Key Points
Question
Is the combination of erlotinib and bevacizumab superior to erlotinib alone to treat epidermal growth factor receptor (EGFR)–mutant non–small cell lung cancer?
Findings
This phase 2 randomized clinical trial found that compared with erlotinib alone, the combination of erlotinib and bevacizumab did not result in superior progression-free survival.
Meaning
The combination of erlotinib and bevacizumab does not have superior efficacy compared with erlotinib alone.
This phase 2 multicenter randomized clinical trial including 88 patients compares progression-free survival, and secondarily overall survival, overall response rate, and adverse events, in patients with stage 4 EGFR-mutant non–small cell lung cancer treated with erlotinib plus bevacizumab vs erlotinib alone.
Abstract
Importance
Erlotinib is a standard first-line therapy for patients with epidermal growth factor receptor (EGFR)–mutant non–small cell lung cancer (NSCLC). Median progression-free survival (PFS) with erlotinib is approximately 10 months.
Objective
To determine whether adding bevacizumab to erlotinib treatment results in superior progression-free survival compared with erlotinib alone.
Design, Setting, and Participants
This phase 2 randomized clinical trial compared erlotinib plus bevacizumab with erlotinib alone in EGFR-mutant NSCLC. The trial was conducted in 17 US academic and community medical centers among 88 patients with EGFR exon 19 deletion or exon 21 L858R mutation based on local testing and stage 4 NSCLC who were eligible for bevacizumab. Patients were enrolled between November 2, 2012, and August 22, 2016, and followed up for a median (range) of 33 (0.7-62.5) months. Data were analyzed on August 28, 2018, and included data from November 2, 2012, to August 20, 2018.
Interventions
Patients were randomized with equal allocation to 150 mg of oral erlotinib daily alone or with 15 mg/kg of intravenous bevacizumab every 3 weeks. Study therapy continued until disease progression, unacceptable adverse event, or withdrawal of consent.
Main Outcomes and Measures
The primary outcome was PFS as assessed by the investigator; secondary outcomes were objective response rate (ORR), adverse events, and overall survival (OS). Analysis was designed to detect a hazard ratio (HR) of 0.667 for PFS (an improvement from a median PFS of 10 to 15 months).
Results
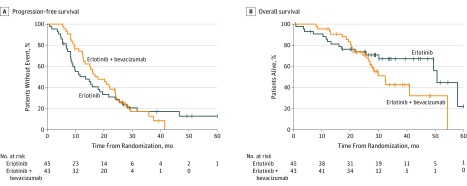
Among 88 patients enrolled, the median (range) age was 63 (31-84) years; 62 patients (70%) were female; 75 (85%) were white, 8 (9%) were African American, 3 (3%) were Asian, and for 2 (2%), data on race were not available. Forty-eight patients (55%) were never smokers, 45 patients (51%) were of Eastern Cooperative Oncology Group performance status 1, and 59 patients (67%) had EGFR exon 19 deletion. Compared with erlotinib, the combination did not result in a significant difference in PFS (HR, 0.81; 95% CI, 0.50-1.31; P = .39; median PFS 17.9 [combination] and 13.5 months [erlotinib]), ORR (81% vs 83%; P = .81), and OS (HR, 1.41; 95% CI, 0.71-2.81; P = .33; median OS, 32.4 months [combination] and 50.6 months [erlotinib]). Adverse events of grade 3 or higher observed in 5 or more patients in the combination and erlotinib arms were skin eruption in 11 (26%) vs 7 (16%) patients, diarrhea in 4 (9%) vs 6 (13%) patients, hypertension in 17 (40%) vs 9 (20%) patients, and proteinuria in 5 (12%) vs 0 (0%) patients.
Conclusions and Relevance
Erlotinib plus bevacizumab compared with erlotinib did not result in a significant improvement in PFS in EGFR-mutant NSCLC.
Trial Registration
ClinicalTrials.gov identifier: NCT01532089.
