Key Points
Question
Can a multifaceted quality improvement intervention increase the adherence to 10 evidence-based performance measures for patients with acute ischemic stroke and transient ischemic attack in Latin America?
Findings
In this cluster randomized clinical trial that included 1624 patients from 36 hospitals in 3 countries, hospitals in the intervention group had a composite adherence score of 85.3% for the evidence-based performance measures, and those in the control group had a score of 77.8%, a difference that was not statistically significant. In a planned secondary analysis, improvement in adherence to all 10 performance measures was greater in the intervention group than in the control group using an all-or-none approach for the outcome.
Meaning
In this cohort of patients with acute ischemic stroke and transient ischemic attack, a multifaceted intervention did not demonstrate an overall improvement in adherence to evidence-based performance measures compared with standard of care, yet individual care elements improved.
This cluster randomized clinical trial assesses the effect of a multifaceted quality improvement intervention compared with routine care on adherence to evidence-based therapy among patients with acute ischemic stroke and transient ischemic attack.
Abstract
Importance
Translating evidence into clinical practice in the management of acute ischemic stroke (AIS) and transient ischemic attack (TIA) is challenging, especially in low- and middle-income countries.
Objective
To assess the effect of a multifaceted quality improvement intervention on adherence to evidence-based therapies for care of patients with AIS and TIA.
Design, Setting and Participants
This 2-arm cluster-randomized clinical trial assessed 45 hospitals and 2336 patients with AIS and TIA for eligibility before randomization. Eligible hospitals were able to provide care for patients with AIS and TIA in Brazil, Argentina, and Peru. Recruitment started September 12, 2016, and ended February 26, 2018; follow-up ended June 29, 2018. Data were analyzed using the intention-to-treat principle.
Interventions
The multifaceted quality improvement intervention included case management, reminders, a roadmap and checklist for the therapeutic plan, educational materials, and periodic audit and feedback reports to each intervention cluster.
Main Outcomes and Measures
The primary outcome was a composite adherence score for AIS and TIA performance measures. Secondary outcomes included an all-or-none composite end point of performance measures, the individual process measure components of the composite end points, and clinical outcomes at 90 days after admission (stroke recurrence, death, and disability measured by the modified Rankin scale).
Results
A total of 36 hospitals and 1624 patients underwent randomization. Nineteen hospitals were randomized to the quality improvement intervention and 17 to routine care. The overall mean (SD) age of patients enrolled in the study was 69.4 (13.5) years, and 913 (56.2%) were men. Overall mean (SD) composite adherence score for the 10 performance measures in the intervention group hospitals compared with control group hospitals was 85.3% (20.1%) vs 77.8% (18.4%) (mean difference, 4.2%; 95% CI, −3.8% to 12.2%). As a secondary end point, 402 of 817 patients (49.2%) at intervention hospitals received all the therapies that they were eligible for vs 203 of 807 (25.2%) in the control hospitals (odds ratio, 2.59; 95% CI, 1.22-5.53; P = .01).
Conclusions and Relevance
A multifaceted quality improvement intervention did not result in a significant increase in composite adherence score for evidence-based therapies in patients with AIS or TIA. However, when using an all-or-none approach, the intervention resulted in improved adherence to evidence-based therapies.
Trial Registration
ClinicalTrials.gov identifier: NCT02223273
Introduction
Stroke represents the second leading cause of death and disability globally.1,2,3 Large-scale randomized evidence has established the efficacy of interventions for acute ischemic stroke (AIS) and transient ischemic attack (TIA), such as intravenous recombinant tissue plasminogen activator (rt-PA),4,5 antiplatelet therapy,6,7,8 and anticoagulation for patients with atrial fibrillation.9 Nevertheless, the implementation of these therapies in clinical practice remains suboptimal, especially in low- and middle-income countries.10,11,12,13,14,15,16
To date and to our knowledge, most studies assessing the effect of quality improvement tools such as reminders, audit and feedback, case management, and distribution of educational materials to health care professionals for the care of patients with stroke have been conducted in developed countries.17,18,19 Quality improvement interventions have rarely been rigorously evaluated in lower-resource settings20 such as Latin America, where the burden of cerebrovascular diseases remains very high.3 Thus, we conducted a cluster randomized trial to assess the effect of a multifaceted quality improvement intervention on the adherence to evidence-based performance measures in patients with AIS or TIA in Brazil, Argentina, and Peru.
