Key Points
Question
Are chronic active multiple sclerosis (MS) lesions linked to patient disability and poor long-term lesion outcomes?
Findings
In this in vivo cohort study, the association was shown of chronic active/slowly expanding/smoldering MS lesions, which are detectable on high-field susceptibility-based magnetic resonance imaging (MRI) by their characteristic paramagnetic rims, with aggressive disease course and poor clinical outcomes despite approved disease-modifying therapy. Over time, rim lesions do not shrink slowly as other lesions do, but typically remain stable or even enlarge due to ongoing demyelination (confirmed pathologically in one of the patients who later came to autopsy).
Meaning
These data provide in vivo evidence that inflammation in chronic active plaques is a prominent feature of MS that is linked to disability accumulation, suggesting a path forward for new susceptibility-based MRI clinical trials to test new types of treatment to ameliorate this process.
Abstract
Importance
In multiple sclerosis (MS), chronic active lesions, which previously could only be detected at autopsy, can now be identified on susceptibility-based magnetic resonance imaging (MRI) in vivo as non–gadolinium-enhancing lesions with paramagnetic rims. Pathologically, they feature smoldering inflammatory demyelination at the edge, remyelination failure, and axonal degeneration. To our knowledge, the prospect of long-term in vivo monitoring makes it possible for the first time to determine their contribution to disability and value as a treatment target.
Objective
To assess whether rim lesions are associated with patient disability and long-term lesion outcomes.
Design, Setting, Participants
We performed 3 studies at the National Institutes of Health Clinical Center: (1) a prospective clinical/radiological cohort of 209 patients with MS (diagnosis according to the 2010 McDonald revised MS criteria, age ≥18 years, with 7-T or 3-T susceptibility-based brain MRI results) who were enrolled from January 2012 to March 2018 (of 209, 17 patients [8%] were excluded because of uninterpretable MRI scans); (2) a radiological/pathological analysis of expanding lesions featuring rims; and (3) a retrospective longitudinal radiological study assessing long-term lesion evolution in 23 patients with MS with yearly MRI scans for 10 years or more (earliest scan, 1992).
Main Outcomes and Measures
(1) Identification of chronic rim lesions on 7-T or 3-T susceptibility-based brain MRI in 192 patients with MS and the association of rim counts with clinical disability (primary analysis) and brain volume changes (exploratory analysis). (2) Pathological characterization of 10 expanding lesions from an adult with progressive MS who came to autopsy after 7 years of receiving serial in vivo MRI scans. (3) Evaluation of annual lesion volume change (primary analysis) and T1 times (exploratory analysis) in 27 rim lesions vs 27 rimless lesions.
Results
Of 209 participants, 104 (50%) were women and 32 (15%) were African American. One hundred seventeen patients (56%) had at least 1 rim lesion regardless of prior or ongoing treatment. Further, 84 patients (40%) had no rims (mean [SD] age, 47 [14] years), 66 (32%) had 1 to 3 rims (mean [SD] age, 47 [11] years), and 42 (20%) had 4 rims or more (mean [SD] age, 44 [11] years). Individuals with 4 rim lesions or more reached motor and cognitive disability at an earlier age. Normalized volumes of brain, white matter, and basal ganglia were lower in those with rim lesions. Whereas rimless lesions shrank over time (−3.6%/year), rim lesions were stable in size or expanded (2.2%/year; P < .001). Rim lesions had longer T1 times, suggesting more tissue destruction, than rimless lesions. On histopathological analysis, all 10 rim lesions that expanded in vivo had chronic active inflammation.
Conclusions and Relevance
Chronic active lesions are common, are associated with more aggressive disease, exert ongoing tissue damage, and occur even in individuals treated with effective disease-modifying therapies. These results prompt the planning of MRI-based clinical trials aimed at treating perilesional chronic inflammation in MS.
This cohort study examines association between chronic active lesions and clinical outcomes in US patients with multiple sclerosis.
Introduction
Multiple sclerosis (MS) is a chronic neuroinflammatory and neurodegenerative disease.1 Despite the availability of multiple treatment options, many patients live with disability or experience progressive symptoms that limit their quality of life and longevity. Even if pathobiological causes of disability accrual and disease progression are not completely understood, individual lesion-type patterns might account for clinical and prognostic variability.2,3,4 After the acute phase of inflammatory demyelination at lesion onset, chronic lesions can show different pathological and repair outcomes, namely “chronic active/slowly expanding/smoldering,” “chronic inactive,” and, when repair is successful, “remyelinated.”5,6 As personalized treatment paradigms emerge, imaging the distribution of different lesion types within individuals may prove critical.
Among different pathological lesion types, chronic active/slowly expanding/smoldering lesions (termed chronic active) are of special clinical and biological interest for their accumulation of microglia and/or macrophages at the lesion edge, subtle opening of the blood-brain barrier, and repair/remyelination failure with axonal loss.2,4,6,7,8,9 In 2 large pathological studies,2,4 chronic active lesions accounted for approximately 30% of all analyzed lesions and prevailed in patients with higher disability. Several recent magnetic resonance imaging (MRI) pathological studies8,9,10,11,12,13 reported that chronic active lesions, which previously could only be detected at autopsy, can be identified on 7-T8,9,10,11,12,13,14,15,16,17,18 and 3-T19 susceptibility-based MRI in vivo as non–gadolinium-enhancing chronic lesions with a paramagnetic rim. A recent 3-T MRI and carbon 11–labeled-PK11195 positron emission tomography (PET) study also confirmed that rim lesions show an uptake of PET tracers sensitive to the presence of activated microglia/macrophages.20 In in vivo MRI studies with limited follow-up duration, paramagnetic rims tend to persist, suggesting steady chronic inflammation.8,9,15 However, the long-term effects of rim persistence in vivo and whether rim lesions are modified by existing disease-modifying treatments are still uncertain.
In this article, we address 2 questions: (1) do patients with MS with multiple chronic active lesions have a more aggressive disease course that engenders earlier disability? and (2) do chronic rim lesions expand over time? We assessed whether rim lesions are associated with patient disability in a clinical cohort analysis of 192 patients with MS. One patient from the cohort came to autopsy after a 7-year longitudinal in vivo MRI characterization showing expanding lesions featuring a paramagnetic rim. This motivated us to explore more in-depth long-term lesion outcomes (rim vs rimless lesions) for the patients in the cohort who had received yearly MRI scans for 10 years or longer.
