Key Points
Question
Can summary measures be defined characterizing brain and clinical changes to better understand disease trajectories in Huntington disease?
Findings
Among 2065 visits from 443 participants, this study identifies motor-cognitive and white matter–ventricle summary measures with similar nonlinear trajectories across the disease span and a strong association with CAG. In contrast, the striatum shows a near-linear trajectory with strong CAG dependence, and gray matter has a slow, nonlinear pattern with a weaker dependence on CAG.
Meaning
This work aids understanding of the association between brain changes and clinical manifestation in Huntington disease and their dependence on age and CAG repeat length, which may have utility in disease-modifying clinical trials.
This study analyzes the decades-long early progression of Huntington disease and its dependence on CAG repeat length.
Abstract
Importance
In Huntington disease (HD), mutation severity is defined by the length of the CAG trinucleotide sequence, a well-known predictor of clinical onset age. The association with disease trajectory is less well characterized. Quantifiable summary measures of trajectory applicable over decades of early disease progression are lacking. An accurate model of the age-CAG association with early progression is critical to clinical trial design, informing both sample size and intervention timing.
Objective
To succinctly capture the decades-long early progression of HD and its dependence on CAG repeat length.
Design, Setting, and Participants
Prospective study at 4 academic HD treatment and research centers. Participants were the combined sample from the TRACK-HD and Track-On HD studies consisting of 290 gene carriers (presymptomatic to stage II), recruited from research registries at participating centers, and 153 nonbiologically related controls, generally spouses or friends. Recruitment was targeted to match a balanced, prespecified spectrum of age, CAG repeat length, and diagnostic status. In the TRACK-HD and Track-On HD studies, 13 and 5 potential participants, respectively, failed study screening. Follow-up ranged from 0 to 6 years. The study dates were January 2008 to November 2014. These analyses were performed between December 2015 and January 2019.
Main Outcomes and Measures
The outcome measures were principal component summary scores of motor-cognitive function and of brain volumes. The main outcome was the association of these scores with age and CAG repeat length.
Results
We analyzed 2065 visits from 443 participants (247 female [55.8%]; mean [SD] age, 44.4 [10.3] years). Motor-cognitive measures were highly correlated and had similar CAG repeat length–dependent associations with age. A composite summary score accounted for 67.6% of their combined variance. This score was well approximated by a score combining 3 items (total motor score, Symbol Digit Modalities Test, and Stroop word reading) from the Unified Huntington’s Disease Rating Scale. For either score, initial progression age and then acceleration rate were highly CAG repeat length dependent. The acceleration continues through at least stage II disease. In contrast, 3 distinct patterns emerged among brain measures (basal ganglia, gray matter, and a combination of whole-brain, ventricular, and white matter volumes). The basal ganglia pattern showed considerable change in even the youngest participants but demonstrated minimal acceleration of loss with aging. Each clinical and brain summary score was strongly associated with the onset and rate of decline in total functional capacity.
Conclusions and Relevance
Results of this study suggest that succinct summary measures of function and brain loss characterize HD progression across a wide disease span. CAG repeat length strongly predicts their decline rate. This work aids our understanding of the age and CAG repeat length–dependent association between changes in the brain and clinical manifestations of HD.
Introduction
Understanding the natural trajectory of Huntington disease (HD) is central to the quest for disease-modifying treatments that may eventually prevent or delay symptom onset using early intervention. Huntington disease is a slowly progressing, inherited neurological disorder caused by an expansion of the CAG trinucleotide sequence in the huntingtin gene (OMIM 613004). It presents as a triad of motor, cognitive, and neuropsychiatric impairments, with subtle signs developing many years before clinical onset. This onset is typically in middle age but varies depending on the length of the CAG repeat, with longer expansions hastening onset.1 Because predictive genetic testing is available, we have a unique opportunity to track HD progression many years before clinically substantial impairment and gain insights into the timelines with which impairments emerge and in turn gauge the optimal time to provide treatments.
Previous prospective studies2,3,4,5 have highlighted the progression of individual cognitive, motor, and neuropsychiatric features over time in HD. Unlike onset age, the association between CAG repeat length and lifetime trajectory of decline within this triad is less well characterized. There is contradictory evidence regarding the role of CAG repeat length in HD symptoms progression after clinical onset. For example, some studies found that time from clinical onset to death6 and the progression rate after diagnosis,7 when measured by total functional capacity (TFC) from the Unified Huntington Disease Rating Scale (UHDRS),8 are not CAG repeat length dependent, while other evidence suggests the reverse to be true.9 It is unclear whether there is CAG dependency for the age at which subtle pathology is first detectable or for its progression rate during the very earliest premanifest phase.
