Key Points
Question
Can serum neurofilament light chain measurements serve as a reliable biomarker of disease worsening for patients with multiple sclerosis?
Findings
In this cohort study of 607 patients with multiple sclerosis, serum neurofilament light chain levels increased significantly faster in those experiencing disability worsening than in those who remained clinically stable. Serum neurofilament light chain level was associated with brain fraction loss, whereas this was less the case for clinical outcomes such as relapses or EDSS worsening.
Meaning
The association of serum neurofilament light chain level with changes in relevant clinical and neuroimaging outcomes in multiple sclerosis was confirmed, strengthening the potential of this biomarker as a measure of disease activity in multiple sclerosis; however, the clinically useful prognostic value of serum neurofilament light chain level for the individual patient was limited.
This cohort study compares changes in serum levels of neurofilament light chains with progression of disease among patients with multiple sclerosis.
Abstract
Importance
Blood sample–based biomarkers that are associated with clinically meaningful outcomes for patients with multiple sclerosis (MS) have not been developed.
Objective
To evaluate the potential of serum neurofilament light chain (sNFL) measurements as a biomarker of disease activity and progression in a longitudinal MS data set.
Design, Setting, and Participants
Single-center, ongoing, prospective observational cohort study of 607 patients with MS from the longitudinal EPIC (Expression, Proteomics, Imaging, Clinical) study at the University of California, San Francisco from July 1, 2004, through August 31, 2017. Clinical evaluations and sample collection were performed annually for 5 years, then at different time points for up to 12 years, with a median follow-up duration of 10 (interquartile range, 7-11) years. Serum NFL levels were measured using a sensitive single molecule array platform and compared with clinical and magnetic resonance imaging variables with the use of univariable and multivariable analyses.
Main Outcomes and Measures
The main outcomes were disability progression defined as clinically significant worsening on the Expanded Disability Status Scale (EDSS) score and brain fraction atrophy.
Results
Mean (SD) age of the 607 study participants at study entry was 42.5 (9.8) years; 423 (69.7%) were women; and all participants were of non-Hispanic European descent. Of 3911 samples sequentially collected, 3904 passed quality control for quantification of sNFL. Baseline sNFL levels showed significant associations with EDSS score (β, 1.080; 95% CI, 1.047-1.114; P < .001), MS subtype (β, 1.478; 95% CI, 1.279-1.707; P < .001), and treatment status (β, 1.120; 95% CI, 1.007-1.245; P = .04). A significant interaction between EDSS worsening and change in levels of sNFL over time was found (β, 1.015; 95% CI, 1.007-1.023; P < .001). Baseline sNFL levels alone were associated with approximately 11.6% of the variance in brain fraction atrophy at year 10. In a multivariable analysis that considered sex, age, and disease duration, baseline sNFL levels were associated with 18.0% of the variance in brain fraction atrophy at year 10. After 5 years’ follow-up, active treatment was associated with lower levels of sNFL, with high-potency treatments associated with the greater decreases in sNFL levels compared with platform therapies (high-potency vs untreated: β, 0.946; 95% CI, 0.915-0.976; P < .001; high-potency vs platform: β, 0.972; 95% CI, 0.948-0.998; P = .04).
Conclusions and Relevance
This study found that statistically significant associations of sNFL with relevant clinical and neuroimaging outcomes in MS were confirmed and extended, supporting the potential of sNFL as an objective surrogate of ongoing MS disease activity. In this data set of patients with MS who received early treatment, the prognostic power of sNFL for relapse activity and long-term disability progression was limited. Further prospective studies are necessary to assess the assay’s utility for decision-making in individual patients.
Introduction
Treatment options for multiple sclerosis (MS) have expanded considerably during the past quarter century,1,2 but decisions about whom to treat, the duration of treatment, and when to change therapy are typically based on tolerability and presumptions of likely long-term efficacy. The lack of sensitive laboratory measures of worsening, progression, and treatment response represent an important unmet need. Few biomarkers are validated, and fewer have been translated into clinical practice.3 In fact, only cerebrospinal fluid (CSF) oligoclonal bands and measurements of intrathecal IgG synthesis (both more than a half-century old in clinical practice) can meaningfully assist with a diagnosis of multiple sclerosis (MS). More recently, antibody titer for the JC polyomavirus has been associated with risk for progressive multifocal leukoencephalopathy in patients treated with natalizumab,4 and anti–aquaporin 4 antibodies can help distinguish MS from neuromyelitis optica spectrum disorder.5
Neuronal and axonal loss, thought to be present from the earliest stages of MS, are the primary mechanism contributing to irreversible neurologic disability.6 As a result, structural cellular proteins including neurofilaments are released into the extracellular space and can be detected in cerebrospinal fluid (CSF). The neurofilament light chain (NFL) and neurofilament heavy chain subunits are elevated in CSF samples from persons with MS7,8,9,10 and persons with other neurologic diseases.11,12,13 However, the use of repeated lumbar punctures for assessment of biomarkers is impractical. The development of a blood-based measure would represent a substantial advancement in MS management. Laboratory assays to detect neurofilament levels from the peripheral blood have been developed, and initial reports indicate that serum NFL (sNFL) levels are closely correlated with CSF levels and are associated with clinical and imaging measures of MS disease activity.14,15,16 Because neurofilaments are derived primarily from neurons, their detection in serum reflects diffusion of the molecule from the central nervous system compartment. However, long-term studies of sNFL concentrations and association with disease outcomes are lacking. Recently, the highly sensitive single molecule array (Simoa; Quanterix) technology was optimized for the detection of NFL in serum.8,14,17 In this study, we leverage the sensitivity of the single molecule array assay and access to a well-characterized cohort with up to 12 years of annual follow-up18 to assess the value of sNFL levels as a biomarker of disability worsening, brain tissue damage, and treatment response.