Methods
Clinical Cohort
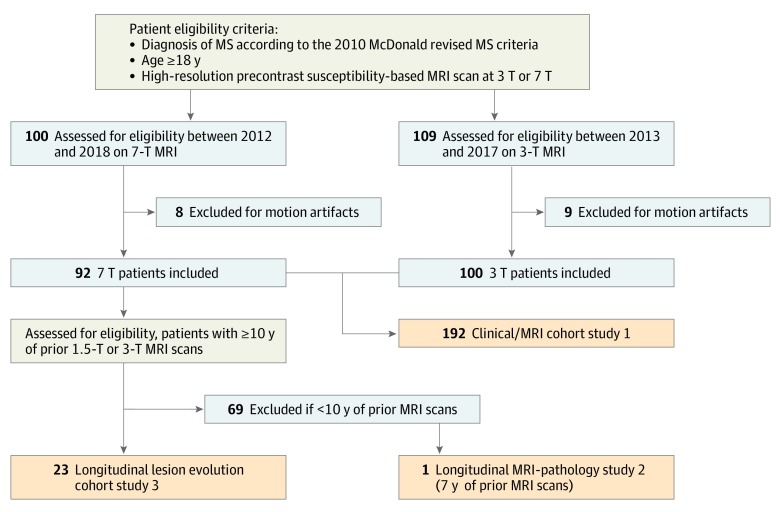
Between January 1, 2012, and March 30, 2018, imaging, laboratory, and clinical data were collected with institutional review board approval from the National Institutes of Health and after patients provided written informed consent. Inclusion criteria were the following: being 18 years or older, diagnosis of MS according to the 2010 McDonald revised MS criteria,21 and availability of a precontrast high-resolution susceptibility-based sequence brain MRI scan at 7T or 3T.19 Experienced MS clinicians collected the clinical history and determined motor and cognitive disability according to the Expanded Disability Status Scale (EDSS),22 MS Severity Scale (MSSS),23 Paced Auditory Symbol Addition Test (PASAT, 3-second version), and Symbol Digit Modality Test (SDMT, paper-based) scores. The Consolidated Standards of Reporting Trials chart is provided in Figure 1.
Figure 1. Consolidated Standards of Reporting Trials Chart.
MRI indicates magnetic resonance imaging; MS, multiple sclerosis.
Susceptibility-based brain MRI sequences were obtained on T2* scanners:
On a Siemens Magnetom 7-T MRI scanner equipped with a birdcage-type transmit coil and a 32-channel receive coil, a precontrast 2-dimensional (D) high-resolution gradient dual-echo sequence providing T2*-weighted (T2*) and phase contrasts (repetition time [TR], 1300 milliseconds; echo time [TE], 15 and 32 milliseconds; 29 axial slices; flip angle [FA], 50°; acquisition time [AT], 8:36; in-plane resolution, 0.2 × 0.2 mm; slice thickness, 1 mm; voxel volume, 0.04 µL); 3 minimally overlapping slabs were acquired to cover most of the supratentorial brain.
On a Siemens Skyra 3-T scanner equipped with a body transmit coil and a 32-channel receive coil, a precontrast whole-brain 3-D segmented echo-planar imaging sequence24 providing T2* and phase contrasts (TR, 64 milliseconds; TE, 35 milliseconds; FA, 10°; echo-train length, 15; AT, 5:46; 256 sagittal slices; 0.65-mm isotropic voxels; voxel volume, 0.27 µL).
The MRI sequences at both magnetic field strengths are capable of detecting lesions with paramagnetic rims in vivo.19 Additional standard MRI sequences, including postcontrast 3-D T2 fluid-attenuated inversion recovery at 3-T as well as precontrast and postcontrast 3-D T1-weighted at 3T and 7T were also acquired.25
In each individual, the number of supratentorial chronic nonenhancing lesions with paramagnetic rims on unwrapped phase images and gadolinium-enhancing lesions were determined by consensus of 2 raters. A chronic lesion was defined as “rim-positive” when it showed a hypointense rim on phase images and internal isointensity to extralesional white matter.12 Infratentorial lesions were excluded a priori from the rim analysis because of the presence of susceptibility artifacts in that region. Brain structures were segmented with Lesion TOADS software.26 Volumes of supratentorial white matter lesions, brain, white matter, cerebral cortex, basal ganglia, and cerebrospinal fluid (the last 5 normalized to the intracranial volume) were recorded.
MRI-Pathology of Rim Lesions That Slowly Expanded In Vivo
One case from the 7-T clinical cohort, a man in his late 50s with clinically progressive MS (disease duration, 21 years; EDSS score, 6.5; treated with interferon beta-1a between ages 49–51 years) came to autopsy after 7 years of undergoing serial in vivo MRI characterization. His brain was attained at autopsy after consent was provided from the next of kin. Neuropathological evaluation results of the formalin-fixed brain focused on 10 lesions (5 frontal, 3 parietal, 2 occipital) with paramagnetic rims seen on in vivo and postmortem 7-T MRI (eMethods in the Supplement). The MRI-matched histological sections were achieved via an MRI-designed, 3-D–printed cutting box.27 Sections (5-10 μm thick) were stained with Luxol fast blue/periodic acid Schiff (LFB-PAS myelin), Bielschowsky (axons), and diaminobenzidine (DAB)-enhanced Turnbull (iron) methods. Immunostains included myelin proteolipid protein, cluster of differentiation 68 (CD68; mononuclear phagocytes), transmembrane protein 119 (TMEM119; microglia), myeloid-related protein 14 (MRP 14; early activated peripheral macrophages), CD8 (T lymphocytes), glial fibrillary acid proteins (GFAP; reactive astrocytes), fibrinogen, and SMI32 nonphosphorylated neurofilaments. Selected slides were double stained for CD68/TMEM119, DAB-Turnbull/CD68, and DAB-Turnbull/GFAP. For all primary antibodies, dilution, source, and catalog numbers are provided in the eMethods in the Supplement. Axonal density was quantified using Bielschowsky staining and optical density (ImageJ/Fiji software; https://imagej.nih.gov) in 3 randomly taken regions of interest within the lesion core and 3 within the surrounding white matter.
Longitudinal Lesion Evolution
Of the patients who underwent 7-T MRI, 23 (11%) had received archival yearly 1.5-T or 3-T MRI scans for 10 years or more (eTable 1 in the Supplement). On 7-T susceptibility-based images, 27 discrete supratentorial chronic lesions with a rim and 27 comparable lesions without a rim were selected for a retrospective yearly evaluation of lesion volume (mean [SD] lesion follow-up, 14 [5] years). When possible, at least 1 lesion with a rim and 1 without a rim were selected for each individual (eTable 1 in the Supplement) to control within-participant variability. To maintain contrast consistency over time,28 unregistered, unnormalized proton density images were implemented for yearly lesion volume analysis using Jim, version 7.0 software (Xinapse Systems), allowing lesion volume correction as previously described.29 Finally, for each lesion, T1 times at 7T were determined (eMethods in the Supplement).