Introduction
Lung cancer is the leading cause of cancer death in the United States, and approximately 85% of these patients have non–small cell lung cancer (NSCLC) subtypes. Approximately 60% of patients with NSCLC have metastatic disease at the time of diagnosis.1,2 Historically, patients with metastatic disease received a platinum-based doublet as first-line therapy. Epidermal growth factor receptor (EGFR)–activating mutations, defined as an exon 19 deletion or exon 21 L858R mutation, are associated with benefit from EGFR tyrosine kinase inhibitors (TKIs).3,4 In the United States and Europe, approximately 10% to 15% of NSCLC with adenocarcinoma histologic type harbor an activating EGFR mutation.5,6,7 Phase 3 clinical trials compared erlotinib with platinum-based doublet in patients with advanced NSCLC with EGFR exon 19 or exon 21 L858R mutation and found that patients in the erlotinib arms experienced a statistically significant higher objective response rate (ORR) and longer progression-free survival (PFS).8,9 These phase 3 trials established erlotinib as a standard first-line therapy for patients with metastatic NSCLC with EGFR exon 19 and exon 21 L858R mutations. Additional phase 3 clinical trials comparing EGFR TKIs with chemotherapy demonstrated an improvement in ORR and PFS with the EGFR TKIs, and the improvement in PFS was considered clinically meaningful and sufficient for regulatory approval worldwide.8,9,10,11,12
The development of erlotinib as a first-line therapy was an important advance, but there was interest in improving the efficacy of erlotinib. The Cancer and Leukemia Group B trial 30406 was a randomized phase 2 clinical trial that investigated erlotinib alone or with carboplatin and paclitaxel in patients with a history of never or light smoking.13 The subset of patients with EGFR-mutant NSCLC in the chemotherapy plus erlotinib arm experienced a modest improvement in PFS; however, the addition of chemotherapy resulted in a higher rate of adverse events. Based on these results, we were interested in combining erlotinib with a biologic therapeutic agent rather than chemotherapy for the next clinical trial. A phase 1/2 clinical trial that investigated the combination of erlotinib and bevacizumab revealed activity and an acceptable rate of adverse events.14 A phase 3 clinical trial compared erlotinib alone with erlotinib plus bevacizumab in patients who had disease progression after platinum-based therapy. In that trial, patients were not enrolled based on EGFR mutation status. Overall survival (OS) did not differ between the 2 treatment arms in the study population. In a subset of analysis of 30 patients with EGFR-mutant NSCLC, patients in the combination arm experienced a longer PFS (hazard ratio [HR], 0.52; 95% CI, 0.19-1.38; median PFS, 17.1 vs 9.7 months) and OS (HR, 0.44; 95% CI. 0.11-1.67; P = .18; median OS, 20.2 vs not reached).15,16 Although these results were intriguing, they were not definitive owing to the small sample size and potential imbalances in prognostic factors and EGFR mutation subtype. Preclinical data indicated that the combination of EGFR and vascular endothelial growth factor inhibition could delay the development of resistance, overcome acquired resistance to EGFR TKI, and delay tumor growth in xenograft models.17,18,19 Thus, a retrospective subset analysis and preclinical data suggested a benefit to the combination of EGFR TKI and antiangiogenesis therapy in EGFR-mutant NSCLC.
Methods
Patients
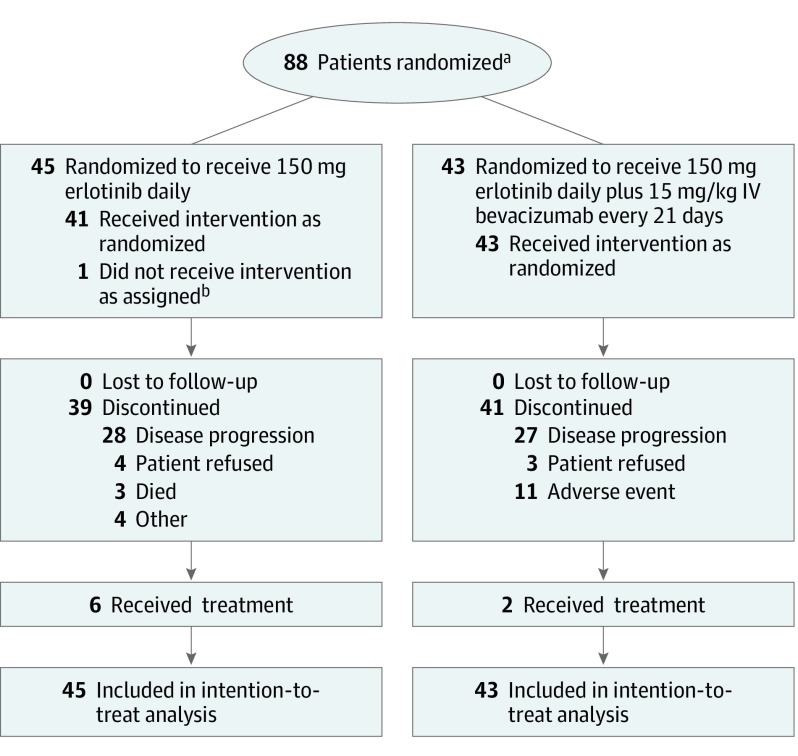