Clinical assessment of HD severity typically is focused on the total motor score (TMS) and TFC from the UHDRS. However, the reliability of the TMS is imperfect, and it is far from clear whether the extent of motor disability should be the sole focus of a severity measure. Total functional capacity has obvious face validity but also has ceiling effects that limit its utility for characterizing earliest progression. To understand more fully the nature of HD progression and its association with CAG repeat length, more sensitive measures are required. Parsimonious summaries of these measures are obviously desirable if empirically justified. If a wide range of clinical measures are substantially correlated, and if at least some of these are sensitive to the subtlest deficits detectable long before overt clinical illness, then a score combining the measures would be suitable for defining clinical severity.
The association of CAG repeat length with brain atrophy is somewhat clearer. Robust evidence suggests that CAG repeat length alters rates of brain atrophy in the pre-HD10 and manifest11,12,13 stages. A recent analysis of the PREDICT-HD study of pre-HD identified “change points” at which magnetic resonance imaging–derived brain volumes started to decline in relation to the age-CAG product.14 These findings involve multiple measurements within the brain, and reasoning similar to the above applies if we desire but a few key composite imaging measures of changes observable in HD.
The international, multicenter, natural history TRACK-HD study15 used annual quantitative assessments of symptoms (ie, motor, cognitive, and neuropsychiatric function) together with brain volumetrics to identify measures sensitive to disease progression, including progression many years before expected clinical onset. The subsequent Track-On HD study16 similarly followed predominantly pre-HD gene carriers across 3 annual time points. Combining annual measures from both studies provides detailed phenotypic data for as long as 72 months, presenting an opportunity to characterize disease trajectories in multiple modalities.
Methods
Study Design
A full description of TRACK-HD procedures, data collection, and data storage has been reported.15 Four academic HD treatment and research centers (London, United Kingdom; Leiden, the Netherlands; Paris, France; and Vancouver, British Columbia, Canada) recruited and assessed cohorts of 123 healthy controls, 120 pre-HD gene carriers, and 123 patients with early HD at baseline and annually for 3 years from 2008 to 2011. In 2010, 18 additional pre-HD participants were added to replace those lost to follow-up.
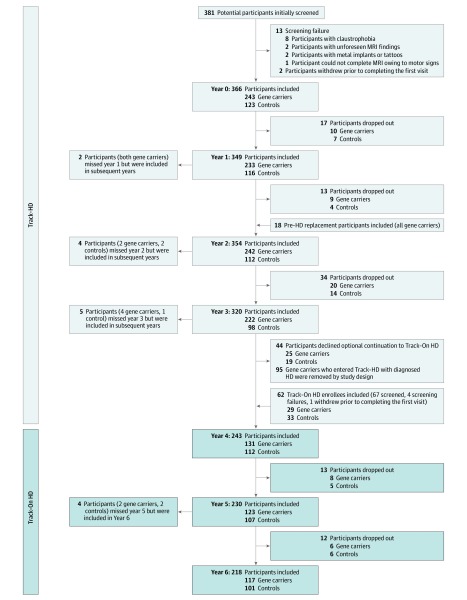
Track-On HD was an extension of the TRACK-HD study and included 112 healthy controls, 109 pre-HD gene carriers, and 22 participants with HD assessed annually over 3 time points. All participants with HD had begun the original TRACK-HD study as pre-HD. A full description of procedures, data collection, and storage has been published previously.16 Figure 1 shows participant numbers and dropout across both studies. The maximum follow-up time was 6 years, with a mean (SD) follow-up per participant of 3.71 (1.98) years. The study dates were January 2008 to November 2014. These analyses were performed between December 2015 and January 2019. Local ethics committees approved both studies, and all participants signed written informed consent.
Figure 1. Participant Entry and Loss in the Combined TRACK-HD and Track-On HD Studies.
HD indicates Huntington disease.
We considered only variables common to both studies and that had been designated a priori as measures of primary interest. The clinical variables ultimately used for the severity summaries included the TMS, Symbol Digit Modalities Test (SDMT), and Stroop word reading (all part of the UHDRS), plus the Spot the Change task and the rate and variability measures from paced and speeded tapping quantitative motor tasks.17 Imaging measures included intracranial volume ratios of caudate, lateral ventricular, and total brain volumes derived from the MIDAS software,18,19 BRAINS2-derived putamen,20 and total gray and total white matter generated using statistical parametric mapping.21 We initially considered several other neuropsychiatric, cognitive, and motor variables that we eventually excluded from the main analysis for reasons described in the eMethods in the Supplement.
Statistical Analysis
We used principal component analysis (PCA) to derive summary measures separately for nonimaging and imaging variables. The first principal component (PC) score, which is our focus, is the weighted sum of the original set of variables that has the highest possible mean correlation with those same variables. Scores are normalized so that a value of 0 represents the sample mean score, and the unit of measurement is within-sample standard deviations. Further explanation is available in the eMethods in the Supplement. By design, the study sample was almost evenly balanced between pre-HD and early diagnosed HD. Therefore, we expect the transition to diagnosed HD to occur near 0, the sample mean score.