Statistical Analysis
Based on preliminary observations, we arbitrarily classified individuals in the clinical cohort into 3 groups according to the number (0, 1–3, and ≥4) of chronic lesions with paramagnetic rims. To examine the association of demographic and clinical variables with rim lesion group, statistical tests were determined based on variable type and corrected for multiple comparisons (eMethods in the Supplement). The primary analysis tested the association between rims and disability scores; an exploratory analysis included rim associations with other clinical and MRI variables. A forward stepwise regression was used to identify a set of independent variables (predictors) associated with log-transformed EDSS scores, using P < .10 for entry and retention in the model; model residuals had a normal distribution.
For the longitudinal lesion evolution analysis, the primary analysis tested whether there was a significant difference between lesions with and without rims in the estimated slope of adjusted log-volume change over time. We also calculated the compound annual growth rate (CAGR) of adjusted log-lesion volumes. Because there was no clear evidence for a bimodal distribution, we arbitrarily classified lesions as shrinking (CAGR, ≤−1%), steady (−1% to 1%), or expanding (≥1%). Statistical comparisons used the Mann-Whitney U test, analysis of variance (ANOVA) with correction for multiple comparisons, and linear mixed models as appropriate. Multiple lesions from the same individual were treated as independent because the effect on the participant was not significant (logistic regression model). Details are provided in the eMethods in the Supplement. Statistical analyses used Prism, version 7.0 (GraphPad), and SAS, version 9.3 (SAS Institute), and statistical significance was set at P < .05.
Results
Clinical Cohort: Association of the Presence of Rim Lesions With Worse Clinical and MRI Outcome Measures
Of 209 consecutive eligible patients with MS, data from 192 (92%) were included in this article (17 [8%] were excluded for motion-associated MRI artifacts; Figure 1). Population demographic and clinical characteristics are described in the Table and eTable 2 in the Supplement (for the 3-T and 7-T cohorts, respectively).
Table. Cohort Characteristics of 192 Patients With Multiple Sclerosis in the Cross-Sectional Cohort.
| Rim Category | No Detected Rims | 1-3 Rims | ≥4 Rims | Statistical Analysisa |
|---|---|---|---|---|
| Demographic and Clinical Data | ||||
| No. (%) | 84 (44) | 66 (34) | 42 (22) | NA |
| Clinical phenotype, No. (%) | ||||
| CIS/RR | 61 (73) | 46 (70) | 24 (57) | Fisher 2 × 3 P = .20, NS |
| SP | 16 (19) | 14 (21) | 10 (24) | |
| PP | 7 (8) | 6 (9) | 8 (19) | |
| Sex, Female, No. (%) | 59 (70) | 45 (68) | 28 (67) | Fisher 2 × 3 P = .90, NS |
| Age, mean (SD), y | 47.3 (14.5) | 47.2 (11.4) | 44.3 (11.1) | ANOVA P = .40, NS |
| Disease duration, mean (SD), y | 13.4 (12.5) | 12.9 (9.9) | 12.2 (8.3) | ANOVA P = .80, NS |
| Patients never treated, No. (%) | 27/84 (32) | 11/66 (17) | 5/42 (12) | Fisher 2 × 3 P = .01 |
| African American, No. (%) | 10 (12) | 12 (18) | 10 (24) | Fisher 2 × 3 P = .20, NS |
| HLA-DRB1*15:01, No. (%) | 29/64 (45) | 15/54 (28) | 13/33 (41) | Fisher 2 × 3 P = .10, NS |
| EDSS score, median (range) | 1.5 (0-7.5)* | 2 (0-8)& | 3 (1-7.5)*& | ANOVA P = .002 |
| MSSS score, mean (SD) | 3.0 (2.5)* | 3.4 (2.5)& | 4.9 (2.5)*& | ANOVA P < .001 |
| PASAT score, mean (SD) | 49.9 (8.6)* | 48.4 (9.9) | 44.6 (11.9)* | ANOVA P = .03 |
| SDMT score, mean (SD) | 53.4 (12.3)* | 48.3 (13.4) | 43.7 (17.8)* | ANOVA P = .001 |
| MRI Data | ||||
| Normalized brain volume, mean (SD) | 0.699 (0.031) | 0.686 (0.032) | 0.683 (0.056) | ANOVA P = .04 |
| Normalized cortical volume, mean (SD) | 0.352 (0.030) | 0.349 (0.028) | 0.354 (0.033) | ANOVA P = .70, NS |
| Normalized WM volume, mean (SD) | 0.274*$ (0.020) | 0.265$ (0.023) | 0.258* (0.020) | ANOVA P = .001 |
| Total lesion volume, mean (SD), mL | 5.7*$ (6.7) | 10.0&$ (8.4) | 17.1*& (10.4) | ANOVA P < .001 |
| No. of rim lesions, median (range) | 0 | 2 (1-3) | 6 (4-16) | NA |
| Rim lesion volume, mean (SD), ml | NA | 2.70 (4.10) | 6.19 (5.86) | NA |
| Rim/total lesion volume, % | NA | 25.7 | 36.5 | NA |
| Normalized thalamic volume, mean (SD) | 0.0129*$ (0.0033) | 0.0091$ (0.0025) | 0.0080* (0.0014) | ANOVA P < .001 |
| Normalized caudate volume, mean (SD) | 0.0062*$ (0.0018) | 0.0041$ (0.0012) | 0.0036* (0.0019) | ANOVA P <.0001 |
| Normalized putamen volume, mean (SD) | 0.0091*$ (0.0019) | 0.0071$ (0.0015) | 0.0068* (0.0012) | ANOVA P < .001 |
| Normalized ventricular CSF volume, mean (SD) | 0.0196* (0.0095) | 0.0234 (0.0101) | 0.0259* (0.0123) | ANOVA P = .001 |
| Normalized sulcal CSF volume, mean (SD) | 0.2812 (0.0243) | 0.2907 (0.0267) | 0.2924 (0.0529) | ANOVA P = .10, NS |
| Patients with ≥1 Gad+ lesions, No. (%) | 17/84 (20) | 12/66 (18) | 6/42 (14) | Fisher 2 × 3 P = .70, NS |
Abbreviations: ANOVA, analysis of variance; CIS, clinically isolated syndrome; CSF, cerebrospinal fluid; EDSS, Expanded Disability Status Scale; Gad, gadolinium; GM, gray matter; HLA, human leukocyte antigen; MRI, magnetic resonance imaging; MSSS, Multiple Sclerosis Severity Score; NA, not applicable; NS, not significant; PASAT, Paced Auditory Serial Addition Test; PP, primary progressive; RR, relapsing-remitting; SDMT, Symbol Digit Modalities Test; SP, secondary progressive; WM, supratentorial white matter.
