Key Points
Question
Is there an association between ambient air pollutants and progression of emphysema and changes in lung function in the general population?
Findings
In this cohort study conducted between 2000 and 2018 that included 5780 participants in 6 US metropolitan regions followed up for a median of 10 years, there was a statistically significant association between baseline ambient concentrations of ambient ozone (O3), fine particulate matter (PM2.5), oxides of nitrogen (NOx), and black carbon with greater increases in emphysema assessed quantitatively using computed tomographic (CT) imaging. Concentrations of O3 and NOx, but not concentrations of PM2.5, over study follow-up were also associated with increases in emphysema. Baseline ambient O3 was significantly associated with a faster decline in forced expiratory volume in the first second (FEV1).
Meaning
Long-term exposure to ambient air pollutants, especially O3, was significantly associated with increasing emphysema assessed quantitatively using CT imaging and with worsening lung function.
Abstract
Importance
While air pollutants at historical levels have been associated with cardiovascular and respiratory diseases, it is not known whether exposure to contemporary air pollutant concentrations is associated with progression of emphysema.
Objective
To assess the longitudinal association of ambient ozone (O3), fine particulate matter (PM2.5), oxides of nitrogen (NOx), and black carbon exposure with change in percent emphysema assessed via computed tomographic (CT) imaging and lung function.
Design, Setting, and Participants
This cohort study included participants from the Multi-Ethnic Study of Atherosclerosis (MESA) Air and Lung Studies conducted in 6 metropolitan regions of the United States, which included 6814 adults aged 45 to 84 years recruited between July 2000 and August 2002, and an additional 257 participants recruited from February 2005 to May 2007, with follow-up through November 2018.
Exposures
Residence-specific air pollutant concentrations (O3, PM2.5, NOx, and black carbon) were estimated by validated spatiotemporal models incorporating cohort-specific monitoring, determined from 1999 through the end of follow-up.
Main Outcomes and Measures
Percent emphysema, defined as the percent of lung pixels less than −950 Hounsfield units, was assessed up to 5 times per participant via cardiac CT scan (2000-2007) and equivalent regions on lung CT scans (2010-2018). Spirometry was performed up to 3 times per participant (2004-2018).
Results
Among 7071 study participants (mean [range] age at recruitment, 60 [45-84] years; 3330 [47.1%] were men), 5780 were assigned outdoor residential air pollution concentrations in the year of their baseline examination and during the follow-up period and had at least 1 follow-up CT scan, and 2772 had at least 1 follow-up spirometric assessment, over a median of 10 years. Median percent emphysema was 3% at baseline and increased a mean of 0.58 percentage points per 10 years. Mean ambient concentrations of PM2.5 and NOx, but not O3, decreased substantially during follow-up. Ambient concentrations of O3, PM2.5, NOx, and black carbon at study baseline were significantly associated with greater increases in percent emphysema per 10 years (O3: 0.13 per 3 parts per billion [95% CI, 0.03-0.24]; PM2.5: 0.11 per 2 μg/m3 [95% CI, 0.03-0.19]; NOx: 0.06 per 10 parts per billion [95% CI, 0.01-0.12]; black carbon: 0.10 per 0.2 μg/m3 [95% CI, 0.01-0.18]). Ambient O3 and NOx concentrations, but not PM2.5 concentrations, during follow-up were also significantly associated with greater increases in percent emphysema. Ambient O3 concentrations, but not other pollutants, at baseline and during follow-up were significantly associated with a greater decline in forced expiratory volume in 1 second per 10 years (baseline: 13.41 mL per 3 parts per billion [95% CI, 0.7-26.1]; follow-up: 18.15 mL per 3 parts per billion [95% CI, 1.59-34.71]).
Conclusions and Relevance
In this cohort study conducted between 2000 and 2018 in 6 US metropolitan regions, long-term exposure to ambient air pollutants was significantly associated with increasing emphysema assessed quantitatively using CT imaging and lung function.
This cohort study examines the association between long-term exposure to ambient ozone, black carbon, and other air pollutants and progression of CT-defined percent emphysema and decline in lung function in adults in 6 US metropolitan regions.
Introduction
Chronic lower respiratory disease is the fourth leading cause of death in the United States and third leading cause of death worldwide.1,2 Most of this mortality is due to chronic obstructive pulmonary disease (COPD), defined by persistent airflow limitation, measured via spirometry, and pulmonary emphysema, defined by destruction of the lung parenchyma.3 Emphysema can be measured quantitatively on computed tomographic (CT) imaging and can be observed without spirometric COPD.4,5 The percentage of emphysema-like lung on CT scans (“percent emphysema”) is associated with the degree of lung function impairment and symptoms as well as mortality in patients with COPD.6 Percent emphysema is also associated with dyspnea and disease exacerbations independent of lung function among smokers7 and with incident COPD and all-cause mortality among individuals without COPD in the general population.4,8
Ambient air pollution is a major risk factor for poor health worldwide.9 The relationship of air pollution exposures with respiratory morbidity and mortality has been documented in the short-term10 and, by a more limited number of prospective cohorts, in the long-term.11,12 There is elevated risk of death associated with ozone (O3) and fine particulate matter less than 2.5 microns in aerodynamic diameter (PM2.5) exposure in patients with COPD11 and a faster decline in lung function associated with long-term exposure to traffic-related air pollutants in healthy populations.13 No longitudinal studies of air pollution and emphysema progression on CT scan, to our knowledge, have been reported.
This cohort study examined the hypothesis that long-term exposure to air pollutants is associated with progression of percent emphysema shown on CT imaging, as well as decline in lung function, in a well-characterized, multiethnic cohort of adults.
Methods
Study Population
The Multi-Ethnic Study of Atherosclerosis (MESA) recruited participants aged 45 to 84 years without clinical cardiovascular disease from 2000 to 2002 in 6 US metropolitan areas. Investigators recruited white, African American, Hispanic, and Chinese American participants to achieve prespecified recruitment goals by clinical site; race/ethnicity was based on participant self-identification. The MESA study included 4 race/ethnic categories to create a more representative and diverse population than was represented in prior National Heart, Lung, and Blood Institute cohort studies. The MESA Air Pollution Study (MESA Air) recruited additional participants with comparable ages and without cardiovascular disease from 2005 to 2007.14 The MESA Lung Study enrolled a subsample of 3813 MESA participants from 2004 to 2006,15 additional MESA participants from 2010 to 2012, and selected all MESA participants in 2017 to 2018. No selection criteria were related to risk or presence of lung disease. The current analysis includes all MESA participants with valid air pollutant exposure estimates, outcomes, and covariates.
The protocols were approved by the institutional review boards of the collaborating institutions. Written informed consent was obtained from all participants.
Percent Emphysema on CT
Percent emphysema was calculated based on all usable CT scans, including cardiac and full-lung scans. All MESA participants underwent a cardiac CT scan at baseline from 2000 to 2002. Cardiac CT scans were acquired at suspended full inspiration.16 A second CT scan was conducted for 84% of participants in the cohort between 2002 and 2005 and for a subset of participants who did not have a prior scan in 2004 or 2005 and consented to a scan at this examination from 2005 to 2007. Participants in the MESA Lung Study underwent a full-lung CT scan at suspended full inspiration following a standardized protocol from 2010 to 201217 and from 2017 to 2018 with modification to account for scanner evolution.