We excluded UHDRS TFC from PCA. This is because of both the ceiling effect in pre-HD and our intent to use it later for validation.
We used mixed-effects linear modeling to estimate the score dependence on age and CAG repeat length and to examine the association between PC scores of clinical and brain volume measures. The models contain quadratic age and CAG repeat length terms and interactions because of their good empirical fit. Correlated random intercepts and slopes were used at the participant level. Regardless of statistical significance, we also fit quadratic age models for the controls to more closely mirror the HD models.
The nonlinear association of the brain vs nonbrain scores was more complicated and was estimated using a restricted cubic spline model with 3 knots placed a priori at the 10th, 50th, and 90th percentiles of the clinical scores.22 Additional knots were not significant.
Models estimating TFC pose a challenge because of the ceiling effect at the level of normal healthy functioning. To model predicted TFC, we treated TFC scores as ordered categorical variables and applied cumulative logit models. We fit both mixed-effects models with random participant terms and generalized estimating equation models. Predictions from the 2 models have different interpretations, which are both useful as we explain in the Results section. We transformed the model coefficient estimates into expected TFC scores and estimated 95% CIs via a delta method covariance transform of the original model coefficient estimates. We also used the delta method to estimate standard errors for the ages of first detectable change.
Missing data occurred due to study dropout (Figure 1) but also occurred in processed brain scan measurements due to a combination of scan intolerance and inadequate scan quality (eTable 1A and eTable 1B in the Supplement). Our mixed-effects models provide unbiased statistical coefficient estimates under the assumption that such data are missing at random, conditional on the values of the other data used in the model. This was plausibly the case. The missing scan rate increased with disease severity, but this severity was strongly predicted by age, CAG repeat length, and previous scan values (when available). Incomplete nonimaging data occurred at less than 3% of visits.
Results
We analyzed 2065 visits from 443 participants. Their mean (SD) age was 44.4 (10.3) years, and 247 (55.8%) were female.
PC Scores
For the nonimaging variables, the first PC correlated highly with all motor-cognitive measures (Table 1), accounting for 67.6% of their combined variance. The PC correlation magnitudes with individual measures were between 0.770 and 0.918; only Spot the Change had a weaker correlation of −0.582. We refer to the first PC scores from this analysis as the motor-cognitive score.
Table 1. Correlations of Observed Measurements to the First PC Scoresa.
| Variable | Motor-Cognitive Score | UHDRS Motor-Cognitive Score | White Matter–Ventricle Score | Caudate-Putamen Score |
|---|---|---|---|---|
| Symbol Digit Modalities Test, No. correct | −0.795 | −0.903 | NA | NA |
| Stroop word reading, No. correct | −0.770 | −0.879 | NA | NA |
| Spot the Change 5 s, corrected for guessing | −0.582 | NA | NA | NA |
| UHDRS total motor score | 0.872 | 0.891 | NA | NA |
| Paced tapping 3-Hz SD of intertap intervals, log | 0.808 | NA | NA | NA |
| Q-Motor speeded tapping intertap interval, mean | 0.829 | NA | NA | NA |
| Q-Motor speeded tapping interonset interval, log SD | 0.885 | NA | NA | NA |
| Q-Motor speeded tapping tap duration, log SD | 0.889 | NA | NA | NA |
| Q-Motor speeded tapping interonset interval, log mean | 0.918 | NA | NA | NA |
| Putamen volume, ratio to ICV | NA | NA | NA | −0.947 |
| Caudate volume, ratio to ICV | NA | NA | NA | −0.947 |
| Total brain volume, ratio to ICV | NA | NA | −0.926 | NA |
| Ventricle volume, ratio to ICV | NA | NA | 0.818 | NA |
| White matter volume, ratio to ICV | NA | NA | −0.870 | NA |
| Gray matter volume, ratio to ICVb | NA | NA | NA | NA |
| Variance explained by the first PC, % | 67.6 | 79.4 | 76.1 | 89.7 |
Abbreviations: ICV, intracranial volume; NA, not applicable (indicating that the corresponding variable was not used in that PC analysis); PC, principal component; UHDRS, Unified Huntington’s Disease Rating Scale.
Columns represent first PCs of 4 separate analyses. All tapping tasks were performed with the nondominant hand.
Gray matter volume is treated separately rather than as part of any PC (see the PC Scores subsection within the Results section for explanation).
We also performed a PCA using only the motor, SDMT, and Stroop word reading scores from the UHDRS (a subset of the above items). The first PC accounted for 79.4% of the joint variance, with correlation magnitudes tightly clustered between 0.879 and 0.903. We refer to the corresponding PC score as the UHDRS motor-cognitive score.