Statistical significance at the P < .05 level in the Bonferroni-corrected post hoc analysis was referred with the following symbols: * for the comparison no rim vs 4 or more rims group; $ for the comparison no rim vs 1 to 3 rims group; and & for the comparison 1 to 3 rims vs 4 or more rims group. Specific treatments are shown in eFigure 1 in the Supplement.
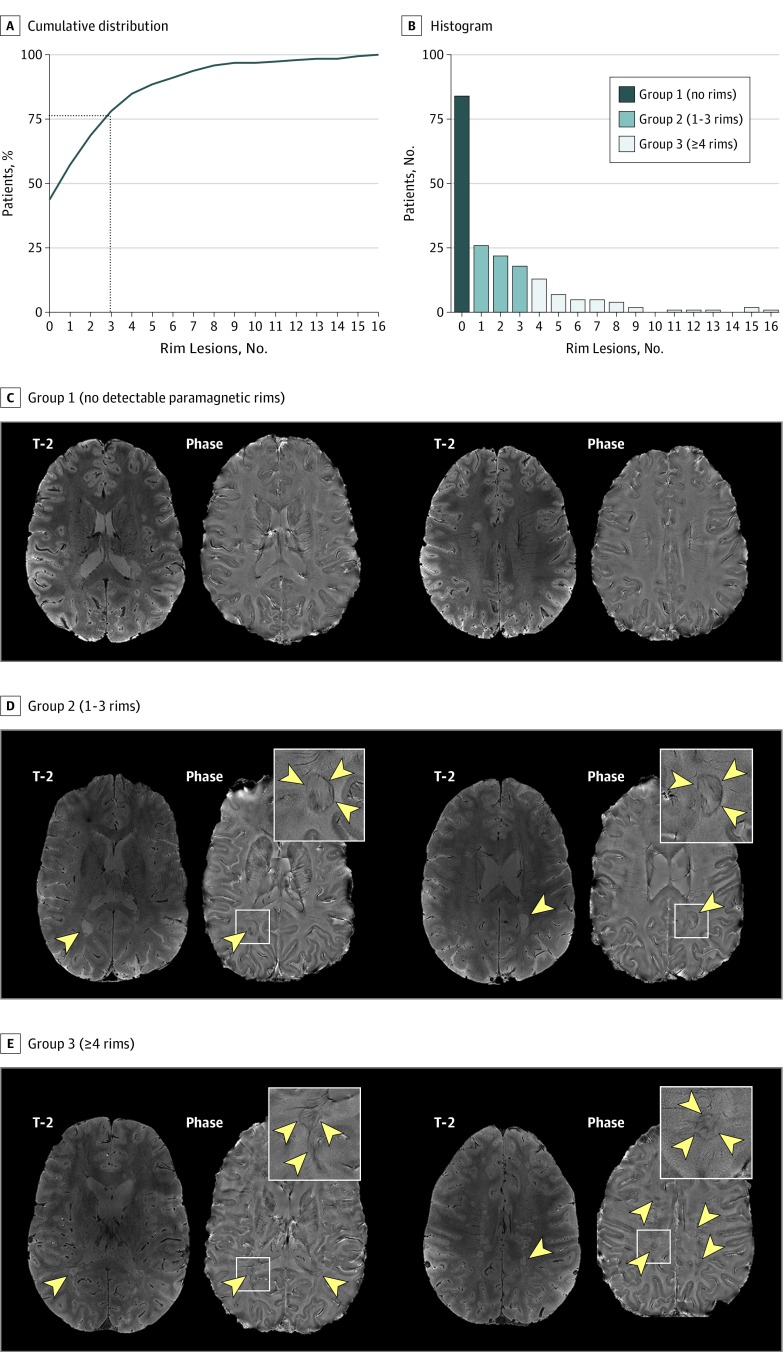
Overall, 117 individuals (56%) had at least 1 chronic lesion with a paramagnetic rim. Based on the number and distribution of chronic rim lesions (mean [SD], 2 [3]; median, 1 [range, 0–16; interquartile range, 3]; Figure 2), cases were further classified into 3 groups: (1) 84 (44%) had no rims, (2) 66 (34%) had 1 to 3 rims, and (3) 42 (22%) had 4 or more rims. Representative examples are shown in Figure 2 and eFigure 1 in the Supplement. The prevalence of clinically progressive MS was 1.6-fold higher in individuals with 4 or more chronic rim lesions (18/42 cases [43%]) than in those without a rim (23/84 cases [27%]) (P = .03). Rim lesions were present in the setting of treatment with most currently available disease-modifying therapies, including natalizumab and ocrelizumab (eFigure 2 in the Supplement).
Figure 2. Cumulative Distribution and Histogram.
A and B, Cumulative distribution and histogram of the frequency of chronic nonenhancing lesions with paramagnetic rims. The representative susceptibility-based 7-T magnetic resonance imaging scans of individuals were classified into 3 groups based on the number of rim lesions. C, Group 1 (no detectable rims) is a woman in her mid-20s with relapsing multiple sclerosis (MS). D, Group 2 (detection of 1–3 rims) is a man in his late 50s with relapsing MS and 2 rim lesions. E, Group 3 (detection of ≥4 rims) is a man in his early 30s with relapsing MS and 16 rim lesions. Rim lesions are indicated with arrowheads, and the insets show representative magnified views.
Individuals with 4 or more chronic rim lesions reached motor (EDSS and MSSS) and cognitive (SDMT and PASAT) disability at a younger age (Table). When only individuals younger than 50 years (112/192 cases [58], post hoc analysis) were considered, those with 4 or more chronic rim lesions had higher disability (median EDSS score, 1.5 [range, 0-6.5]; 1.5 [range, 0-8], and 2.5 [range, 1-7.5], respectively; ANOVA, P < .001; mean [SD] MSSS score, 3.1 [2.4]; 3.2 [2.4], and 4.5 [2.3], respectively; P = .03; mean [SD] SDMT score, 58.7 [9.8]; 52.2 [14.2], and 48.4 [18.7], respectively; P = .01; mean [SD] PASAT score, 51.0 [6.9]; 49.9 [9.5], and 44.7 [11.8], respectively; P = .02). In this younger subgroup, the prevalence of clinically progressive MS was 3.2–fold higher in individuals with 4 or more chronic rim lesions (8/29 patients [28%]) than in those without rims (4/45 patients [9%]) (P < .05).