This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. Patients were required to have stage 4 NSCLC using the International Association for the Study of Lung Cancer 7th edition staging system (defined as contralateral pulmonary metastases, malignant pleural or pericardial effusion, or metastases to an extrathoracic organ), an EGFR exon 19 or exon L858R mutation confirmed at a Clinical Laboratory Improvement Amendment–certified laboratory, and nonsquamous histologic subtype.20 Between November 2, 2012, and August 22, 2016, 88 patients were enrolled (Figure 1) at 17 academic and community medical centers in the United States. Patients were required to have measurable disease according to Response Evaluation for Criteria for Solid Tumors (RECIST) 1.1, an Eastern Cooperative Oncology Group performance status of 0 or 1, adequate organ function, and proteinuria of less than 2+ urine dipstick or urine protein to creatinine ratio of 1.0 or lower.21 Patients could not have received systemic therapy for stage 4 disease. Patients with uncontrolled hypertension, history of bleeding diathesis, coagulopathy, or hemoptysis of grade 2 or higher (defined as bright red blood of at least 2.5 mL) were ineligible. Patients with treated brain metastases were eligible, which was consistent with standard practice with erlotinib and bevacizumab.22,23,24 Patients receiving anticoagulation therapy were initially ineligible, consistent with previous bevacizumab trials, although we amended the trial to permit enrollment of patients taking anticoagulation medication after additional safety data for patients receiving bevacizumab and anticoagulation became available.25,26 Data were analyzed on August 28, 2018, and included data from November 2, 2012, to August 20, 2018. The institutional review board at each center approved the protocol, and patients were required to provide written informed consent before any study tests or procedures. The trial protocol is provided in Supplement 1.
Figure 1. CONSORT Diagram.
aPrescreening data were not collected, and patients were registered only once they were determined to be eligible. Thus, only data for the registered and randomized patients were available.
bPatient received erlotinib plus bevacizumab rather than single-agent erlotinib.
Study Treatments and Assessments
Patients were randomized with equal allocation to 150 mg of oral erlotinib daily or 150 mg of oral erlotinib daily plus 15 mg of intravenous bevacizumab every 3 weeks. One cycle was 21 days, and patients underwent assessment for adverse events at each cycle using the National Cancer Institute Terminology Criteria for Adverse Events version 4.0.27 Erlotinib dosage reductions and parameters for withholding erlotinib were included in the protocol, as well as recommendations for supportive care for skin eruption, diarrhea, and keratitis. Bevacizumab dosage reductions were not permitted. Dosage delays for certain bevacizumab-associated adverse events were included in the protocol. If study therapy was delayed for more than 42 days, all study therapy was permanently discontinued. Patients underwent imaging to assess disease status every 2 cycles (6 weeks) for the first 18 months, and then every 4 cycles (12 weeks). Response was assessed using RECIST 1.1 by the investigator, and central radiology review was not performed. Patients continued therapy until disease progression, unacceptable adverse event, or withdrawal of consent.
Cell-Free DNA Analysis
Collection of a peripheral blood sample at baseline, at the time of disease assessment, and at the time of disease progression was included in the protocol. Exploratory analyses assessed the sensitivity of plasma DNA for the EGFR mutation at baseline and at the time of disease progression for the presence of the EGFR T790M mutation.28 The assay used droplet digital polymerase chain reaction tested for EGFR exon 19 deletion and exon 21 L858R mutation; if an EGFR mutation was detected, testing for the EGFR T790M mutation was performed.
