Key Points
Question
Is National Comprehensive Cancer Network (NCCN)–recommended posttreatment clinical surveillance in patients with human papillomavirus (HPV)–associated oropharyngeal squamous cell carcinoma (SCC) associated with improvements in recurrence detection and survival outcomes?
Findings
In this cohort study of 233 patients with HPV–associated oropharyngeal SCC, only 1 asymptomatic recurrence was detected of 3358 posttreatment clinical surveillance visits, and adherence to the recommended schedule did not seem to improve survival.
Meaning
The findings of this study suggest that reduction of the NCCN-recommended posttreatment clinical surveillance schedule for HPV–associated oropharyngeal SCC is warranted.
Abstract
Importance
National Comprehensive Cancer Network (NCCN) guidelines recommend routine clinical follow-up as posttreatment surveillance for patients with head and neck cancer (HNC). Human papillomavirus–associated oropharyngeal squamous cell carcinoma (HPV-associated OPSCC) is a unique subset of HNC, associated with fewer recurrences and improved survival. The utility of this guideline in this patient population is unknown.
Objective
To determine adherence to the NCCN clinical follow-up guideline, frequency of recurrence detection method, classified as symptom-directed, physician-detected, or imaging-detected, and survival benefit associated with adherence to the NCCN guideline.
Design, Setting, and Participants
Retrospective cohort study of patients with HPV-associated OPSCC diagnosed between January 1, 2011, and April 30, 2014, at a large integrated health care system. Multivariable analyses were conducted using the Cox proportional hazards regression model, with patient adherence to NCCN visit guidelines constructed as a time-dependent variable. All data analyses were complete on September 1, 2018.
Exposures
Posttreatment clinical and imaging surveillance.
Main Outcomes and Measures
Recurrence and overall survival. Secondary outcome was salvage therapy.
Results
Of the 233 study patients with HPV-associated OPSCC, the mean (SD) age at diagnosis was 60.5 (8.7) years; 201 (86.3%) were male, 189 (81.1%) were white, and 109 (46.8%) had a positive smoking history. Median follow-up time through recurrence or all-cause mortality was 4.5 years (interquartile range, 3.8-5.6). Patients demonstrated 83.0% (180 of 217) adherence to NCCN surveillance guidelines in year 1, 52.7% (106 of 201) in year 2, 73.4% (141 of 192) in year 3, 62.3% (96 of 154) in year 4, and 52.9% (45 of 85) in year 5. A total of 3358 clinical surveillance examinations were performed with 22 patients having recurrences. There were 10 symptom-directed, 1 physician-detected, and 11 imaging-detected recurrences. Of the symptom-directed recurrences, salvage therapy was attempted in 5; at the study end date, 1 was alive. Salvage neck dissection was attempted in the physician-detected recurrence; this patient subsequently died. All locoregional recurrences occurred within the first 2 years, and all salvageable recurrences within the first year. Adherence to NCCN guidelines was not protective against all-cause mortality in the multivariable Cox proportional hazards regression model (hazard ratio, 0.76; 95% CI, 0.28-2.05).
Conclusions and Relevance
Among patients with HPV-associated OPSCC, clinical surveillance is of limited utility. Nearly all clinically detected recurrences were elicited by patient symptoms that prompted earlier presentation to the clinician. Adherence to the current schedule does not appear to confer survival advantage, and locoregional recurrences are almost never detected beyond 2 years. These findings support reduction of posttreatment clinical surveillance in this population.
This cohort study evaluates the association between National Comprehensive Cancer Network–recommended posttreatment clinical surveillance adherence and outcomes in a cohort of patients with of human papilloma virus–associated oropharyngeal squamous cell carcinoma.