Methods
The University of California, San Francisco EPIC (Expression, Proteomics, Imaging, Clinical) data set was established in 2004 to study the natural history of MS in the treatment era and prospectively collected samples and clinical outcomes from 607 participants.18 Patients were enrolled beginning July 1, 2004; for the present study, and the data set was closed on August 31, 2017. Clinical evaluations and sample collection were performed annually for 5 years, then at different time points for up to 12 years, with a median follow-up duration of 10 (interquartile range [IQR], 7-11) years (eAppendix 1 in the Supplement). At baseline, all participants satisfied the following inclusion criteria: (1) diagnosis of clinically isolated syndrome (CIS), relapsing-remitting MS (RRMS), primary progressive MS (PPMS), or secondary progressive MS (SPMS) by 2001 International Panel Diagnostic Criteria19; (2) no relapse or treatment with glucocorticoids during the 30 days before the first visit; and (3) availability of demographic and clinical data at the time of sample collection, including information on relapses and disability scores as measured by the standardized Expanded Disability Status Scale (EDSS). Scores on the EDSS may range from 0 to 10, and higher scores are defined by impairment to ambulation and eventual death due to MS. Disability worsening was defined by a clinically significant increase in the EDSS score from baseline to years 4 to 6 and confirmed at years 9 to 11. A clinically significant increase in EDSS score was defined according to the baseline EDSS score as previously described1: a 1.5-point or greater increase in the EDSS score was required for participants with a baseline EDSS score of 0, a 1.0-point or greater increase for scores between 1.0 to 5.0, and a 0.5-point or greater increase for scores greater than 5.0. The Committee on Human Research at the University of California, San Francisco, approved the protocol, and written informed consent was obtained from all participants.
sNFL Measurements
Serum samples were processed immediately after phlebotomy procedure and stored at −80 °C. Serum NFL levels were assessed in duplicate using a single molecule array assay.14 A total of 3911 samples were tested, and those with values below the lower limit of quantification (1.28 pg/mL) or with a coefficient of variation greater than 20% were excluded from the analysis (7 samples [0.18%]). Interassay coefficients of variation were 13% for a low (mean, 8.0 pg/mL), 8% for a medium (mean, 20.9 pg/mL), and 7% for a high (mean, 92.9 pg/mL) concentration quality control serum sample measured in duplicates in every run. All samples were measured in duplicate in every run. Persons performing sNFL measurements were blinded to the clinical measures. The details of the method have been previously described.14
Magnetic Resonance Imaging
Annual brain magnetic resonance imaging with 10 years’ follow-up and measurable clinical progression was available for a subset of 372 patients with RRMS and CIS. The images were acquired with standardized head positioning and pulse sequences, as previously reported8 and described in eAppendix 2 in the Supplement.
Statistical Analysis
In all analyses, sNFL levels were transformed using a natural logarithm to meet normal distribution (eAppendix 3 in the Supplement). A linear regression model was used to assess cross-sectional association between sNFL levels and demographic and clinical variables at the first and last time point available for each participant. Estimates were back-transformed to the original scale and represented multiplicative effects on the geometric mean of sNFL. In addition, a linear regression model was used to estimate the association between sNFL levels and time from the last clinical exacerbation (relapses). A linear mixed-effects model was used to determine the pattern of sNFL levels change over time between active and inactive participants based on the presence or absence of clinical exacerbation among patients with CIS and RRMS from baseline to year 5 of the study, to assess the pattern of sNFL levels change over time relative to disability worsening (progressors vs nonprogressors), and to evaluate the effect of treatments on sNFL levels. A naive Bayes classifier model was used to evaluate baseline sNFL levels as predictors of the clinical outcome (presence of relapse or sustained EDSS worsening) during different follow-up periods. Receiver operating characteristic curves were used to visualize the model performance. A generalized estimating equation model was used to determine the association between sNFL levels and magnetic resonance imaging markers and to determine the association between sNFL levels and brain parenchymal fraction over time, comparing participants with CIS and those with RRMS above and below the established sNFL percentiles at baseline with covariates age, sex, and disease duration. Finally, least squares regression model with sex, age at baseline, and disease duration was used to assess the association of baseline sNFL levels with brain atrophy over different time periods. Detailed statistical methods are presented in eAppendix 3 in the Supplement. All statistical analyses were computed using code written in R version 3.4.3 (R Foundation). In all analyses, sNFL levels were natural logarithm–transformed to meet normal distribution. An α = .05 was used as the cutoff for significance. All tests were 2-sided.