Study Design and Statistical Analysis
The primary objective was to compare the PFS of the combination of erlotinib plus bevacizumab with erlotinib alone. Secondary objectives were the ORR, OS, adverse event rate, and PFS in the EGFR exon 19 deletion and exon 21 L858R subsets. The original sample size was 112 randomized patients. The study was originally designed to detect an improvement in PFS from 13 months with erlotinib alone to 18.6 months with the combination, for an HR of 0.70 with approximately 80% power using a stratified log-rank test at a 1-sided significance level of 0.20. When the results of a trial with a similar design became available,29 the study design was revised to reflect a better estimate of the PFS in the standard and the investigational arms. The amended sample size was 86 patients. The amended design had approximately 81% power to detect an improvement in median PFS from 10 months with erlotinib to 15 months with the combination, an HR of 0.667, at a 1-sided significance level of 0.20. This amendment was approved June 26, 2015, after 38 patients had been randomized. For the duration of the study, patients were randomized at a 1:1 ratio to the 2 arms using the Pocock and Simon dynamic allocation algorithm with the following stratification factors: sex (male vs female) and mutation (exon 19 vs exon 21 L858R).30
The Kaplan-Meier product limit estimator was used to derive median PFS and 12-month PFS rate and their 95% CIs.31 Comparisons of PFS between arms were conducted using a stratified log-rank test. Cox proportional hazards model was used to estimate the adjusted HRs with 95% CIs of the combination relative to erlotinib, adjusting for significant baseline prognostic factors.32 We evaluated the association of baseline prognostic factors with PFS and OS in a multivariable analysis. The association of treatments with binary end points, such as ORR, was evaluated by χ2 test. The ORR difference between treatments and its CI were calculated. All P values reported were 2-sided and no adjustment for multiple comparisons was performed. The sample size was justified to have adequate power for testing better PFS for the combination therapy over erlotinib alone at a 1-sided significance level of 0.20. We used a 2-sided significance level of 0.05 to declare statistical significance unless explicitly stated otherwise. Covariate balance between arms was assessed with descriptive P values from appropriate statistical tests. The statistical analysis was performed in SAS statistical software version 9.4 (SAS Inc).
Results
Between November 2, 2012, and August 22, 2016, 88 eligible patients enrolled and were included in the analysis (Figure 1). The median (range) age was 63 (31-84) years; 62 (70%) of the patients were female; 75 (85%) were white, 8 (9%) were African American, 3 (3%) were Asian, and for 2 (2%), data on race were not available. Forty-eight patients (55%) were never smokers, 45 patients (51%) were of Eastern Cooperative Oncology Group performance status 1, and 59 patients (67%) had an EGFR exon 19 deletion (Table 1). Median (range) follow-up at time of analysis was 33 (0.7-62.5) months, and 71 patients (81%) had experienced disease progression or death. Treatment with erlotinib plus bevacizumab compared with erlotinib did not result in a significant difference in PFS (HR, 0.81; 95% CI, 0.50-1.31; P = .39; median PFS 17.9 months vs 13.5 months; Figure 2). Two-sided P = .39, corresponding to a 1-sided P = .20, was equal to the 1-sided significance level of 0.20 that we used to determine the trial size based on the primary end point, PFS. The ORR was similar in the combination and erlotinib arms (35 patients [81%] in the combination arm vs 35 patients [83%] in the erlotinib arm; P = .81) (eTable 1 in Supplement 2). At the time of the OS analysis, 38 (43%) of patients had died. Treatment with the combination compared with erlotinib did not result in a significant difference in OS (HR, 1.41; 95% CI, 0.71-2.81; P = .33; median OS, 32.4 months in the combination arm vs 50.6 months in the erlotinib arm; Figure 2). Multivariable Cox proportional hazards modeling for PFS (eFigure in Supplement 2) revealed that female sex was associated with a better PFS (HR, 0.53; 95% CI, 0.29-0.94; P = .03). Multivariable Cox proportional hazards modeling for OS (eFigure in Supplement 2) revealed female sex (HR, 0.31; 95% CI, 0.14-0.69; P < .001) and EGFR exon 19 deletion mutation (HR, 34; 95% CI, 0.16-0.72; P < .001) were associated with better OS; baseline Eastern Cooperative Oncology Group performance status of 1 compared with 0 was associated with worse OS (HR, 2.58; 95% CI, 1.14-5.81; P = .02).
Table 1. Patient Characteristics.