Introduction
The National Comprehensive Cancer Network (NCCN) guidelines recommend clinical surveillance for patients with head and neck cancer (HNC) after treatment completion. The recommended schedule is every 1 to 3 months for the first year, every 2 to 6 months for the second year, every 4 to 8 months for years 3 to 5, and annually thereafter.1 Previous studies show that most recurrences in HNC are discovered in the presence of patients’ symptoms, with no survival advantage to those who adhered to the recommended clinical follow-up schedule or whose recurrence was diagnosed while asymptomatic.2,3,4,5,6,7,8 The utility of this guideline in detecting recurrences across different cancer phenotypes, sites, and behaviors is unknown.
There is evidence that the utility of posttreatment clinical surveillance of HNC may differ according to cancer characteristics. For example, oral cavity squamous cell carcinoma is linked to alcohol and tobacco use, which is accompanied by a field cancerization effect9 in which frequent examination may be helpful to detect recurrent or metachronous lesions. Human papillomavirus-associated oropharyngeal squamous cell carcinoma (HPV-associated OPSCC) is a cancer phenotype on the other end of the spectrum with no field cancerization effect10 and excellent locoregional control rates.11 A 2016 study of HPV-associated OPSCC found that most recurrences were distant and that most locoregional recurrences occurred within 6 months in symptomatic patients. The authors therefore proposed de-escalation of the NCCN follow-up schedule.12
The objectives of this retrospective study of patients with HPV-associated OPSCC were to determine (1) adherence to the NCCN clinical follow-up guidelines in a large integrated health care system; (2) frequency of recurrence detection method, classified as symptom-directed, physician-detected, or imaging-detected; and (3) whether there was a survival advantage associated with adhering to the NCCN clinical follow-up guidelines.
Methods
This study population consisted of members of Kaiser Permanente Northern California (KPNC), a large, integrated health care delivery system. There was a mean of 3.35 million KPNC members per year for the duration of the study. Comprehensive patient data such as radiology, inpatient, emergency department, and outpatient visits, social history, and diagnosis data are contained within the electronic health record (EHR). The Kaiser Foundation Research Institute’s Institutional Review Board approved this study with waiver of consent because data were deidentified.
Database Creation
Identification of the final cohort of 233 patients with HPV-associated OPSCC has been described previously.13 Briefly, the KPNC tumor registry was searched for all patients aged 18 to 89 years having OPSCC who were treated from January 1, 2011 to April 30, 2014 (n = 307); potential participants were identified using the following International Classification of Diseases for Oncology, Third Edition codes: C019, C024, C090, C091, C098, C099, C100, C102, C108, and C109. All data analyses were complete on September 1, 2018. Medical record review of potential patients was then conducted to confirm p16 and/or HPV positivity and that treatment was administered with curative intent (n = 248). We also obtained the following additional variables from record review: treatment modalities, posttreatment imaging tests, and salvage therapy. The following clinical and demographic variables were electronically extracted from the EHR: age, race/ethnicity, sex, smoking history, TNM (tumor, node, metastasis) stage (according to the AJCC Cancer Staging Manual 8th edition [AJCC 8]), posttreatment clinical visits, and membership. Fifteen patients were excluded for metastatic disease or synchronous cancer at presentation, insufficient follow-up, or treatment without curative intent.
Outcomes
Recurrences and second primary cancers were obtained from record review. All-cause mortality and dates of death were obtained by linking to the State of California Death Certificates database, also described previously.14
Adherence to NCCN Guidelines
Visits to medical oncology, radiation oncology, and the head and neck surgery departments within KPNC were counted for each patient to determine adherence to NCCN guidelines. We calculated an adherence measurement for each 3-month interval in years 1 and 2, for each 6-month interval in years 3 and 4, and for each 12-month interval in years 5 and later after completion of treatment. We determined that patients were in compliance with guidelines when there was at least 1 visit per interval, allowing for a 45-day grace period. Visits were only counted once; when a grace period visit was counted toward the immediately preceding interval, it was not also counted toward the current interval.
