role of oxidative stress in metabolic syndrome and cardiovascular diseases: important role of Rac/NADPH oxidase, J. Pathol 231 (2013) 290–300. [DOI] [PubMed] [Google Scholar]
- [47].Reddy JM, Samuel FG, McConnell JA, Reddy CP, Beck BW, Hynds DL, Non-prenylatable, cytosolic Rac1 alters neurite outgrowth while retaining the ability to be activated, Cell Signal 27 (2015) 630–637. [DOI] [PubMed] [Google Scholar]
- [48].Chen G-P, Zhang X-Q, Wu T, Li L, Han J, Du C-Q, Alteration of mevalonate pathway in proliferated vascular smooth muscle from diabetic mice: possible role in high-glucose-induced atherogenic process, J. Diabetes Res (2015) 379287, doi: 10.1155/2015/379287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kim KW, Chung HH, Chung CW, Kim IK, Miura M, Wang S, et al. , Inactivation of farnesyltransferase and geranylgeranyltransferase I by caspase-3: cleavage of the common alpha subunit during apoptosis, Oncogene 20 (2001) 358–366. [DOI] [PubMed] [Google Scholar]
- [50].Arora DK, Mohammed AM, Kowluru A, Nifedipine prevents etoposide-induced caspase-3 activation, prenyl transferase degradation and loss in cell viability in pancreatic beta-cells, Apoptosis 18 (2013) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Syeda K, Mohammed AM, Arora DK, Kowluru A, Glucotoxic conditions induce endoplasmic reticulum stress to cause caspase 3 mediated lamin B degradation in pancreatic betα-cells: protection by nifedipine, Biochem. Pharmacol 86 (2013) 1338–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wang Y, Gao L, Li Y, Chen H, Sun Z, Nifedipine protects INS-1 beta cell from high glucose-induced ER stress and apoptosis, Int. J. Mol. Sci 12 (2011) 7569–7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Xu G, Chen J, Jing G, Shalev A, Preventing beta cell loss and diabetes with calcium channel blockers, Diabetes 61 (2012) 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kowluru RA, Kowluru A, Veluthakal R, Mohammad G, Syed I, Santos JM, et al. , Tiam1–Rac1 signaling axis-mediated activation of NADPH oxidase-2 initiates mitochondrial damage in the development of diabetic retinopathy, Diabetologia 57 (2014) 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Syed I, Jayaram B, Sunasinghe W, Kowluru A, Tiam1/Rac1 signaling pathway mediates palmitate-induced, ceramide-sensitive generation of superoxides and lipid peroxides and the loss of mitochondrial membrane potential in pancreatic-beta cells, Biochem. Pharmacol 80 (2010) 874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Veluthakal R, Kumar B, Mohammad G, Kowluru A, Kowluru RA, Tiam1– Rac1 axis promotes activation of p38 MAP kinase in the development of diabetic retinopathy: evidence for a requisite role for protein palmitoylation, Cell. Physiol. Biochem 36 (2015) 208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]