| Characteristic | No. (%) | P Valuea | |
|---|---|---|---|
| Erlotinib (n = 45) | Erlotinib + Bevacizumab (n = 43) | ||
| Age, median (range), y | 63 (47-84) | 65 (31-84) | .58 |
| Sex | |||
| Male | 14 (31) | 12 (28) | .74 |
| Female | 31 (69) | 31 (72) | |
| Race | |||
| White | 39 (87) | 36 (84) | .82 |
| African American | 3 (7) | 5 (12) | |
| Asian | 2 (4) | 1 (2) | |
| Not availableb | 1 (2) | 1 (2) | |
| ECOG performance status | |||
| 0 | 19 (42) | 24 (56) | .20 |
| 1 | 26 (58) | 19 (44) | |
| EGFR exon mutation | |||
| Exon 19 deletion | 30 (67) | 29 (67) | .94 |
| Exon 21 L858R | 15 (33) | 14 (33) | |
| Smoking statusc | |||
| Never | 23(51) | 25 (60) | .37 |
| Light | 4 (9) | 3 (7) | |
| Former | 15 (33) | 14 (33) | |
| Current | 3 (7) | 0 | |
| Missing | 0 | 1 | |
| Weight loss in previous 3 mo, % of body weight | |||
| <10 | 40 (91) | 40 (98) | .19 |
| ≥10 | 4 (9) | 1 (2) | |
| Missing | 1 | 2 | |
| Stage | |||
| M1a | 15 (38) | 19 (49) | .36 |
| M1b | 24 (62) | 20 (51) | |
| Missing/recurrenced | 6 | 4 | |
| Brain metastasese | |||
| Yes | 14 (31) | 11 (26) | .57 |
| No | 31 (69) | 32 (74) | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group, EGFR: epidermal growth factor receptor.
Equal variance 2-sample t test used for age and χ2test for other comparisons.
Includes not reported, patient refused, not available.
Never smoking history defined as less than 100 cigarettes in lifetime, light smoker defined as fewer than 10 pack-years and quit at least 15 years ago), former smoker defined as more than 10 pack-years and quit more than 15 years ago), current defined as using tobacco at the time of study enrollment.
Only the patients with M1a or M1b were included in the percentages and the P value. 3 patients in each arm were labeled recurrence. Data are missing from 3 patients in the erlotinib arm and 1 patient in the erlotinib plus bevacizumab arm.
Brain metastases at the time of study enrollment.
Figure 2. Progression-Free and Overall Survival in 88 Patients With Non–Small Cell Lung Cancer Treated With Erlotinib vs Erlotinib Plus Bevacizumab.
We assessed the therapies patients received after discontinuing the study therapy. The poststudy therapies were not specified in the protocol and were at the discretion of the treating physician. In the combination arm, which comprised 43 patients, data were available 43 patients 23 (54%), and in the erlotinib arm, which comprised 45 patients, data were available for 21 (47%) (eTable 2 in Supplement 2). Osimertinib, which became available as a second-line therapy while the study was ongoing, was a subsequent therapy for 10 (23%) patients in the combination arm and 13 (29%) patients in the erlotinib arm, and 1 patient in the erlotinib arm received PF-06747775 (a third-generation EGFR TKI).
The median (range) number of cycles of treatment for patients was 21 (3-55) in the combination arm and 15 (1-87) in the erlotinib arm. The most common reason for treatment discontinuation was disease progression in both arms (Figure 1). Dosage modification (defined as dosage reduction of erlotinib or dose omission of erlotinib or bevacizumab) was common in both arms: 39 of 43 (91%) patients in the combination arm and 34 of 45 patients (76%) in the erlotinib arm had a dosage modification. In the combination arm, 24 patients (56%) and in the erlotinib arm, 19 of 45 patients (42%) had a dosage reduction or omission of erlotinib (P = .20). Of the 43 patients in the combination arm, 11 (26%) discontinued owing to an adverse event. The reasons for treatment-related adverse event–associated treatment discontinuation were skin eruption in 3 patients (7%), hypertension in 3 patients (7%), proteinuria in 2 patients (5%), diarrhea in 1 patient (2%), and pulmonary hemorrhage in 1 patient (2%). The grade 3 or greater adverse events, regardless of attribution, that occurred in 3 or more patients in either arm are presented in Table 2. Adverse events of grade 3 or higher observed in 3 or more patients in the combination and erlotinib arms were skin eruption in 11 (26%) vs 7 (16%) patients, diarrhea in 4 (9%) vs 6 (13%) patients, hypertension in 17 (40%) vs 9 (20%) patients, and proteinuria in 5 (12%) vs 0 (0%) patients. Three grade 5 adverse events were observed in the erlotinib arm (1 thromboembolic event and 2 sudden deaths without further specification), although none was attributed to the study therapy by the study investigator. No grade 5 adverse events were observed in the erlotinib plus bevacizumab arm.