Results
Demographic and Clinical Features of the MS Cohort
At study baseline, the MS cohort consisted of 607 participants: 93 with CIS, 435 with RRMS, 25 with PPMS, and 54 with SPMS. The mean (SD) age of participants at study entry was 42.5 (9.8) years; 423 (69.7%) were women; and all study participants were of non-Hispanic European descent. Clinical and demographic characteristics of the cohort are summarized in Table 1. The number of samples available for each time point is shown in eFigure 1 in the Supplement. Altogether, sNFL concentrations were obtained for 3904 samples.
Table 1. Clinical and Demographic Characteristics of the MS Cohort at Baseline and at the Last Visit.
| Variable | Baseline (n = 607) | Last Visit (n = 607) | P Value |
|---|---|---|---|
| Age at examination, mean (SD), y | 42.5 (9.8) | 51.0 (10.4) | NA |
| Female, No. (%) | 423 (69.7) | 423 (69.7) | NA |
| Disease course, No. (%) | |||
| CIS | 93 (15.3) | 32 (5.3) | <.001 |
| RRMS | 435 (71.7) | 435 (71.7) | |
| SPMS | 54 (8.9) | 115 (19.0) | |
| PPMS | 25 (4.1) | 25 (4.1) | |
| Disease duration, mean (SD) | 8.6 (8.7) | 17.4 (9.4) | NA |
| EDSS score, mean (SD) | 2.0 (1.6) | 3.1 (1.9) | <.001 |
| Treatment, No. (%)a | |||
| Untreated | 236 (38.9) | 247 (40.7) | <.001 |
| Platform | 352 (58.0) | 209 (34.4) | |
| High-potency | 19 (3.1) | 151 (24.9) |
Abbreviations: CIS, clinically isolated syndrome; EDSS, Expanded Disability Status Scale20; MS, multiple sclerosis; NA, not applicable; PPMS, primary progressive MS; RRMS, relapsing-remitting MS; SPMS, secondary progressive MS.
Platform treatment (interferon beta-1b, interferon beta-1a, and glatiramer acetate, monthly pulsed dose glucocorticoids, azathioprine, mycophenolate mofetil, and teriflunomide); high-potency treatment (natalizumab, rituximab, mitoxantrone, cyclophosphamide, fingolimod, and dimethyl fumarate).
Associations Between sNFL and Demographic and Clinical Variables
The median sNFL concentration at baseline was 25.5 pg/mL (IQR, 17.7-38.5 pg/mL). Consistent with previous reports,14 baseline sNFL levels were positively associated with age at sampling (β, 1.008; 95% CI, 1.003-1.014; P = .002), but not with sex (eTable 1 in the Supplement). Baseline sNFL levels also showed significant associations with EDSS score (β, 1.08; 95% CI, 1.047-1.114; P < .001 [ie, 8.0% higher sNFL levels per EDSS step]), MS subtype (higher values in progressive vs CIS or RRMS), presence of relapse in the 90 days before sampling (β, 1.478; 95% CI, 1.279-1.707; P < .001), and treatment status (β, 1.120; 95% CI, 1.007-1.245; P = .04) (eTable 1 and eFigure 2 in the Supplement). No effect of copy number of HLA-DRB1*15:01 with sNFL level was observed (eTable 1 in the Supplement). In the multivariable analysis, all associations remained significant except for disease subtypes.
Similarly, at the last visit available for each study participant, sNFL levels showed a univariable association with age (β, 1.022; 95% CI, 1.018-1.026; P < .001), EDSS score (β, 1.095; 95% CI, 1.071-1.120; P < .001), disease subtype (β, 1.469; 95% CI, 1.331-1.621; P < .001), and treatment with high-potency drugs (β, 0.871; 95% CI, 1.331-1.621; P = .01), but not with the presence of relapse in the 90 days before sampling (β, 1.031; 95% CI, 0.817-1.300 P = .80 (eTable 2 and eFigure 2 in the Supplement). In the multivariable analysis, the association with age, EDSS, disease subtype, and treatment with high-potency drugs remained significant. Although at baseline, treatment with platform therapies was associated with slightly higher sNFL levels, at the last visit, treatment with high-potency therapies was associated with decreased levels of sNFL. This effect remained significant in the multivariable analysis (data not shown).
sNFL Levels Across Time, Relapse Activity, and Disease Worsening
Participants were classified into 2 groups, according to whether they had experienced 0 (inactive) or 1 or more relapses from baseline to year 5 of the study (active). Significantly higher levels of sNFL at baseline were observed in the active participants relative to the inactive group (β, 2.234; P < .001) (Table 2). However, there was no difference in the change of sNFL levels over time between the 2 groups (Table 2). Similar results were observed with the analysis restricted to 1-year periods.