Mean normalized volumes of the brain, white matter, thalamus, putamen, and caudate nucleus were lower in cases with 4 or more rims, but this was not true for normalized volumes of the cortex (Table). Interestingly, mean normalized volumes of ventricular, but not sulcal, cerebrospinal fluid were higher in cases with 4 rims or more. The same results were obtained when only relapsing cases (131/192 cases [68.2%]) were considered (eTable 3 in the Supplement). Mean supratentorial lesion volumes were highest in cases with 4 or more rims (P < .001). Rim lesions accounted for approximately one-third of the total supratentorial lesion volume (26% in cases with 1–3 rims and 36% in those with ≥4 rims).
On multivariable analysis (excluding the clinical phenotype), the presence of 4 or more rim lesions, supratentorial lesion volume, deep gray matter volume (thalamus and putamen), age, and male sex were independently associated with EDSS score (model R2=0.3). Univariable correlations are reported in eTable 4 in the Supplement.
MRI-Pathology Association of Rim Lesions That Slowly Expanded In Vivo
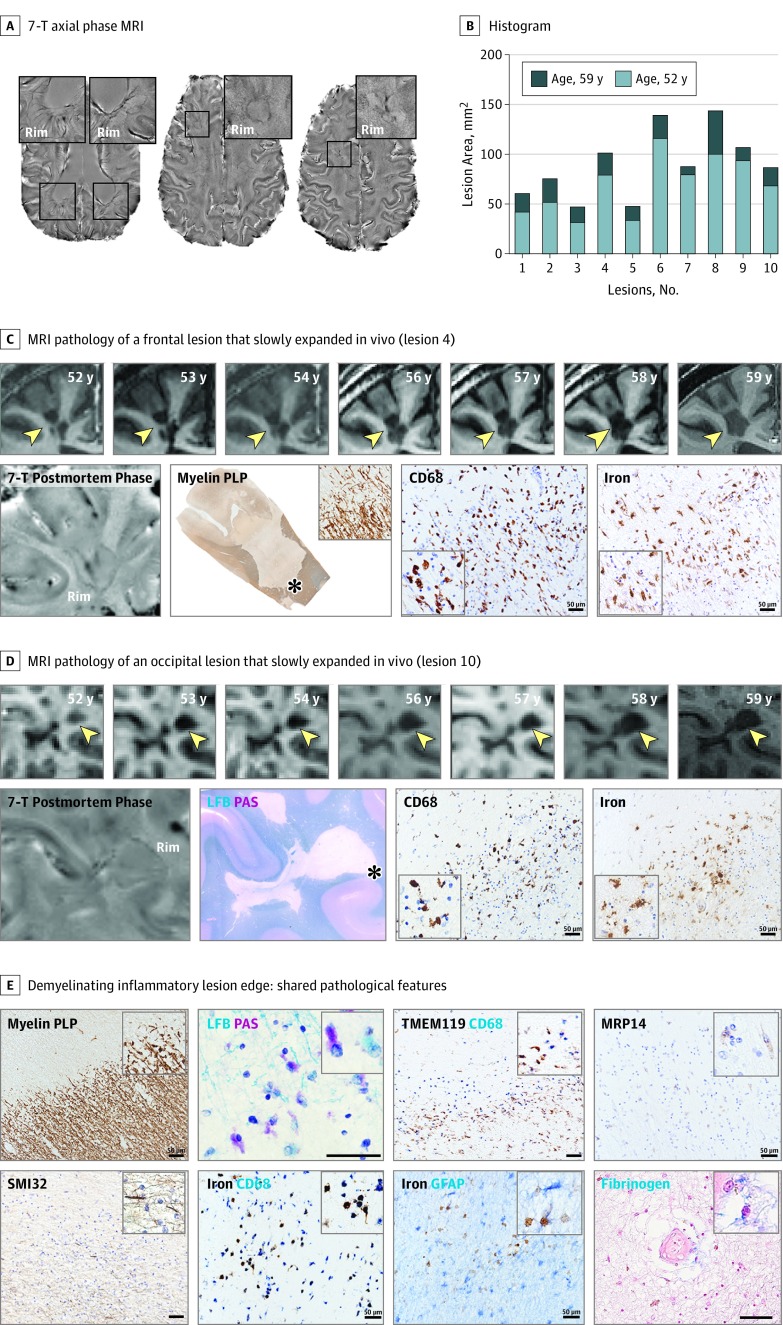
The results from the analysis of the individual who came to autopsy are shown in Figure 3. Almost all supratentorial lesions showed a 7-T paramagnetic rim in vivo (this individual was included in the group of ≥4 rim lesions). The 10 chronic rim lesions selected for pathological assessment expanded on annual MRI scans (7-year follow-up; Video 1) and were chronic active lesions according to pathology results (Figure 3 and eFigure 4 in the Supplement). These 10 rim lesions consistently showed a fully demyelinated lesion core with axonal loss and an inflammatory demyelinating edge, characterized by iron-laden phagocytes with axonal transection and subtle opening of the blood-brain barrier (BBB). The mean (SD, range) intralesional axonal reduction was reduced by 25% (10%; 14%–42%) compared with the surrounding nondemyelinated white matter.
Figure 3. Man in His Late 50s With Progressive Multiple Sclerosis and Expanding Rim Lesions.
A, In vivo 7-T axial phase magnetic resonance imaging (MRI) acquired at age 59 years showing representative supratentorial rim lesions (magnified views are in the insets). B, Histogram of the 10-rim lesions analyzed by MRI and histopathology. The lesion area was measured on in vivo coregistered T1-weighted scans at ages 52 and 59 years. All 10-rim lesions expanded over 7 years. C and D, MRI-histopathology comparison of individual rim lesion evolution and pathology. Serial in vivo coronal T1-weighted MRI scans and relative patient age are shown in the first row of each section. Clearly expanding confluent areas are indicated by arrowheads. All expanding rim lesions were chronic-active by pathology results. An accumulation of iron-laden phagocytes (cluster of differentiation [CD] 68 and iron staining) was seen at the lesion edge (asterisks on myelin proteolipid protein [PLP] and Luxol fast blue–periodic acid–Schiff [LFB-PAS] staining). The MRI-pathology comparison for lesions 2 and 3 was previously published (Figure 6 and eFigure 3 in the Supplement, respectively, in Absinta et al8). E, The paramagnetic rim localizes inflammatory demyelination. Smoldering demyelination can be inferred by the copresence of early (LFB+) and late (PAS+) myelin degradation products within phagocytes at the lesion edge. Most CD68+ cells contain iron and showed a downregulation of the homeostatic microglia marker transmembrane protein (TMEM) 119. A few perivascular CD8+ T lymphocytes (not shown here) and early activated peripheral macrophages (myeloid-related protein [MRP] 14+) can be seen at the edge. Reactive astrocytes were negative for iron. Some vessels at the lesion edge, but not within the lesion center, show fibrinogen deposition within the perivascular space, suggesting a subtle opening of the blood-brain barrier. Some degenerating axons are marked by staining for nonphosphorylated neurofilaments (SMI32+). Magnified views are shown in the insets. Scale bar, 50 μm. GFAP indicates glial fibrillary acid protein. Black font indicates the DAB staining method; blue font indicates the AP method.
