Key Points
Question
Is intensive blood pressure treatment associated with less progression of small vessel ischemic disease, as reflected by cerebral white matter lesion volume?
Findings
In this substudy of a randomized clinical trial of 449 hypertensive patients with longitudinal brain magnetic resonance imaging, intensive blood pressure management to a target of less than 120 mm Hg, vs less than 140 mm Hg, was associated with a smaller increase in white matter lesion volume (0.92 cm3 vs 1.45 cm3).
Meaning
More intensive blood pressure management was associated with less progression of cerebral small vessel ischemic disease, although the difference was small.
Abstract
Importance
The effect of intensive blood pressure lowering on brain health remains uncertain.
Objective
To evaluate the association of intensive blood pressure treatment with cerebral white matter lesion and brain volumes.
Design, Setting, and Participants
A substudy of a multicenter randomized clinical trial of hypertensive adults 50 years or older without a history of diabetes or stroke at 27 sites in the United States. Randomization began on November 8, 2010. The overall trial was stopped early because of benefit for its primary outcome (a composite of cardiovascular events) and all-cause mortality on August 20, 2015. Brain magnetic resonance imaging (MRI) was performed on a subset of participants at baseline (n = 670) and at 4 years of follow-up (n = 449); final follow-up date was July 1, 2016.
Interventions
Participants were randomized to a systolic blood pressure (SBP) goal of either less than 120 mm Hg (intensive treatment, n = 355) or less than 140 mm Hg (standard treatment, n = 315).
Main Outcomes and Measures
The primary outcome was change in total white matter lesion volume from baseline. Change in total brain volume was a secondary outcome.
Results
Among 670 recruited patients who had baseline MRI (mean age, 67.3 [SD, 8.2] years; 40.4% women), 449 (67.0%) completed the follow-up MRI at a median of 3.97 years after randomization, after a median intervention period of 3.40 years. In the intensive treatment group, based on a robust linear mixed model, mean white matter lesion volume increased from 4.57 to 5.49 cm3 (difference, 0.92 cm3 [95% CI, 0.69 to 1.14]) vs an increase from 4.40 to 5.85 cm3 (difference, 1.45 cm3 [95% CI, 1.21 to 1.70]) in the standard treatment group (between-group difference in change, −0.54 cm3 [95% CI, −0.87 to −0.20]). Mean total brain volume decreased from 1134.5 to 1104.0 cm3 (difference, −30.6 cm3 [95% CI, −32.3 to −28.8]) in the intensive treatment group vs a decrease from 1134.0 to 1107.1 cm3 (difference, −26.9 cm3 [95% CI, 24.8 to 28.8]) in the standard treatment group (between-group difference in change, −3.7 cm3 [95% CI, −6.3 to −1.1]).
Conclusions and Relevance
Among hypertensive adults, targeting an SBP of less than 120 mm Hg, compared with less than 140 mm Hg, was significantly associated with a smaller increase in cerebral white matter lesion volume and a greater decrease in total brain volume, although the differences were small.
Trial Registration
ClinicalTrials.gov Identifier: NCT01206062
This substudy of the SPRINT randomized clinical trial evaluates the association between intensive (systolic blood pressure <120 mm Hg) vs standard (<140 mm Hg) blood pressure control and changes in cerebral white matter lesion and total brain volumes among hypertensive adults.
Introduction
In older adults at risk of vascular disease, the effect of intensive systolic blood pressure (SBP) control on brain heath is uncertain, despite its proven efficacy for reducing cardiovascular disease morbidity and mortality.1 Epidemiologic data have identified hypertension as a primary risk factor for cerebral small vessel ischemic disease (SVID), particularly development of white matter lesions (WMLs).2,3 Observational studies have increasingly suggested that SVID is associated with cognitive decline and the pathogenesis of Alzheimer disease and related dementias.4,5 WMLs seen on brain magnetic resonance imaging (MRI) are an independent risk factor for cognitive decline and dementia.6,7 It has been estimated that 30% to 60% of patients with Alzheimer disease and related dementias have vascular findings contributing to their cognitive impairment, while Alzheimer disease pathology may be present in 40% to 80% of patients with dementia for which the primary etiology is classified as vascular.8,9,10
There is limited evidence supporting a beneficial effect of antihypertensive treatment in slowing the progression of SVID in the brain.11 To the contrary, some hypothesize that intensive SBP control may adversely affect the brain through mechanisms such as decreasing cerebral perfusion.12 The Systolic Blood Pressure Intervention Trial (SPRINT) tested the effect of intensive SBP control (SBP target <120 mm Hg) vs a standard SBP treatment goal of less than 140 mm Hg. Cognitive results from that trial indicated a lower rate of mild cognitive impairment with intensive SBP control, with an inconclusive effect on probable dementia.13 Brain MRI scans were obtained in a subgroup of participants to test the primary hypothesis that the increase in WML volume, a measure of SVID progression, would be lower in participants randomized to intensive SBP control. The secondary hypothesis was that declines in total brain volume (TBV) would be less in the intensive treatment group.
Methods
Study Participants
The overall trial design and primary outcome results have been described,1,14 and the study protocol is provided in Supplement 1. The trial and MRI substudy were approved by the institutional review board at each participating site, and each participant provided written informed consent.