Trained readers performed percent emphysema measurements at a central reading center, without knowledge of other participant information, using modified Pulmonary Analysis Software Suite for cardiac scans and the updated version, Apollo 1.2 (VIDA Diagnostics), for full-lung scans. For cardiac CT scans, which were performed in duplicate at each examination, the scan with the greater air volume was used except in cases in which 1 scan failed to meet quality control criteria.18
Percent emphysema was defined as the percentage of lung pixels below −950 Hounsfield units (HU), a threshold selected based on pathologic comparisons5 and prognostic significance in this cohort.4 The −950-HU threshold on all scans was adjusted for attenuation of air outside the chest to account for scanner variation.18 Because cardiac CT images approximate 66% of lung volume (from the carina to the lung bases), the upper-third portion of full-lung scans was excluded to follow the same lung region over time. Percent emphysema on cardiac scans was reproducible (scan-rescan intraclass correlation coefficient [ICC], 0.91), comparable between scanners (ICC, 0.94), and valid compared with full-lung CT scans (ICC, 0.93).18
CT technology changed over the 18 years of the study. Therefore, sensitivity analyses were performed using a hidden Markov measure field approach to the measurement of percent emphysema, which reduces interscanner and interprotocol variability by adapting the characteristics of each image under varying CT imaging protocols and differences in intensity distributions due to inspiration level.19 In additional sensitivity analyses, scans with inspiratory levels less than 80% of the participant’s maximum lung volume on scans were excluded and an alternative metric, the HU of the lowest 15th percentile of the lung frequency distribution (PD15), was examined in each participant. A higher PD15 value indicates less emphysema.
Lung Function
Spirometry was conducted in the MESA Lung Study from 2004 to 2006 and repeated twice from 2010 to 2012 and 2017 to 2018 in accordance with American Thoracic Society guidelines and with the same dry-rolling seal spirometers (Occupational Marketing Inc).15 All results were reviewed by 1 investigator. Airflow obstruction was defined at the first measurement of lung function as forced expiratory volume in the first second (FEV1)/forced vital capacity (FVC) ratio less than 0.70.
Exposure Assessment
The method used to estimate long-term outdoor air pollution concentrations at each participant’s home address has been previously described in detail.20,21 In brief, spatiotemporal exposure models were developed for O3, PM2.5, and oxides of nitrogen (NOx) in each study region based on measurements (1999-2018) from the US Environmental Protection Agency (EPA) Air Quality System and the spatially dense cohort-specific monitoring performed for MESA Air.22 Mean outdoor concentrations of black carbon were estimated only for the years 2006 to 2008 because comparable air quality system data were not available at other times (the EPA’s measurement method for black carbon was not stable over follow-up). City-specific models incorporated a large number of variables covering a wide diversity of geographic features, such as traffic, industrial emissions, population density, and land use. The performance of these models ranged from good to excellent as assessed by the overall cross-validation R2 evaluated at participant residence locations (range, 0.59-0.92).
To account for strong temporal trends in some air pollutants and the knowledge that prior exposure can alter ventilatory function trajectories even after exposure stops (as seen with smoking23), 2 approaches were used to assign long-term mean exposure to each participant: (1) baseline exposure, assessed as pollutant concentration for the year of the baseline examination in 2000 (or 2006-2008 for black carbon) because this exposure measure more closely approximates prior epochs of this exposure and (2) exposure over follow-up, assessed as time-varying mean concentrations between the year of the baseline examination and follow-up time of the repeated outcome measure.
Statistical Analysis
The principal interest of the study was to assess the longitudinal relationship between long-term exposure to air pollutants and progression of percent emphysema and decline in lung function over time (assessed as the interaction between air pollutant and time in the model). As described in more detail in the Supplement, these associations were estimated using linear mixed effects models with random intercept (and slope for the emphysema model).
The primary adjusted model for the percent emphysema and pulmonary function outcomes included cross-sectional terms, including baseline age, sex, race/ethnicity, study region, education, height, weight, body mass index (time-varying), smoking (status, pack-years, cigarettes per day for current smokers, and secondhand smoke exposure [both time-varying]), long-term mean city-specific temperature (time-varying), income, employment outside the home, physical activity, neighborhood socioeconomic status (SES) index,24 and the interaction between SES and study region; longitudinal terms for interactions between these variables and study time (years since baseline); and a transient effects term. While most key covariates were 99% to 100% complete, 5% of participants were missing complete information to calculate pack-years of smoking. We assigned those participants a value of zero and adjusted for an indicator variable reflecting missing pack-years data; we performed sensitivity analyses to confirm that results were consistent between this approach and alternative approaches (excluding missing subjects and assigning the median value for pack-years). Scanner parameters (eg, CT scanner model, milliampere-second adjustment, pixel size) were also included in the percent emphysema model, while the model of lung function additionally controlled for occupational exposure to fumes and gases. Each of the air pollution exposure metrics (at baseline or over follow-up as described above) was investigated separately and included as a main effect and in interaction with time. The associations of exposure to air pollutants with outcomes were compared with the association of pack-years in the same model. In secondary analyses, the regression models were extended by also adjusting for some self-reported transient respiratory conditions (eg, cold, flu, bronchitis, pneumonia) in the lung function models and for high-attenuation areas on CT imaging (percentage of imaged lung volume with attenuation values between −600 and −250 HU) in the emphysema model.
Effect modification of the associations with air pollutants was examined for age, sex, race/ethnicity, obesity, smoking status, SES level, baseline airflow obstruction, study region, and scanner type using 3-way interaction terms between study time, the effect modifier, and exposure concentration. A significant difference between subgroups was indicated by a P value less than .05 for the F test of the 3-way interaction.
To examine whether pollutants acted independently or jointly on lung outcomes, a model with linear combinations was implemented based on estimates from the fully adjusted models that included all the pollutants simultaneously. Concentration-response curves were generated using a thin plate regression spline with 4 degrees of freedom for long-term exposure to the air pollutants assessed at baseline and during follow-up.
The significance threshold was .05 and all tests were 2-sided. Additional sensitivity analyses were conducted by (1) including copollutants to assess independent, combined, and multiplicative associations on the study outcomes, (2) adding an additional random intercept for neighborhood clusters, (3) examining the relationship between pollutants and relative progression of percent emphysema using a log-transformed outcome, (4) using alternative definitions for emphysema, (5) excluding data with low lung volume on CT scan, (6) examining the effect of missing data by excluding participants who underwent only 1 examination, and (7) restricting to participants who had both CT and spirometry measurements. Analyses were performed using SAS version 9.4 (SAS Institute).
Results
Study Participants and Air Pollutant Concentrations
This study included 7071 participants (6814 MESA participants and 257 MESA Air study participants). Of 7069 participants with percent emphysema measures, 6860 were assigned outdoor residential air pollution concentrations in the year of their baseline examination and during the follow-up period (eFigure 1 in the Supplement). Of these participants, 5780 (84%) had at least 1 follow-up CT scan over a median (range) of 10 (1-18) years. Air pollution concentrations could be assigned to 3636 of 3813 MESA Lung participants with at least 1 spirometric lung function assessment, 2772 (76%) of whom had a second spirometric assessment at a median of 10 years.
Of the 6860 participants at baseline, 3126 (46%) were lifelong nonsmokers, 2595 (38%) were white, and 4344 (63%) reported at least some college education (Table). Baseline median (interquartile range [IQR]) percent emphysema was 3% (4.5%) and the mean rate of change was 0.58 percentage points (95% CI, 0.38-0.78) over 10 years. The rate of change varied by site and exposure levels (eTable 1 in the Supplement). Regarding lung function, 826 of 3636 participants (22%) had airflow obstruction at baseline (eTable 2 in the Supplement) and the cohort exhibited a mean decline in FEV1 of 309 mL (95% CI, 299-319) and in FVC of 331 mL (95% CI, 317-345) over 10 years.