Our 6 brain volume measures could not be satisfactorily condensed to a single summary score because they showed 3 distinct patterns of dependence on age and CAG repeat length. Therefore, we combined only measures that had similar patterns. Whole-brain, ventricular, and white matter volumes showed consistent age and CAG repeat length dependencies. The first PC in this white matter–ventricle PCA accounted for 76.1% of the shared variance (Table 1). In the caudate-putamen PCA, the first PC accounted for 89.7% of the combined variance. Finally, gray matter volume had a unique association with age and CAG repeat length, and we kept this as a separate measure. However, for consistency with PC scores, we standardized gray matter volume to have a mean (SD) of 0 (1), with higher scores representing smaller volumes.
Association Between Huntington Disease PC Scores and Age and CAG Repeat Length
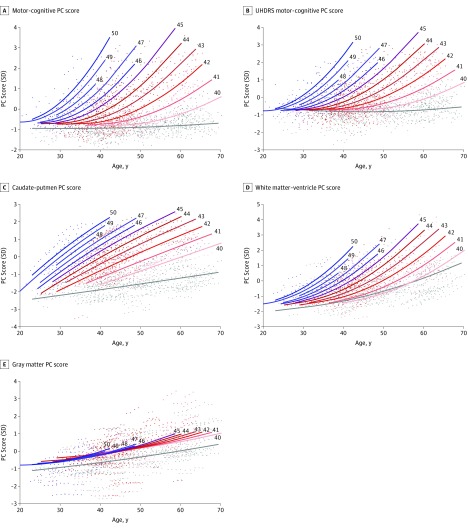
In the gene carriers, the scores all had accelerating nonlinear associations with age and CAG repeat length (Figure 2). The data were well fit by quadratic regressions (eTable 2A in the Supplement), implying for a given CAG repeat length that acceleration rate of worsening scores increases linearly with age (eFigure 1 in the Supplement). The rate also increases quadratically with CAG repeat length. We note that this association is more complex than can be described by the often-used disease burden23 or HD CAG-product (CAP) scores.24
Figure 2. CAG Repeat Length–Dependent PC Associations With Age.
A-E, The CAG repeat length associated with each prediction curve is indicated at the upper right of the curve. Underlying data are color coded to match the curve of the corresponding CAG repeat length. Each prediction curve spans only the observed age range for data with the corresponding CAG repeat length. The association with age is highly CAG repeat length dependent for all scores except gray matter. Underlying prediction models are listed in eTable 2 in the Supplement. Age-dependent patterns and the underlying data for controls are in gray. PC indicates principal component; UHDRS, Unified Huntington Disease Rating Scale.
For the motor-cognitive score (Figure 2A), the rate of motor-cognitive change continues to accelerate in the oldest (mainly early diagnosed HD) participants, suggesting that CAG dependency remains after diagnosis. The simplified UHDRS motor-cognitive PC closely mirrors the original motor-cognitive pattern (Figure 2B). In contrast, the controls’ age association with both motor-cognitive scores is weak, despite a statistically significant linear component (eTable 2B in the Supplement).
Figure 2D shows that the accelerating white matter–ventricle pattern mimics that of the motor-cognitive scores. In contrast, for fixed CAG repeat length, caudate-putamen change is almost linear (Figure 2C). Finally, the gray matter pattern shows a statistically significant but far weaker dependence on CAG repeat length (Figure 2E), accelerating slightly with age.
We assume our quadratic models are only valid after the age at which they begin to predict worsening scores. We tentatively interpret this as the mean age of the first detectable changes in gene carriers (more details are available in eResults in the Supplement). In Table 2, we list CAG repeat length–specific estimates of these ages. For example, with 40 CAG repeats, the estimated mean (SE) age of initial motor-cognitive score change is 42.46 (2.82) years. For 45 CAG repeats, the estimate is 26.65 (1.38) years, and it is 18.49 (2.08) years for 50 CAG repeats. The estimates are almost identical for the motor-cognitive and UHDRS motor-cognitive scores but are younger for the white matter–ventricle score. However, nonnegligible brain volume loss is also occurring in healthy controls. Adjusting for this, the white matter–ventricle estimates are much closer to those of the clinical measures. We estimate no minimum within the observed age range at which the caudate-putamen scores initially increase. The weak dependency of gray matter estimates on CAG would yield very wide 95% CIs; therefore, they are not represented.