Briefly, participants were 50 years or older with SBP between 130 and 180 mm Hg at the screening visit and had increased cardiovascular risk. Participants were considered to have increased cardiovascular risk if they had clinical or subclinical cardiovascular disease, chronic kidney disease (defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2), a 10-year Framingham cardiovascular disease risk of 15% or greater, or were 75 years or older. Individuals residing in a nursing home, persons with a diagnosis of dementia (based on medical record review), or those treated with medications primarily used for dementia therapy were excluded, as were persons with prevalent diabetes mellitus or a history of stroke. Between November 2010 and March 2013, a total of 9361 participants were randomized by the data coordinating center in a 1:1 allocation to an intensive treatment strategy with an SBP goal of less than 120 mm Hg or a standard treatment strategy with an SBP goal of less than 140 mm Hg. The algorithms and formulary for hypertension treatment are listed in the study protocol (Supplement 1).
A subset of participants (n = 2913) were recruited into a substudy to more extensively evaluate the effects of intensive SBP control on specific domains of cognitive function, the results of which are not presented here. MRI scans were obtained in a further subset of these participants to evaluate brain structure. All participants accessible to 1 of 7 designated MRI sites (27 clinic sites) were screened for the MRI substudy, and eligible participants provided written informed consent. Exclusion criteria for the MRI substudy included presence of a pacemaker, defibrillator, neurostimulator or other implanted electrical device, ferro-magnetic or unknown cerebral aneurysm clip, cochlear or other otologic implant, unknown metallic foreign bodies or exposure to metal fragments in or around the eyes, or severe claustrophobia.
Study Measures
Baseline measures, including age, sex, prior cardiovascular disease, and education, were collected via self-report; participants brought their antihypertensive medications to the baseline visit, where the medications were recorded. Race and ethnicity were collected via self-report using fixed categories to satisfy the National Institutes of Health Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research. Blood pressure was measured via standardized techniques as follows.15
All sites were provided with the Professional Digital Blood Pressure Monitor (Omron Healthcare; model 907XL) for BP measurement. Training in BP measurement technique emphasized proper positioning of participants, measurement of arm circumference and use of a proper cuff size, and the importance of a 5-minute rest period before obtaining the 3 seated automated BP values. During the rest and BP measurement periods, the protocol called for the participant to not be completing questionnaires, talking, or texting. Visit BP was the mean of 3 readings. A central laboratory, located in Minneapolis, Minnesota, analyzed all blood and urine samples.
MRI Study
MRIs were planned at baseline and 48 months after randomization. Several different MRI scanner models were used: 3T Phillips Achieva 3.2 (University of Alabama at Birmingham, Boston University, and Vanderbilt University), 3T Siemens Skyra VD11B (Wake Forest University), 3T Siemens Tim Trio VB17 (University of Miami and University of Pennsylvania), and 3T Siemens Verio VB17 (Case Western Reserve University).
Structural MRI of the brain included 1-mm isotropic T1, T2, and fluid-attenuated inversion recovery (FLAIR) imaging. Imaging parameters were as follows: T1 (repetition time = 1900 ms, echo time = 2.89 ms, field of view = 250 mm, thickness = 1 mm, slices = 176, native resolution = 1 mm isotropic), T2 (repetition time = 3200 ms, echo time = 409 ms, field of view = 250 mm, thickness = 1 mm, slices = 176, native resolution = 1 mm isotropic), and FLAIR (repetition time = 6000 ms, inversion time = 2200 ms, echo time = 285 ms, field of view = 258 mm, thickness = 1 mm, slices = 160, native resolution = 1 mm isotropic).
Scanner performance was monitored with quarterly Alzheimer Disease Neuroimaging Initiative and Function Biomedical Informatics Research Network phantom acquisition, with all scanners showing stability of phantom measurements throughout the trial.
MRI Processing
Image processing was performed by the Center for Biomedical Image Computing and Analytics in the Department of Radiology at the University of Pennsylvania. All image analysts were blinded to treatment group. An automated pipeline was applied for preprocessing structural MRI scans, including correction of inhomogeneity16 and extraction of the intracranial tissues using multiatlas skull stripping.17 Anatomical regions of interest were identified using a multiatlas label fusion method18 and used to segment supratentorial gray matter and white matter tissues, with the sum of gray matter and white matter defining TBV. WMLs were identified from inhomogeneity-corrected and coregistered FLAIR and T1-weighted images using a deep learning–based segmentation method built on the UNet architecture,19 with the convolutional layers in the network replaced by an Inception ResNet architecture.20 The model was trained using a separate training set with human-validated segmentation of WML. WML segmentations were quality inspected by a neuroradiologist blinded to treatment group.
Thirteen participants with baseline scans showing structural brain lesions (large areas of encephalomalacia [8], tumors [3], subdural hematoma [1], prior brain resection [1]) and 3 patients who developed large strokes (2) or a subdural hematoma at follow-up were excluded from analyses.
Duration of Follow-up
On August 20, 2015, the director of the National Heart, Lung, and Blood Institute accepted a recommendation from the data and safety monitoring board to inform the investigators and participants of the cardiovascular outcome results and initiated the process to end the trial intervention. Because of this, the majority of follow-up MRI scans with a WML volume passing quality control (n = 428, 95.3%) occurred during a closeout period (from August 20, 2015, to July 1, 2016), when participants were either having, or transitioning to having, their BP managed by their primary care clinician, although antihypertensive medications were still being provided by the study. The median time between August 20, 2015, and the follow-up MRI scan for those conducted after August 20, 2015, was 196 days (interquartile range, 145-244 days). Adverse events during the intervention phase of the trial have been published.1,21,22
Assessment of Cognitive Status
Methods for neuropsychological testing of cognitive status and adjudication have been described13 and are also described in the trial protocol (Supplement 1). For this report, cognitive assessments were only included through the trial closeout period (through July 1, 2016), as this corresponds to the timing of the follow-up MRI scans.