Table. Descriptive Characteristics of Study Participants at Baseline in 2000 and Unadjusted Longitudinal Outcomes by Study Areas in the Analysis of Percent Emphysema.
| Baseline Characteristics | Winston-Salem, NC | New York, NY | Baltimore, MD | St. Paul, MN | Chicago, IL | Los Angeles, CA |
|---|---|---|---|---|---|---|
| No. of participants | ||||||
| Baseline (n = 6860) | 1037 | 1182 | 1054 | 1021 | 1142 | 1424 |
| Follow-up (n = 5780) | 928 | 1031 | 821 | 914 | 1016 | 1070 |
| Age, mean (SD), y | 62.6 (9.8) | 61.7 (10.3) | 63.2 (10) | 60.4 (10.5) | 62.4 (10.1) | 62.7 (10.5) |
| Sex, No. (%) | ||||||
| Female | 553 (53.3) | 662 (56.0) | 569 (54.0) | 516 (50.5) | 611 (53.5) | 725 (50.9) |
| Male | 484 (46.7) | 520 (44.0) | 485 (46.0) | 505 (49.5) | 531 (46.5) | 699 (49.1) |
| Race/ethnicity, No. (%) | ||||||
| White | 552 (53.2) | 236 (20.0) | 521 (49.4) | 596 (58.4) | 542 (47.5) | 148 (10.4) |
| Chinese | 0 | 2 (0.2) | 0 | 0 | 300 (26.4) | 521 (36.6) |
| Black | 482 (46.5) | 409 (34.6) | 533 (50.6) | 0 | 299 (26.2) | 171 (12.1) |
| Hispanic | 3 (0.3) | 534 (45.2) | 0 | 425 (41.6) | 0 | 584 (41.0) |
| Education, No. (%) | ||||||
| ≤High school | 304 (29.3) | 526 (44.5) | 339 (32.2) | 417 (40.8) | 175 (15.3) | 755 (53.0) |
| ≥Some college | 733 (70.7) | 656 (55.5) | 715 (67.8) | 604 (59.2) | 967 (84.7) | 669 (47.0) |
| Smoking status, No. (%) | ||||||
| Never | 403 (38.9) | 532 (45.0) | 436 (41.4) | 414 (40.5) | 545 (47.7) | 796 (55.9) |
| Former | 464 (44.7) | 472 (39.9) | 456 (43.3) | 438 (42.9) | 464 (40.6) | 474 (33.3) |
| Current | 170 (16.4) | 177 (15.0) | 161 (15.3) | 169 (16.6) | 134 (11.7) | 154 (10.8) |
| Pack-years of smoking in ever-smokers, median (25th percentile, 75th percentile) | 19 (5, 37) | 12 (3, 30) | 17 (5, 35) | 14 (3, 33) | 16 (4, 35) | 11 (2, 28) |
| Secondhand smoking, No. (%)a | 590 (56.9) | 518 (43.8) | 531 (50.4) | 627 (61.4) | 592 (51.8) | 410 (28.8) |
| Current employment at baseline, No. (%) | 661 (63.7) | 671 (56.8) | 628 (59.6) | 712 (69.7) | 762 (66.7) | 658 (46.2) |
| BMI <30, No. (%) | 658 (63.5) | 766 (64.8) | 637 (60.4) | 602 (59.0) | 762 (78.5) | 1081 (75.9) |
| Airflow obstruction, No. (%) | 256 (24.7) | 273 (23.1) | 278 (26.4) | 211 (20.7) | 274 (24.0) | 263 (18.5) |
| Air pollution at baseline, mean (SD)b | ||||||
| O3, ppb | 25.2 (1.6) | 14 (2.0) | 20.4 (1.3) | 23.2 (1.3) | 18.3 (1.6) | 16.6 (2.1) |
| PM2.5, μg/m3 | 17 (0.4) | 17.5 (1.8) | 16.2 (0.8) | 12 (0.6) | 16.2 (1.2) | 22.7 (2.6) |
| NOx, ppb | 24.2 (6.7) | 83.4 (21.6) | 42.7 (12.1) | 26.1 (5.6) | 47.5 (8.0) | 73.4 (18.7) |
| Black carbon, μg/m3 | 0.5 (0.1) | 1.4 (0.3) | 0.7 (0.2) | 0.5 (0.1) | 0.7 (0.1) | 1.1 (0.1) |
| Air pollution over follow-up, mean (SD)c | ||||||
| O3, ppb | 25.7 (2.4) | 15 (1.6) | 22.2 (2.2) | 22.5 (1.6) | 21.4 (2.3) | 18.3 (2.4) |
| PM2.5, μg/m3 | 13.5 (1.3) | 14.7 (1.8) | 13.2 (1.9) | 9.7 (0.6) | 13.6 (1.6) | 18.4 (3.9) |
| NOx, ppb | 16.5 (6.0) | 66.7 (17.2) | 30.2 (11.6) | 20.6 (4.8) | 35.5 (10.0) | 55.4 (14.9) |
| Percent emphysema, mean, (SD)d | ||||||
| Baseline | 3.26 (4.85) | 4.70 (4.29) | 4.04 (3.95) | 3.50 (4.09) | 5.37 (4.46) | 4.08 (4.07) |
| Progression/y | 0.07 (0.28) | 0.08 (0.55) | 0.09 (0.42) | 0.06 (0.23) | 0.11 (0.57) | 0.01 (0.45) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NOx, oxides of nitrogen; O3, ozone; PM2.5, fine particulate matter; ppb, parts per billion
Secondhand smoking was defined as participants, including never and ever smokers, who contacted other people smoking at home, in a car, or at work in the past year.
Baseline exposures were assessed as pollutant concentration for the year of the baseline examination in 2000 or for the years 2006 to 2008 (for black carbon) for a total of 18902 scans in 6860 participants.
Exposure over follow-up was the mean exposures for each participant aggregated from the year of the baseline examination to that of the follow-up clinic examination within the years 2000 to 2018 for a total of 18574 scans in 6860 participants.
The percentage of lung pixels below −950 Hounsfield units.