Table 2. Grade 3 or Higher Adverse Events Occurring in 3 or More Patients in Either Treatment Arma.
| Adverse Event, No. (%) | Erlotinib | Erlotinib + Bevacizumab | ||
|---|---|---|---|---|
| Grade 3 | Grade 4/5 | Grade 3 | Grade 4/5 | |
| Abdominal pain | 1 (2) | 0 | 3 (7) | 0 |
| Acneiform rash | 7 (16) | 0 | 11 (26) | 0 |
| Diarrhea | 6 (13) | 0 | 4 (9) | 0 |
| Dyspnea | 0 | 0 | 3 (7) | 0 |
| Fatigue | 1 (2) | 0 | 3 (7) | 0 |
| Hypertension | 9 (20) | 0 | 17 (40) | 0 |
| Proteinuria | 0 | 0 | 5 (12) | 0 |
Grading based on National Cancer Institute Terminology Criteria for Adverse Events version 4.0.21
Cell-free DNA blood samples at baseline and at time of disease progression were available for 36 of the 71 patients (51%) with progressive disease. The canonical EGFR mutation was detected in 12 of the 36 samples and the resistance EGFR exon 20 T790M mutation was detected in 5 of the 12 cell-free DNA blood samples at the time of disease progression.
Discussion
Our trial did not meet the primary end point of demonstrating a significant improvement in PFS with the combination of erlotinib and bevacizumab. Additional studies that have investigated this combination in EGFR-mutant NSCLC include a single-arm phase 2 trial by Rosell and colleagues (BELIEF), a randomized phase 2 trial by Seto and colleagues (JO25567), and a randomized phase 3 trial by Furuya and colleagues (NEJ026) (eTable 3 in Supplement 2).29,33,34,35 Although cross-trial comparison is hazardous, the median PFS observed with the combination in our study is similar to the median PFS observed in the previous randomized trials and it is longer than the median PFS trial in the single-arm phase 2 trial by Rosell and colleagues (13.2 months). Thus, the performance of the combination in our trial was similar to contemporary trials. The median PFS observed in the erlotinib arm in our study is similar to the median PFS observed with erlotinib by independent radiologic review (13.3 months) in the recent phase 3 trial.35
One consideration is that our trial used investigator assessment of response and disease progression, whereas previous randomized trials used blinded independent radiologic review.29,35 Variation in the assessment or investigator bias may have influenced our results in regard to ORR and PFS, but it is unknown whether these factors would have affected one arm disproportionately. In the phase 3 trial, investigator and independent radiologic review assessment of PFS were consistent.35
Limitations
The OS data should be interpreted cautiously given the small number of events, and a subset of patients with durable benefit from erlotinib may be contributing to the OS results. An analysis of OS of the Japanese randomized phase 2 trial revealed similar OS with the combination and erlotinib (HR, 0.81; 95% CI, 0.53-1.23; P = .33; median, 47.0 months for combination therapy vs 47.4 months for erlotinib), and the median OS observed in the erlotinib arm is to the findings of our trial.34 We investigated the receipt of subsequent therapies as a potential factor in the OS results. The receipt of subsequent therapies was similar in the 2 arms, and the rate of second-line osimertinib was similar in both arms. The receipt of second-line osimertinib is similar to the receipt of second-line osimertinib observed in the phase 3 trial of osimertinib compared with first-generation EGFR TKIs.36 The rate of subsequent immunotherapy was low, and the modest activity of immunotherapy in EGFR mutant NSCLC make it unlikely an imbalance in subsequent immunotherapy is a confounding factor. We did not have efficacy data on the subsequent therapies, and some patients received multiple subsequent therapies, which made it difficult to assess the influence of poststudy therapy on the OS results.
The limited number of circulating free DNA blood samples collected at baseline and at time of disease progression severely limits the utility of this analysis. It is possible that some patients experienced disease progression at a time other than scheduled sample collection (eg, unscheduled clinic or emergency department visit), but suboptimal collection of the study samples was a contributing factor as well. The circulating free DNA analysis detected the original EGFR mutation in 12 of 36 samples, and this analysis is inconclusive owing to the small sample size.
The single-arm phase 2 trial by Rosell and colleagues investigated the outcomes of patients with pretreatment T790M mutation using a peptide nucleic acid polymerase chain reaction testing. This assay identified a pretreatment T790M mutation in approximately one-third of patients.33 This study found a longer PFS in the patients with a T790M mutation detected in pretreatment specimen. This observation has not been validated in other studies. None of the patients enrolled in this trial had a known T790M mutation, and we were unable to assess differential efficacy based on baseline T790M status.