Outcomes
The trial protocol originally defined the primary MRI outcome as change in total SVID lesion volume, which encompasses lesions in the white matter, gray matter, and basal ganglia. Before any data analyses, the primary outcome was modified to consist solely of change in WML volume. This was done because WML volume is a more standard measurement of SVID and because the automated segmentation algorithm used in this trial was specifically trained to detect WMLs. Change in TBV was a secondary outcome.
Subgroups
Prespecified subgroups included age (<75 years vs ≥75 years), sex, race (nonblack vs black), chronic kidney disease (estimated glomerular filtration rate <60 vs ≥60 mL/min/1.73 m2), history of cardiovascular disease, baseline tertiles of SBP (≤129, 129 to <143, ≥143 mm Hg), and orthostatic hypotension.
Statistical Analysis
Based on the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, the standard deviations for change in WML volume and TBV (40 months after randomization) were 2.77 cm3 and 16.45 cm3, adjusted for baseline values and intracranial volume.23 With 640 participants (320 participants in each treatment group), assuming 3% loss to follow-up per year, and a 2-sided significance level of .05, we estimated that we would be able to detect group differences over 4 years of 0.65 cm3 (WML volume) and 3.9 cm3 (TBV) with 80% power and of 0.76 cm3 (WML volume) and 4.5 cm3 (TBV) with 90% power.
Because of the skewed distribution for WML volume, we first applied an inverse hyperbolic sine transformation (asinh), which is similar to a log transformation but can accommodate values of zero.24 Linear mixed models, including random effects for participant and MRI facility, were used to estimate the change in WML volume and TBV between the treatment groups, including time since randomization (in days) and intracranial volume as covariates. Because the inverse hyperbolic sine transformation is nonlinear, and given the context of a mixed-effects model, back-transformation to the original scale of cm3 is difficult. Therefore, we also present estimates for WML volume based on a robust linear mixed model to aid in interpretation.25 Intuitively, this approach down-weights observations with large residuals or random effects, reducing their influence on model estimates. However, all formal hypothesis testing was based on the linear mixed models fit to transformed WML volumes. Interactions between treatment group and prespecified subgroups were assessed with a likelihood ratio test.
We also conducted 2 sets of sensitivity analyses. The first used inverse probability weighting and multiple imputation to examine the effect of selective entry into the MRI substudy and missing follow-up MRI scans (eMethods in Supplement 2). The second mimicked the primary analyses based on linear mixed models to examine the association between cognitive status during follow-up (no impairment, mild cognitive impairment, or probable dementia) with change in WML volume and TBV.
All analyses were performed using SAS version 9.4 (SAS Institute Inc) and R version 3.4.2 (R Project for Statistical Computing [http://www.r-project.org]). All hypothesis tests were 2-sided, and P values less than .05 were considered statistically significant. No adjustments for multiple comparisons were made, so the interpretation of secondary analyses should be considered exploratory.
Results
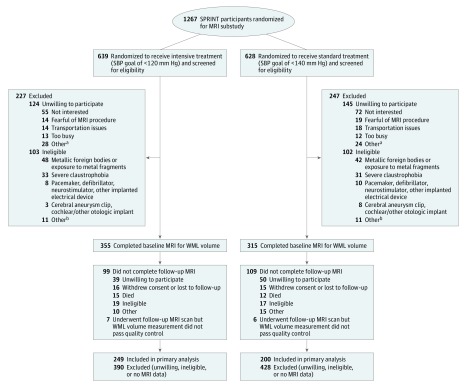
Of the 1267 SPRINT participants screened for the MRI substudy, 998 were willing to participate, 793 were eligible, and 670 completed a baseline MRI scan with a measurement of WML volume that passed quality control (Figure 1). An analogous depiction for the secondary outcome of TBV is shown in eFigure 1 in Supplement 2. The major reasons for ineligibility included the presence of metal fragments (43.9%), claustrophobia (31.2%), or a pacemaker (8.8%).
Figure 1. Eligibility, Randomization, and Follow-up for Participants in the MRI Substudy.
MRI indicates magnetic resonance imaging; SBP, systolic blood pressure; SPRINT, Systolic Blood Pressure Intervention Trial; WML, white matter lesion.
aOther reasons for unwillingness to participate include participant could not lie flat for extended period (n = 7), participant reported metal in body before formal screening (n = 8), participant concerns about stent or other cardiac device (n = 8), concerns about body size (n = 2), other reasons (n = 7), and unknown reason (n = 22).
bOther reasons for ineligibility include participant concerns about stent or other cardiac device (n = 8), use of pain pump (n = 2), prolonged hospitalization (n = 2), participant declined consent (n = 2), participant too large for MRI scanner (n = 1), and other reasons (n = 7).
Characteristics of the 670 participants who completed a baseline MRI scan did not differ between treatment groups (Table 1). At baseline, the mean age overall was 67.3 (SD, 8.2) years; 40.4% were women, and 33.1% were black. Mean SBP was 138.0 (SD, 16.6) mm Hg and mean diastolic BP was 77.8 (SD, 11.4) mm Hg. Additional baseline characteristics of participants are reported in eTable 1 in Supplement 2. Compared with participants not in the MRI substudy, those in the substudy were more likely to be women, black, and younger; had a lower SBP at baseline; and were less likely to have a history of cardiovascular disease (eTable 2 in Supplement 2). Participants in the MRI substudy also had higher scores on the Montreal Cognitive Assessment compared with the remaining participants in the trial.
