Key Points
Question
Are specific midlife to late-life longitudinal blood pressure patterns associated with increased risk of dementia among older adults?
Finding
In this prospective cohort study that included 4761 participants with 24-year follow-up and blood pressure measurements at midlife and at late life, those with midlife and late-life hypertension (hazard ratio, 1.49) and those with midlife hypertension and late-life hypotension (hazard ratio, 1.62) had higher risk for incident dementia compared with those who remained normotensive.
Meaning
Patterns of blood pressure in midlife and late life may be associated with differing risks for incident dementia.
Abstract
Importance
The association between late-life blood pressure (BP) and cognition may depend on the presence and chronicity of past hypertension. Late-life declines in blood pressure following prolonged hypertension may be associated with poor cognitive outcomes.
Objective
To examine the association of midlife to late-life BP patterns with subsequent dementia, mild cognitive impairment, and cognitive decline.
Design, Setting, and Participants
The Atherosclerosis Risk in Communities prospective population-based cohort study enrolled 4761 participants during midlife (visit 1, 1987-1989) and followed-up over 6 visits through 2016-2017 (visit 6). BP was examined over 24 years at 5 in-person visits between visits 1 and 5 (2011-2013). During visits 5 and 6, participants underwent detailed neurocognitive evaluation. The setting was 4 US communities: Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and Minneapolis, Minnesota. Follow-up ended on December 31, 2017.
Exposures
Five groups based on longitudinal patterns of normotension, hypertension (>140/90 mm Hg), and hypotension (<90/60 mm Hg) at visits 1 to 5.
Main Outcomes and Measures
Primary outcome was dementia onset after visit 5, based on Ascertain Dementia-8 informant questionnaires, Six-Item Screener telephone assessments, hospital discharge and death certificate codes, and the visit 6 neurocognitive evaluation. Secondary outcome was mild cognitive impairment at visit 6, based on the neurocognitive evaluation.
Results
Among 4761 participants (2821 [59%] women; 979 [21%] black race; visit 5 mean [SD] age, 75 [5] years; visit 1 mean age range, 44-66 years; visit 5 mean age range, 66-90 years), there were 516 (11%) incident dementia cases between visits 5 and 6. The dementia incidence rate for participants with normotension in midlife (n = 833) and late life was 1.31 (95% CI, 1.00-1.72 per 100 person-years); for midlife normotension and late-life hypertension (n = 1559), 1.99 (95% CI, 1.69-2.32 per 100 person-years); for midlife and late-life hypertension (n = 1030), 2.83 (95% CI, 2.40-3.35 per 100 person-years); for midlife normotension and late-life hypotension (n = 927), 2.07 (95% CI, 1.68-2.54 per 100 person-years); and for midlife hypertension and late-life hypotension (n = 389), 4.26 (95% CI, 3.40-5.32 per 100 person-years). Participants in the midlife and late-life hypertension group (hazard ratio [HR], 1.49 [95% CI, 1.06-2.08]) and in the midlife hypertension and late-life hypotension group (HR, 1.62 [95% CI, 1.11-2.37]) had significantly increased risk of subsequent dementia compared with those who remained normotensive. Irrespective of late-life BP, sustained hypertension in midlife was associated with dementia risk (HR, 1.41 [95% CI, 1.17-1.71]). Compared with those who were normotensive in midlife and late life, only participants with midlife hypertension and late-life hypotension had higher risk of mild cognitive impairment (37 affected individuals (odds ratio, 1.65 [95% CI, 1.01-2.69]). There was no significant association of BP patterns with late-life cognitive change.
Conclusions and Relevance
In this community-based cohort with long-term follow-up, sustained hypertension in midlife to late life and a pattern of midlife hypertension and late-life hypotension, compared with midlife and late-life normal BP, were associated with increased risk for subsequent dementia.
Over 24 years plus follow-up, this community-based cohort study monitored individuals categorized by midlife and late-life blood pressure levels to evaluate association between blood pressure levels and incident dementia.
Introduction
High blood pressure, and in certain circumstances, low blood pressure, have been associated with cognitive decline and dementia in community-based studies, suggesting that blood pressure may serve as a viable target for primary or secondary dementia prevention.1 Accumulating evidence2,3 suggests that hypertension during midlife may be a risk factor for cognitive decline and dementia. While the relationship of elevated blood pressure during the 7th, 8th, and 9th decades of life with cognitive outcomes is less clear,1 several studies indicate that optimal blood pressure ranges for older adults may depend on earlier blood pressure characteristics.4,5 However, few large-scale studies have examined dynamic midlife to late-life blood pressure patterns longitudinally in relation to subsequent cognitive decline and dementia, making it difficult to draw firm conclusions.4,6
An improved understanding of the evolving relationship between late-life blood pressure, past hypertension, and cognitive functioning must be established before recommendations can be made with regard to blood pressure targets for lowering the risk of dementia in older adults. The Atherosclerosis Risk in Communities (ARIC) study has recorded blood pressure information for its cohort over 24 years, including midlife to late-life. Using this community-based sample of mostly black and white participants, the current study examined the association of midlife to late-life blood pressure patterns with incident dementia, mild cognitive impairment, and late-life cognitive change. This study tested the hypothesis that individuals with an extended duration of midlife hypertension followed by low blood pressure later in life are at higher risk for dementia in older age.
Methods
Participants
This ongoing community-based cohort study initially enrolled 15 792 participants (aged 45-65 years) from 4 US communities: Washington County, Maryland; Forsyth County, North Carolina; northwestern suburbs of Minneapolis, Minnesota; and Jackson, Mississippi from 1987 to 1989.7 Participants were evaluated in person every 3 years until visit 4 (1996-1998) (Figure 1). Fifteen years later, participants were invited back for visit 5 (2011-2013). Participants returned for visit 6 between 2016 and 2017. At visits 5 and 6, participants underwent a comprehensive cognitive and functional assessment. This analysis included all participants who received a baseline cognitive battery and functional assessment at visit 5, excluding participants who met criteria for dementia before or at visit 5 and those missing data on important variables (eFigure 1 in the Supplement). The study protocols were approved by the institutional review boards at each participating center. All participants gave written informed consent at each study visit; proxies (usually next of kin or other family members) provided consent for participants who were judged to lack capacity.
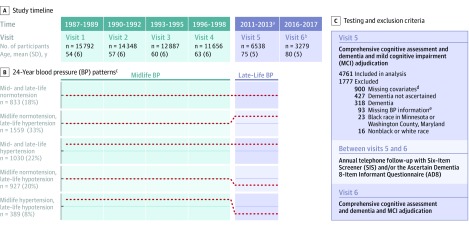
Figure 1. Study Timeline and Longitudinal Blood Pressure Patterns.
aOf the participants who attended visit 4, 3304 died before visit 5 and 2247 did not attend visit 5. There were 433 participants who did not attend visit 4 but attended visit 5.
bBetween visits 5 and 6, 1777 participants in the analytic sample were excluded (see panel C), 198 died, and 1284 did not attend visit 6.
cBlood pressure pattern groups based on blood pressure levels at Visits 1 to 5. Dotted lines represent the approximate 24-year blood pressure pattern. Hypertension was defined as systolic blood pressure >140 mm Hg, or diastolic blood pressure >90 mm Hg, or use of antihypertensive medication. Hypotension was defined as systolic blood pressure <90 or diastolic blood pressure <60, irrespective of current antihypertensive medication use or hypertension diagnosis. Blood pressure patterns were defined using the standard hypertension definition. Midlife hypertension was defined as meeting hypertension criteria for two consecutive visits between Visits 1 and 4; persons not meeting this criterion were classified as midlife normotensive. Late-life normotension, hypertension, and hypotension were defined at Visit 5. Participants with midlife hypertension/late-life normotension were not included in the standard hypertension definition analyses because of small sample size (n = 23).
dParticipants were excluded for missing information regarding the following: smoking status (n = 687), drinking status (n = 321), body mass index (n = 185), coronary heart disease (n = 100), total cholesterol (n = 85), total high-density lipoprotein cholesterol (n = 85), diabetes (n = 56), previous stroke (n = 10), and education (n = 9). A subset of participants were missing information for more than 1 covariate; these values are not mutually exclusive.
eParticipants missing information (blood pressure or antihypertensive medication use) necessary to determine visit 5 hypertension status using the standard hypertension criteria were excluded.