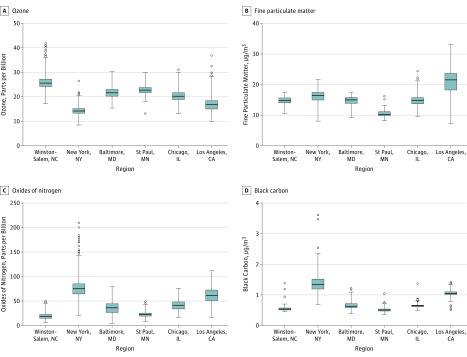
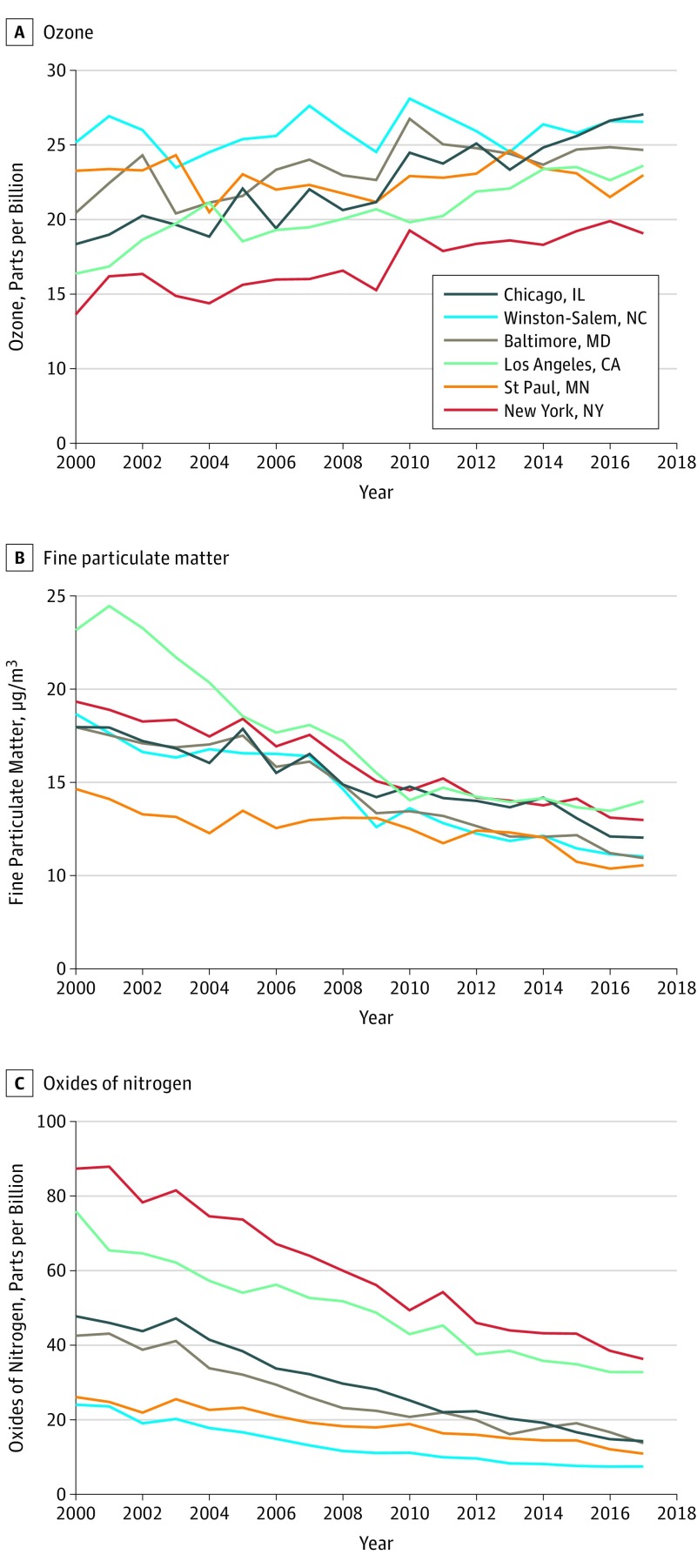
Air pollution concentrations varied substantially across the study regions over 18 years (Figure 1 and Figure 2). While annual mean concentrations of PM2.5 and NOx declined over follow-up, O3 concentrations did not (Figure 2). Correlations of residential ambient O3 (2000-2018) with residential ambient PM2.5 and NOx within each study region were negative and of moderate degree (r = −0.32 for PM2.5 and −0.42 for NOx) (eTable 3 in the Supplement).
Figure 1. Distribution of Air Pollution Exposure at Baseline and Over Follow-up Examinations From 2000 to 2018 by Study Area .
Boxplots included baseline air pollution concentrations in 2000 or for the years of 2006-2008 (for black carbon) and follow-up air pollution exposures aggregated from the year of the baseline examination to that of the follow-up clinic examination from 2000 to 2018 for each participant at each scan. The boxes indicate the interquartile range (IQR) and the line in the center indicates the median concentration. Whiskers extend to 1.5 times the IQR to the most distant observation within that distance (ie, the largest or smallest observations within quartile 3 + 1.5 × IQR or quartile 1 − 1.5 × IQR). Outlier observations are shown as circles. Exposure data are shown by study areas, which included 1037 participants (2837 scans) in Winston-Salem, NC; 1182 (3227 scans) in New York, NY, and Rockland County, NY; 1054 (2842 scans) in Baltimore, MD; 1021 (2933 scans) in St Paul, MN; 1142 (3396 scans) in Chicago, IL; and 1424 (3339 scans) in the Los Angeles Basin area.
Figure 2. Annual Mean Air Pollution Concentrations per Year Based on Location.
Metropolitan area temporal trends in air pollution exposures are shown based on annual mean concentrations for all participants in each area from continuous 2-week average concentrations from 2000 through follow-up. The exposure data included 1037 participants in Winston-Salem, NC; 1182 in New York, NY, and Rockland County, NY; 1054 in Baltimore, MD; 1021 in St Paul, MN; 1142 in Chicago, IL; and 1424 in the Los Angeles Basin area.
Air Pollution and Longitudinal Change in Percent Emphysema
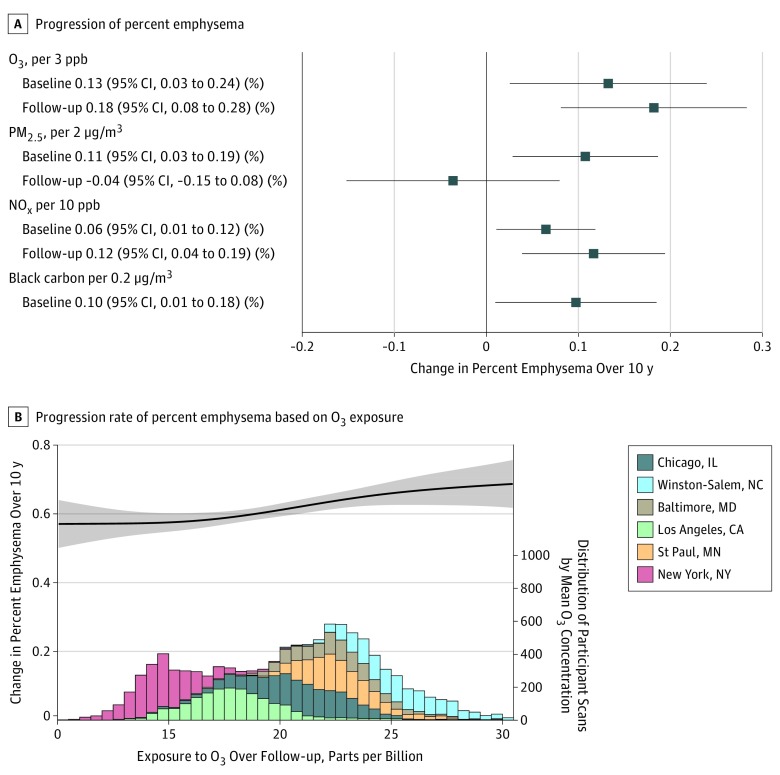
Among the 6860 included participants, greater exposures to O3, PM2.5, and NOx assessed at study baseline and to black carbon averaged through 2006 to 2008 were all significantly associated with an increased progression of percent emphysema over 10 years, per rounded IQR increment (O3: 0.13 per 3 parts per billion [ppb] [95% CI, 0.03-0.24]; PM2.5: 0.11 per 2 μg/m3 [95% CI, 0.03-0.19]; NOx: 0.06 per 10 ppb [95% CI, 0.01 to 0.12]; black carbon: 0.10 per 0.2 μg/m3 [95% CI, 0.01-0.18]).