Table 2. Estimated Initial Age for Huntington Disease–Related Score Decline as a Function of CAG Repeat Length.
| CAG Repeats | Estimated Age (SE), y | |||
|---|---|---|---|---|
| Motor-Cognitive Score | UHDRS Motor-Cognitive Score | White Matter–Ventricle Score | Control-Corrected White Matter–Ventricle Score | |
| 40 | 42.46 (2.82) | 42.68 (2.50) | 33.32 (2.73) | 45.93 (2.74) |
| 41 | 37.44 (2.34) | 38.09 (2.14) | 30.32 (2.22) | 39.29 (2.60) |
| 42 | 33.86 (1.90) | 34.58 (1.81) | 27.78 (1.82) | 34.28 (2.53) |
| 43 | 31.06 (1.58) | 31.70 (1.55) | 25.58 (1.58) | 30.34 (2.46) |
| 44 | 28.71 (1.41) | 29.25 (1.41) | 23.66 (1.53) | 27.14 (2.48) |
| 45 | 26.65 (1.38) | 27.07 (1.41) | 21.96 (1.65) | 24.48 (2.62) |
| 46 | 24.80 (1.45) | 25.10 (1.51) | 20.43 (1.85) | 22.22 (2.83) |
| 47 | 23.09 (1.58) | 23.28 (1.67) | 19.05 (2.10) | 20.26 (3.10) |
| 48 | 21.48 (1.74) | 21.57 (1.87) | 17.78 (2.36) | 18.54 (3.37) |
| 49 | 19.96 (1.91) | 19.96 (2.09) | 16.62 (2.62) | 17.01 (3.65) |
| 50 | 18.49 (2.08) | 18.41 (2.31) | 15.55 (2.86) | 15.64 (3.91) |
Abbreviation: UHDRS, Unified Huntington’s Disease Rating Scale.
Association Between Huntington Disease PC Scores and TFC
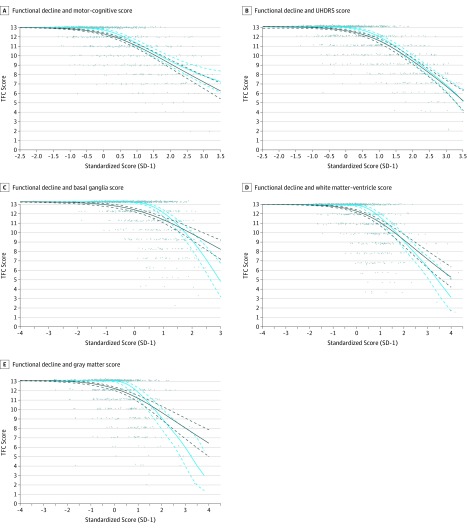
Despite its clinical relevance, ceiling effects discouraged us from incorporating TFC into the PCA. However, as shown in Figure 3, once TFC decline starts, it progresses almost linearly with the PC severity scores.
Figure 3. Total Functioning Capacaity (TFC) Loss as Predicted by Clinical Scores and Brain Volume Scores.
A-E, Marginal means and 95% CIs from generalized estimating equation models are in green, while random-effects model means and 95% CIs are in blue. The random-effects means are also estimates of the marginal median values. We discuss the difference between these estimates further in the eResults in the Supplement. After TFC scores begin to decline, there is almost a linear association between TFC decline and increasing PC scores. The point when this happens corresponds roughly to so-called clinical onset in HD. The linearity after this point gives clinimetric support to addition of the TFC score to the motor-cognitive PC score or the UHDRS motor-cognitive PC score when assessing progression in manifest HD. The above plots are derived from ordinal logistic regression models of the probabilities of observing various TFC scores given the PC scores. HD indicates Huntington disease; PC, principal component; and UHDRS, Unified Huntington’s Disease Rating Scale.
Association Between Brain Volume Loss and Motor-Cognitive Scores
eFigure 2 in the Supplement shows the nonlinear association between brain volume loss and motor-cognitive scores. There is little concomitant increase in disease severity with the earliest brain volume losses, although there is some increase with white matter–ventricle scores. This association shifts abruptly around the mean score of 0 and is then almost linear with a substantial slope. The pattern is similar for all brain scores. The estimated mean age associations of the motor-cognitive and three brain scores are superimposed in eFigure 3 in the Supplement and are discussed further in eResults in the Supplement.
Discussion
We have characterized the trajectory of HD progression from earliest detectable signs to moderately severe illness. We derived a single summary measure capturing the motor-cognitive phenotype and showed that the accelerating progression of the phenotype with aging is highly CAG repeat length dependent (ie, those with higher CAG decline earlier and faster). Contrary to some previous assertions,6,7,25 this CAG dependence continues well past the onset of clinical illness.
We did not detect evidence of distinct motor-cognitive phenotypes in early HD. However, we do not know the extent to which the apparent unidimensionality of motor-cognitive decline is due to motor impairment interference with cognitive test performance. Such potential confounding remains an ongoing challenge in the study of HD cognitive decline.
A simplified UHDRS motor-cognitive score, limited to the TMS, SDMT, and Stroop word reading, approximated this phenotype score well and showed a similar CAG repeat length–dependent trajectory. Once daily function begins to decline, the association with TFC and the UHDRS motor-cognitive score is almost linear. This justifies addition of TFC to the UHDRS motor-cognitive score when working exclusively with early-stage, diagnosed individuals. Such a score was recently proposed as an outcome in early manifest HD trials.26
Changes in brain volumes were not well summarized by a single dimension. Instead, we identified distinctive caudate-putamen, white matter–ventricle, and gray matter patterns with age and CAG repeat length.