Blood Pressure Assessment, Hypertension, and Hypotension
Sitting diastolic and systolic blood pressure (SBP) levels were assessed at visits 1 through 5 using a random zero sphygmomanometer. Blood pressure was defined as the mean of the last 2 measurements (>30-second interval between each measurement). Hypertension was defined as SBP above 140 mm Hg or diastolic blood pressure (DBP) above 90 mm Hg. Hypotension was defined at visit 5 as SBP lower than 90 mm Hg or DBP lower than 60 mm Hg, irrespective of current antihypertensive medication use or hypertension diagnosis.8,9,10 Midlife hypertension was defined as meeting hypertension criteria for 2 consecutive visits between visits 1 and 4; persons not meeting this criterion were classified as midlife normotensive. Late-life normotension, hypertension, and hypotension were defined at visit 5. To define 24-year blood pressure patterns, participants were grouped into one of the 5 categories based on blood pressure measured at visits 1 to 5 (Figure 1): midlife and late-life normotension; midlife normotension and late-life hypertension; midlife and late-life hypertension; midlife normotension and late-life hypotension; and midlife hypertension and late-life hypotension. Two approaches were used to define these blood pressure categories. The primary approach used the standard hypertension definition, which classified a participant as hypertensive if the participant met hypertension criteria based on measured blood pressure or if the participant was taking blood pressure medication at that visit, even if the individual’s blood pressure was in the normal range. In a post hoc exploratory analysis, the measured hypertension definition was used, which classified participants as hypertensive based on their measured blood pressure value only (eFigure 2 in the Supplement).
Outcomes
Primary Outcome of Dementia
For participants who attended visit 6, a comprehensive neuropsychological battery and an informant interview were used to assess dementia.11 An initial algorithmic dementia diagnosis at visit 6 was defined when 3 criteria were met: Functional Activities Questionnaire greater than 5 or Clinical Dementia Rating sum of boxes greater than 3; at least 2 cognitive domain scores greater than 1.5 standard deviations below the normative mean; and an overall decline from visit 5 on the study’s cognitive battery of greater than 0.055 standard deviations per year (the approximate rate of cognitive decline in neurologically healthy older adults).12,13 Dementia diagnoses were reviewed and confirmed by an expert committee (unaware of blood pressure measurement or hypertension status) based on criteria set by the National Institute on Aging and the Alzheimer’s Association14 and the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition).11,15 Between visits 5 and 6, participants were contacted annually by telephone and administered the Six-Item Screener.16 When indicated by low Six-Item Screener scores or when proxies were needed for the annual calls, the participant’s informant was administered the Ascertain Dementia 8-Item Informant Questionnaire.17 These measures were used to estimate date of dementia onset for participants who attended visit 6. For participants who did not attend visit 6, the Six-Item Screener, Ascertain Dementia 8-Item Informant Questionnaire, hospital discharge codes, and death certificates were used to define dementia diagnosis and date dementia of onset (eTable 1 in the Supplement).
Secondary Outcome of Mild Cognitive Impairment
Among participants without dementia, mild cognitive impairment at visit 6 was defined as at least 1 cognitive domain score greater than 1.5 standard deviations below the cohort’s normative mean (described in the next section),18 a Clinical Dementia Rating sum of boxes (≥0.5 and ≤3), a Functional Activities Questionnaire score of 5 or less, and an overall decline from visit 5 on the full cognitive battery of greater than 0.055 standard deviations per year.
Secondary Outcome of Cognitive Change
A battery of neuropsychological tests was administered to assess memory, processing speed and executive function, and language (eMethods in the Supplement). Using a latent variable approach described in the eMethods,19 a global cognitive z score (observed range, −3.7 [worst] to 2.9 [best]) was generated using all neuropsychological tests at visits 5 and 6. This global cognitive z score was used to estimate 5-year cognitive change.
Covariates
Analyses were adjusted for the following set of potential confounding variables: sex, race (selected from several choices), and years of education, defined at visit 1 based on the participant’s self-report. Race was examined given previous results suggesting race may moderate the association between vascular risk factors and dementia.20 All other covariates were assessed at visit 5, concurrent with the baseline cognitive assessment. Age and cigarette smoking and alcohol use status (current vs former vs never) were defined based on self-report. Body mass index (calculated as weight in kilograms divided by height in meters squared) was calculated, total cholesterol21 and high-density lipoprotein cholesterol were measured,22 and history of coronary heart disease, heart failure, and stroke (yes/no) was adjudicated. Diabetes was defined based on self-report of physician diagnosis, diabetes medication use, or HbA1c level of 6.5% or greater. The TaqMan assay was used to assess apolipoprotein E (APOE) genotype (0 vs ≥1 APOEε4 vs missing [n = 139]; Applied Biosystems, Foster City, California).
Statistical Analysis
The midlife and late-life normotension group was used as the referent to which each of the groups was compared on cognitive outcomes (Figure 1). Primary analyses examined the association of 24-year blood pressure patterns with time to onset of dementia for all participants who attended visit 5. This outcome, which is not conditional on visit 6 attendance, minimizes bias related to attrition and the competing risk of death after visit 5. A secondary outcome included only participants who attended visits 5 and 6. Participants without a dementia diagnosis were censored at the latest date of the visit 6 assessment or phone follow-up assessment with adequate information from the Six-Item Screener and the Ascertain Dementia 8-Item Informant Questionnaire. Analyses of incident dementia used Cox proportional hazards regression models. The Schoenfeld residuals test verified that the proportional hazards assumption was met. The association between blood pressure patterns and mild cognitive impairment was examined using logistic regression. Mild cognitive impairment analyses were restricted to participants who were cognitively normal at visit 5 who attended visit 6. Goodness-of-fit for logistic regression models was assessed using the Hosmer-Lemeshow test. Model diagnostics confirmed adequate model fit. Model assumptions related to collinearity and a sufficient number of events per variable were met. To determine whether rates of cognitive change differed by 24-year blood pressure pattern, generalized estimating equations with an exchangeable correlation matrix and robust variance were used to describe cognitive functioning at visits 5 and 6. An interaction term between blood pressure pattern and time was included to determine whether rate of cognitive change differed according to blood pressure pattern. Multiple imputation by chained equations was used to estimate visit 6 cognitive scores for participants who did not attend visit 6 (eMethods in the Supplement).
Analyses were adjusted for the demographic factors, physiological variables, vascular risk factors, and medical comorbidity described in the previous section. Cox proportional hazards regression models for incident dementia in the full sample included interactions of time with age, age squared, body mass index, heart failure, and stroke to account for changes in the association of each variable with dementia incidence over time. Generalized estimating equations models were also adjusted for an age × time interaction term. Multiplicative interaction terms were used to assess potential effect modification by age (median split at visit 5, <74 years vs ≥74 years), APOEε4 status (0 vs ≥1), and race (black vs white). A 2-sided P value of less than .05 was used as the cutoff for statistical significance. Because of the potential for type 1 error due to multiple comparisons, findings from analyses of secondary end points should be interpreted as exploratory. Analyses were conducted using Stata Version 14 (StataCorp).