In the analyses of air pollutant exposure over follow-up, a 3 ppb–higher long-term mean O3 exposure assessed over follow-up was significantly associated with an increased progression of 0.18 percentage points in percent emphysema over 10 years (95% CI, 0.08-0.28) (Figure 3). This increase is equal to the association of 29 pack-years of smoking (each 10 pack-years of smoking was significantly associated with an increased progression of 0.06 percentage points [95% CI, 0.02-0.11] of percent emphysema) or 3 years of aging in this cohort. The association between pollutant concentrations over follow-up and faster progression of percent emphysema was also statistically significant for NOx (increase, 0.12 percentage points per 10 ppb [95% CI, 0.04-0.19]), but not for PM2.5 (increase, −0.04 per 2 μg/m3 [95% CI, −0.15 to 0.08]).
Figure 3. Longitudinal Associations of Exposure to Air Pollution With Progression of Percent Emphysema Over 10 Years in 6860 Participants .
A, The linear longitudinal association of baseline exposure to O3, PM2.5, and NOx in 2000 or 2006 to 2008 for black carbon (18 902 scans) and mean follow-up air pollution exposures from the year of the baseline examination to that of the follow-up clinic examination from 2000 to 2018 (18 574 scans) for each scan. Progression of percent emphysema shown via computed tomographic (CT) imaging (percentage of lung pixels less than −950 Hounsfield units) over 10 years shown from linear mixed model. Associations were presented per rounded interquartile range increment of exposure to O3, PM2.5, NOx, and black carbon. Models are adjusted for age, sex, race/ethnicity, height, weight, temperature, smoking status, secondhand smoking, pack-years, cigarettes per day, body mass index, physical activity, income, employment status, education, neighborhood socioeconomic status (SES) index, study region, the interaction between SES and study region, and interactions of these variables with time as well as variables without interactions with time, such as CT scanners, pixel size, and baseline exposure. B, The concentration-response curve with 95% CI for the overall change of percent emphysema (progression rate associated with O3 concentrations over follow-up) with the same model adjustments as in panel A. The curve is based on a mixed model that includes a thin plate regression spline with 4 degrees of freedom to more flexibly assess the potentially nonlinear relationship. The highest and lowest 1% of overall concentrations have been trimmed for visualization because the relationship at the extremes is uncertain and might rely on concentrations from a single region. The histogram shows the distribution of O3 concentration over follow-up in this cohort, with the contribution of each area to the total distribution indicated by color; the height of each bar in the histogram represents the number of scans analyzed in each 0.5 parts per billion (ppb) bin of O3 concentration.
Results for O3, PM2.5, and NOx were not sensitive to accounting for neighborhood clusters, use of log-transformed outcome or alternative definitions of quantitatively assessed emphysema (ie, hidden Markov measure field, PD15), restriction to participants having both percent emphysema and lung function measures, or exclusion of data with low scan lung volume or participants with a single examination (eTables 4 and 5 in the Supplement). Addition of PM2.5, NOx, or black carbon as a covariate similarly did not weaken the associations with exposure to O3 at baseline or over follow-up, nor did addition of O3 affect the association with baseline PM2.5.
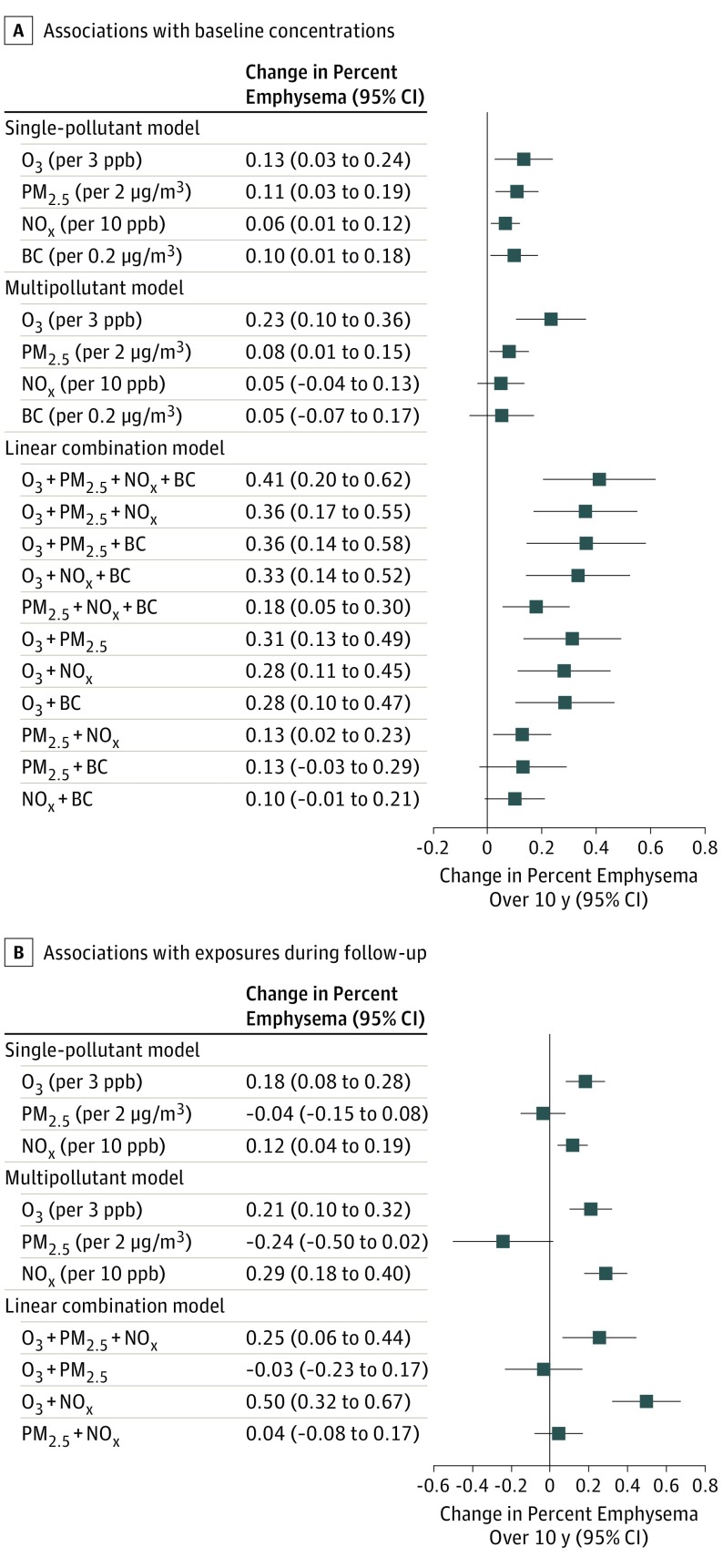
Combining the associations of the multiple air pollutants on change in percent emphysema resulted in a greater magnitude of associations than when assessing associations of individual pollutants (Figure 4 and eTable 6 in the Supplement). The concentration-response function between long-term O3 exposure assessed over follow-up and progression of percent emphysema has a sigmoidal appearance, most strongly linear in the area with the most participants (where CIs are narrower) and with suggestion of attenuation of the association at both lower and higher concentrations (where CIs are wider) (Figure 3). While there was no clear-cut evidence for effect modification across the subgroups studied (eTables 7-9 in the Supplement), greater progression of emphysema with O3 assessed over follow-up was observed in the group with airflow limitation at baseline (increase of 0.35 percentage points [95% CI, 0.18-0.51]) compared with the group without airflow limitation (increase, 0.15 percentage points [95% CI, 0.03-0.27]).
Figure 4. Effect Estimates for the Associations Between Air Pollutants and Progression of Percent Emphysema .