Classification and Frequency of Recurrence Detection
Recurrences were classified as (1) symptom-directed, (2) physician-detected, or (3) imaging-detected. A recurrence was classified as symptom-directed when it was the patient’s symptom that prompted the workup and then ultimately diagnosis. A recurrence was classified as physician-detected when the clinician detected an examination result that led to the diagnosis in an otherwise asymptomatic patient. Last, a recurrence was classified as imaging-detected when an abnormal finding on a posttreatment imaging test in an asymptomatic patient led to the recurrence diagnosis. The last date of treatment was used as baseline when assessing time to recurrence. Only a patient’s first recurrence was counted. Recurrences after this were excluded from outcomes analyses because these recurrences were considered to have a significantly higher pretest probability for subsequent recurrence than the rest of the cohort.
Statistical Analyses
Adherence to NCCN guidelines was analyzed in descriptive analyses by 3- to 12-month intervals. Adherence to NCCN guidelines was also analyzed by year to show adherence in years 1 through 5 posttreatment.
For analyses with the outcome of recurrence, patients were followed until recurrence, death, loss to follow-up, or until the study end date. For analyses with the outcome of all-cause mortality, patients were followed until death, loss to follow-up, or study end date.
Multivariable analysis for the outcome all-cause mortality was conducted using the Cox proportional hazards regression model adjusted for age, sex, race/ethnicity, smoking status, AJCC 8 stage, treatment modality, as well as patient adherence to NCCN posttreatment clinic visit guidelines, which was constructed as a time-dependent variable.
As patients’ adherence to NCCN guidelines varied, adherence to guidelines was constructed as a time-dependent variable. To create the variable adherence to NCCN guidelines, 3-month intervals were created for patients for all months of follow-up. For univariate analyses, intervals were the length of time during which a patient was required to have 1 visit (3-month intervals in years 1 and 2, 6-month intervals in years 3 and 4, and 12-month intervals thereafter). However, for the multivariable Cox proportional hazards regression model, we created intervals of equal length across all years of follow-up. For example, patients meeting NCCN guidelines during one 6-month interval (including grace period) in year 3 of follow-up were counted as adherent for two 3-month intervals. A patient was considered to be adherent if they were adherent in the prior interval; in other words, in this model we assessed whether adherence in the immediately preceding interval was associated with all-cause mortality during the current interval. Age was also constructed as a time-dependent covariate; age was calculated for each interval. All other covariates were constructed as fixed covariates. For the model assessing the association between adherence and all-cause mortality for the full cohort, data were truncated at 5 years of follow-up.
Adjusted hazard ratios were calculated as maximum likelihood estimates with sandwich variance estimates. Proportional hazards assumptions were met for all Cox proportional hazards regression models. Covariates included in the multivariable model were selected for clinical significance. Variables in multivariable models were assessed for interaction; all were found to be independent.
Statistical analyses were performed using SAS 9.4 (SAS Institute Inc). Analyses were 2-tailed and unpaired with statistical significance set at <.05.
Results
Of the 233 study patients with HPV-associated OPSCC, the mean (SD) age at diagnosis was 60.5 (8.7) years; 201 (86.3%) were male, 189 (81.1%) were white, and 109 (46.8%) had a positive smoking history. One hundred fifty-four patients (66.1%) had stage I disease, 61 (26.2%) stage II, and 18 (7.7%) stage III. There were no patients with stage IV disease (Table 1).
Table 1. Cohort Demographic and Clinical Characteristics.