Sensitivity Analyses
To mitigate potential reverse causation (ie, neurodegeneration causing a change in late-life blood pressure), analyses were repeated including only participants who were cognitively normal at visit 5. Additional analyses were performed using alternative methods to define late-life hypotension (>25% blood pressure decline), and propensity score matching was used to determine how blood pressure patterns related to dementia risk in a subset of participants with overlapping demographic, physiological, and clinical characteristics. Propensity scores for each 24-year blood pressure pattern (vs midlife and late-life normotension) were calculated using multivariable logistic regression. Participants in each 24-year blood pressure group were matched to a similar participant in the midlife and late-life normotension (reference) group based on the calculated propensity scores using a 1:1 nearest-neighbor matching procedure. Differences in dementia risk between the reference blood pressure group and the matched participant subset from each comparison group were examined using individual Cox proportional hazard regression models (eMethods in the Supplement). Analyses were also incorporated to determine whether the results were similar when midlife and late-life blood pressure were modeled as continuous variables. A post hoc analysis was conducted to determine whether the 2 blood pressure groups with midlife hypertension significantly differed with regard to dementia risk. Using a Cox regression model, this analysis compared a combined midlife hypertension group (the midlife and late-life hypertension group combined with the midlife hypertension and late-life hypotension group) to the midlife and late-life normotension (reference) group and incorporated a midlife hypertension × late-life blood pressure (hypertension/hypotension) interaction term to directly assess between-group heterogeneity. Additionally, a post hoc analysis was conducted using inverse probability weighting to examine the effects of participant attrition before visit 5. This technique, which uses regression weighting to correct for sampling bias, assigns larger weights to participants with characteristics associated with study dropout. Attrition weights were calculated from predicted probabilities derived using logistic regression (with demographic, physiological, and clinical covariate information) to model the probability of dropout due to study withdrawal between visits 1 and 5 (these methods are described in detail in the eMethods section in the Supplement).
Results
Participant characteristics are presented in Table 1; eTable 2 in the Supplement. A total of 4761 participants were included in the analytic sample (2821 [59%] women; 979 [21%] black race; visit 5 mean [SD] age, 75 [5] years; visit 1 mean age range, 44-66 years; visit 5 mean age range, 66-90 years). Compared with participants included in the study, participants who dropped out before visit 5 (eTable 3 in the Supplement) and those who were excluded (eTable 4 in the Supplement) were older and and more commonly identified as black, had higher blood pressure, poorer cardiovascular health, and poorer cognitive health. At visit 5, 21% (n = 1006) of participants met mild cognitive impairment criteria; all others were cognitively normal. The mean (SD) time between visit 5 and visit 6 was 4.9 (0.6) years. A subset of study participants (n = 1534) were missing visit 6 cognitive data. The prevalence of each blood pressure pattern is presented in Figure 1B.
Table 1. Visit 5 Participant Characteristics Stratified by 24-Year BP Patterns Using the Standard Hypertension Definition.
| Characteristic | 24-y Longitudinal Blood Pressure Patterns, No. (%)a | ||||
|---|---|---|---|---|---|
| Midlife and Late-Life Normotension | Midlife Normotension, Late-Life Hypertension | Midlife and Late-Life Hypertension | Midlife Normotension, Late-Life Hypotension | Midlife Hypertension, Late-Life Hypotension | |
| No.b | 833 | 1559 | 1030 | 927 | 389 |
| Demographics | |||||
| Age, mean (SD), y | 73.8 (4.4) | 74.9 (4.9) | 75.2 (4.9) | 76.0 (5.2) | 77.9 (5.2) |
| Men | 369 (44.3) | 639 (41.0) | 382 (37.1) | 374 (40.4) | 168 (43.2) |
| Women | 464 (55.7) | 920 (59.0) | 648 (62.9) | 553 (59.7) | 221 (56.8) |
| Black | 93 (11.2) | 299 (19.2) | 429 (41.7) | 68 (7.3) | 79 (20.3) |
| White | 740 (88.8) | 1260 (80.8) | 601 (58.4) | 859 (92.7) | 310 (79.7) |
| Center | |||||
| Minneapolis, Minnesota | 315 (37.8) | 507 (32.5) | 248 (24.1) | 358 (38.6) | 114 (29.3) |
| Washington County, Maryland | 183 (22.0) | 401 (25.7) | 215 (20.9) | 329 (35.5) | 158 (40.6) |
| Forsyth County, North Carolina | 250 (30.0) | 371 (23.8) | 162 (15.7) | 177 (19.1) | 41 (10.5) |
| Jackson, Mississippi | 85 (10.2) | 280 (18.0) | 405 (39.3) | 63 (6.8) | 76 (19.5) |
| Education | |||||
| <High school | 59 (7.1) | 196 (12.6) | 173 (16.8) | 89 (9.6) | 61 (15.7) |
| High school/GED/vocational | 326 (39.1) | 664 (42.7) | 438 (42.4) | 405 (43.7) | 182 (46.8) |
| College/graduate/professional | 448 (53.8) | 699 (44.9) | 419 (40.6) | 433 (46.7) | 146 (37.5) |
| Apolipoprotein E ε4 allelesc | |||||
| 0 (lowest AD risk) | 594 (73.3) | 1111 (73.4) | 700 (70.1) | 652 (72.6) | 265 (69.9) |
| 1 (moderate AD risk) | 204 (25.2) | 373 (24.6) | 269 (27.0) | 228 (25.4) | 107 (28.2) |
| 2 (highest AD risk) | 12 (1.5) | 32 (2.1) | 27 (2.7) | 18 (2.0) | 7 (1.9) |
| Physiological and laboratory variables, mean (SD) | |||||
| Body mass indexd | 27.5 (4.7) | 28.8 (5.2) | 30.8 (6.3) | 27.3 (5.6) | 29.4 (5.7) |
| Systolic BP, mm Hge | 124.3 (9.5) | 137.2 (16.4) | 138.1 (18.6) | 117.1 (14.3) | 121.1 (16.3) |
| Diastolic BP, mm Hge | 68.5 (6.1) | 71.8 (8.3) | 71.9 (8.6) | 53.9 (4.6) | 53.8 (4.8) |
| Total cholesterol, mg/dL | 195.3 (41.5) | 183.1 (41.8) | 179.7 (38.9) | 178.2 (40.4) | 165.8 (37.4) |
| HDL-C, mg/dL | 54.5 (14.0) | 52.5 (14.1) | 51.1 (13.3) | 53.3 (14.2) | 49.0 (12.0) |
| LDL-C, mg/dL | 116.2 (35.0) | 105.1 (34.7) | 102.6 (32.5) | 101.3 (32.7) | 91.2 (31.0) |
| Cardiovascular disease | |||||
| Diabetes | 100 (12.0) | 401 (25.7) | 396 (38.5) | 238 (25.7) | 172 (44.2) |
| Coronary heart disease | 37 (4.4) | 182 (11.7) | 143 (13.9) | 166 (17.9) | 105 (27.0) |
| Heart failure | 12 (1.4) | 52 (3.3) | 61 (5.9) | 55 (5.9) | 34 (8.7) |
| Medication | |||||
| Antihypertensive | 0 | 1266 (81.2) | 1004 (97.5) | 538 (58.0) | 373 (95.9) |
| No. of BP–lowering medication, No. (%)f | 0.25 (0.5) | 1.2 (1.1) | 2.1 (1.3) | 1.1 (1.2) | 2.3 (1.4) |
| 0 | 661 (79.4) | 490 (31.4) | 94 (9.1) | 367 (39.6) | 32 (8.2) |
| 1 | 142 (17.1) | 520 (33.4) | 277 (26.9) | 256 (27.6) | 85 (21.9) |
| 2 | 25 (3.0) | 361 (23.2) | 336 (32.6) | 181 (19.5) | 101 (26.0) |
| ≥3 | 5 (0.6) | 188 (12.1) | 323 (31.4) | 123 (13.3) | 171 (44.0) |
| Cholesterol lowering | 322 (38.9) | 890 (57.2) | 623 (60.8) | 532 (57.6) | 280 (72.2) |
| Cigarette smoking statusg | |||||
| Current | 47 (5.6) | 93 (6.0) | 52 (5.1) | 79 (8.5) | 19 (4.9) |
| Former | 421 (50.5) | 833 (53.4) | 507 (49.2) | 471 (50.8) | 198 (50.9) |
| Never | 365 (43.8) | 633 (40.6) | 471 (45.7) | 377 (40.7) | 172 (44.2) |
| Alcohol consumptionh | |||||
| Current | 497 (59.7) | 832 (53.4) | 417 (40.5) | 486 (52.4) | 165 (42.4) |
| Former | 185 (22.2) | 422 (27.1) | 324 (31.5) | 272 (29.3) | 144 (37.0) |
| Never | 151 (18.1) | 305 (19.6) | 289 (28.1) | 169 (18.2) | 80 (20.6) |
| Visit 6 attendance status | |||||
| Attended | 616 (74.0) | 1096 (70.3) | 690 (67.0) | 647 (69.8) | 216 (55.5) |
| Alive but did not attend | 198 (23.8) | 418 (26.8) | 287 (27.9) | 234 (25.2) | 141 (36.3) |
| Death before visit 6 | 19 (2.3) | 45 (2.9) | 53 (5.2) | 46 (5.0) | 32 (8.2) |
Abbreviations: AD, Alzheimer disease; BP, blood pressure; GED, general education diploma; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
SI conversion factor: to convert cholesterol level to mmol/L, multiply by 0.0259.