Table 2. Association of sNFL Levels With Disease Activitya.
| Disease Activity | Baseline to Year 5 | Baseline to Year 1 | Years 1-2 | Years 2-3 | Years 3-4 | Years 4-5 |
|---|---|---|---|---|---|---|
| No. of patients | ||||||
| Relapse | 165 | 97 | 77 | 42 | 33 | 37 |
| No relapse | 155 | 305 | 273 | 259 | 232 | 238 |
| Intercept estimateb | ||||||
| Relapse | 13.158 | 18.916 | 8.499 | 13.957 | 11.078 | 8.240 |
| No relapse | 10.924 | 16.151 | 8.820 | 11.953 | 8.256 | 7.360 |
| Group, coefficient (95% CI) | 1.205 (1.085-1.336) |
1.171 (1.021-1.344) |
0.964 (0.748-1.240) |
1.168 (0.690-1.975) |
1.342 (0.737-2.442) |
1.120 (0.500-2.507) |
| Group, P valuec | <.001 | .02 | .77 | .56 | .34 | .78 |
| Slope estimated | ||||||
| Relapse | 0.946 | 0.677 | 1.228 | 0.931 | 0.983 | 1.041 |
| No relapse | 0.967 | 0.658 | 1.150 | 0.919 | 0.988 | 1.030 |
| Group by time, P valuee | .08 | .71 | .39 | .90 | .96 | .91 |
Abbreviation: sNFL, serum neurofilament light chain.
Analysis of the interaction between sNFL levels and disease activity (presence of relapse).
Intercept estimate is the adjusted value for the mean sNFL levels in each group.
Group P value is the comparison of sNFL levels at baseline between patients with and without the outcome.
Slope estimate is the calculated slope for each group.
Group by time P value is the comparison of sNFL levels change over time between disease activity groups.
Significant associations between sNFL levels and contemporaneous EDSS scores were found (eTables 1 and 2 and eFigure 2 in the Supplement). To assess the association of disease worsening over time with sNFL levels, once again participants were classified in 2 groups: those who experienced EDSS-defined worsening from baseline to years 4 through 6, with worsening sustained at years 9 through 11 of the study (“progressors”) (n = 159), and those who did not experience EDSS worsening (“nonprogressors”) (n = 248). At baseline, there was no difference in sNFL levels between the 2 groups (β, 0.409; P = .69; Table 3). A significant interaction between EDSS worsening and change in levels of sNFL over time was found (β, 0.015; P < .001; Table 3), indicating a steeper trajectory of sNFL levels in progressors. This result remained significant after correction for age, sex, and disease duration.
Table 3. Association of sNFL Levels With Disease Progressiona.
| Disease Progression | Baseline to Year 12 |
|---|---|
| Patients with EDSS worsening, No. | 159 |
| Patients with No EDSS worsening, No. | 248 |
| Intercept estimate, baseline sNFLb | |
| EDSS score worsening | 21.758 |
| No EDSS score worsening | 21.349 |
| Group, coefficient (95% CI) | 0.981 (0.890-1.080) |
| Group, P valuec | .69 |
| Slope estimated | |
| EDSS score worsening | 1.017 |
| No EDSS score worsening | 1.002 |
| Group by time, coefficient (95% CI) | 0.985 (0.977-0.993) |
| Group by time, P valuee | <.001 |
Abbreviations: EDSS, Expanded Disability Status Scale; sNFL, serum neurofilament light chain.
Analysis of the interaction between time and disease progression (EDSS score worsening from baseline to years 4 to 6 and confirmed at year 9 to 11 of the study) over time.
Intercept estimate is the adjusted value for the mean sNFL levels in each group.
Group P value is the comparison of sNFL levels at baseline between patients with and without the outcome.
Slope estimate is the calculated slope for each group.
Group by time P value is the comparison of sNFL level change over time between patients with and without the outcome.
sNFL Level and Long-term Relapse Activity and Disability Worsening
Two approaches were used to assess the capacity of sNFL level measured at a specific time point to compare with disease activity (clinical relapse) and disability worsening in the subsequent year. The first consisted of using a continuous measure of sNFL level, the second was based on stratifying samples according to extreme values of sNFL level relative to the total cohort.
Based on area under the curve (AUC) values, sNFL levels at baseline were not associated with clinical relapses in the subsequent years (AUC range, 0.59-0.72; eFigure 3 in the Supplement). When samples where categorized according to different percentiles of NFL levels, we observed that higher sNFL levels were associated with a greater risk of having experienced a relapse in the 60 and 360 days before sampling compared with lower levels (eTable 3A and B and eTable 4A and B in the Supplement). However, extreme levels of sNFL were not associated with future relapses (eTables 4A and 4B in the Supplement). Considering that potential bias caused by the requirement that patients experiencing an acute relapse wait at least 30 days before being enrolled in the study, the analysis of factors associated with relapse was repeated after omitting baseline samples and showed similar results (eTable 5 in the Supplement).