Table 1. Characteristics of Participants in the Magnetic Resonance Imaging Substudy.
| Variable | Completed Baseline Scan | Completed Follow-up Scan | ||
|---|---|---|---|---|
| Intensive Treatment (n = 355) | Standard Treatment (n = 315) | Intensive Treatment (n = 249) | Standard Treatment (n = 200) | |
| Age, mean (SD), y | 67.7 (8.0) | 66.9 (8.5) | 67.8 (7.7) | 66.3 (7.8) |
| Age ≥75 y, No. (%) | 83 (23.4) | 67 (21.3) | 55 (22.1) | 36 (18.0) |
| Sex, No. (%) | ||||
| Men | 200 (56.3) | 199 (63.2) | 151 (60.6) | 131 (65.5) |
| Women | 155 (43.7) | 116 (36.8) | 98 (39.4) | 69 (34.5) |
| Black race, No. (%)a | 116 (32.7) | 106 (33.7) | 71 (28.5) | 67 (33.5) |
| Race/ethnicity, No. (%) | ||||
| White | 222 (62.5) | 184 (58.4) | 169 (67.9) | 118 (59.0) |
| Black | 114 (32.1) | 104 (33.0) | 69 (27.7) | 65 (32.5) |
| Hispanicb | 14 (3.9) | 22 (7.0) | 9 (3.6) | 13 (6.5) |
| Otherc | 5 (1.4) | 5 (1.6) | 2 (0.8) | 4 (2.0) |
| History of CVD, No. (%) | 48 (13.5) | 45 (14.3) | 31 (12.4) | 21 (10.5) |
| Systolic BP, mean (SD), mm Hg | 138.2 (17.6) | 137.8 (15.5) | 136.0 (17.0) | 138.2 (15.8) |
| Tertile, No. (%) | ||||
| ≤129 | 130 (36.6) | 99 (31.4) | 96 (38.6) | 63 (31.5) |
| >129 to <143 | 102 (28.7) | 105 (33.3) | 77 (30.9) | 67 (33.5) |
| ≥143 | 123 (34.6) | 111 (35.2) | 76 (30.5) | 70 (35.0) |
| Diastolic BP, mean (SD), mm Hg | 77.3 (10.9) | 78.5 (12.0) | 76.5 (10.7) | 79.3 (12.1) |
| Orthostatic hypotension, No. (%)d | 26 (7.3) | 17 (5.4) | 20 (8.0) | 15 (7.5) |
| eGFR, mean (SD), mL/min/1.73 m2,e | 72.0 (20.0) | 72.6 (21.3) | 71.7 (19.2) | 73.3 (20.7) |
| eGFR <60 mL/min/1.73 m2, No. (%)e | 94 (26.6) | 90 (28.7) | 66 (26.5) | 55 (27.5) |
| Montreal Cognitive Assessment, median (IQR)f | 24 (21-26) | 24 (21-26) | 24 (21-27) | 24 (22-27) |
| Intracranial volume, mean (SD), cm3 | 1372.1 (148.3) | 1391.7 (145.1) | 1384.3 (142.3) | 1398.6 (146.7) |
| Total brain volume, mean (SD), cm3 | 1126.9 (113.6) | 1141.0 (114.7) | 1136.7 (110.4) | 1150.5 (113.2) |
| WML volume, median (IQR), cm3 | 3.0 (1.5-6.2) | 3.3 (1.6-6.2) | 2.9 (1.5-5.9) | 3.2 (1.7-6.2) |
| Transformed WML volume, mean (SD), asinh(cm3) | 2.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | 1.9 (0.9) |
Abbreviations: BP, blood pressure; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; IQR, interquartile range; WML, white matter lesion; asinh, inverse hyperbolic sine transformation, f(x) = log(x + (x2 + 1)0.5).
Black race includes Hispanic black and black as part of multiracial identification.
Hispanic race/ethnicity encompasses a self-report of being of Spanish, Hispanic, or Latino origin, independent of any other race/ethnicity designation.
Includes categories of Asian, American Indian/Alaskan Native, Native Hawaiian/Pacific Islander, or other.
Defined as a value of −20 mm Hg or less for standing systolic BP minus seated systolic BP or of −10 mm Hg or less for standing diastolic BP minus seated diastolic BP.
Based on the 4-variable Modification of Diet in Renal Disease equation.
Scores range from 0 to 30, with higher scores denoting better cognitive function.
There was a sustained between-group difference in SBP among participants in the MRI substudy. Mean SBP through the end of active intervention was 120.7 mm Hg in the intensive treatment group vs 134.9 mm Hg in the standard treatment group (difference, 14.2 mm Hg [95% CI, 13.1 to 15.3 mm Hg]) (eFigure 2 in Supplement 2). During the transitional closeout period from August 20, 2015, to July 1, 2016, mean SBPs increased slightly, to 122.1 for the intensive treatment group and 136.1 mm Hg for the standard treatment group, with only a small change to the SBP difference (14.0 mm Hg [95% CI, 9.3 to 18.8 mm Hg]) between groups.
Follow-up MRI scans were performed at a median of 3.97 years (range, 2.81-4.75 years) after randomization, after a median intervention period of 3.40 years (range, 2.46-4.30 years). Of the 670 participants with WML volume measurement at baseline, 462 completed the follow-up MRI (Figure 1). Image quality control requirements were not met for 13 participants with a follow-up MRI scan, resulting in a sample of 449 adults (249 in the intensive treatment group and 200 in the standard treatment group). The 221 participants who did not have a follow-up scan included 89 (13.3% of baseline population) who were unwilling to participate, 36 (5.4%) who were ineligible, 31 (4.6%) who withdrew from the trial or were lost to follow-up, 27 (4.0%) who died, and 25 (3.7%) for other reasons. Participants who did not have a follow-up MRI scan were more likely to be women, have a baseline history of cardiovascular disease, have higher SBP, have lower physical quality of life, and have lower baseline cognitive scores (eTable 3 in Supplement 2).