Associations are presented as rounded interquartile range (IQR) increment of exposure to O3, PM2.5, NOx, and black carbon (BC). A, Results of a single-pollutant model derived from main analyses for the associations between each of the air pollutants and progression of percent emphysema (percentage of lung pixels less than −950 Hounsfield units) are shown. The multipollutant model presents the associations of the fully adjusted model with all measured air pollutants modeled simultaneously. Linear combination models were implemented by combining the associations from any pairs of the air pollutants, based on associations from the multi-pollutant model.
A, The associations with baseline air pollution exposure in 2000 or 2006 to 2008 (for black carbon) in 18 902 scans. B, The associations with follow-up air pollution exposures aggregated from the year of the baseline examination to that of the follow-up clinic examination from 2000 to 2018 in 18 574 scans. Details of the exact numbers for the associations from the single-pollutant, multipollutant and linear combination models are shown in eTable 6 in the Supplement.
Air Pollution and Longitudinal Change in Lung Function
Among 3636 participants, 3 ppb in long-term O3 exposure assessed over follow-up was significantly associated with a 18.15-mL greater decline in FEV1 (95% CI, 1.59-34.71) and a 40.19-mL greater decline in FVC (95% CI, 17.88-62.49) over 10 years (eTable 10 and eFigure 3 in the Supplement). These associations were robust when additionally adjusted for baseline respiratory symptoms. The associations were generally not sensitive to inclusion of copollutants. Associations between O3 and FEV1 and FVC were of greater magnitude among current smokers; associations for FEV1 were of greater magnitude among participants with airflow obstruction at baseline (eTable 11 in the Supplement).
There was no significant association between other air pollutants and lung function decline or between O3 and FEV1/FVC ratio (eTable 10 in the Supplement). No indication of combined association was observed for FEV1 and FVC with other pollutants (eFigure 4 in the Supplement). Associations with FEV1 and FVC were statistically significant for exposure to O3 at baseline, but not for the other pollutants (eTable 12 in the Supplement).
Discussion
Higher residential concentrations of O3, PM2.5, and NOx at study baseline, of black carbon averaged from 2006 to 2008, and of O3 and NOx assessed over study follow-up were significantly associated with greater increases in percent emphysema, assessed via CT imaging, over a median of 10 years. Findings were most robust and of greatest magnitude for O3. Because percent emphysema is related to respiratory symptoms, hospitalizations,7 and mortality even among individuals without airflow obstruction,4,6 these associations in a community-based population demonstrate novel evidence that air pollution contributes to worsening lung health.
Despite existing regulations to prevent short-term excursions of O3 levels, long-term average concentrations of O3, which were associated with changes in lung structure and function, did not decline during the years of observation. Absent new control strategies, these levels are not expected to decline as climate change advances.25 This contrasts with the observed trends in PM2.5 and NOx that highlight the success of past regulatory initiatives to control these 2 pollutants. Because long-term concentrations of O3 at current levels were strongly and consistently associated with both progression of emphysema and decline in lung function in this study, more effective control strategies to reduce O3 concentrations may be needed to protect lung health.
Ground-level O3 is a powerful oxidizing agent and common air pollutant worldwide. Toxicologic studies have shown that O3 causes persistent pulmonary inflammation and structural alterations throughout the lung that may play a role in chronic lower respiratory disease, including emphysema.26 In a mouse model, exposure to O3 for 6 weeks induced a chronic inflammatory process, with increased protease expression, epithelial apoptosis and alveolar enlargement, and destruction, mimicking emphysema.27 In animals, PM2.5 exposure leads to neutrophilic pulmonary inflammation and oxidative stress,28 and in healthy adults, controlled exposure to PM2.5 leads to increased systemic inflammation and endothelial microparticles, including those of lung origin.29 This may be relevant because endothelial apoptosis causes emphysema in animals and endothelial microparticles have been linked to lower lung function and, specifically, percent emphysema in individuals with mild COPD and smokers without COPD.30
The current study found that faster progression of percent emphysema and decline in FEV1 were significantly associated with long-term O3 concentrations among the subset of participants with airflow limitation at baseline. This finding is consistent with prior studies demonstrating worse outcomes related to air pollution in patients with lung disease31 and supports the conclusion in the 2013 EPA Integrated Science Assessment that individuals with lung disease are a high-risk group for O3-related health effects.32 Findings for O3 and loss of lung function were greater among current smokers, perhaps because of additive effects of airway inflammation and oxidative stress.33
Higher exposures to PM2.5 and NOx at baseline and NOx (and no other pollutants) over follow-up were also significantly associated with faster emphysema progression. When all pollutants were considered together, there was evidence that the association for O3 was of the greatest magnitude, which is consistent with other literature,12 and for an additive effect of copollutants, which is consistent with animal studies.33
Unlike O3 and NOx, PM2.5 exposure over follow-up was not significantly associated with emphysema progression. Development of emphysema can be a slow, lifelong process and, once initiated, additional areas of emphysema in the adjacent lung can be produced from biomechanical forces, even in the absence of further exposures, such as cigarette smoke.34 These findings might explain the increased rate of emphysema progression significantly associated with PM2.5 at baseline but not during follow-up. Baseline measures likely reflect exposure at and prior to baseline, particularly for PM2.5 concentrations, which declined substantially over the 10 years of follow-up. In addition, the changes in scanner technology over follow-up as PM2.5 concentrations decreased may have made the follow-up analyses less sensitive for emphysema progression than the baseline analyses.
While O3 exposure was significantly associated with decline in lung function, the other pollutants were not; statistical power for spirometry was smaller than for percent emphysema on CT because the number of observations for spirometry were less than half those for percent emphysema and occurred during the latter period of follow-up when nonozone pollutant concentrations had declined. While air pollutants, including O3, PM2.5, NOx, and black carbon, have been associated consistently with respiratory events,10,12 relatively few studies have reported a longitudinal association between O3, PM2.5, and NOx and decline in lung function in older adults, and findings have not been completely consistent. Single-site studies have reported longitudinal associations between PM2.513 and black carbon35 and a faster decline in lung function, and a larger multi-center study in Europe found PM10 to be associated with a faster lung function decline.36 However, another large European study did not find any longitudinal associations between air pollutants and lung function decline.37
To our knowledge, this is the first longitudinal study to assess the association between long-term exposure to air pollutants and progression of percent emphysema on CT in a large, community-based multiethnic cohort. Other strengths of this study include the large sample size, the long period of follow-up, and the fine-scale assessment of residential-level outdoor air pollution concentrations from cohort-specific monitoring and advanced statistical modeling.
Limitations
This study has several limitations. First, outdoor air pollution concentrations, especially in the case of O3, may not fully reflect individual air pollution exposures and dose in all microenvironments38; outdoor concentrations do not explain all variation in indoor concentrations, and most individuals spend the majority of their time indoors.39 Second, percent emphysema was measured only in the lower two-thirds of the lung. However, percent emphysema measured in the lower two-thirds of the lung correlates well with full-lung percent emphysema (ICC, 0.93) in this cohort and a cohort of smokers,18 and percent emphysema measured on cardiac scans was associated with dyspnea, spirometric obstruction, and mortality in this cohort.4,8 Third, CT scanners changed over the 18 years of data collection; however, analyses using an advanced image processing approach designed to account for scanner variation yielded similar results, as did sensitivity analyses using various approaches to adjust for stratification by scanner type (eTables 4 and 5 in the Supplement). There is debate about the optimal parameterization of the histogram of the lung attenuation; however, sensitivity analyses using PD15 yielded consistent results. Fourth, no volume correction of CT data was performed,40 given the debate over its utility and the partial lung imaging; however, results were consistent when participants with a difference greater than 20% in lung volume on CT scans were excluded.