Likewise, sNFL levels at different time points were not associated with long-term disability progression (AUC range, 0.54-0.59; eFigure 4 in the Supplement). Serum NFL levels categorized according to extreme percentiles were also not associated with subsequent EDSS worsening, nor were they associated with previous EDSS worsening (eTable 3C and eTable 4C in the Supplement).
sNFL Levels After Treatment
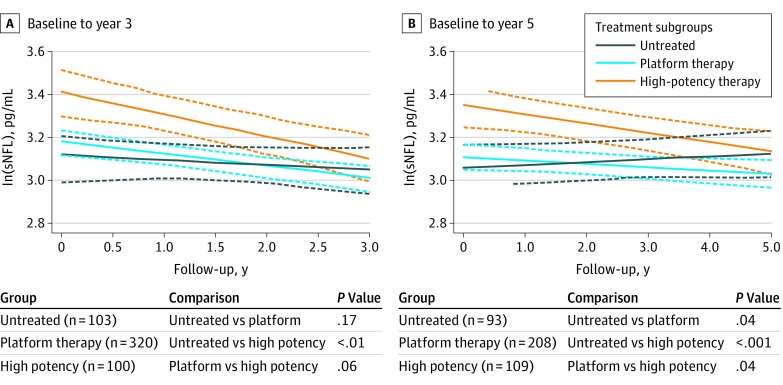
Given the cross-sectional association between sNFL levels and treatment status, the association of platform and high-potency therapies with sNFL levels over time was compared. Participants were stratified into 3 treatment arms: untreated, treated with platform therapies, or treated with intermediate or high-potency therapies during the initial 3 and 5 years of the study, and changes in sNFL levels over time were compared between groups. Clinical and demographic characteristics of the patients included in the study are detailed in eTable 6 in the Supplement. Participants treated with high-potency therapies during the first 3 years of the study showed a more significant decrease of sNFL levels over time compared with those who were untreated (β, 0.922; 95% CI, 0.868-0.980; P < .01; Figure 1). The same analysis over a period of 5 years was repeated, and a differential association of high-potency therapies relative to no treatment or platform therapies was observed, suggesting that drugs with greater effectiveness produced more robust changes for sNFL levels. After 5 years’ follow-up, active treatment was associated with lower levels of sNFL, with high-potency treatments associated with the greater decreases in sNFL levels compared with platform therapies (high-potency vs untreated: β, 0.946; 95% CI, 0.915-0.976; P < .001; high-potency vs platform: β, 0.972; 95% CI, 0.948-0.998; P = .04). The high-potency treatment group consisted of participants who initiated treatment before baseline (19 participants for the 3-year and 15 for the 5-year follow-up points) as well as participants who switched from platform therapies to high-potency therapy during follow-up (81 participants for the 3-year and 94 for the 5-year follow-up points). Participants were subsequently stratified according to their treatment status at baseline (eFigure 5 in the Supplement). Clinically active participants switching to high-potency treatments (ie, treat to target) showed higher levels of sNFL at baseline, and the decrease of sNFL over time was more pronounced compared with the other treatment groups (eFigure 5 in the Supplement).
Figure 1. Change Over Time of Serum Neurofilament Light Chain (sNFL) Levels in Patients With Multiple Sclerosis (MS) in Different Treatment Groups.
The graphs represent the group means of sNFL over time. Levels of sNFL show a different rate of change over time in patients treated with high-potency therapies compared with those receiving platform therapy or those who were untreated during a period of 3 and 5 years. The analysis includes patients with relapsing-remitting multiple sclerosis, clinically isolated syndrome, secondary progressive MS, and primary progressive MS, analyzed as a single group. Dashed lines represent 95% CIs.
sNFL Levels and Magnetic Resonance Imaging Markers
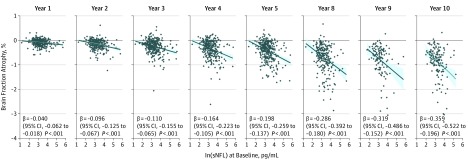
To maximize power, we restricted the analysis to 372 study participants who started as RRMS or CIS and completed at least 9 years of annual follow-ups including magnetic resonance imaging. Here, T2 lesion volume was associated with sNFL levels over time (β, 3.361; 95% CI, 2.300-4.420; P = 5.8 × 10−10), and there was an association between sNFL levels and brain fraction (β, 2.0 × 10−4; 95% CI, 4 × 10−6-0.000396; P = .02) (eTable 7A in the Supplement). These associations remained significant when the analyses were adjusted for covariates (eTable 7B in the Supplement). Baseline sNFL levels were associated with brain fraction atrophy at the different time points analyzed (Figure 2 and eTable 8 in the Supplement). Furthermore, when the data set was stratified into extreme percentiles according to the levels of sNFL at baseline, we observed an interaction between percentile of sNFL levels and time (β = 0.00028; 95% CI, 0.00014-0.00042; P < .001; eTable 9 in the Supplement), which may mean that the change of brain parenchymal fraction over time differs between patients with sNFL levels above and below the threshold percentiles. Baseline sNFL levels accounted for approximately 11.6% (univariable) and 18.0% (multivariable) of the variance in brain fraction atrophy at year 10 (eTable 10 in the Supplement).
Figure 2. Association of Serum Neurofilament Light Chain (sNFL) Level at Baseline With Percentage of Brain Fraction Change Over Time.