Longitudinal BP patterns are determined by 24-year patterns of normotension, hypertension, and hypotension measured at visits 1 to 5, as defined in Figure 1. Participants with midlife hypertension and late-life normotension were not included in the standard hypertension definition analyses because of small sample size (n = 23).
The number with missing data are 9 for systolic and diastolic BP, 24 for LDL-C, and 19 for cholesterol-lowering medication use.
A greater number of apolipoprotein E ε4 alleles is associated with a higher risk of late-onset Alzheimer disease. The number with missing data are 139 for the apolipoprotein E genotype.
Calculated as weight in kilograms divided by height in meters squared.
All participants missing systolic and diastolic BP met standard hypertension criteria at visit 5 based on antihypertensive medication use.
Includes medication not taken for hypertension that secondarily lowers BP.
Current indicates a participant report of current cigarette use. Former indicates any report of cigarette use at a previous study visit or of past cigarette use.
Current indicates any alcohol consumption within the last 6 months. Former indicates any previous alcohol consumption if none was consumed within the last 6 months.
Incident Dementia
A total of 516 (11%) participants progressed to dementia after visit 5 through to the end of visit 6 (December 31, 2017). In the primary analysis (using the standard hypertension definition), dementia incidence per 100 person-years was 1.31 (95% CI, 1.00-1.72) for the midlife and late-life normotension group, 1.99 (95% CI, 1.69-2.32) for the midlife normotension and late-life hypertension group, 2.83 (95% CI, 2.40-3.35) for the midlife and late-life hypertension group, 2.07 (95% CI, 1.68-2.54) for the midlife normotension and late-life hypotension group, and 4.26 (95% CI, 3.40-5.32) for the midlife hypertension and late-life hypotension group.
In the primary analysis of the entire visit 5 sample, patterns defined by midlife and late-life hypertension (adjusted hazard ratio [HR], 1.49 [95% CI, 1.06-2.08]) and midlife hypertension and late-life hypotension (adjusted HR, 1.62 [95% CI, 1.11-2.37 ]) were significantly associated with increased risk of incident dementia compared with the group that remained normotensive after adjusting for confounders (Table 2 and Figure 2). Results were similar for the midlife hypertension and late-life hypotension group when a 25% decrease in either SBP or DBP from late midlife (visit 4) to late life (visit 5) was used to define late-life hypotension (adjusted HR, 1.74 [95% CI, 1.18-2.56; eTable 5, eTable 6, and eTable 7 in the Supplement). These results were further supported in sensitivity analyses, which incorporated inverse probability weighting to account for differential attrition before visit 5 (eTable 8 in the Supplement) and in analyses that examined the continuous association between late-life SBP and subsequent dementia risk in persons with normal vs elevated midlife SBP (≥120 mm Hg) (Figure 3; presented stratified according to medication status in eFigure 3, eFigure 4, and eFigure 5 in the Supplement). In sensitivity analyses that used propensity score matching to further control for confounding (eTable 9 in the Supplement), only the pattern of midlife hypertension and late-life hypotension was associated with dementia risk. When participants with mild cognitive impairment at visit 5 were excluded from the analyses, the association of midlife and late-life hypertension (adjusted HR, 1.15 [95% CI, 0.73-1.82]) with dementia was attenuated and not statistically significant. The association of midlife hypertension and late-life hypotension with dementia also was not statistically significant but was essentially unchanged (adjusted HR, 1.64 [95% CI, 0.98-2.75]; eTable 10 in the Supplement). The associations were attenuated and statistically nonsignificant for all 24-year blood pressure patterns in a secondary analysis which used the measured hypertension definition to define blood pressure patterns (Table 2; eFigure 6 in the Supplement).
Table 2. The Association of 24-Year Blood Pressure Patterns with Dementia, Mild Cognitive Impairment, and Cognitive Changea.
| Incident Dementia (Full Sample) | Incident Dementia (Attended Visit 6) | Visit 6 Mild Cognitive Impairment | Cognitive Change | |||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | No./Total No. (%) | Hazard Ratio (95% CI) | No./Total No. (%) | Odds Ratio (95% CI) | No./Total No. (%) | β (95% CI)b | No. | |
| 24-y Blood Pressure Patterns Defined Using the Standard Hypertension Criteriac | ||||||||
| No. | 4738 | 3264 | 2584 | 4403 | ||||
| Midlife and late-life normotension | 1 [Reference] | 53/833 (6) | 1 [Reference] | 25/616 (4) | 1 [Reference] | 68/509 (13) | 0 [Reference] | 811 |
| Midlife normotension, late-life hypertension | 1.16 (0.84 to 1.61) | 149/1559 (10) | 0.95 (0.58 to 1.56) | 51/1096 (5) | 1.32 (0.96 to 1.82) | 153/874 (18) | −0.01 (−0.06 to 0.03) | 1462 |
| Midlife and late-life hypertension | 1.49 (1.06 to 2.08) | 138/1030 (13) | 1.10 (0.64 to 1.89) | 47/690 (7) | 1.18 (0.80 to 1.73) | 84/530 (16) | −0.01 (−0.06 to 0.03) | 905 |
| Midlife normotension, late-life hypotension | 1.11 (0.78 to 1.57) | 91/927 (10) | 0.93 (0.54 to 1.61) | 32/647 (5) | 1.19 (0.83 to 1.70) | 93/519 (18) | −0.01 (−0.05 to 0.04) | 884 |
| Midlife hypertension, late-life hypotension | 1.62 (1.11 to 2.37) | 77/389 (20) | 1.45 (0.76 to 2.75) | 21/215 (10) | 1.65 (1.01 to 2.69) | 37/152 (24) | −0.05 (−0.13 to 0.02) | 341 |
| 24-y Blood Pressure Patterns Defined Using the Measured Hypertension Criteriad | ||||||||
| No. | 4752 | 3273 | 2590 | 4409 | ||||
| Midlife and late-life normotension | 1 [Reference] | 181/2116 (9) | 1 [Reference] | 68/1525 (4) | 1 [Reference] | 182/1234 (15) | 0 [Reference] | 1998 |
| Midlife normotension, late-life hypertension | 1.13 (0.89 to 1.44) | 113/938 (12) | 1.19 (0.80 to 1.78) | 39/635 (6) | 1.34 (1.01 to 1.77) | 96/494 (19) | −0.01 (−0.06 to 0.03) | 856 |
| Midlife and late-life hypertension | 1.39 (0.95 2.04) | 33/202 (16) | 1.37 (0.69 to 2.70) | 10/123 (8) | 0.94 (0.50 to 1.75) | 14/90 (16) | −0.01 (−0.10 to 0.08) | 176 |
| Midlife normotension, late-life hypotension | 1.06 (0.84 to 1.33) | 135/1186 (11) | 1.08 (0.72 to 1.61) | 44/789 (6) | 1.14 (0.87 to 1.50) | 117/621 (19) | 0.01 (−0.03 to 0.05) | 1111 |
| Midlife hypertension, late-life hypotension | 1.37 (0.93 to 2.02) | 33/130 (25) | 1.73 (0.83 to 3.60) | 9/73 (12) | 1.48 (0.75 to 2.93) | 13/50 (26) | −0.05 (−0.16 to 0.06) | 111 |
| Midlife hypertension, late-life normotension | 1.12 (0.69 to 1.80) | 19/180 (11) | 1.02 (0.46 to 2.25) | 7/128 (5) | 1.14 (0.64 to 2.02) | 16/101 (16) | −0.05 (−0.14 to 0.04) | 157 |
Abbreviations: APOE, apolipoprotein E; GEE, generalized estimating equation.