MRI Outcomes
At the follow-up MRI assessment, the intensive treatment group had a significantly smaller increase in transformed WML volume compared with the standard treatment group. The mean transformed WML volume in the intensive treatment group increased from 1.99 asinh(cm3) at baseline to 2.14 asinh(cm3) at follow-up, while the standard treatment group increased from 1.96 to 2.25 asinh(cm3) (between-group difference, −0.13 asinh[cm3] [95% CI, −0.19 to −0.07]) (Table 2). Based on a robust linear mixed model, this approximately corresponds to a between-group difference for the change in WML volume of −0.54 cm3 (95% CI, −0.87 to −0.20 cm3) (Table 2).
Table 2. Estimated Changes in Structural Magnetic Resonance Imaging Outcomes by Treatment Groupa.
| Outcome | Volume (95% CI), cm3 | P Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Intensive Treatment | Standard Treatment | Estimated Difference in Change | ||||||
| Baseline | Follow-up | Change | Baseline | Follow-up | Change | |||
| WML volume, asinh | 1.99 (1.86 to 2.13) |
2.14 (2.01 to 2.28) |
0.15 (0.11 to 0.19) |
1.96 (1.82 to 2.10) |
2.25 (2.10 to 2.39) |
0.28 (0.24 to 0.33) |
−0.13 (−0.19 to −0.07) |
<.001 |
| WML volume | 4.57 (4.00 to 5.14) |
5.49 (4.91 to 6.07) |
0.92 (0.69 to 1.14) |
4.40 (3.80 to 5.00) |
5.85 (5.23 to 6.47) |
1.45 (1.21 to 1.70) |
−0.54 (−0.87 to −0.20) |
|
| Annualized change | 0.23 (0.17 to 0.29) |
0.37 (0.30 to 0.43) |
||||||
| Total brain volume | 1134.5 (1125.1 to 1144.0) |
1104.0 (1094.5 to 1113.4) |
−30.6 (−32.3 to −28.8) |
1134.0 (1124.4 to 1143.6) |
1107.1 (1097.4 to 1116.8) |
−26.9 (−28.8 to −24.9) |
−3.7 (−6.3 to −1.1) |
.006 |
| Annualized change | −7.7 (−8.1 to −7.3) |
−6.8 (−7.3 to −6.3) |
||||||
Abbreviations: WML, white matter lesion; asinh, inverse hyperbolic sine transformation, f(x) = log(x + (x2 + 1)0.5).
Estimates based on a linear mixed model, adjusting for intracranial volume and days since randomization, with random effects for participant and magnetic resonance imaging facility. All estimates computed using the baseline mean intracranial volume of 1382.03 cm3, with follow-up estimates computed at 1452 days (3.98 years) after randomization. For change estimates, negative values denote decreases from baseline; positive values, increases from baseline. Difference in change represents intensive treatment group minus standard treatment group.
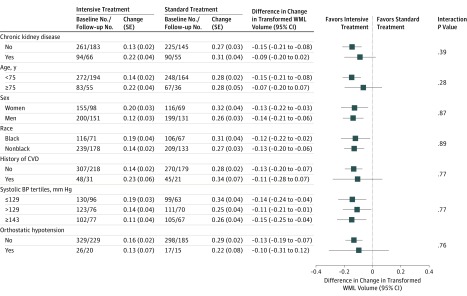
Results were similar using whole brain output from the lesion segmentation algorithm, not restricted to supratentorial white matter (eTable 4 in Supplement 2). There was no evidence of subgroup heterogeneity with regard to the change in WML volume (P > .05 for interaction for all) (Figure 2). The intensive treatment group had a significantly greater decrease in TBV compared with the standard treatment group. In the intensive treatment group, mean TBV decreased from 1134.5 cm3 at baseline to 1104.0 cm3 at follow-up, compared with 1134.0 to 1107.1 cm3 in the standard treatment group. This represents a between-group difference in mean change for TBV of −3.7 cm3 (95% CI, −6.3 to −1.1).
Figure 2. Change in Transformed White Matter Lesion Volume According to Subgroups.
Estimates denote the estimated change for transformed white matter lesion (WML) volume at 1452 days (3.98 years) among participants with WML measurement at baseline and follow-up, based on a linear mixed model, adjusting for intracranial volume and days since randomization, with random effects for participant and magnetic resonance imaging facility. Negative values denote decreases from baseline; positive values, increases from baseline. Differences represent intensive treatment group minus standard treatment group. WML volumes were transformed using inverse hyperbolic sine transformation, f(x) = log(x + (x2 + 1)0.5). BP indicates blood pressure; CVD, cardiovascular disease; SE, standard error.
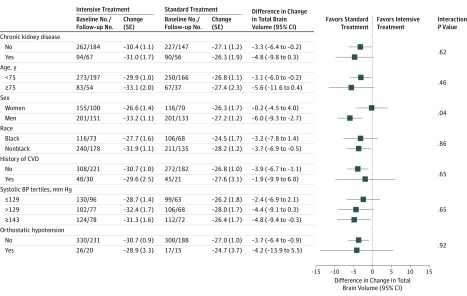
In subgroup analyses for TBV, there was a significant interaction between treatment group and sex for the change in TBV (P = .04 for interaction) (Figure 3). Compared with women, who showed no difference for change in TBV by treatment group, men randomized to intensive treatment experienced greater declines in TBV compared with those in the standard treatment group.
Figure 3. Change in Total Brain Volume According to Subgroups.
Estimates denote the estimated change in total brain volume at 1452 days (3.98 years) among participants with WML measurement at baseline and follow-up, based on a linear mixed model, adjusting for intracranial volume and days since randomization, with random effects for participant and magnetic resonance imaging facility. Differences represent intensive treatment group minus standard treatment group. BP indicates blood pressure; CVD, cardiovascular disease; SE, standard error.