Striatal atrophy has already been identified from the very earliest premanifest disease stages,4,27,28 as has white matter degeneration.4,29 We have shown that striatal brain volume loss is almost linear throughout the wide range of HD that we studied. This linear decline is consistent with a recent study30 examining regional variation in atrophy rates. In contrast, white matter–ventricle scores showed a nonlinear trajectory similar to the clinical scores. The accelerating white matter loss presumably accompanies breakdown of brain connectivity and thus brain function. This is consistent with the close association between this volume score and the clinical scores, which appears even before clinical disease onset (eFigure 2 in the Supplement). Global gray matter loss does not have a strong association with CAG at any age and shows a slow, nonlinear mean course. Nonetheless, it has been previously shown that faster gray matter loss is predictive of new diagnosis and clinical progression shortly thereafter.31 Accelerating gray matter loss around the time of clinically important symptom onset may thus be driven by proximal etiological factors other than CAG repeat length. These distinct brain loss patterns show that a single measure cannot reflect a consistent association among brain volumes over a wide range of disease evolution.
Although both behavioral and brain imaging changes have been previously documented many years before symptom onset,15,28,32 we are unaware of previous estimates of age of earliest detectable change. Our model suggests that the first motor-cognitive signs typically begin at approximately age 42 years in people with a CAG expansion of 40. The estimate is age 27 years for 45 repeats and approximately 18 years for 50 repeats. After adjusting for normal brain atrophy in controls, the estimated ages are also similar for the white matter–ventricle score. We must emphasize that there will be considerable person-to-person variability around these mean ages and that these initial changes are quite subtle, should not alter daily functioning, and are not likely to be noticeable except in collective analyses of grouped data.
In contrast, we estimated no minimal age at which caudate-putamen atrophy starts, suggesting that this begins much earlier. This is consistent with evidence from the lowest-risk participants in the PREDICT-HD study.28 Studies in younger gene carriers further from disease onset will address whether these values are ever within the control range.
There is considerable debate regarding where HD pathology is initiated because many imaging studies report both striatal loss and white matter degeneration in the earliest premanifest stages.15,27,28 Our estimates of earliest detectable change suggest that striatal atrophy occurs first, followed by white matter loss, which may be in response to the breakdown of striatal connections. As connectivity is further compromised, this is likely in turn to affect loss of gray matter and cortical functioning.30 However, this is speculative, and our data were not designed to establish pathological cause and effect. Volumetric measures are likely to be insensitive to early microstructural damage, which is more effectively highlighted using techniques like diffusion imaging. Studies examining earlier cohorts with multimodal imaging may provide more insight.
Limitations
We acknowledge limitations in our analyses. The entry and continuation criteria for the studies may have introduced bias. In TRACK-HD, pre-HD participants were required to have a screening UHDRS TMS of 5 or less and a minimal combination of age and CAG repeat length such that they had nontrivial risk for progression. Manifest TRACK-HD participants needed a TFC score of 7 or greater. Therefore, for some age-CAG combinations, a nontrivial fraction of the sickest individuals may have been excluded. Furthermore, the diagnosed participants in the initial TRACK-HD sample had shorter follow-up because they did not further participate in Track-On HD, which focused on the pre-HD group from the TRACK-HD study.
To combine longitudinal data across participants in a wide age range, our models assume equivalence for within-participant and between-participant age differences. This assumption may be violated if longitudinal measurements are altered by study participation. Practice effects and regression to the mean are examples. However, in the absence of decades-long longitudinal analysis of a random sample from the entire population with the HD CAG expansion, we regard our models as plausible starting points.
Conclusions
To date, these are the first reported models that we know of in HD of scores that synthesize severity across a broad range of disease progression. We have demonstrated the marked consequences of the genetic burden (ie, CAG repeat length) on these measures and now have CAG models of progression similar to the well-established CAG models of onset age. We have also demonstrated 3 distinct patterns (basal ganglia, gray matter, and a combination of whole-brain, ventricular, and white matter volumes) of CAG repeat length–dependent progression among commonly used brain volume measurements and describe the association between such brain changes and clinical progression. By characterizing these CAG repeat length–dependent disease trajectories, we provide insights into disease progression that may guide future therapeutic approaches and identify the most appropriate intervention ages to prevent clinical decline.
eMethods. Supplemental Methods
eTable 1. A, Missing Data Rates in the Combined TRACK-HD and Track-On Studies. B, Missing Data by HD Stage in TRACK-HD and Track-On Studies
eTable 2. A, Regression Coefficients for Principal Component Score Models in Cases. B, Regression Coefficients for Principal Component Score Models in Controls
eFigure 1. Estimated Acceleration Rates by CAG Repeat Length
eFigure 2. Nonlinear Relationship Between the Motor-Cognitive Score and Brain Imaging Measures
eFigure 3. Comparison of Age-Dependent Motor-Cognitive and Brain Volume PC Scores in 3 groups
eResults. Supplemental Results
eReferences.