Cox proportional hazard, logistic regression, and GEE regression (with a Gaussian distribution to model the continuous outcome of cognitive performance) models were adjusted for baseline age, sex, race+center (a combined variable was used because center [study site], in many cases, determined race; this method was used instead of coding participants for race and center separately for analytic reasons), education, and APOEε4 status, and visit 5 body mass index, total cholesterol, high-density lipoprotein cholesterol, cigarette smoking status, alcohol use status, prevalent diabetes, coronary heart disease, heart failure, and previous stroke, as defined at visit 5.
Unstandardized estimates of additional 5-year cognitive change (z score) associated with blood pressure pattern. These estimates were derived from GEE models adjusted for baseline age, an age × time interaction term, sex, race+center (see footnote a for definition), education, APOEε4 status, and visit 5 body mass index, total cholesterol, high-density lipoprotein cholesterol, cigarette smoking status, alcohol use status, prevalent diabetes, coronary heart disease, heart failure, and previous stroke, as defined at visit 5.
The standard hypertension definition classified a participant as hypertensive if the participant met hypertension criteria based on measured blood pressure or if the participant was taking blood pressure medication at that visit, even if the blood pressure was in the normal range. Participants with midlife hypertension and late-life normotension were not included in the standard hypertension definition analyses because of small sample size (n = 23).
The measured hypertension definition classified a participant as hypertensive if the measured blood pressure value was greater than the threshold for hypertension at that visit. Participants with missing systolic or diastolic blood pressure at visit 5 (n = 9) were not included in the measured hypertension definition analyses.
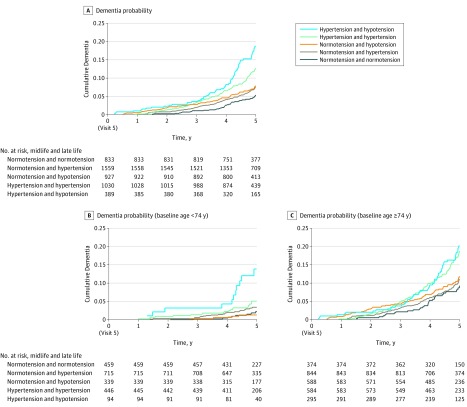
Figure 2. Kaplan-Meier Curves for Time to Dementia Onset for Standard Hypertension Definition Blood Pressure Groups.
Kaplan-Meier curves for time to dementia onset in the full analytic sample (N = 4738), and among younger (n = 2053) and older (n = 2685) subgroups based on 24-year midlife to late-life blood pressure patterns. Younger and older groups were defined based on median split (<74 vs ≥74 years of age). The median interquartile range (IQR) follow-up times for the midlife and late-life group were 5.0 (4.4-5.3) for normotension and normotension, 4.9 (4.4-5.3) for normotension and hypertension, 4.9 (4.3-5.3) for hypertension and hypertension, 4.9 (4.3-5.2) for hypertension and normotension, and 4.9 (4.2-5.2) years for the hypertension and hypotension group. The median (IQR) follow-up time for the groups with participants younger than 74 years of age were 5.0 (4.5-5.3) for normotension and normotension, 5.0 (4.4-5.3) for normotension and hypertension, 4.9 (4.4-5.3) for hypertension and hypertension, 5.0 (4.5-5.4) for hypertension and normotension, and 4.9 (4.3-5.3) years for the hypertension and hypotension group. The median (IQR) follow-up times for the groups with participants aged 74 years or older were 4.9 (4.3-5.2) for normotension and normotension, 4.9 (4.3-5.2) for normotension and hypertension, 4.8 (4.2-5.2) for hypertension and hypertension, 4.9 (4.2-5.2) for hypertension and normotension, and 4.9 (4.2-5.2) years for the hypertension and hypotension group. Participants with midlife hypertension and late-life normotension were not included in the standard hypertension definition analyses because of small sample size (n = 23).
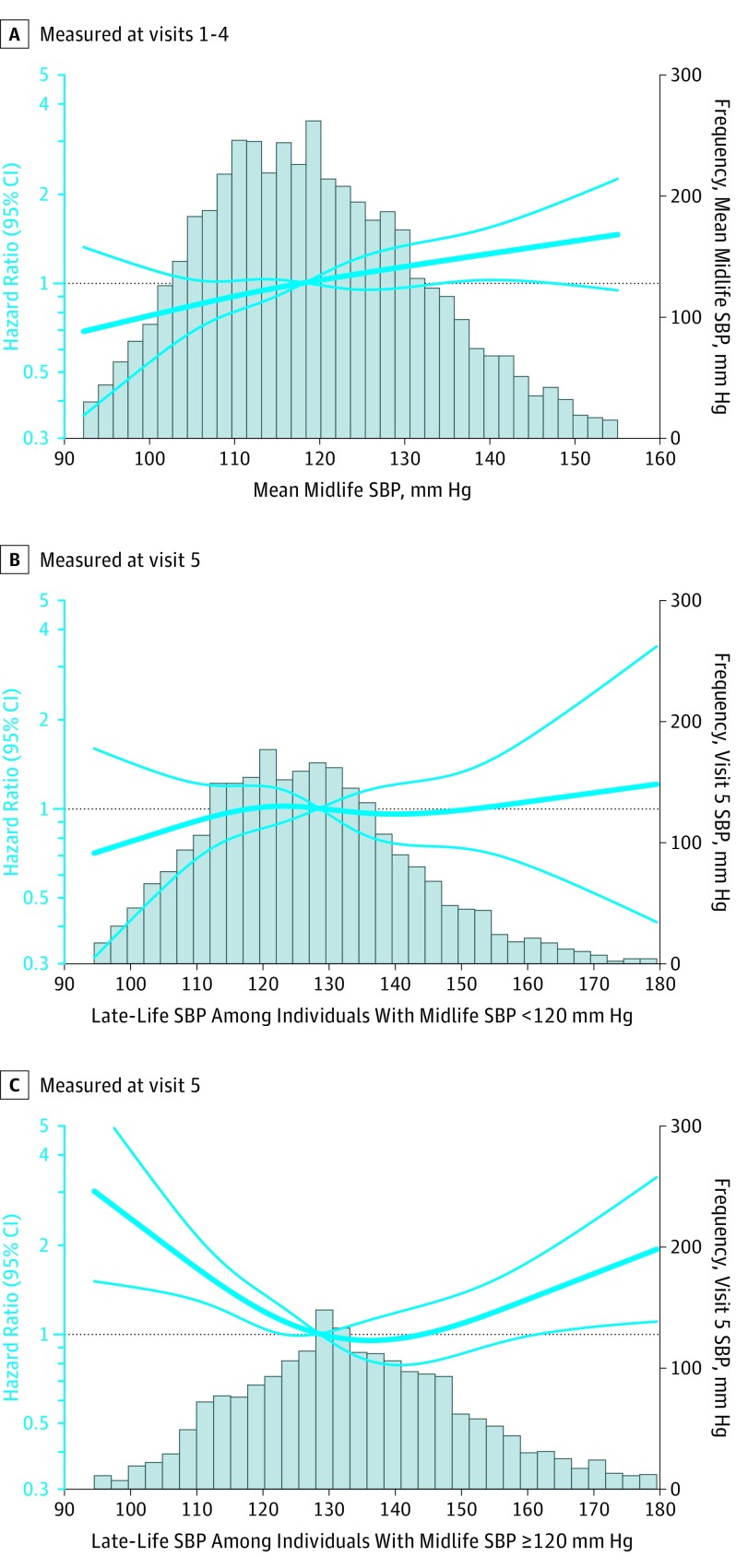
Figure 3. Adjusted Hazard Ratios (95% CI) for the Association of Midlife and Late-Life Systolic Blood Pressure With Incident Dementia.