Video 1. Time-lapse Magnetic Resonance Imaging (MRI) From a Patient With Multiple Sclerosis (MS) and Expanding Rim Lesions Characterized Neuropathologically at Autopsy.
This time-lapse video, constructed from 10 coregistered intensity normalized axial MRI scans (proton density [PD] sequence) obtained over 7 years from a patient with MS, demonstrates expanding lesions with a paramagnetic rim on susceptibility-phase images. Ten chronic rim lesions selected for pathological assessment showed a fully demyelinated lesion core with axonal loss and an inflammatory demyelinating edge. The finding with follow-up from other radiographic cohorts suggest chronic active lesions on MRI are common in patients with MS, exert ongoing tissue damage, and are associated with more aggressive disease despite effective disease-modifying therapy. Read the article here: https://ja.ma/2MdoE0H.
Longitudinal Study: Dichotomous Evolution of Chronic MS Lesions With and Without Rims
Chronic lesions with and without rims had a mean CAGR of 2.5% per year (expansion) and −4.7% per year (shrinkage), respectively (P < .001; primary analysis). At the time of the last MRI, lesions with rims were bigger than those without (mean [SD] adjusted log-lesion volume, 5.8 [0.8] and 4.7 [0.6], respectively; P < .001), but not at baseline (5.5 [0.8] and 5.2 [0.5], respectively; P = .08]. The age at MRI baseline was similar for cases with and without rims (mean [SD], 36 [6] and 40 [9] years, respectively; P = .20). Of 54 analyzed lesions, 30 lesions (56%) shrank (7 with rims and 23 without), 9 (20%) were steady (8 with rims and 1 without), and 15 (28%) expanded over time (12 with rims and 3 without). Rim lesions were more frequently steady or expanding (Fisher exact test, P < .001). Eight of 12 expanding rim lesions (67%) showed a re-enhancement of the lesion edge (6 lesions) or subsumed adjacent small white matter abnormalities (2 lesions) during follow-up; none of the rimless lesions re-enhanced. Time-lapse examples of representative expanding and shrinking lesions are shown in Video 2.
Video 2. Time-lapse Magnetic Resonance Imaging (MRI) From a Patient With Multiple Sclerosis (MS) Showing an Expanding Rim+ and a Shrinking Rim− Lesion.
This time-lapse video, constructed from 118 coregistered intensity normalized MRI scans (proton density [PD] sequence) obtained over 29 years (average, 4 scans per year) from a man in his 50s with relapsing MS, shows examples of an expanding (red box) and a shrinking (blue box) lesion. Not shown here, the expanding lesion had a paramagnetic rim (was “rim+”) at 7T MRI. For the expanding lesion, static MRI snapshots (both PD and postcontrast T1-weighted images) are also provided every 5 years. This progression with follow-up from other radiographic cohorts suggests chronic active lesions on MRI are common in patients with MS, exert ongoing tissue damage, and are associated with more aggressive disease despite effective disease-modifying therapy. Read the article here: https://ja.ma/2MdoE0H.
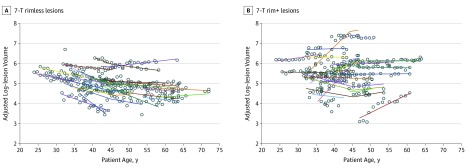
We performed a second analysis in which all 617 yearly times were evaluated to assess the evolution of 54 lesions. Using linear mixed-effects modeling, the estimated slope of adjusted log-volume change was 2.2% per year (expansion) in lesions with rims and −3.6% per year (shrinkage) in lesions without rims (P < .001; Figure 4 and eTable 5 in the Supplement).
Figure 4. Chronic Multiple Sclerosis Lesions With and Without Paramagnetic Rim Evolve Dichotomously.
A and B, Adjusted log-transformed lesion volume was tracked over time for all lesions (617 yearly points). The nonlinear spline regression model fit is shown for each lesion (solid curves). Raw data are represented as circles.
At 7T, rim lesions had longer T1 times than rimless lesions (mean [SD], 2475 [386] and 2076 [340] milliseconds, respectively; P = .002). Regardless of the rim, expanding (2603 [310] milliseconds) and steady lesions (2427 [350] milliseconds) had longer mean T1 times than shrinking lesions (2062 [345] milliseconds; ANOVA, P < .001; Tukey post hoc, P < .001 and P = .03, respectively).
Discussion
In vivo MRI- and PET-based visualization of smoldering inflammation in MS has only recently become possible. This study shows an association between chronic active lesions and clinical outcomes in a cohort of 192 patients with MS, reifying limited autopsy data2,4 and confirming the long-term detrimental effects of these lesions.
The most relevant and novel findings of this research can be summarized as follows: first, independently of clinical phenotype and disease-modifying therapy, most patients with MS (56%) had at least 1 chronic active lesion, supporting the notion that microglia/macrophage–mediated inflammation is a prominent feature of MS that is not the primary target of current treatments. Second, individuals with multiple chronic active lesions had more aggressive disease (higher lesion load and ventricular volumes and lower white matter and basal ganglia volumes) and reached higher motor and cognitive disability or transitioned to disease progression at younger age, despite treatment (Table and eTable 3 and eFigure 2 in the Supplement). In line with a recent pathological report,2 we found that the number of chronic active lesions and overall high lesion accrual was independently associated with disability. Finally, the neuropathology evaluation, consistent with previous results,2,4,6,7,8,9 showed that chronic active lesions are destructive at core (pronounced axonal loss resulting in fewer axons to remyelinate) and edge (smoldering inflammation and demyelination affecting surrounding tissue), which corresponds to our in vivo MRI findings in terms of size (approximately one-third of the volume of all MRI-visible lesions), long T1 times at 7-T MRI, and the tendency not to shrink (as other lesions do)28,30 but rather remain stable or expand over long periods (Figures 3 and 4 and eTable 5 and eFigure 3 in the Supplement; Videos 1 and 2).