In a multivariable model including age, disease duration, and sex, baseline sNFL levels were significantly associated with the percentage of brain fraction change across time.
Discussion
The past few years have seen real progress in defining the pathologic and etiologic underpinnings of MS, reflected by a robust pipeline of disease-modifying drugs that control inflammation and prevent or delay the neurodegenerative phase of the disease.1,2 Pairing these advances with the development of biomarkers for disease progression to inform and individualize treatment decisions represents an important unmet need in the field. A number of recent studies have proposed the use of NFL level, obtained from CSF or serum, as a biomarker of disease activity and worsening in MS. In the present study, sNFL levels in a well-characterized cohort of 607 patients with MS with up to 12 years’ follow-up were analyzed to assess their association with disease activity, treatment, and prognostic power with respect to disability and brain fraction atrophy. At study baseline, we found that sNFL levels were associated with age, disease subtype, relapses, and treatment status. Consistent with previously reported studies,9,14,21,22,23,24,25 levels of sNFL in this cohort were associated with EDSS scores at the time of sampling. However, in contrast to others, we did not detect an association with relapse activity over time. This was an actively monitored and treated cohort, with relatively few relapses recorded during the annual follow-up visits, and the study was not designed to closely assess relapse activity but rather to monitor the long-term behavior of MS in the treatment era. In this regard, we were also unable to replicate the finding that levels of sNFL are associated with future EDSS worsening.14,25 Possible explanations for these discrepancies may be unidentified confounders inherent to each data set, the lack of a healthy control data set in our study for calculating the sNFL percentiles, or therapeutic practices that could attenuate sNFL levels as well as clinical outcomes. However, we interpret our data as representative of inherent variability in the behavior of MS over time. The fact that we did not find an association of sNFL levels with future EDSS worsening could be due to the lag in evolution of disability after neuronal injury.26 On the other hand, we show for the first time, to our knowledge, that participants who experienced disability worsening as measured by a clinically significant increase in EDSS have a different rate of sNFL change over time compared with those not showing worsening.
It was previously shown that some disease-modifying treatments decrease NFL levels in CSF27,28,29,30 and blood.14,31,32 At baseline, participants receiving treatment with platform therapies showed higher levels of sNFL than those receiving high-potency disease-modifying treatments; at the last visit, participants treated with high-potency therapies showed decreased levels compared with untreated subjects. These results suggest that high-potency therapies are associated with a greater reduction in sNFL levels over time compared with other treatment options. These data could also represent confounding by indication, whereby it is possible that, at study entry, patients with more aggressive disease were more likely to have started disease-modifying treatments. These observations were further confirmed in the longitudinal analysis, where we found that high-potency treatment, especially in those participants switching from platform treatment, was associated with a greater reduction in sNFL levels than platform therapies.33 This study was not designed to assess the association of treatment with NFL levels; however, results were in line with recent findings from retrospective analyses from randomized clinical trials.34 The potential of sNFL to serve as a surveillance biomarker for treatment response should be further defined by including sNFL levels as an outcome in treatment-specific clinical trials.
Consistent with recent reports,25,35 sNFL levels at baseline may be associated, albeit modestly, with future brain fraction atrophy as early as 1 year after sampling. This result was observed at the different time points analyzed. We also observed a significantly different atrophy rate over time between patients with sNFL levels greater than and less than established percentiles, further supporting the potential of sNFL levels as biomarkers of brain damage.
Limitations
This study has limitations worth highlighting. First, samples were acquired on an annual basis. More frequent sample acquisition would be useful to provide a broader understanding of the dynamics of sNFL levels, especially with respect to changes associated with clinical relapses. Second, even though the number of participants and samples acquired was substantial, the study lacks power for assessment of the outcomes of individual therapies. We grouped therapies into tiers based on limited comparative efficacy data, and much larger numbers of participants would be needed to achieve adequate statistical power to assess the impact of individual therapies and how switching between therapies affects sNFL levels. Another limitation of the study was the lack of data on NFL stability and serially sampled healthy control participants. However, the detection of NFL did not appear to be altered by storage time; therefore, this variable should not change the results.14,31
Conclusions
Our findings from a large observational cohort followed up for 12 years at a single center suggest that (1) sNFL levels are associated with brain atrophy, (2) changes in sNFL levels are associated with disability worsening, and (3) sNFL levels may be influenced by treatment. For an individual patient, the biomarker prognostic power of sNFL level for clinical and magnetic resonance imaging outcomes was limited.