A, The association of mean midlife systolic blood pressure (SBP) (measured at visits 1-4) with dementia risk (n=4662). B, The association of late-life SBP with dementia risk among individuals with a mean midlife SBP lower than 120 mm Hg (n=2583). C, The association of late-life SBP with dementia risk among individuals with a mean midlife SBP greater than or equal to 120 mm Hg (n=2068). Hazard ratios (indicated by a bold blue line) and 95% CIs (lighter-faced blue lines) are derived from Cox proportional hazard regression models adjusted for baseline age, sex, race-center, education, apolipoprotein E ε4 (APOEε4) status, and body mass index, total cholesterol, high-density lipoprotein cholesterol, cigarette smoking status, alcohol use status, prevalent diabetes, coronary heart disease, heart failure, and previous stroke defined at visit 5. Models used to examine late-life blood pressure were also adjusted for mean midlife SBP. SBP values were centered at the sample median and modeled using a restricted cubic spline with knots at the 5th, 35th, 65th, and 95th percentiles. Histograms of time-averaged SBP are displayed as solid bars. Participants with extreme blood pressure values (in the bottom first and top 99th percentile) were excluded from these analyses.
Midlife hypertension and higher midlife SBP were associated with higher dementia risk irrespective of late-life blood pressure level (Figure 3A; eTable 11 and eFigure 7 in the Supplement). In a post hoc analysis that examined whether dementia risk differed between the 2 midlife hypertension groups (ie, the midlife and late-life hypertension group and the midlife hypertension and late-life hypotension group) compared with the midlife and late-life normotension reference group, the interaction between midlife hypertension and late-life hypertension or late-life hypotension was not statistically significant (P for interaction = .48), suggesting that the midlife and late-life hypertension group and the midlife hypertension and late-life hypotension group did not differ significantly with regard to dementia risk.
There was evidence of effect modification by age (P for interaction = .02; eTable 12 in the Supplement), whereby younger participants with midlife hypertension and late-life hypotension had higher risk for dementia (adjusted HR, 2.73 [95% CI, 1.20-6.20]), but this association was not statistically significant among older participants (adjusted HR, 1.43 [95% CI, 0.93-2.20]; Figure 2). Effect modification by race was also found (P for interaction = .02; eTable 13 in the Supplement), whereby white participants with midlife hypertension and late-life hypotension had a greater risk of incident dementia (adjusted HR, 1.77 [95% CI, 1.16-2.71]) compared with black participants with this same blood pressure pattern (adjusted HR, 1.06 [95% CI, 0.45-2.48]). There was no statistically significant effect modification by APOEε4 status. Some but not all associations were attenuated and no longer significant when analyses were restricted to participants who attended visit 6 (Table 2; eTable 5, eTable 6, and eTable 7 in the Supplement).
Mild Cognitive Impairment
A total of 2584 participants who were cognitively normal at visit 5 and attended visit 6 were included. Of these, 435 (17%) met mild cognitive impairment criteria at visit 6. There was a significant association between a pattern of midlife hypertension/late-life hypotension and mild cognitive impairment risk (adjusted OR, 1.65 [95% CI, 1.01-2.69]; Table 2). However, this association was not statistically significant in sensitivity analyses that used alternative definitions to define late-life hypotension (eTable 5, eTable 6, and eTable 7 in the Supplement).
Cognitive Change
Participants with 1 or more cognitive domain scores in the bottom 5th percentile at visit 5 were excluded from this analysis to avoid floor effects (7%; n = 318). There was no significant association between 24-year blood pressure pattern and cognitive change between visits 5 and 6 (Table 2; eTable 5, eTable 6, eTable 7, and eTable 14 in the Supplement).
Discussion
In this analysis of a community-based cohort, a pattern of sustained hypertension from middle to late life and a pattern of midlife hypertension followed by late-life hypotension were associated with an increased risk for subsequent dementia, compared with participants who maintained normal blood pressure. The association of a pattern of midlife hypertension and late-life hypotension with incident dementia, which occurred regardless of whether a standard threshold or a relative decline in blood pressure was used to define late-life hypotension, was more strongly associated with incident dementia among a younger group of older adults (<74 years of age) compared with an older group of older adults (for which there was no significant association), and among white participants compared with black participants (for which there was no significant association). A pattern of midlife hypertension followed by late-life hypotension was also associated with incident mild cognitive impairment.
Previous research examining the association of high vs low late-life blood pressure with cognition has been inconsistent.23,24,25 Discrepant findings may be accounted for, in part, by a failure to consider whether4,5,6 and for how long26 an individual has been hypertensive previously. The current results highlight the importance of considering past hypertension chronicity, as late-life hypertension and late-life hypotension were associated with increased dementia risk, but only among individuals with hypertension during middle adulthood. Given the population prevalence of hypertension, the size of the at-risk subgroup, and the effect size for dementia risk (nearing that of having an APOEε4 allele or diabetes; eTable 15 in the Supplement),20 there may be a potentially large subset of the population (30% in the current study) for whom there is potential for prevention.
The association of midlife and late-life hypertension and midlife hypertension and late-life hypotension with dementia risk was no longer statistically significant in analyses that classified individuals according to measured blood pressure irrespective of antihypertension medication use. It is possible that this analysis was underpowered, as far fewer participants were classified as having midlife and late-life hypertension (4%) and midlife hypertension and late-life hypotension (4%) using the measured hypertension criteria. Differences may also be accounted for by the handling of antihypertensive medication use because normotension status with vs without antihypertensive treatment likely has different implications in terms of underlying physiology and dementia risk.
The current findings are consistent with previous work that has demonstrated a relationship between chronic hypertension and reduced cognition in older adults.26,27 Several studies have also demonstrated that blood pressure tends to decline in the years immediately preceding the onset of dementia.28,29 However, it remains unclear whether these declines occur as a consequence of neurodegeneration or act as a risk factor for later cognitive decline. The current study adds to the existing literature by demonstrating the following: (1) late-life declines in blood pressure occurred at high rates among individuals without dementia who were normotensive and hypertensive during middle adulthood; and (2) these blood pressure declines, at least in a subset of individuals, preceded the onset of mild cognitive impairment and dementia. The results, therefore, provide temporal support for the hypothesis that a pattern of midlife hypertension and late-life hypotension may represent a risk factor for later cognitive decline and dementia. However, given what is known about the gradual progression of neurodegenerative disease, the possibility that early neurological changes may be responsible for late-life declines in blood pressure cannot be ruled out.