We previously reported that not all new active lesions become chronically active after the closure of the BBB, which occurs roughly 2 to 6 weeks after initial demyelination.8,31 On MRI, different scenarios of early lesion evolution can be identified; a newly forming active enhancing lesion can become chronic active (rim positive, approximately 20% of lesions), chronic inactive (rim negative), and partially or completely repaired (rim negative).8 Importantly, lesion fate is dictated, approximately within the first 3 months after the acute phase of inflammatory demyelination.8 Over the ensuing months and years, most nonchronic active lesions contract in size,28,30 likely because of diverse and potentially contrasting pathological events, such as edema resorption, remyelination (usually starting at the lesion edge), astrogliosis, and axonal degeneration (collapse of the lesion core). The MRI data from this study show that, in chronic active lesions, the general tendency of lesions to collapse, likely due to prominent axonal loss (consistent with long T1 times), is counterbalanced by ongoing inflammatory demyelination at the lesion edge. The volumes of these lesions over more than 10 years of follow-up are therefore steadier than those of inactive lesions (Figure 4), although both lesion types harbor marked injury at the core. In a few chronic active lesions during the follow-up, a reopening of the BBB, seen as an incomplete or subtle gadolinium enhancement at the edge, was associated with a clear stepwise increase in lesion volume (Figure 4), reaffirming the smoldering inflammatory environment at the margins of these lesions.
These results prompt research questions for future neuropathological studies. Why do some individuals develop chronic active lesions, whereas others do not? Do individuals who are prone to forming multiple chronic active lesions have microglia-specific checkpoint dysregulation32 or sensitization, which might lead to an increased sensitivity of the central nervous tissue to MS-associated inflammatory insults (eg, excessive microglia activation and a reduced ability of microglia to return to homeostasis)?33 This might contribute to remyelination failure and the ongoing damage to previously spared tissue that is often seen in chronic active lesions. Older age at the time of lesion formation might contribute to this process and to the overall worse lesion outcome, as we previously suggested.8 This aligns with the notion that inflammation in MS does not decrease with age but rather tends to compartmentalize within the central nervous system and shift to a prevalently innate immune response.34 Unfortunately, in this analysis of lesion evolution, it was not possible to analyze lesions from their time of onset, which may have been many years prior to the earliest scan analyzed. Although we investigated potentially important associations of chronic active lesions with proposed risk factors for aggressive disease course, such as African American race/ethnicity35,36 and the presence of the allele HLA-DRB1*15:01,37,38 neither was definitively true in this cohort.
Limitations
Future studies should address the association between presence of chronic rim lesions and other mediators of disease progression, including spinal cord,39 infratentorial, and cortical lesions.40 Further prospective studies should address the negative prognostic value of chronic rim lesions and define evidence-based thresholds for discriminating expansion from shrinkage. Additionally, although this study was performed in the context of a natural history MS protocol, it is potentially affected by external referral bias, recruitment, and enrollment biases, which occur commonly in research institutes. The longitudinal analysis is potentially biased by the relatively small lesion sample size and the classification of rim presence/absence only at the terminal point. Ultimately, it will be critical to test, in dedicated studies, the efficacy of current and future disease-modifying treatments in limiting microglia/macrophage-mediated chronic inflammation.
Conclusions
Patient-specific accrual of multiple chronic active lesions is a negative prognostic marker in MS. Together with prior work showing that microglia/macrophage-mediated inflammation is a major barrier for remyelination,8 this study confirms that chronic active lesions, seen on in vivo MRI, can exert ongoing damage on the surrounding white matter. These results support using paramagnetic rim lesion development, or the resolution of such lesions, as outcome measures in MRI-based clinical trials aimed at treating perilesional chronic inflammation and its potential effects on remyelination. Such trials will probably require treatments distinct from those currently used to limit MS relapses.
eMethods.
eTable 1. Cohort characteristics of 7T patients with archival 3T yearly NIH scans for ≥10 years
eTable 2. Cohort characteristics by MRI scanner
eTable 3. Cohort characteristics - only CIS/relapsing MS cases
eTable 4. Simple correlations with EDSS
eTable 5. Dichotomous evolution of chronic MS lesions with and without 7T rim in the longitudinal cohort: linear and nonlinear regression models
eFigure 1. Representative susceptibility-based 3T MRI images of patients
eFigure 2. Distribution of disease-modifying treatments at the time of MRI by patient groups
eFigure 3. Expanding confluent lesion with paramagnetic rim
eFigure 4. Different evolution and pathology of chronic MS lesions with and without 7T rim
References
- 1.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169-180. doi: 10.1056/NEJMra1401483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frischer JM, Weigand SD, Guo Y, et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol. 2015;78(5):710-721. doi: 10.1002/ana.24497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Absinta M, Sati P, Reich DS. Advanced MRI and staging of multiple sclerosis lesions. Nat Rev Neurol. 2016;12(6):358-368. doi: 10.1038/nrneurol.2016.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luchetti S, Fransen NL, van Eden CG, Ramaglia V, Mason M, Huitinga I. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol. 2018;135(4):511-528. doi: 10.1007/s00401-018-1818-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lassmann H, Raine CS, Antel J, Prineas JW. Immunopathology of multiple sclerosis: report on an international meeting held at the Institute of Neurology of the University of Vienna. J Neuroimmunol. 1998;86(2):213-217. doi: 10.1016/S0165-5728(98)00031-9 [DOI] [PubMed] [Google Scholar]
- 6.Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133(1):13-24. doi: 10.1007/s00401-016-1653-y [DOI] [PubMed] [Google Scholar]
- 7.Popescu BF, Lucchinetti CF. Pathology of demyelinating diseases. Annu Rev Pathol. 2012;7:185-217. doi: 10.1146/annurev-pathol-011811-132443 [DOI] [PubMed] [Google Scholar]
- 8.Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126(7):2597-2609. doi: 10.1172/JCI86198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7T magnetic resonance imaging. Acta Neuropathol. 2017;133(1):25-42. doi: 10.1007/s00401-016-1636-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67(7):812-818. doi: 10.1001/archneurol.2010.148 [DOI] [PubMed] [Google Scholar]