Since this trial was initiated, the treatment landscape for EGFR-mutant NSCLC has changed. A recent phase 3 trial revealed superior PFS with osimertinib compared with first-generation EGFR TKIs (erlotinib or gefitinib), and osimertinib has become the preferred first-line therapy.36 Consequently, there is interest in combining osimertinib with bevacizumab as first-line therapy. A single arm phase 1/2 clinical trial of osimertinib and bevacizumab (NCT02803203) and a randomized phase 2 clinical trial of osimertinib alone and with bevacizumab (NCT03133546) are ongoing. Given the longer PFS observed with osimertinib, the combination treatment would likely lead to a longer duration of bevacizumab therapy. In our study in the combination arm, disease progression was the most common reason for treatment discontinuation, followed by adverse events. In our study and other studies, approximately 20% to 40% of patients had to discontinue bevacizumab because of adverse events.29,33,35 With improved first-line EGFR TKIs, the frequency of treatment discontinuation owing to disease progression should be reduced, and it will be interesting to observe whether bevacizumab is tolerable and efficacious in combination with osimertinib.
Recently, a phase 3 clinical trial compared carboplatin, paclitaxel, and bevacizumab alone with carboplatin, paclitaxel, bevacizumab, and atezolizumab, an antibody against programmed cell death-ligand 1, in patients with stage 4 NSCLC with nonsquamous histologic subtype.37 Patients with EGFR-mutant NSCLC with progressive disease while taking an EGFR TKI were enrolled, and in a retrospective subset analysis, the 4-drug combination resulted in a higher ORR and longer PFS. This subset analysis should be interpreted cautiously; approval by the United Sates Food and Drug Administration does not include patients with EGFR-mutant NSCLC.
Conclusions
Our study, unlike previous randomized clinical trials, did not reveal a significant improvement in PFS with the combination of erlotinib and bevacizumab. Future studies will investigate novel osimertinib combinations and molecular markers to identify patients most likely to experience disease progression with single-agent EGFR TKIs.
Trial Protocol.
eTable 1. Best Response to Study Therapy
eTable 2. Post-Study Therapy
eTable 3. Prospective Phase 2 or 3 Trials of Erlotinib and Bevacizumab in EGFR mutant NSCLC
eFigure 1. Cox Proportional Hazard Modeling for Progression-Free Survival and Overall Survival
Data Sharing Statement.
References
- 1.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539-4544. doi: 10.1200/JCO.2005.04.4859 [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute Surveillance, Epidemiology, and Ends Results Program. Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed March 4, 2019.
- 3.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497-1500. doi: 10.1126/science.1099314 [DOI] [PubMed] [Google Scholar]
- 4.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129-2139. doi: 10.1056/NEJMoa040938 [DOI] [PubMed] [Google Scholar]
- 5.Rosell R, Moran T, Queralt C, et al. ; Spanish Lung Cancer Group . Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958-967. doi: 10.1056/NEJMoa0904554 [DOI] [PubMed] [Google Scholar]
- 6.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998-2006. doi: 10.1001/jama.2014.3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barlesi F, Mazieres J, Merlio JP, et al. ; Biomarkers France contributors . Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet. 2016;387(10026):1415-1426. doi: 10.1016/S0140-6736(16)00004-0 [DOI] [PubMed] [Google Scholar]
- 8.Rosell R, Carcereny E, Gervais R, et al. ; Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica . Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi: 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi: 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 10.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi: 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 11.Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141-151. doi: 10.1016/S1470-2045(14)71173-8 [DOI] [PubMed] [Google Scholar]
- 12.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327-3334. doi: 10.1200/JCO.2012.44.2806 [DOI] [PubMed] [Google Scholar]
- 13.Jänne PA, Wang X, Socinski MA, et al. Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 trial. J Clin Oncol. 2012;30(17):2063-2069. doi: 10.1200/JCO.2011.40.1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbst RS, Johnson DH, Mininberg E, et al. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23(11):2544-2555. doi: 10.1200/JCO.2005.02.477 [DOI] [PubMed] [Google Scholar]