The finding that a pattern of midlife hypertension and late-life hypotension showed a stronger relationship with incident dementia in the younger group of older adults supports studies suggesting that hypertension at a younger age (and closer to midlife) is especially deleterious,20 and further suggests that significant blood pressure declines following hypertension may be more pathogenic at a younger age. However, the effect modification by age and race may also be driven by a survival bias (greater attrition among older and black participants [eTable 3 the Supplement]), so the findings related to effect modification should be interpreted with caution. The majority of participants with late-life declines in blood pressure were using antihypertensive medication as older adults (Table 1) and also had an increase in the number of prescribed antihypertensive medications from midlife to late life (eTable 16 in the Supplement), suggesting a potential role for hypertension overtreatment. Other potential causes of late-life blood pressure declines, include cardiocirculatory dysfunction, blunted autonomic signaling, and arterial stiffening.30
While chronic hypertension has been associated with cerebral small vessel disease, reduced white matter integrity, and other factors that are known to have detrimental effects on cognition,1,31 the dementia risk associated with the pattern of midlife hypertension and late-life hypotension may also be explained by the deleterious effect of chronic hypertension on the brain’s autoregulatory capacity. Although low cerebral blood flow is not a universal finding among individuals with hypertension,32 impaired cerebral autoregulation can result in a reduced ability to maintain consistent steady blood flow when systemic blood pressure is too low or too high. Individuals with impaired cerebral autoregulation are believed to be especially prone to reductions of cerebral blood flow in the context of low systemic blood pressure.33 Even modest reductions in cerebral blood flow have been associated with pathogenic brain changes.34,35
Strengths of this study include the use of a large, population-based sample, the identification of and adjustment for potentially confounding medical comorbidity, and the extensive dementia surveillance.
Limitations
This study has several limitations. First, the increased propensity for study dropout among participants with higher blood pressure and poorer cognition during midlife (eTable 3 in the Supplement) may have biased the current findings. Given that high blood pressure is a known risk factor for cardiovascular events and mortality,36 participants who would have otherwise maintained elevated blood pressure throughout midlife and late life may be especially vulnerable to attrition. For participants with midlife hypertension, in particular, the selective attrition of individuals at risk for dementia may have biased estimates.
Second, analyses to assess the competing risk of mortality were not performed. Despite the careful survey methods to ascertain the outcome, there is the potential that some participants who died without having dementia may have been censored before dementia was observed. Given the association between hypertension and mortality,36 a competing risk of mortality may have biased the current results. Although a large proportion of the study sample died before the start of the baseline cognitive examination (visit 5), the findings of this study may be generalizable to individuals who are alive and without dementia in their 70s and 80s.
Third, the current study was unable to identify dementia etiology and determine whether associations differ according to dementia type. This needs further investigation as previous research has found that specific blood pressure characteristics exert unique effects on vascular- and Alzheimer-specific pathology31 and therefore, may differentially influence risk for Alzheimer and cerebrovascular pathologies.
Fourth, given the number of blood pressure pattern groups, it is possible that the analyses, especially interactions and stratified analyses, were underpowered. Limited power, and potentially differences in dementia classification methods, may account for nonsignificant associations in analyses including only participants who attended the final visit (visit 6).
Fifth, there may have been residual confounding from unmeasured variables. Sixth, the findings may lack generalizability to all regions and racial and ethnic groups within the United States. Seventh, there is not a well-established definition of clinically important difference for the measure of cognitive change used in this study.
Conclusions
In this community-based cohort with long-term follow-up, a pattern of sustained hypertension from midlife to late life and a pattern of midlife hypertension followed by late-life hypotension, compared with midlife and late-life normal blood pressure, were associated with an increased risk for subsequent dementia.
eMethods. Neuropsychological measures used to estimate cognitive change, Classification of medication use patterns, Summary of methods for multiple imputation by chained equations (MICE), Derivation of the cognitive factor scores, Summary of methods of inverse probability weighting, Summary of methods for propensity score matching
eTable 1. Algorithm for classification of incident dementia
eTable 2. Visit 5 participant characteristics stratified by 24-year measured hypertension definition blood pressure patternsa
eTable 3. Visit 1 participant characteristics stratified according to study inclusion, dropout, and death
eTable 4. Visit 5 participant characteristics stratified according to study inclusion
eTable 5. The association of mid- to late-life standard hypertension definition blood pressure patterns with cognitive outcomes using a 25% systolic or diastolic blood pressure decline to define hypotension
eTable 6. The association of mid- to late-life standard hypertension definition blood pressure patterns with cognitive outcomes using a 25% systolic blood pressure decline to define hypotension
eTable 7. The association of mid- to late-life standard hypertension definition blood pressure patterns with cognitive outcomes using a 25% diastolic blood pressure decline to define hypotension
eTable 8. The association of mid- to late-life standard hypertension definition blood pressure patterns with dementia after incorporating inverse probability weighting
eTable 9. The association of mid- to late-life standard hypertension definition blood pressure patterns with dementia using a propensity score-matched sample
eTable 10. The association of mid- to late-life standard hypertension definition blood pressure patterns with dementia and cognitive change among participants cognitively normal at baseline (Visit 5)
eTable 11. The association of mid- to late-life standard hypertension definition blood pressure patterns with dementia after combining 24-year blood pressure groups with similar mid- and late-life blood pressure patterns
eTable 12. The age-stratified association of mid- to late-life standard hypertension definition blood pressure patterns with dementia
eTable 13. The race-stratified association of mid- to late-life standard hypertension definition blood pressure patterns with dementia
eTable 15. Cox proportional hazard regression models of dementia risk associated with individual covariates
eTable 16. Visit 4 to 5 antihypertensive medication use according to mid- to late-life standard hypertension definition blood pressure patterns
eFigure 1. Study flowchart with detailed study attrition and exclusion information
eFigure 2. Study flowchart and longitudinal measured hypertension definition blood pressure patterns
eFigure 3. Adjusted hazard ratios (95% CI) for the association of midlife and late-life blood pressure with incident dementia among participants who did not use antihypertensive medication during midlife or late-life
eFigure 4. Adjusted hazard ratios (95% CI) for the association of midlife and late-life blood pressure with incident dementia among participants who only used antihypertensive medication during late-life
eFigure 5. Adjusted hazard ratios (95% CI) for the association of midlife and late-life blood pressure with incident dementia among participants who used antihypertensive medication during midlife and late-life
eFigure 7. Adjusted hazard ratios (95% CI) for the association of mean midlife diastolic blood pressure with incident dementia
eReferences
References
- 1.Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr Hypertens Rep. 2017;19(3):24. doi: 10.1007/s11906-017-0724-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottesman RF, Schneider ALC, Albert M, et al. . Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014;71(10):1218-1227. doi: 10.1001/jamaneurol.2014.1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottesman RF, Schneider ALC, Zhou Y, et al. . Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317(14):1443-1450. doi: 10.1001/jama.2017.3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller M, Sigurdsson S, Kjartansson O, et al. . Joint effect of mid- and late-life blood pressure on the brain. Neurology. 2014;82(24):2187-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glodzik L, Rusinek H, Pirraglia E, et al. . Blood pressure decrease correlates with tau pathology and memory decline in hypertensive elderly. Neurobiol Aging. 2014;35(1):64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Power MC, Schneider ALC, Wruck L, et al. . Life-course blood pressure in relation to brain volumes. Alzheimers Dement. 2016;12(8):890-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687-702. [PubMed] [Google Scholar]