Conclusions
In the United States between 2000 and 2018, long-term exposure to ambient air pollutants was significantly associated with increases in emphysema assessed quantitatively via CT imaging and lung function.
eMethods. Statistical modeling
eTable 1 Unadjusted percent emphysema progression and lung function decline per year by quartile of O3 exposure concentrations in the MESA cohort
eTable 2 Descriptive characteristics [mean (SD) or %] of study participants at baseline and unadjusted longitudinal outcomes by study areas in the analysis of lung function
eTable 3 Pearson correlation coefficients between predicted air pollution exposures at the MESA participants’ homes within the MESA cities (N=6860)
eTable 4 Main and sensitivity analyses of effect estimates (95% CIs) of air pollution concentrations, assessed at baseline, with longitudinal changes of percent emphysema on CT, per 10 years from staged models
eTable 5 Main and sensitivity analyses of effect estimates (95% CIs) of long-term air pollution concentrations, assessed during follow-up, with longitudinal change of percent emphysema on CT over 10 years from staged models
eTable 6 Effect estimates (95% CIs) for the associations between air pollutant exposures at baseline or over follow-up and progression of percent emphysema in single-pollutant, multiple-pollutant and linear combination models, per IQR increment for O3 (3 ppb), PM2.5 (2 µg/m3), NOx (10 ppb) and black carbon (0.2 µg/m3).
eTable 7 Longitudinal changes in percent emphysema (95% CI) per over ten years per increase of O3, PM2.5, NOx and black carbon, assessed at baseline, stratified by potential effect modifiers
eTable 8 Longitudinal changes in percent emphysema (95% CI) over ten years per increase of long-term O3, PM2.5, NOx concentration, assessed during following, stratified by potential effect modifiers
eTable 9 Multiplicative interactions between air pollutant exposures for effect of air pollution on progression of percent emphysema over ten years (N=6860)
eTable 10 Main and sensitivity analyses of effect estimates (95% CIs) of long-term air pollution concentrations, assessed during following with longitudinal changes of lung function over 10 years from staged models
eTable 11 Longitudinal changes in lung function (95% CI) per 3 ppb increase of O3 assessed during following over 10 years stratified by personal factors
eTable 12 Main and sensitivity analyses of effect estimates (95% CIs) of exposure to air pollution concentrations assessed at baseline with longitudinal changes of lung function over 10 years from staged models
eFigure 1 Participant recruitment, retention, and flow of outcome testing in the analysis of percent emphysema and lung function in the Multi-Ethnic Study of Atherosclerosis.
eFigure 2 Concentration-response curves with 95%CI for the overall change of percent emphysema (progression rate associated with air pollution concentrations, assessed at baseline and over follow-up (N=6860)
eFigure 3 Concentration-response curve with 95%CI for the change of FEV1 (rate of change associated with air pollution concentrations, assessed at baseline and over follow-up, N=3636).
eFigure 4 Effect estimates for the associations between air pollutants and lung function decline (N=3636). Results from single-pollutant model, multiple-pollutant model and linear combination model for the effect estimates of multiple air pollutant exposures (A: at baseline for FEV1, B: over follow-up for FEV1, C: at baseline for FVC, and D: over follow-up for FVC), from multi-pollutant models.
References
- 1.Kochanek KD, Murphy SL, Xu JQ, Arias E. Mortality in the United States, 2016. NCHS Data Brief, No. 293. Hyattsville, MD: National Center for Health Statistics; 2017. [PubMed] [Google Scholar]
- 2.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736-1788. doi: 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557-582. doi: 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 4.Oelsner EC, Hoffman EA, Folsom AR, et al. Association between emphysema-like lung on cardiac computed tomography and mortality in persons without airflow obstruction: a cohort study. Ann Intern Med. 2014;161(12):863-873. doi: 10.7326/M13-2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gevenois PA, De Vuyst P, de Maertelaer V, et al. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154(1):187-192. doi: 10.1164/ajrccm.154.1.8680679 [DOI] [PubMed] [Google Scholar]
- 6.Johannessen A, Skorge TD, Bottai M, et al. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med. 2013;187(6):602-608. doi: 10.1164/rccm.201209-1722OC [DOI] [PubMed] [Google Scholar]
- 7.McAllister DA, Ahmed FS, Austin JH, et al. Emphysema predicts hospitalisation and incident airflow obstruction among older smokers: a prospective cohort study. PLoS One. 2014;9(4):e93221. doi: 10.1371/journal.pone.0093221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oelsner EC, Smith BM, Hoffman EA, et al. Associations between emphysema-like lung on CT and incident airflow limitation: a general population-based cohort study. Thorax. 2018;73(5):486-488.doi: 10.1136/thoraxjnl-2017-210842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen AJ, Brauer M, Burnett R, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389(10082):1907-1918. doi: 10.1016/S0140-6736(17)30505-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Sun S, Tang R, et al. Major air pollutants and risk of COPD exacerbations: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2016;11:3079-3091. doi: 10.2147/COPD.S122282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao Y, Balluz L, Strosnider H, Wen XJ, Li C, Qualters JR. Ozone, fine particulate matter, and chronic lower respiratory disease mortality in the United States. Am J Respir Crit Care Med. 2015;192(3):337-341. doi: 10.1164/rccm.201410-1852OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strosnider HM, Chang HH, Darrow LA, Liu Y, Vaidyanathan A, Strickland MJ. Age-specific associations of ozone and fine particulate matter with respiratory emergency department visits in the United States. Am J Respir Crit Care Med. 2019;199(7):882-890. doi: 10.1164/rccm.201806-1147OC [DOI] [PubMed] [Google Scholar]
- 13.Rice MB, Ljungman PL, Wilker EH, et al. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham heart study. Am J Respir Crit Care Med. 2015;191(6):656-664. doi: 10.1164/rccm.201410-1875OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman JD, Adar SD, Allen RW, et al. Prospective study of particulate air pollution exposures, subclinical atherosclerosis, and clinical cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). Am J Epidemiol. 2012;176(9):825-837. doi: 10.1093/aje/kws169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez J, Jiang R, Johnson WC, MacKenzie BA, Smith LJ, Barr RG. The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: a cross-sectional study. Ann Intern Med. 2010;152(4):201-210. doi: 10.7326/0003-4819-152-4-201002160-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35-43. doi: 10.1148/radiol.2341040439 [DOI] [PubMed] [Google Scholar]
- 17.Sieren JP, Newell JD Jr, Barr RG, et al. ; SPIROMICS Research Group . SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194(7):794-806. doi: 10.1164/rccm.201506-1208PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman EA, Jiang R, Baumhauer H, et al. Reproducibility and validity of lung density measures from cardiac CT scans—the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16(6):689-699. doi: 10.1016/j.acra.2008.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hame Y, Angelini ED, Hoffman EA, Barr RG, Laine AF. Adaptive quantification and longitudinal analysis of pulmonary emphysema with a hidden Markov measure field model. IEEE Trans Med Imaging. 2014;33(7):1527-1540. doi: 10.1109/TMI.2014.2317520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M, Keller JP, Adar SD, et al. Development of long-term spatiotemporal models for ambient ozone in six metropolitan regions of the United States: the MESA Air Study. Atmos Environ (1994). 2015;123(A):79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller JP, Olives C, Kim SY, et al. A unified spatiotemporal modeling approach for predicting concentrations of multiple air pollutants in the multi-ethnic study of atherosclerosis and air pollution. Environ Health Perspect. 2015;123(4):301-309. doi: 10.1289/ehp.1408145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen MA, Adar SD, Allen RW, et al. Approach to estimating participant pollutant exposures in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). Environ Sci Technol. 2009;43(13):4687-4693. doi: 10.1021/es8030837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohansal R, Martinez-Camblor P, Agustí A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med. 2009;180(1):3-10. doi: 10.1164/rccm.200901-0047OC [DOI] [PubMed] [Google Scholar]