References
- 1.Langbehn DR, Hayden MR, Paulsen JS; and the PREDICT-HD Investigators of the Huntington Study Group . CAG-repeat length and the age of onset in Huntington disease (HD): a review and validation study of statistical approaches. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(2):397-408. doi: 10.1002/ajmg.b.30992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirkwood SC, Siemers E, Stout JC, et al. Longitudinal cognitive and motor changes among presymptomatic Huntington disease gene carriers. Arch Neurol. 1999;56(5):563-568. doi: 10.1001/archneur.56.5.563 [DOI] [PubMed] [Google Scholar]
- 3.Solomon AC, Stout JC, Weaver M, et al. Ten-year rate of longitudinal change in neurocognitive and motor function in prediagnosis Huntington disease. Mov Disord. 2008;23(13):1830-1836. doi: 10.1002/mds.22097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabrizi SJ, Scahill RI, Durr A, et al. ; TRACK-HD Investigators . Biological and clinical changes in premanifest and early stage Huntington’s disease in the TRACK-HD study: the 12-month longitudinal analysis. Lancet Neurol. 2011;10(1):31-42. doi: 10.1016/S1474-4422(10)70276-3 [DOI] [PubMed] [Google Scholar]
- 5.Epping EA, Kim JI, Craufurd D, et al. ; PREDICT-HD Investigators and Coordinators of the Huntington Study Group . Longitudinal psychiatric symptoms in prodromal Huntington’s disease: a decade of data. Am J Psychiatry. 2016;173(2):184-192. doi: 10.1176/appi.ajp.2015.14121551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keum JW, Shin A, Gillis T, et al. The HTT CAG-expansion mutation determines age at death but not disease duration in Huntington disease. Am J Hum Genet. 2016;98(2):287-298. doi: 10.1016/j.ajhg.2015.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Squitieri F, Cannella M, Simonelli M. CAG mutation effect on rate of progression in Huntington’s disease. Neurol Sci. 2002;23(suppl 2):S107-S108. doi: 10.1007/s100720200092 [DOI] [PubMed] [Google Scholar]
- 8.Kieburtz K; Huntington Study Group . Unified Huntington’s Disease Rating Scale: reliability and consistency. Mov Disord. 1996;11(2):136-142. doi: 10.1002/mds.870110204 [DOI] [PubMed] [Google Scholar]
- 9.Rosenblatt A, Kumar BV, Mo A, Welsh CS, Margolis RL, Ross CA. Age, CAG repeat length, and clinical progression in Huntington’s disease. Mov Disord. 2012;27(2):272-276. doi: 10.1002/mds.24024 [DOI] [PubMed] [Google Scholar]
- 10.Aylward E, Mills J, Liu D, et al. Association between age and striatal volume stratified by CAG repeat length in prodromal Huntington disease. PLoS Curr. 2011;3(1):RRN1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruocco HH, Lopes-Cendes I, Li LM, Santos-Silva M, Cendes F. Striatal and extrastriatal atrophy in Huntington’s disease and its relationship with length of the CAG repeat. Braz J Med Biol Res. 2006;39(8):1129-1136. doi: 10.1590/S0100-879X2006000800016 [DOI] [PubMed] [Google Scholar]
- 12.Ruocco HH, Bonilha L, Li LM, Lopes-Cendes I, Cendes F. Longitudinal analysis of regional grey matter loss in Huntington disease: effects of the length of the expanded CAG repeat. J Neurol Neurosurg Psychiatry. 2008;79(2):130-135. doi: 10.1136/jnnp.2007.116244 [DOI] [PubMed] [Google Scholar]
- 13.Rosas HD, Reuter M, Doros G, et al. A tale of two factors: what determines the rate of progression in Huntington’s disease? a longitudinal MRI study. Mov Disord. 2011;26(9):1691-1697. doi: 10.1002/mds.23762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu D, Faria AV, Younes L, et al. ; PREDICT-HD Investigators and Coordinators of the Huntington Study Group . Mapping the order and pattern of brain structural MRI changes using change-point analysis in premanifest Huntington’s disease. Hum Brain Mapp. 2017;38(10):5035-5050. doi: 10.1002/hbm.23713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabrizi SJ, Langbehn DR, Leavitt BR, et al. ; TRACK-HD Investigators . Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2009;8(9):791-801. doi: 10.1016/S1474-4422(09)70170-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klöppel S, Gregory S, Scheller E, et al. ; Track-On Investigators . Compensation in preclinical Huntington’s disease: evidence from the Track-On HD study. EBioMedicine. 2015;2(10):1420-1429. doi: 10.1016/j.ebiom.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bechtel N, Scahill RI, Rosas HD, et al. Tapping linked to function and structure in premanifest and symptomatic Huntington disease. Neurology. 2010;75(24):2150-2160. doi: 10.1212/WNL.0b013e3182020123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeborough PA, Fox NC, Kitney RI. Interactive algorithms for the segmentation and quantitation of 3-D MRI brain scans. Comput Methods Programs Biomed. 1997;53(1):15-25. doi: 10.1016/S0169-2607(97)01803-8 [DOI] [PubMed] [Google Scholar]