- 8.Zhang Y-P, Zuo X-C, Huang Z-J, et al. . The impact of blood pressure on kidney function in the elderly. Kidney Blood Press Res. 2013;38(2-3):205-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oyetunji TA, Chang DC, Crompton JG, et al. . Redefining hypotension in the elderly. Arch Surg. 2011;146(7):865-869. [DOI] [PubMed] [Google Scholar]
- 10.Gould CE, Beaudreau SA. Association between depression and anxiety on blood pressure dysregulation and pulse in the Health and Retirement Study. Int J Geriatr Psychiatry. 2013;28(10):1045-1053. doi: 10.1002/gps.3926 [DOI] [PubMed] [Google Scholar]
- 11.Knopman DS, Gottesman RF, Sharrett AR, et al. . Mild cognitive impairment and dementia prevalence. Alzheimers Dement (Amst). 2016;2:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayden KM, Reed BR, Manly JJ, et al. . Cognitive decline in the elderly. Age Ageing. 2011;40(6):684-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson RS, Li Y, Bienias JL, Bennett DA. Cognitive decline in old age. Psychol Aging. 2006;21(4):774-789. [DOI] [PubMed] [Google Scholar]
- 14.McKhann GM, Knopman DS, Chertkow H, et al. . The diagnosis of dementia due to Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Psychiatric Association DSM-5: Diagnostic and Statistical Manual of Mental Disorders. 5th ed Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 16.Carpenter CR, DesPain B, Keeling TN, Shah M, Rothenberger M. The Six-Item Screener and AD8 for the detection of cognitive impairment in geriatric emergency department patients. Ann Emerg Med. 2011;57(6):653-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galvin JE, Roe CM, Xiong C, Morris JC. Validity and reliability of the AD8 informant interview in dementia. Neurology. 2006;67(11):1942-1948. [DOI] [PubMed] [Google Scholar]
- 18.Schneider ALC, Sharrett AR, Gottesman RF, et al. . Normative data for 8 neuropsychological tests in older blacks and whites from the atherosclerosis risk in communities (ARIC) study. Alzheimer Dis Assoc Disord. 2015;29(1):32-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross AL, Power MC, Albert MS, et al. . Application of latent variable methods to the study of cognitive decline when tests change over time. Epidemiology. 2015;26(6):878-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottesman RF, Albert MS, Alonso A, et al. . Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol. 2017;74(10):1246-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siedel J, Hägele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem. 1983;29(6):1075-1080. [PubMed] [Google Scholar]
- 22.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28(6):1379-1388. [PubMed] [Google Scholar]
- 23.Reitz C, Tang M-X, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol. 2007;64(12):1734-1740. doi: 10.1001/archneur.64.12.1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandav R, Dodge HH, DeKosky ST, Ganguli M. Blood pressure and cognitive impairment in India and the United States. Arch Neurol. 2003;60(8):1123-1128. [DOI] [PubMed] [Google Scholar]
- 25.Power MC, Weuve J, Gagne JJ, et al. . The association between blood pressure and incident Alzheimer disease. Epidemiology. 2011;22(5):646-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Power MC, Tchetgen EJT, Sparrow D, Schwartz J, Weisskopf MG. Blood pressure and cognition. Epidemiology. 2013;24(6):886-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swan GE, Carmelli D, Larue A. Systolic blood pressure tracking over 25 to 30 years and cognitive performance in older adults. Stroke. 1998;29(11):2334-2340. doi: 10.1161/01.STR.29.11.2334 [DOI] [PubMed] [Google Scholar]
- 28.Skoog I, Lernfelt B, Landahl S, et al. . 15-Year longitudinal study of blood pressure and dementia. Lancet. 1996;347(9009):1141-1145. [DOI] [PubMed] [Google Scholar]
- 29.Qiu C, von Strauss E, Winblad B, Fratiglioni L. Decline in blood pressure over time and risk of dementia. Stroke. 2004;35(8):1810-1815. [DOI] [PubMed] [Google Scholar]
- 30.Ferrari AU. Modifications of the cardiovascular system with aging. Am J Geriatr Cardiol. 2002;11(1):30-33. doi: 10.1111/1467-8446.00044-i1 [DOI] [PubMed] [Google Scholar]
- 31.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723-738. doi: 10.1038/nrn3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glodzik L, Rusinek H, Tsui W, et al. . Different relationship between systolic blood pressure and cerebral perfusion in subjects with and without hypertension. Hypertension. 2019;73(1):197-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller M, van der Graaf Y, Visseren FL, et al. . Hypertension and longitudinal changes in cerebral blood flow. Ann Neurol. 2012;71(6):825-833. [DOI] [PubMed] [Google Scholar]
- 34.Koike MA, Green KN, Blurton-Jones M, Laferla FM. Oligemic hypoperfusion differentially affects tau and amyloid-beta. Am J Pathol. 2010;177(1):300-310. doi: 10.2353/ajpath.2010.090750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gentile MT, Poulet R, Di Pardo A, et al. . Beta-amyloid deposition in brain is enhanced in mouse models of arterial hypertension. Neurobiol Aging. 2009;30(2):222-228. [DOI] [PubMed] [Google Scholar]
- 36.Wright JT Jr, Williamson JD, Whelton PK, et al. . A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Neuropsychological measures used to estimate cognitive change, Classification of medication use patterns, Summary of methods for multiple imputation by chained equations (MICE), Derivation of the cognitive factor scores, Summary of methods of inverse probability weighting, Summary of methods for propensity score matching
eTable 1. Algorithm for classification of incident dementia
eTable 2. Visit 5 participant characteristics stratified by 24-year measured hypertension definition blood pressure patternsa
eTable 3. Visit 1 participant characteristics stratified according to study inclusion, dropout, and death
eTable 4. Visit 5 participant characteristics stratified according to study inclusion
eTable 5. The association of mid- to late-life standard hypertension definition blood pressure patterns with cognitive outcomes using a 25% systolic or diastolic blood pressure decline to define hypotension
eTable 6. The association of mid- to late-life standard hypertension definition blood pressure patterns with cognitive outcomes using a 25% systolic blood pressure decline to define hypotension
eTable 7. The association of mid- to late-life standard hypertension definition blood pressure patterns with cognitive outcomes using a 25% diastolic blood pressure decline to define hypotension
eTable 8. The association of mid- to late-life standard hypertension definition blood pressure patterns with dementia after incorporating inverse probability weighting
eTable 9. The association of mid- to late-life standard hypertension definition blood pressure patterns with dementia using a propensity score-matched sample
eTable 10. The association of mid- to late-life standard hypertension definition blood pressure patterns with dementia and cognitive change among participants cognitively normal at baseline (Visit 5)
eTable 11. The association of mid- to late-life standard hypertension definition blood pressure patterns with dementia after combining 24-year blood pressure groups with similar mid- and late-life blood pressure patterns
eTable 12. The age-stratified association of mid- to late-life standard hypertension definition blood pressure patterns with dementia
eTable 13. The race-stratified association of mid- to late-life standard hypertension definition blood pressure patterns with dementia
eTable 15. Cox proportional hazard regression models of dementia risk associated with individual covariates
eTable 16. Visit 4 to 5 antihypertensive medication use according to mid- to late-life standard hypertension definition blood pressure patterns
eFigure 1. Study flowchart with detailed study attrition and exclusion information
eFigure 2. Study flowchart and longitudinal measured hypertension definition blood pressure patterns
eFigure 3. Adjusted hazard ratios (95% CI) for the association of midlife and late-life blood pressure with incident dementia among participants who did not use antihypertensive medication during midlife or late-life
eFigure 4. Adjusted hazard ratios (95% CI) for the association of midlife and late-life blood pressure with incident dementia among participants who only used antihypertensive medication during late-life
eFigure 5. Adjusted hazard ratios (95% CI) for the association of midlife and late-life blood pressure with incident dementia among participants who used antihypertensive medication during midlife and late-life
eFigure 7. Adjusted hazard ratios (95% CI) for the association of mean midlife diastolic blood pressure with incident dementia
eReferences