- 24.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99-106. doi: 10.1056/NEJM200107123450205 [DOI] [PubMed] [Google Scholar]
- 25.Wilson A, Reich BJ, Nolte CG, Spero TL, Hubbell B, Rappold AG. Climate change impacts on projections of excess mortality at 2030 using spatially varying ozone-temperature risk surfaces. J Expo Sci Environ Epidemiol. 2017;27(1):118-124. doi: 10.1038/jes.2016.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Bree L, Dormans JA, Boere AJ, Rombout PJ. Time study on development and repair of lung injury following ozone exposure in rats. Inhal Toxicol. 2001;13(8):703-718. doi: 10.1080/08958370152409928 [DOI] [PubMed] [Google Scholar]
- 27.Triantaphyllopoulos K, Hussain F, Pinart M, et al. A model of chronic inflammation and pulmonary emphysema after multiple ozone exposures in mice. Am J Physiol Lung Cell Mol Physiol. 2011;300(5):L691-L700. doi: 10.1152/ajplung.00252.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riva DR, Magalhães CB, Lopes AA, et al. Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhal Toxicol. 2011;23(5):257-267. doi: 10.3109/08958378.2011.566290 [DOI] [PubMed] [Google Scholar]
- 29.Pope CA III, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O’Toole T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res. 2016;119(11):1204-1214. doi: 10.1161/CIRCRESAHA.116.309279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomashow MA, Shimbo D, Parikh MA, et al. Endothelial microparticles in mild chronic obstructive pulmonary disease and emphysema: the Multi-Ethnic Study of Atherosclerosis Chronic Obstructive Pulmonary Disease study. Am J Respir Crit Care Med. 2013;188(1):60-68. doi: 10.1164/rccm.201209-1697OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ware LB, Zhao Z, Koyama T, et al. Long-term ozone exposure increases the risk of developing the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2016;193(10):1143-1150. doi: 10.1164/rccm.201507-1418OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ISA Integrated Science Assessment for Ozone and Related Photochemical Oxidants. Washington, DC: US Environmental Protection Agency; 2013:600/R-10/076F. https://www.epa.gov/isa/integrated-science-assessment-isa-ozone-and-related-photochemical-oxidants.
- 33.Yu M, Pinkerton KE, Witschi H. Short-term exposure to aged and diluted sidestream cigarette smoke enhances ozone-induced lung injury in B6C3F1 mice. Toxicol Sci. 2002;65(1):99-106. [DOI] [PubMed] [Google Scholar]
- 34.Bhatt SP, Bodduluri S, Hoffman EA, et al. ; COPDGene Investigators . Computed tomography measure of lung at risk and lung function decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(5):569-576. doi: 10.1164/rccm.201701-0050OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lepeule J, Litonjua AA, Coull B, et al. Long-term effects of traffic particles on lung function decline in the elderly. Am J Respir Crit Care Med. 2014;190(5):542-548. doi: 10.1164/rccm.201402-0350OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Downs SH, Schindler C, Liu LJ, et al. ; SAPALDIA Team . Reduced exposure to PM10 and attenuated age-related decline in lung function. N Engl J Med. 2007;357(23):2338-2347. doi: 10.1056/NEJMoa073625 [DOI] [PubMed] [Google Scholar]
- 37.Adam M, Schikowski T, Carsin AE, et al. Adult lung function and long-term air pollution exposure. ESCAPE: a multicentre cohort study and meta-analysis. Eur Respir J. 2015;45(1):38-50. doi: 10.1183/09031936.00130014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brauer M, Brook JR. Personal and fixed-site ozone measurements with a passive sampler. J Air Waste Manag Assoc. 1995;45(7):529-537. doi: 10.1080/10473289.1995.10467384 [DOI] [PubMed] [Google Scholar]
- 39.Spalt EW, Curl CL, Allen RW, et al. Time-location patterns of a diverse population of older adults: the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). J Expo Sci Environ Epidemiol. 2016;26(4):349-355. doi: 10.1038/jes.2015.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoel BC, Putter H, Bakker ME, et al. Volume correction in computed tomography densitometry for follow-up studies on pulmonary emphysema. Proc Am Thorac Soc. 2008;5(9):919-924. doi: 10.1513/pats.200804-040QC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Statistical modeling
eTable 1 Unadjusted percent emphysema progression and lung function decline per year by quartile of O3 exposure concentrations in the MESA cohort
eTable 2 Descriptive characteristics [mean (SD) or %] of study participants at baseline and unadjusted longitudinal outcomes by study areas in the analysis of lung function
eTable 3 Pearson correlation coefficients between predicted air pollution exposures at the MESA participants’ homes within the MESA cities (N=6860)
eTable 4 Main and sensitivity analyses of effect estimates (95% CIs) of air pollution concentrations, assessed at baseline, with longitudinal changes of percent emphysema on CT, per 10 years from staged models
eTable 5 Main and sensitivity analyses of effect estimates (95% CIs) of long-term air pollution concentrations, assessed during follow-up, with longitudinal change of percent emphysema on CT over 10 years from staged models
eTable 6 Effect estimates (95% CIs) for the associations between air pollutant exposures at baseline or over follow-up and progression of percent emphysema in single-pollutant, multiple-pollutant and linear combination models, per IQR increment for O3 (3 ppb), PM2.5 (2 µg/m3), NOx (10 ppb) and black carbon (0.2 µg/m3).
eTable 7 Longitudinal changes in percent emphysema (95% CI) per over ten years per increase of O3, PM2.5, NOx and black carbon, assessed at baseline, stratified by potential effect modifiers
eTable 8 Longitudinal changes in percent emphysema (95% CI) over ten years per increase of long-term O3, PM2.5, NOx concentration, assessed during following, stratified by potential effect modifiers
eTable 9 Multiplicative interactions between air pollutant exposures for effect of air pollution on progression of percent emphysema over ten years (N=6860)
eTable 10 Main and sensitivity analyses of effect estimates (95% CIs) of long-term air pollution concentrations, assessed during following with longitudinal changes of lung function over 10 years from staged models
eTable 11 Longitudinal changes in lung function (95% CI) per 3 ppb increase of O3 assessed during following over 10 years stratified by personal factors
eTable 12 Main and sensitivity analyses of effect estimates (95% CIs) of exposure to air pollution concentrations assessed at baseline with longitudinal changes of lung function over 10 years from staged models
eFigure 1 Participant recruitment, retention, and flow of outcome testing in the analysis of percent emphysema and lung function in the Multi-Ethnic Study of Atherosclerosis.
eFigure 2 Concentration-response curves with 95%CI for the overall change of percent emphysema (progression rate associated with air pollution concentrations, assessed at baseline and over follow-up (N=6860)
eFigure 3 Concentration-response curve with 95%CI for the change of FEV1 (rate of change associated with air pollution concentrations, assessed at baseline and over follow-up, N=3636).
eFigure 4 Effect estimates for the associations between air pollutants and lung function decline (N=3636). Results from single-pollutant model, multiple-pollutant model and linear combination model for the effect estimates of multiple air pollutant exposures (A: at baseline for FEV1, B: over follow-up for FEV1, C: at baseline for FVC, and D: over follow-up for FVC), from multi-pollutant models.