Sensitivity Analyses
Results of sensitivity analyses based on inverse probability weighting and multiple imputation to address 2 potential sources of bias—selective entry into the MRI substudy and missing follow-up MRI scans—are reported in eTable 4 in Supplement 2. Results for WML volume were generally unchanged, consistently indicating a smaller increase in WML volume for the intensive treatment group. In contrast, the between-group difference for change in TBV was somewhat attenuated and not statistically significant, decreasing from −3.7 cm3 (95% CI, −6.3 to −1.1) to −2.2 cm3 (95% CI, −6.5 to 2.2) (P = .32). There were a small number of participants in this imaging substudy who completed the follow-up MRI scan and were adjudicated as having mild cognitive impairment (n = 23) or probable dementia (n = 6). Participants with probable dementia did exhibit significantly larger increases in transformed WML volume as well as significantly larger decreases in TBV compared with participants adjudicated as having no cognitive impairment (eFigures 3 and 4 in Supplement 2).
Discussion
In this imaging substudy of a randomized clinical trial, intensive SBP control was significantly associated with a smaller increase in mean WML volume compared with standard SBP control. There was also a significant association of intensive SBP control on the secondary outcome of TBV, with participants randomized to intensive treatment exhibiting larger mean declines in TBV. Given that the main structural correlate of hypertension on the brain is abnormal WML volumes, these results suggest that the development of this structural abnormality can be slowed by more intensive treatment of hypertension. However, the anatomical basis and functional significance of the greater brain volume loss in the intensive treatment group is unclear.
Many previous cross-sectional and longitudinal cohort studies have shown that, after age, the strongest risk factor for increased WML volume is hypertension, suggesting that treatment of hypertension might attenuate progression of WMLs.2,3 There are relatively few clinical trial reports, however, on the effects of hypertension treatment on this metric of brain structure. In a cohort study of hypertensive adults, de Leeuw et al26 reported that the successfully treated group (defined as SBP <160 mm Hg) had a slower progression of WMLs, compared with the group with poorly controlled hypertension. In the PROGRESS randomized clinical trial of a predefined antihypertensive regimen vs placebo, the treatment group had a decreased incidence of new WMLs and lesser WML volume increase.11 These studies used traditional BP treatment targets, with both having a posttreatment mean SBP greater than 130 mm Hg in the treated groups. This trial showed that intensive SBP control was associated with smaller WML volume increases compared with standard treatment targets, an association preserved in high-risk subgroups. In terms of more aggressive management of SBP, the ACCORD BP trial compared the same SBP targets as this trial in patients with diabetes and showed significantly less WML volume increase in the intensive treatment group27; however, it was not clear that this result would generalize to populations without diabetes.
While the literature documents a strong association between greater WML volumes and cognitive impairment, there is no defined threshold for WML volume associated with a clinical diagnosis of mild cognitive impairment or probable dementia, either in terms of an absolute threshold or within-person change over time. While associations between WML volume and cognitive performance are evident at the group level, there often is significant overlap comparing groups with different levels of cognitive impairment. Several studies have reported annualized rates of change for WML volume; however, populations in these studies typically differed both in terms of age and burden of vascular disease from participants in this trial.28,29 The annual rates of change for WML volume in this trial were 0.23 cm3 per year for the intensive treatment group and 0.37 cm3 per year for the standard treatment group. As expected, these rates are higher than observed in normal populations; for example, using data from the Alzheimer Disease Neuroimaging Initiative (ADNI), Carmichael et al28 reported an annual WML volume change rate of 0.08 cm3 in normal controls. The rates in this trial were more comparable to rates for participants with mild cognitive impairment and Alzheimer disease in ADNI (0.24 cm3 per year), which may in part be attributable to more comorbid vascular disease in those groups. The Rotterdam study reported an increased annual change of 0.05 cm3 per standard deviation increase in SBP (approximately 18-19 mm Hg),29 a lower change than observed in this trial. It is unclear to what extent these between-study differences reflect variability in the studied populations vs variability in technical factors such as use of multiple MRI scanners in this trial or differences in MRI acquisition and processing.
The relationship of hypertension to TBV is less robust and less well documented, although high BP generally has been associated with decreased brain volumes.30,31,32 Of the above-referenced hypertension treatment studies, only the ACCORD BP trial reported data on TBV, with the intensive BP treatment group also showing greater loss of TBV. In both ACCORD BP and this trial, the between-group difference was small. Jack et al33 reported TBV annual change rates of −0.4% in asymptomatic participants, increasing to −1.4% in participants with neurodegeneration from Alzheimer disease. In this trial, annualized change rates for TBV were on the order of −0.6% to −0.7% per year. It is also unclear whether the between-group difference in TBV change reflects loss of brain tissue or another factor, such as hydration status, potentially related to differences in the antihypertensive intervention.34 There was an unexpected statistical interaction between sex and intensive SBP control for change in TBV, which warrants future investigation.
Limitations
This trial has several limitations. First, the duration of the intervention and follow-up was relatively short, approximately 4 years. Second, the completion rate of follow-up MRIs was lower than expected and was at least partially affected by the early termination of the intervention. Third, given the limited size of this imaging substudy and length of follow-up, informative analyses correlating changes in brain structure with the occurrence of mild cognitive impairment and dementia are not possible. However, in this substudy, the small number of participants adjudicated as having probable dementia did exhibit larger increases in WML volume and larger decreases in TBV. Fourth, generalizability to other hypertensive populations should be considered with caution, as this trial did not include persons with baseline diabetes, stroke, severe heart failure, dementia, or advanced or heavily proteinuric kidney disease or persons who resided in a nursing home. Fifth, regional WML and brain volumes were not evaluated, and it remains possible that there are differential regional associations between therapies, which will be further investigated in future analyses. Sixth, while the multisite nature of the study fosters generalization, the requisite use of different MRI scanners inevitably increased measurement variability.