- 15.Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet. 2011;377(9780):1846-1854. doi: 10.1016/S0140-6736(11)60545-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbst R, Stern H, Amler L, et al. Biomarker evaluation in the phase III, placebo (P)-controlled, randomized BeTa trial of bevacizumab (B) and erlotinib (E) for patients (pts) with advanced non-small cell lung cancer (NSCLC) after failure of standard 1st-line chemotherapy: correlation with treatment outcomes[abstract LB-131]. Cancer Res. 2009;69(9)(suppl):LB-131-LB-131. [Google Scholar]
- 17.Ichihara E, Ohashi K, Takigawa N, et al. Effects of vandetanib on lung adenocarcinoma cells harboring epidermal growth factor receptor T790M mutation in vivo. Cancer Res. 2009;69(12):5091-5098. doi: 10.1158/0008-5472.CAN-08-4204 [DOI] [PubMed] [Google Scholar]
- 18.Naumov GN, Nilsson MB, Cascone T, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res. 2009;15(10):3484-3494. doi: 10.1158/1078-0432.CCR-08-2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Takayama K, Wang S, et al. Addition of bevacizumab enhances antitumor activity of erlotinib against non-small cell lung cancer xenografts depending on VEGF expression. Cancer Chemother Pharmacol. 2014;74(6):1297-1305. doi: 10.1007/s00280-014-2610-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstraw P, Crowley J, Chansky K, et al. ; International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions . The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of Malignant Tumours. J Thorac Oncol. 2007;2(8):706-714. doi: 10.1097/JTO.0b013e31812f3c1a [DOI] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 22.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542-2550. doi: 10.1056/NEJMoa061884 [DOI] [PubMed] [Google Scholar]
- 23.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. ; National Cancer Institute of Canada Clinical Trials Group . Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123-132. doi: 10.1056/NEJMoa050753 [DOI] [PubMed] [Google Scholar]
- 24.Socinski MA, Langer CJ, Huang JE, et al. Safety of bevacizumab in patients with non-small-cell lung cancer and brain metastases. J Clin Oncol. 2009;27(31):5255-5261. doi: 10.1200/JCO.2009.22.0616 [DOI] [PubMed] [Google Scholar]
- 25.Leighl NB, Bennouna J, Yi J, Moore N, Hambleton J, Hurwitz H. Bleeding events in bevacizumab-treated cancer patients who received full-dose anticoagulation and remained on study. Br J Cancer. 2011;104(3):413-418. doi: 10.1038/sj.bjc.6606074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crinò L, Dansin E, Garrido P, et al. Safety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 study. Lancet Oncol. 2010;11(8):733-740. doi: 10.1016/S1470-2045(10)70151-0 [DOI] [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf. Revised May 2009. Accessed March 5, 2019.
- 28.Oxnard GR, Paweletz CP, Kuang Y, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014;20(6):1698-1705. doi: 10.1158/1078-0432.CCR-13-2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15(11):1236-1244. doi: 10.1016/S1470-2045(14)70381-X [DOI] [PubMed] [Google Scholar]
- 30.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103-115. doi: 10.2307/2529712 [DOI] [PubMed] [Google Scholar]
- 31.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(22):457-481. doi: 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 32.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34(2):187-220. [Google Scholar]
- 33.Rosell R, Dafni U, Felip E, et al. ; BELIEF collaborative group . Erlotinib and bevacizumab in patients with advanced non-small-cell lung cancer and activating EGFR mutations (BELIEF): an international, multicentre, single-arm, phase 2 trial. Lancet Respir Med. 2017;5(5):435-444. doi: 10.1016/S2213-2600(17)30129-7 [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto N, Seto T, Nishio M, et al. Erlotinib plus bevacizumab (EB) versus erlotinib alone (E) as first-line treatment for advanced EGFR mutation–positive non-squamous non–small-cell lung cancer (NSCLC): survival follow-up results of JO25567. J Clin Oncol. 2018;36(15_suppl):9007-9007. [DOI] [PubMed] [Google Scholar]
- 35.Furuya N, Fukuhara T, Saito H, et al. Phase III study comparing bevacizumab plus erlotinib to erlotinib in patients with untreated NSCLC harboring activating EGFR mutations: NEJ026. J Clin Oncol. 2018;36(15_suppl):9006-9006. [Google Scholar]
- 36.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113-125.doi: 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 37.Socinski MA, Jotte RM, Cappuzzo F, et al. ; IMpower150 Study Group . Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301. doi: 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eTable 1. Best Response to Study Therapy
eTable 2. Post-Study Therapy
eTable 3. Prospective Phase 2 or 3 Trials of Erlotinib and Bevacizumab in EGFR mutant NSCLC
eFigure 1. Cox Proportional Hazard Modeling for Progression-Free Survival and Overall Survival
Data Sharing Statement.