eAppendix 1. Treatments
eAppendix 2. Brain MRI Scans
eAppendix 3. Statistical Analysis
eFigure 1. Visit Counts and Sample Availability
eFigure 2. Associations Between NFL Levels and Clinical Variables
eFigure 3. NFL Levels and Prediction of Relapse
eFigure 4. NFL Levels and Prediction of Disability Worsening
eFigure 5. Treatment Effect on NFL Levels
eTable 1. Univariate and Multivariate Models to Test the Association Between sNFL Levels at Baseline and Clinical and Demographical Variables
eTable 2. Univariate and Multivariate Models to Test the Association Between sNFL Levels at the Last Visit Available for the Study and Clinical and Demographical Variables
eTable 3. Association of sNFL Levels and Past Relapse Activity and Disability Worsening
eTable 4. Association of sNFL Levels and Future Relapse Activity and Disability Worsening
eTable 5. Sensitivity Analysis of the Association of NFL Levels with Past and Future Relapse Activity
eTable 6. Effect of Treatment on sNFL Levels. Clinical and Demographical Characteristics of the Treatment Groups
eTable 7. Association of NFL Levels and MRI Markers
eTable 8. Baseline NFL Levels as Predictors of Brain Fraction Atrophy
eTable 9. Baseline NFL Percentiles as Predictors of Brain Fraction Atrophy
eTable 10. Baseline NFL Levels as Predictors of Brain Fraction Atrophy Variance
References
- 1.Cross AH, Naismith RT. Established and novel disease-modifying treatments in multiple sclerosis. J Intern Med. 2014;275(4):350-363. doi: 10.1111/joim.12203 [DOI] [PubMed] [Google Scholar]
- 2.Feinstein A, Freeman J, Lo AC. Treatment of progressive multiple sclerosis: what works, what does not, and what is needed. Lancet Neurol. 2015;14(2):194-207. doi: 10.1016/S1474-4422(14)70231-5 [DOI] [PubMed] [Google Scholar]
- 3.Housley WJ, Pitt D, Hafler DA. Biomarkers in multiple sclerosis. Clin Immunol. 2015;161(1):51-58. doi: 10.1016/j.clim.2015.06.015 [DOI] [PubMed] [Google Scholar]
- 4.Reuwer AQ, Heron M, van der Dussen D, Schneider-Hohendorf T, Murk JL. The clinical utility of JC virus antibody index measurements in the context of progressive multifocal leukoencephalopathy. Acta Neurol Scand. 2017;136(suppl 201):37-44. doi: 10.1111/ane.12840 [DOI] [PubMed] [Google Scholar]
- 5.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106-2112. doi: 10.1016/S0140-6736(04)17551-X [DOI] [PubMed] [Google Scholar]
- 6.Bjartmar C, Kidd G, Mörk S, Rudick R, Trapp BD. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol. 2000;48(6):893-901. doi: [DOI] [PubMed] [Google Scholar]
- 7.Petzold A, Eikelenboom MJ, Keir G, et al. Axonal damage accumulates in the progressive phase of multiple sclerosis: three year follow up study. J Neurol Neurosurg Psychiatry. 2005;76(2):206-211. doi: 10.1136/jnnp.2004.043315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lycke JN, Karlsson JE, Andersen O, Rosengren LE. Neurofilament protein in cerebrospinal fluid: a potential marker of activity in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1998;64(3):402-404. doi: 10.1136/jnnp.64.3.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhle J, Barro C, Andreasson U, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med. 2016;54(10):1655-1661. doi: 10.1515/cclm-2015-1195 [DOI] [PubMed] [Google Scholar]
- 10.Petzold A, Steenwijk MD, Eikelenboom JM, Wattjes MP, Uitdehaag BM. Elevated CSF neurofilament proteins predict brain atrophy: a 15-year follow-up study. Mult Scler. 2016;22(9):1154-1162. doi: 10.1177/1352458516645206 [DOI] [PubMed] [Google Scholar]
- 11.Weston PSJ, Poole T, Ryan NS, et al. Serum neurofilament light in familial Alzheimer disease: a marker of early neurodegeneration. Neurology. 2017;89(21):2167-2175. doi: 10.1212/WNL.0000000000004667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skillbäck T, Mattsson N, Blennow K, Zetterberg H. Cerebrospinal fluid neurofilament light concentration in motor neuron disease and frontotemporal dementia predicts survival. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(5-6):397-403. doi: 10.1080/21678421.2017.1281962 [DOI] [PubMed] [Google Scholar]
- 13.Byrne LM, Rodrigues FB, Blennow K, et al. Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington’s disease: a retrospective cohort analysis. Lancet Neurol. 2017;16(8):601-609. doi: 10.1016/S1474-4422(17)30124-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Disanto G, Barro C, Benkert P, et al. ; Swiss Multiple Sclerosis Cohort Study Group . Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857-870. doi: 10.1002/ana.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhle J, Barro C, Disanto G, et al. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult Scler. 2016;22(12):1550-1559. doi: 10.1177/1352458515623365 [DOI] [PubMed] [Google Scholar]
- 16.Kuhle J, Nourbakhsh B, Grant D, et al. Serum neurofilament is associated with progression of brain atrophy and disability in early MS. Neurology. 2017;88(9):826-831. doi: 10.1212/WNL.0000000000003653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gisslén M, Price RW, Andreasson U, et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine. 2015;3:135-140. doi: 10.1016/j.ebiom.2015.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cree BA, Gourraud PA, Oksenberg JR, et al. ; University of California, San Francisco MS-EPIC Team . Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol. 2016;80(4):499-510. doi: 10.1002/ana.24747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127. doi: 10.1002/ana.1032 [DOI] [PubMed] [Google Scholar]