| Characteristic | No. (%) |
|---|---|
| Age, mean (SD), y | 60.5 (8.7) |
| Follow-up, median (IQR), y | 4.5 (3.8-5.6) |
| Sex | |
| Male | 201 (86.3) |
| Female | 32 (13.7) |
| Race/ethnicity | |
| White | 189 (81.1) |
| Black | 19 (18.2) |
| Hispanic | 14 (6.0) |
| Asian | 10 (4.3) |
| Unknown/other | 1 (0.4) |
| Smoking status | |
| Never | 115 (49.3) |
| Current | 34 (14.6) |
| Past | 75 (32.2) |
| Second-hand | 3 (1.3) |
| Unknown | 6 (2.6) |
| Cancer site | |
| Palatine tonsil | 141 (60.5) |
| Tongue base | 87 (37.3) |
| Other | 5 (2.1) |
| AJCC 8 | |
| Stage I | 154 (66.1) |
| Stage II | 61 (26.2) |
| Stage III | 18 (7.7) |
| Stage IV | 0 |
| Initial curative treatment | |
| Definitive concurrent chemoradiotherapy | 159 (68.2) |
| Definitive radiotherapy | 10 (4.3) |
| Surgery | |
| Alone | 16 (6.9) |
| With adjuvant radiotherapy | 29 (12.4) |
| With adjuvant chemoradiotherapy | 19 (8.2) |
Abbreviations: AJCC 8, AJCC Cancer Staging Manual 8th edition; IQR, interquartile range.
Median follow-up time through recurrence or all-cause mortality was 4.5 years (interquartile range [IQR], 3.8-5.6). Of the full cohort, 159 (68.2%) were treated with definitive chemoradiotherapy, 10 (4.3%) with radiotherapy alone, 16 (6.9%) with surgery alone, and 48 (20.6%) with surgery plus adjuvant radiotherapy or chemoradiotherapy (Table 1). Posttreatment surveillance imaging consisted of 403 positron emission tomography (PET)/computed tomography (CT) scans, 17 magnetic resonance imaging (MRI) tests, and 21 computed tomography (CT) tests. At the study end date, 198 (85.0%) patients were alive. One patient developed a second primary cancer in the lung. There were no metachronous HNC recurrences.
Adherence to NCCN Guidelines
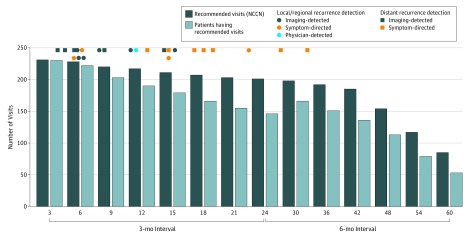
Patients demonstrated 83.0% adherence (180 of 217) to the NCCN surveillance guidelines in year 1, 52.7% (106 of 201) in year 2, 73.4% (141 of 192) in year 3, 62.3% (96 of 154) in year 4, and 52.9% (45 of 85) in year 5. Numbers of patients adhering to NCCN guidelines per 3- to 6-month interval are shown in the Figure. In each interval, the bar on the left indicates the number of expected visits, assuming all patients followed for the full interval were adherent to NCCN guidelines. The bar on the right reflects the number of patients meeting the minimum number of visits needed to be considered adherent. For analyses summarized in Figure, patients were followed until their first recurrence or until death or loss to follow-up. To have been counted in an interval, a patient must have been followed (without censoring, recurrence, or death) for the full interval.
Recurrence Detection
A total of 3358 clinical surveillance examinations were performed to detect recurrences in 10 symptomatic patients (symptom-directed; 4 locoregional and 7 distant, 1 patient experienced simultaneous locoregional and distant recurrences) and 1 asymptomatic patient (physician-detected; locoregional, Table 2). Surveillance-imaging (imaging-detected) identified a further 11 recurrences (10 PET/CT scan and 1 CT scan, Table 2). In sum, 22 patients experienced a cumulative 23 recurrences. Twenty-one of 22 (95.0%) patients came in early for the visit at which their recurrence was detected. All locoregional recurrences occurred within the first 2 years and all salvageable recurrences within the first year (Figure). There were no metachronous HNCs.
Table 2. Method and Site of Recurrence Detection.
| Method of Detection | No. (%) | |||
|---|---|---|---|---|
| Patient (n = 22) | Recurrence | |||
| Locoregional (n = 11) | Distant (n = 12) | Total (n = 23) | ||
| Symptom-directed | 10 (45.5) | 4 (36.3) | 7 (58.3) | 11 (47.8) |
| Physician-detected | 1 (4.5) | 1 (9.1) | 0 (0) | 1 (4.3) |
| Imaging-detected | 11 (50.0) | 6 (54.5) | 5 (41.7) | 11 (47.8) |
Figure. Adherence to NCCN-Recommended Surveillance Schedule.