- 19.Hobbs NZ, Henley SMD, Wild EJ, et al. Automated quantification of caudate atrophy by local registration of serial MRI: evaluation and application in Huntington’s disease. Neuroimage. 2009;47(4):1659-1665. doi: 10.1016/j.neuroimage.2009.06.003 [DOI] [PubMed] [Google Scholar]
- 20.Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WTC, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26(4):251-264. doi: 10.1016/S0895-6111(02)00011-3 [DOI] [PubMed] [Google Scholar]
- 21.Ashburner J, Friston KJ. Voxel-based morphometry: the methods. Neuroimage. 2000;11(6, pt 1):805-821. doi: 10.1006/nimg.2000.0582 [DOI] [PubMed] [Google Scholar]
- 22.Harrell FE. Regression Modeling Strategies. 2nd ed New York, NY: Springer New York; 2015. doi: 10.1007/978-3-319-19425-7 [DOI] [Google Scholar]
- 23.Penney JB Jr, Vonsattel JP, MacDonald ME, Gusella JF, Myers RH. CAG repeat number governs the development rate of pathology in Huntington’s disease. Ann Neurol. 1997;41(5):689-692. doi: 10.1002/ana.410410521 [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Long JD, Mills JA, Warner JH, Lu W, Paulsen JS; PREDICT-HD Investigators and Coordinators of the Huntington Study Group . Indexing disease progression at study entry with individuals at-risk for Huntington disease. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(7):751-763. doi: 10.1002/ajmg.b.31232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kieburtz K, MacDonald M, Shih C, et al. Trinucleotide repeat length and progression of illness in Huntington’s disease. J Med Genet. 1994;31(11):872-874. doi: 10.1136/jmg.31.11.872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schobel SA, Palermo G, Auinger P, et al. ; TRACK-HD, COHORT, CARE-HD, and 2CARE Huntington Study Group Investigators . Motor, cognitive, and functional declines contribute to a single progressive factor in early HD. Neurology. 2017;89(24):2495-2502. doi: 10.1212/WNL.0000000000004743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aylward EH, Sparks BF, Field KM, et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63(1):66-72. doi: 10.1212/01.WNL.0000132965.14653.D1 [DOI] [PubMed] [Google Scholar]
- 28.Paulsen JS, Langbehn DR, Stout JC, et al. ; Predict-HD Investigators and Coordinators of the Huntington Study Group . Detection of Huntington’s disease decades before diagnosis: the Predict-HD study. J Neurol Neurosurg Psychiatry. 2008;79(8):874-880. doi: 10.1136/jnnp.2007.128728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciarmiello A, Cannella M, Lastoria S, et al. Brain white-matter volume loss and glucose hypometabolism precede the clinical symptoms of Huntington’s disease. J Nucl Med. 2006;47(2):215-222. [PubMed] [Google Scholar]
- 30.Johnson EB, Ziegler G, Penny W, et al. Dynamics of cortical degeneration over a decade in Huntington’s disease. bioRxiv. 2019;537977. doi: 10.1101/537977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tabrizi SJ, Scahill RI, Owen G, et al. ; TRACK-HD Investigators . Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol. 2013;12(7):637-649. doi: 10.1016/S1474-4422(13)70088-7 [DOI] [PubMed] [Google Scholar]
- 32.Domínguez D JF, Egan GF, Gray MA, et al. Multi-modal neuroimaging in premanifest and early Huntington’s disease: 18 month longitudinal data from the IMAGE-HD study. PLoS One. 2013;8(9):e74131. doi: 10.1371/journal.pone.0074131 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods
eTable 1. A, Missing Data Rates in the Combined TRACK-HD and Track-On Studies. B, Missing Data by HD Stage in TRACK-HD and Track-On Studies
eTable 2. A, Regression Coefficients for Principal Component Score Models in Cases. B, Regression Coefficients for Principal Component Score Models in Controls
eFigure 1. Estimated Acceleration Rates by CAG Repeat Length
eFigure 2. Nonlinear Relationship Between the Motor-Cognitive Score and Brain Imaging Measures
eFigure 3. Comparison of Age-Dependent Motor-Cognitive and Brain Volume PC Scores in 3 groups
eResults. Supplemental Results
eReferences.