- 20.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452. doi: 10.1212/WNL.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 21.Teunissen CE, Iacobaeus E, Khademi M, et al. Combination of CSF N-acetylaspartate and neurofilaments in multiple sclerosis. Neurology. 2009;72(15):1322-1329. doi: 10.1212/WNL.0b013e3181a0fe3f [DOI] [PubMed] [Google Scholar]
- 22.Norgren N, Sundström P, Svenningsson A, Rosengren L, Stigbrand T, Gunnarsson M. Neurofilament and glial fibrillary acidic protein in multiple sclerosis. Neurology. 2004;63(9):1586-1590. doi: 10.1212/01.WNL.0000142988.49341.D1 [DOI] [PubMed] [Google Scholar]
- 23.Kuhle J, Leppert D, Petzold A, et al. Neurofilament heavy chain in CSF correlates with relapses and disability in multiple sclerosis. Neurology. 2011;76(14):1206-1213. doi: 10.1212/WNL.0b013e31821432ff [DOI] [PubMed] [Google Scholar]
- 24.Khalil M, Enzinger C, Langkammer C, et al. CSF neurofilament and N-acetylaspartate related brain changes in clinically isolated syndrome. Mult Scler. 2013;19(4):436-442. doi: 10.1177/1352458512458010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain. 2018;141(8):2382-2391. doi: 10.1093/brain/awy154 [DOI] [PubMed] [Google Scholar]
- 26.Giovannoni G, Cutter G, Sormani MP, et al. Is multiple sclerosis a length-dependent central axonopathy? the case for therapeutic lag and the asynchronous progressive MS hypotheses. Mult Scler Relat Disord. 2017;12:70-78. doi: 10.1016/j.msard.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 27.Gunnarsson M, Malmeström C, Axelsson M, et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann Neurol. 2011;69(1):83-89. doi: 10.1002/ana.22247 [DOI] [PubMed] [Google Scholar]
- 28.Axelsson M, Malmeström C, Gunnarsson M, et al. Immunosuppressive therapy reduces axonal damage in progressive multiple sclerosis. Mult Scler. 2014;20(1):43-50. doi: 10.1177/1352458513490544 [DOI] [PubMed] [Google Scholar]
- 29.Kuhle J, Disanto G, Lorscheider J, et al. Fingolimod and CSF neurofilament light chain levels in relapsing-remitting multiple sclerosis. Neurology. 2015;84(16):1639-1643. doi: 10.1212/WNL.0000000000001491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novakova L, Axelsson M, Khademi M, et al. Cerebrospinal fluid biomarkers as a measure of disease activity and treatment efficacy in relapsing-remitting multiple sclerosis. J Neurochem. 2017;141(2):296-304. doi: 10.1111/jnc.13881 [DOI] [PubMed] [Google Scholar]
- 31.Piehl F, Kockum I, Khademi M, et al. Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult Scler. 2018;24(8):1046-1054. doi: 10.1177/1352458517715132 [DOI] [PubMed] [Google Scholar]
- 32.Novakova L, Zetterberg H, Sundström P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology. 2017;89(22):2230-2237. doi: 10.1212/WNL.0000000000004683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arrambide G, Espejo C, Eixarch H, et al. Neurofilament light chain level is a weak risk factor for the development of MS. Neurology. 2016;87(11):1076-1084. doi: 10.1212/WNL.0000000000003085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology. 2019;92(10):e1007-e1015. doi: 10.1212/WNL.0000000000007032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chitnis T, Gonzalez C, Healy BC, et al. Neurofilament light chain serum levels correlate with 10-year MRI outcomes in multiple sclerosis. Ann Clin Transl Neurol. 2018;5(12):1478-1491. doi: 10.1002/acn3.638 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Treatments
eAppendix 2. Brain MRI Scans
eAppendix 3. Statistical Analysis
eFigure 1. Visit Counts and Sample Availability
eFigure 2. Associations Between NFL Levels and Clinical Variables
eFigure 3. NFL Levels and Prediction of Relapse
eFigure 4. NFL Levels and Prediction of Disability Worsening
eFigure 5. Treatment Effect on NFL Levels
eTable 1. Univariate and Multivariate Models to Test the Association Between sNFL Levels at Baseline and Clinical and Demographical Variables
eTable 2. Univariate and Multivariate Models to Test the Association Between sNFL Levels at the Last Visit Available for the Study and Clinical and Demographical Variables
eTable 3. Association of sNFL Levels and Past Relapse Activity and Disability Worsening
eTable 4. Association of sNFL Levels and Future Relapse Activity and Disability Worsening
eTable 5. Sensitivity Analysis of the Association of NFL Levels with Past and Future Relapse Activity
eTable 6. Effect of Treatment on sNFL Levels. Clinical and Demographical Characteristics of the Treatment Groups
eTable 7. Association of NFL Levels and MRI Markers
eTable 8. Baseline NFL Levels as Predictors of Brain Fraction Atrophy
eTable 9. Baseline NFL Percentiles as Predictors of Brain Fraction Atrophy
eTable 10. Baseline NFL Levels as Predictors of Brain Fraction Atrophy Variance