In each interval, the bar on the left indicates the number of expected visits, assuming all patients followed for the full interval were adherent to NCCN guidelines. The bar on the right reflects the number of patients meeting the minimum number of visits needed to be considered adherent. Patients were followed until their first recurrence or until death or loss to follow-up. To have been counted in an interval, a patient must have been followed (without censoring, recurrence, or death) for the full interval. NCCN indicates National Comprehensive Cancer Network.
Survival Outcomes
Of the 11 clinically detected recurrences (symptom-directed and physician-detected), salvage treatment was attempted in 6 patients (3 local resections, 2 neck dissections, and 1 metastasectomy). As of the study end date, 1 was alive, 4 dead, and 1 lost to follow-up. The patient with the physician-detected recurrence died. Of patients having recurrence, 16 (72.7%) died by the end of follow-up; median survival time after recurrence diagnosis was 2.6 years (IQR 1.7-3.9).
Of the 22 patients having recurrence, 19 (86.4%) adhered to NCCN guidelines in the interval prior to diagnosis. In a multivariable Cox proportional hazards regression model assessing the association between adherence to visit guidelines and all-cause mortality among patients having recurrence, adherence to guidelines in the interval immediately preceding detection of recurrence did not confer a survival advantage (adjusted hazard ratio [aHR], 0.62; 95% CI, 0.17-2.30). This model was adjusted for cancer stage at diagnosis.
In a multivariable Cox proportional hazards regression model assessing the association between adherence to NCCN guidelines (as a time-dependent variable) and all-cause mortality among the full cohort, adherence to guidelines was not associated with survival (referent: no adherence, aHR 0.76; 95% CI, 0.28-2.05, Table 3). Additionally, no covariates were significantly associated with survival.
Table 3. Association Between Adherence to NCCN Surveillance Guidelines and All-Cause Mortality in 230 Patientsa.
| Independent Variable | Adjusted Hazard Ratio (95% CI)b |
|---|---|
| Adherence to clinic guidelines in prior interval | 0.76 (0.28-2.05) |
| Age (every 10 y) | 1.75 (0.95-3.19) |
| Male | 0.83 (0.22-3.19) |
| Female | 1 [Reference] |
| Race/ethnicity | |
| White | 1 [Reference] |
| Asian | 1.32 (0.14-12.05) |
| Black | 2.22 (0.65-7.60) |
| Hispanic | 0.28 (0.04-2.12) |
| Smoking | |
| Non-smoker | 1 [Reference] |
| Ever smoked | 1.00 (0.43-2.32)) |
| Missing smoking data | 3.26 (0.27-38.95) |
| AJCC 8 stage | |
| Stage I | 1 [Reference] |
| Stage II | 2.04 (0.84-5.00) |
| Stage III | 3.21 (0.90-11.40) |
| Treatment modality | |
| Radiation therapy and chemotherapy, but no surgery | 1 [Reference] |
| Chemotherapy, surgery, and radiotherapy | 2.11 (0.57-7.83) |
| Surgery and radiotherapy (no chemotherapy) | 0.56 (0.12-2.74) |
| Radiotherapy only | 0.44 (0.03-7.03) |
| Surgery only | 0.36 (0.04-3.46) |
Abbreviations: AJCC 8, AJCC Cancer Staging Manual 8th edition; NCCN, National Comprehensive Cancer Network.
Two patients having less than 1 full interval of follow-up and 1 patient having unknown/other indication for race were excluded from this analysis.
Adjusted for age, sex, race/ethnicity, smoking status, AJCC 8 stage, treatment modality, as well as patient adherence to NCCN posttreatment clinic visit guidelines, which was constructed as a time-dependent variable.