- 11.Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134(pt 12):3602-3615. doi: 10.1093/brain/awr278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao B, Bagnato F, Matsuura E, et al. Chronic multiple sclerosis lesions: characterization with high-field-strength MR imaging. Radiology. 2012;262(1):206-215. doi: 10.1148/radiol.11110601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagemeier J, Heininen-Brown M, Poloni GU, et al. Iron deposition in multiple sclerosis lesions measured by susceptibility-weighted imaging filtered phase: a case control study. J Magn Reson Imaging. 2012;36(1):73-83. doi: 10.1002/jmri.23603 [DOI] [PubMed] [Google Scholar]
- 14.Hammond KE, Metcalf M, Carvajal L, et al. Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann Neurol. 2008;64(6):707-713. doi: 10.1002/ana.21582 [DOI] [PubMed] [Google Scholar]
- 15.Bian W, Harter K, Hammond-Rosenbluth KE, et al. A serial in vivo 7T magnetic resonance phase imaging study of white matter lesions in multiple sclerosis. Mult Scler. 2013;19(1):69-75. doi: 10.1177/1352458512447870 [DOI] [PubMed] [Google Scholar]
- 16.Walsh AJ, Lebel RM, Eissa A, et al. Multiple sclerosis: validation of MR imaging for quantification and detection of iron. Radiology. 2013;267(2):531-542. doi: 10.1148/radiol.12120863 [DOI] [PubMed] [Google Scholar]
- 17.Wisnieff C, Ramanan S, Olesik J, Gauthier S, Wang Y, Pitt D. Quantitative susceptibility mapping (QSM) of white matter multiple sclerosis lesions: interpreting positive susceptibility and the presence of iron. Magn Reson Med. 2015;74(2):564-570. doi: 10.1002/mrm.25420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison DM, Li X, Liu H, et al. Lesion heterogeneity on high-field susceptibility MRI is associated with multiple sclerosis severity. AJNR Am J Neuroradiol. 2016;37(8):1447-1453. doi: 10.3174/ajnr.A4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Absinta M, Sati P, Fechner A, Schindler MK, Nair G, Reich DS. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39(7):1233-1238. doi: 10.3174/ajnr.A5660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaunzner UW, Kang Y, Zhang S, et al. Quantitative susceptibility mapping identifies inflammation in a subset of chronic multiple sclerosis lesions. Brain. 2019;142(1):133-145. doi: 10.1093/brain/awy296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302. doi: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452. doi: 10.1212/WNL.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 23.Roxburgh RH, Seaman SR, Masterman T, et al. Multiple sclerosis severity score: using disability and disease duration to rate disease severity. Neurology. 2005;64(7):1144-1151. doi: 10.1212/01.WNL.0000156155.19270.F8 [DOI] [PubMed] [Google Scholar]
- 24.Sati P, Thomasson DM, Li N, et al. Rapid, high-resolution, whole-brain, susceptibility-based MRI of multiple sclerosis. Mult Scler. 2014;20(11):1464-1470. doi: 10.1177/1352458514525868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Absinta M, Vuolo L, Rao A, et al. Gadolinium-based MRI characterization of leptomeningeal inflammation in multiple sclerosis. Neurology. 2015;85(1):18-28. doi: 10.1212/WNL.0000000000001587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiee N, Bazin PL, Ozturk A, Reich DS, Calabresi PA, Pham DL. A topology-preserving approach to the segmentation of brain images with multiple sclerosis lesions. Neuroimage. 2010;49(2):1524-1535. doi: 10.1016/j.neuroimage.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Absinta M, Nair G, Filippi M, et al. Postmortem magnetic resonance imaging to guide the pathologic cut: individualized, 3-dimensionally printed cutting boxes for fixed brains. J Neuropathol Exp Neurol. 2014;73(8):780-788. doi: 10.1097/NEN.0000000000000096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reich DS, White R, Cortese IC, et al. Sample-size calculations for short-term proof-of-concept studies of tissue protection and repair in multiple sclerosis lesions via conventional clinical imaging. Mult Scler. 2015;21(13):1693-1704. doi: 10.1177/1352458515569098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sethi V, Nair G, Absinta M, et al. Slowly eroding lesions in multiple sclerosis. Mult Scler. 2017;23(3):464-472. doi: 10.1177/1352458516655403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dwyer MG, Bergsland N, Ramasamy DP, Jakimovski D, Weinstock-Guttman B, Zivadinov R. Atrophied brain lesion volume: a new imaging biomarker in multiple sclerosis. J Neuroimaging. 2018;28(5):490-495. doi: 10.1111/jon.12527 [DOI] [PubMed] [Google Scholar]
- 31.Absinta M, Sati P, Gaitán MI, et al. Seven-tesla phase imaging of acute multiple sclerosis lesions: a new window into the inflammatory process. Ann Neurol. 2013;74(5):669-678. doi: 10.1002/ana.23959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deczkowska A, Amit I, Schwartz M. Microglial immune checkpoint mechanisms. Nat Neurosci. 2018;21(6):779-786. doi: 10.1038/s41593-018-0145-x [DOI] [PubMed] [Google Scholar]
- 33.Zrzavy T, Hametner S, Wimmer I, Butovsky O, Weiner HL, Lassmann H. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain. 2017;140(7):1900-1913. doi: 10.1093/brain/awx113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9):545-558. doi: 10.1038/nri3871 [DOI] [PubMed] [Google Scholar]
- 35.Cree BA, Reich DE, Khan O, et al. Modification of multiple sclerosis phenotypes by African Ancestry at HLA. Arch Neurol. 2009;66(2):226-233. doi: 10.1001/archneurol.2008.541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kister I, Chamot E, Bacon JH, et al. Rapid disease course in African Americans with multiple sclerosis. Neurology. 2010;75(3):217-223. doi: 10.1212/WNL.0b013e3181e8e72a [DOI] [PubMed] [Google Scholar]
- 37.Okuda DT, Srinivasan R, Oksenberg JR, et al. Genotype-phenotype correlations in multiple sclerosis: HLA genes influence disease severity inferred by 1HMR spectroscopy and MRI measures. Brain. 2009;132(pt 1):250-259. doi: 10.1093/brain/awn301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yates RL, Esiri MM, Palace J, Mittal A, DeLuca GC. The influence of HLA-DRB1*15 on motor cortical pathology in multiple sclerosis. Neuropathol Appl Neurobiol. 2015;41(3):371-384. doi: 10.1111/nan.12165 [DOI] [PubMed] [Google Scholar]
- 39.Gass A, Rocca MA, Agosta F, et al. ; MAGNIMS Study Group . MRI monitoring of pathological changes in the spinal cord in patients with multiple sclerosis. Lancet Neurol. 2015;14(4):443-454. doi: 10.1016/S1474-4422(14)70294-7 [DOI] [PubMed] [Google Scholar]
- 40.Calabrese M, Magliozzi R, Ciccarelli O, Geurts JJ, Reynolds R, Martin R. Exploring the origins of grey matter damage in multiple sclerosis. Nat Rev Neurosci. 2015;16(3):147-158. doi: 10.1038/nrn3900 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Cohort characteristics of 7T patients with archival 3T yearly NIH scans for ≥10 years
eTable 2. Cohort characteristics by MRI scanner
eTable 3. Cohort characteristics - only CIS/relapsing MS cases
eTable 4. Simple correlations with EDSS
eTable 5. Dichotomous evolution of chronic MS lesions with and without 7T rim in the longitudinal cohort: linear and nonlinear regression models
eFigure 1. Representative susceptibility-based 3T MRI images of patients
eFigure 2. Distribution of disease-modifying treatments at the time of MRI by patient groups
eFigure 3. Expanding confluent lesion with paramagnetic rim
eFigure 4. Different evolution and pathology of chronic MS lesions with and without 7T rim