Conclusions
Among hypertensive adults, targeting an SBP of less than 120 mm Hg, compared with less than 140 mm Hg, was significantly associated with a smaller increase in cerebral white matter lesion volume and a greater decrease in total brain volume, although the differences were small.
Trial Protocol
eMethods. Multiple Imputation and Inverse Probability Weighting to Assess the Influence of Selection Into the Magnetic Resonance Imaging (MRI) Substudy and Missing Follow-up MRI Scans
eTable 1. Additional Baseline Characteristics of Participants With a Measured White Matter Lesion Volume From the Baseline Magnetic Resonance Imaging Scan, by Treatment Group
eTable 2. Baseline Characteristics of Participants in the MRI Substudy Versus Remaining Trial Participants
eTable 3. Baseline Characteristics of Participants in the MRI Substudy With and Without a Follow-up MRI With Measured White Matter Lesion Volume
eTable 4. Changes in Small Vessel Ischemic Disease Lesion Volume by Treatment Group
eTable 5. Sensitivity Analyses for Magnetic Structural Outcomes Based on Inverse Probability Weighting and Multiple Imputation
eTable 6. Association of Change in Structural Magnetic Resonance Imaging Outcomes by Adjudicated Cognitive Status
eFigure 1. Eligibility, Randomization, and Follow-up for Participants in the MRI Substudy for the Secondary Outcome of Total Brain Volume
eFigure 2. Mean Systolic Blood Pressure by Treatment Group for Participants in MRI Substudy
eFigure 3. Association Between Change in White Matter Lesion Volume and Adjudicated Cognitive Status
eFigure 4. Association Between Change in Total Brain Volume and Adjudicated Cognitive Status
Data Sharing Statement
References
- 1.Wright JT Jr, Williamson JD, Whelton PK, et al. ; SPRINT Research Group . A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liao D, Cooper L, Cai J, et al. . Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control: the ARIC study. Stroke. 1996;27(12):2262-2270. doi: 10.1161/01.STR.27.12.2262 [DOI] [PubMed] [Google Scholar]
- 3.Basile AM, Pantoni L, Pracucci G, et al. ; LADIS Study Group . Age, hypertension, and lacunar stroke are the major determinants of the severity of age-related white matter changes: the LADIS (Leukoaraiosis and Disability in the Elderly) study. Cerebrovasc Dis. 2006;21(5-6):315-322. doi: 10.1159/000091536 [DOI] [PubMed] [Google Scholar]
- 4.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828. doi: 10.1016/S1474-4422(11)70072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorelick PB, Scuteri A, Black SE, et al. ; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia . Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672-2713. doi: 10.1161/STR.0b013e3182299496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verdelho A, Madureira S, Ferro JM, et al. ; LADIS Study . Differential impact of cerebral white matter changes, diabetes, hypertension and stroke on cognitive performance among non-disabled elderly: the LADIS study. J Neurol Neurosurg Psychiatry. 2007;78(12):1325-1330. doi: 10.1136/jnnp.2006.110361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MMB. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215-1222. doi: 10.1056/NEJMoa022066 [DOI] [PubMed] [Google Scholar]
- 8.Kalaria RN, Ballard C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord. 1999;13(suppl 3):S115-S123. doi: 10.1097/00002093-199912003-00017 [DOI] [PubMed] [Google Scholar]
- 9.Kalback W, Esh C, Castaño EM, et al. . Atherosclerosis, vascular amyloidosis and brain hypoperfusion in the pathogenesis of sporadic Alzheimer’s disease. Neurol Res. 2004;26(5):525-539. doi: 10.1179/016164104225017668 [DOI] [PubMed] [Google Scholar]
- 10.Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer’s disease—lessons from pathology. BMC Med. 2014;12:206. doi: 10.1186/s12916-014-0206-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dufouil C, Chalmers J, Coskun O, et al. ; PROGRESS MRI Substudy Investigators . Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) magnetic resonance imaging substudy. Circulation. 2005;112(11):1644-1650. doi: 10.1161/CIRCULATIONAHA.104.501163 [DOI] [PubMed] [Google Scholar]
- 12.Saper CB. How low can you go? Ann Neurol. 2015;78(5):665-666. doi: 10.1002/ana.24530 [DOI] [PubMed] [Google Scholar]
- 13.Williamson JD, Pajewski NM, Auchus AP, et al. ; SPRINT MIND Investigators for the SPRINT Research Group . Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321(6):553-561. doi: 10.1001/jama.2018.21442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrosius WT, Sink KM, Foy CG, et al. ; SPRINT Study Research Group . The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11(5):532-546. doi: 10.1177/1740774514537404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson KC, Whelton PK, Cushman WC, et al. ; SPRINT Research Group . Blood pressure measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension. 2018;71(5):848-857. doi: 10.1161/HYPERTENSIONAHA.117.10479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87-97. doi: 10.1109/42.668698 [DOI] [PubMed] [Google Scholar]
- 17.Doshi J, Erus G, Ou Y, Gaonkar B, Davatzikos C. Multi-atlas skull-stripping. Acad Radiol. 2013;20(12):1566-1576. doi: 10.1016/j.acra.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doshi J, Erus G, Ou Y, et al. ; Alzheimer’s Neuroimaging Initiative . MUSE: MUlti-atlas region Segmentation utilizing Ensembles of registration algorithms and parameters, and locally optimal atlas selection. Neuroimage. 2016;127:186-195. doi: 10.1016/j.neuroimage.2015.11.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ronneberger O, Fischer P, Brox T. U-Net: convolutional networks for biomedical image segmentation In: Navab N, Hornegger J, Wells W, Frangi A, eds. Medical Image Computing and Computer-Assisted Intervention—MICCAI 2015. Basel, Switzerland: Springer; 2015:234-241. [Google Scholar]