Discussion
In this exclusively HPV-associated OPSCC cohort, adherence rates to the NCCN surveillance guidelines were high during the first posttreatment year (83.0%) and then decreased each subsequent year (52%-73%). Adherence to these guidelines is not well documented. In 2 other studies of patients with HNC, adherence ranged from 81.0% to 98.5%.6,15 In our integrated health care system with an insured patient population, one might expect a higher adherence rate. The reasons for nonadherence were not captured in our study, but we hypothesize that they could be due to physician, patient, or logistical factors.
Among our cohort of patients with HPV-associated OPSCC, just 1 asymptomatic recurrence was diagnosed by examination of 3358 clinical visits. Furthermore, no metachronous HNCs were identified. Similar findings were reported in 2 studies, with just 1 asymptomatic recurrence of 232 patients12 and no recurrences over 340 clinical visits.16 The reasons for this low number of asymptomatic recurrence detections are several. First, for HNC of all subsites, most recurrences are detected in the presence of patient symptoms.17 Second, HPV-associated OPSCC carries a better prognosis with low risk of locoregional recurrence compared with other types of HNC.11 Third, when recurrences do occur in HPV-associated OPSCC, a substantial proportion of them are distant18 and unlikely to be detected in the absence of symptoms. Last, in our cohort a significant proportion of asymptomatic recurrences are detected by the first posttreatment PET/CT scan, and therefore may be more accurately characterized as radiotherapy failures rather than recurrence.
To our knowledge, the current study is the first to assess the association between posttreatment clinical surveillance adherence and mortality in a cohort of patients having HPV-associated OPSCC. We found that adherence to current guidelines is not associated with improved survival, with all locoregional recurrences identified within the first 2 years of follow-up and all salvageable recurrences within the first year. Recurrence was associated with worse outcomes; 72.7% of the patients died by the end of follow-up. Researchers have recommended reducing posttreatment clinical surveillance schedules to 3 visits in the first year, 2 in the second year, and then annually for years 3-5.12,16 Based on this study’s findings, results support the above recommended schedule for routine clinical surveillance. Patients should be educated on warning signs for locoregional recurrence and have ready access to physicians when symptoms arise. Such a modification to the surveillance schedule would decrease the burden of visits on both patients and the health care system without compromising recurrence detection.
Strengths and Limitations
The strengths of this study are its relatively large cohort size and follow-up time frame. Limitations of this study include its retrospective nature. The sample size and low recurrence rate may render the survival analyses underpowered to detect statistically significant hazard ratios. The utility of posttreatment clinical visits for psychological reassurance and management of surgical complications or radiotherapy toxic effects is not addressed, but undoubtedly these are important considerations of the posttreatment clinical schedule and should not be overlooked.
Conclusions
For patients with HPV-associated OPSCC, the currently recommended clinical surveillance regimen almost never detects an asymptomatic recurrence. The findings of this study suggest that adherence to this schedule is not associated with improvements in survival, and locoregional recurrences were not detected beyond 2 years. These findings appear support reduction in the number of posttreatment clinical surveillance visits for patients with HPV-associated OPSCC.
References
- 1.National Comprehensive Cancer Network NCCN guidelines. Head and neck cancer. http://www.nccn.org/professionals/physician_gls/ pdf/head-and-neck.pdf. Accessed March 23, 2019.