- 20.Szegedy C, Ioffe S, Vanhoucke V, Alemi AA Inception-v4, Inception-ResNet and the impact of residual connections on learning. In: Proceedings of the Thirty-First AAAI Conference on Artificial Intelligence (AAAI-17) Menlo Park, CA: Association for the Advancement of Artificial Intelligence; 2017:4278-4284. [Google Scholar]
- 21.Williamson JD, Supiano MA, Applegate WB, et al. ; SPRINT Research Group . Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315(24):2673-2682. doi: 10.1001/jama.2016.7050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sink KM, Evans GW, Shorr RI, et al. . Syncope, hypotension, and falls in the treatment of hypertension: results from the randomized clinical systolic blood pressure intervention trial. J Am Geriatr Soc. 2018;66(4):679-686. doi: 10.1111/jgs.15236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williamson JD, Launer LJ, Bryan RN, et al. ; Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Investigators . Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: a randomized clinical trial. JAMA Intern Med. 2014;174(3):324-333. doi: 10.1001/jamainternmed.2013.13656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burbidge JB, Magee L, Robb AL. Alternative transformations to handle extreme values of the dependent variable. J Am Stat Assoc. 1988;83(401):123-127. doi: 10.1080/01621459.1988.10478575 [DOI] [Google Scholar]
- 25.Koller M. robustlmm: An R package for robust estimation of linear mixed-effects models. J Stat Softw. 2016;75(6):1-24. doi: 10.18637/jss.v075.i06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Leeuw F-E, de Groot JC, Oudkerk M, et al. . Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125(pt 4):765-772. doi: 10.1093/brain/awf077 [DOI] [PubMed] [Google Scholar]
- 27.Murray AM, Hsu F-C, Williamson JD, et al. ; Action to Control Cardiovascular Risk in Diabetes Follow-On Memory in Diabetes (ACCORDION MIND) Investigators . ACCORDION MIND: results of the observational extension of the ACCORD MIND randomised trial. Diabetologia. 2017;60(1):69-80. doi: 10.1007/s00125-016-4118-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carmichael O, Schwarz C, Drucker D, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch Neurol. 2010;67(11):1370-1378. doi: 10.1001/archneurol.2010.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verhaaren BFJ, Vernooij MW, de Boer R, et al. . High blood pressure and cerebral white matter lesion progression in the general population. Hypertension. 2013;61(6):1354-1359. doi: 10.1161/HYPERTENSIONAHA.111.00430 [DOI] [PubMed] [Google Scholar]
- 30.Enzinger C, Fazekas F, Matthews PM, et al. . Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology. 2005;64(10):1704-1711. doi: 10.1212/01.WNL.0000161871.83614.BB [DOI] [PubMed] [Google Scholar]
- 31.Vlek ALM, Visseren FLJ, Kappelle LJ, et al. ; SMART Study Group . Blood pressure and progression of cerebral atrophy in patients with vascular disease. Am J Hypertens. 2009;22(11):1183-1189. doi: 10.1038/ajh.2009.166 [DOI] [PubMed] [Google Scholar]
- 32.Jochemsen HM, Muller M, Visseren FL, et al. ; SMART Study Group . Blood pressure and progression of brain atrophy: the SMART-MR Study. JAMA Neurol. 2013;70(8):1046-1053. doi: 10.1001/jamaneurol.2013.217 [DOI] [PubMed] [Google Scholar]
- 33.Jack CR Jr, Shiung MM, Gunter JL, et al. . Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62(4):591-600. doi: 10.1212/01.WNL.0000110315.26026.EF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duning T, Kloska S, Steinsträter O, Kugel H, Heindel W, Knecht S. Dehydration confounds the assessment of brain atrophy. Neurology. 2005;64(3):548-550. doi: 10.1212/01.WNL.0000150542.16969.CC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Multiple Imputation and Inverse Probability Weighting to Assess the Influence of Selection Into the Magnetic Resonance Imaging (MRI) Substudy and Missing Follow-up MRI Scans
eTable 1. Additional Baseline Characteristics of Participants With a Measured White Matter Lesion Volume From the Baseline Magnetic Resonance Imaging Scan, by Treatment Group
eTable 2. Baseline Characteristics of Participants in the MRI Substudy Versus Remaining Trial Participants
eTable 3. Baseline Characteristics of Participants in the MRI Substudy With and Without a Follow-up MRI With Measured White Matter Lesion Volume
eTable 4. Changes in Small Vessel Ischemic Disease Lesion Volume by Treatment Group
eTable 5. Sensitivity Analyses for Magnetic Structural Outcomes Based on Inverse Probability Weighting and Multiple Imputation
eTable 6. Association of Change in Structural Magnetic Resonance Imaging Outcomes by Adjudicated Cognitive Status
eFigure 1. Eligibility, Randomization, and Follow-up for Participants in the MRI Substudy for the Secondary Outcome of Total Brain Volume
eFigure 2. Mean Systolic Blood Pressure by Treatment Group for Participants in MRI Substudy
eFigure 3. Association Between Change in White Matter Lesion Volume and Adjudicated Cognitive Status
eFigure 4. Association Between Change in Total Brain Volume and Adjudicated Cognitive Status
Data Sharing Statement