- 2.Schwartz LH, Ozsahin M, Zhang GN, et al. . Synchronous and metachronous head and neck carcinomas. Cancer. 1994;74(7):1933-1938. doi: [DOI] [PubMed] [Google Scholar]
- 3.Cooney TR, Poulsen MG. Is routine follow-up useful after combined-modality therapy for advanced head and neck cancer? Arch Otolaryngol Head Neck Surg. 1999;125(4):379-382. doi: 10.1001/archotol.125.4.379 [DOI] [PubMed] [Google Scholar]
- 4.Schwartz DL, Barker J Jr, Chansky K, et al. . Postradiotherapy surveillance practice for head and neck squamous cell carcinoma: too much for too little? Head Neck. 2003;25(12):990-999. doi: 10.1002/hed.10314 [DOI] [PubMed] [Google Scholar]
- 5.Agrawal A, deSilva BW, Buckley BM, Schuller DE. Role of the physician versus the patient in the detection of recurrent disease following treatment for head and neck cancer. Laryngoscope. 2004;114(2):232-235. doi: 10.1097/00005537-200402000-00011 [DOI] [PubMed] [Google Scholar]
- 6.Agrawal A, Hammond TH, Young GS, Avon AL, Ozer E, Schuller DE. Factors affecting long-term survival in patients with recurrent head and neck cancer may help define the role of post-treatment surveillance. Laryngoscope. 2009;119(11):2135-2140. doi: 10.1002/lary.20527 [DOI] [PubMed] [Google Scholar]
- 7.Flynn CJ, Khaouam N, Gardner S, et al. . The value of periodic follow-up in the detection of recurrences after radical treatment in locally advanced head and neck cancer. Clin Oncol (R Coll Radiol). 2010;22(10):868-873. doi: 10.1016/j.clon.2010.05.016 [DOI] [PubMed] [Google Scholar]
- 8.Kothari P, Trinidade A, Hewitt RJD, Singh A, O’Flynn P. The follow-up of patients with head and neck cancer: an analysis of 1,039 patients. Eur Arch Otorhinolaryngol. 2011;268(8):1191-1200. doi: 10.1007/s00405-010-1461-2 [DOI] [PubMed] [Google Scholar]
- 9.Malik A, Sahu A, Singh SP, et al. . In vivo Raman spectroscopy-assisted early identification of potential second primary/recurrences in oral cancers: An exploratory study. Head Neck. 2017;39(11):2216-2223. doi: 10.1002/hed.24884 [DOI] [PubMed] [Google Scholar]
- 10.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. . Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294-4301. doi: 10.1200/JCO.2011.36.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ang KK, Harris J, Wheeler R, et al. . Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frakes JM, Naghavi AO, Demetriou SK, et al. . Determining optimal follow-up in the management of human papillomavirus-positive oropharyngeal cancer. Cancer. 2016;122(4):634-641. doi: 10.1002/cncr.29782 [DOI] [PubMed] [Google Scholar]
- 13.Corpman DW, Masroor F, Carpenter DM, Nayak S, Gurushanthaiah D, Wang KH. Posttreatment surveillance PET/CT for HPV-associated oropharyngeal cancer. Head Neck. 2019;41(2):456-462. doi: 10.1002/hed.25425 [DOI] [PubMed] [Google Scholar]
- 14.Newman TB, Brown AN. Use of commercial record linkage software and vital statistics to identify patient deaths. J Am Med Inform Assoc. 1997;4(3):233-237. doi: 10.1136/jamia.1997.0040233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagh A, Vedtofte T, Lynggaard CD, et al. . The value of routine follow-up after treatment for head and neck cancer: a national survey from DAHANCA. Acta Oncol. 2013;52(2):277-284. doi: 10.3109/0284186X.2012.741324 [DOI] [PubMed] [Google Scholar]
- 16.Ilmarinen T, Keski-Säntti H, Markkanen-Leppänen M, et al. . De-escalation of post-treatment surveillance in oropharyngeal cancer. Head Neck. 2019;41(5):1457-1462. doi: 10.1002/hed.25593 [DOI] [PubMed] [Google Scholar]
- 17.Denaro N, Merlano MC, Russi EG. Follow-up in head and neck cancer: do more does it mean do better? a systematic review and our proposal based on our experience. Clin Exp Otorhinolaryngol. 2016;9(4):287-297. doi: 10.21053/ceo.2015.00976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bledsoe TJ, Noble AR, Hunter GK, et al. . Oropharyngeal squamous cell carcinoma with known human papillomavirus status treated with definitive chemoradiotherapy: patterns of failure and toxicity outcomes. Radiat Oncol. 2013;8(1):174. doi: 10.1186/1748-717X-8-174 [DOI] [PMC free article] [PubMed] [Google Scholar]