Methods
Study Design
The detailed trial methods have been published previously,21 and the full protocol and the statistical analysis plan are available in Supplement 1. In brief, the Brazilian Intervention to Increase Evidence Usage in Practice–Stroke (BRIDGE-Stroke) study was a pragmatic international, 2-arm, cluster randomized clinical trial with blinded outcome adjudication. The main objective was to evaluate whether a multifaceted quality improvement intervention could improve the adherence to in-hospital evidence-based therapies for patients with AIS or TIA. All hospitals (clusters) submitted the protocol for approval by their research ethics boards; written informed consent was obtained at the cluster and patient levels. The enrollment period was from September 12, 2016, through February 26, 2018. Follow-up was completed June 29, 2018.
Clusters and Patients
We included 36 public or private hospitals from Brazil (n = 26), Argentina (n = 6), and Peru (n = 4) offering 24-hour emergency care with at least 1 physician in charge of the emergency care unit for 24 hours, at least 1 on-call neurologist, and available central nervous system imaging and alteplase therapy. At participating sites, we enrolled consecutive patients with AIS or TIA who were admitted within 24 hours from symptom onset as soon as they presented in the emergency department. We excluded patients with hemorrhagic stroke, those with expansive lesions and central nervous system infections, and those for whom presumptive admission diagnosis of AIS or TIA was not confirmed. Detailed eligibility criteria are shown in eMethods 1 in Supplement 2.
Baseline Survey
We conducted a prerandomization survey in all clusters using the same eligibility criteria for patient inclusion. The main objective was to assess whether clusters were comparable with regard to prescription rates of evidence-based therapies and to obtain reliable estimates for our sample size calculation. Methods and results of the survey are presented in eTable 1 in Supplement 2.
Randomization and Allocation Concealment
Hospitals were randomized (1:1) to a quality improvement intervention or to routine practice. Randomization was stratified in tertiles according to baseline performance. The randomization list was generated at once by a statistician (L.P.D.) using a central web-based randomization system before enrollment of the first patient.
Blinding
Patients and investigators were not blinded to the allocation of treatment. Outcome assessors and statisticians were blinded to the nature of the intervention.
Intervention
The quality improvement intervention included case management, reminders, a roadmap and checklist of the therapeutic plan, educational materials, and audit and feedback. Case management was conducted by a physician leader and trained nurses from each cluster. Case managers were responsible for the timely delivery of study materials and for checking the implementation of evidence-based therapies.
Reminders (colored wristbands) and a roadmap of the therapeutic plan were designed to be implemented in sequence during patient management. The wristband helped to promptly identify patients with a potential AIS or TIA. Once the diagnosis was confirmed, the case managers prompted the attending physicians and provided them with a roadmap of the therapeutic plan. This tool guided the physicians from appropriate AIS or TIA diagnosis confirmation to the recommended therapies needed until discharge. The treatment plan also required that the attending physician complete a checklist to confirm the implementation of all recommended interventions.
Educational materials included an rt-PA kit case, a bedside dysphagia screening test, the National Institutes of Health Stroke Scale, a medication brochure, and a patient educational brochure. Periodic audit and feedback reports on performance were provided to encourage the teams to seek continuous improvement.
Hospitals randomized to the intervention received on-site training visits complemented by web-based and telephone training. In addition, 2 health care professionals from each of these clusters attended a workshop on how to implement the intervention. The detailed methods of the workshops are provided in eMethods 2 in Supplement 2.
Data Collection
In all hospitals, data were collected prospectively by a trained research coordinator not involved in patient care. Adherence to therapies was assessed by a medical record review, patient files, and medical prescriptions. Quality control was guaranteed by automated data entry checks, on-site monitoring, and central statistical checks.
Outcomes
The primary outcome was as a composite adherence score for evidence-based therapies (early antithrombotic therapy, deep venous thrombosis prophylaxis, intravenous rt-PA among patients with ischemic stroke arriving within 3.5 hours and treated within 4.5 hours, door-to-needle time of 60 minutes or less, dysphagia screening, assessment for rehabilitation, antithrombotics at discharge, statins for patients with low-density lipoprotein levels of 100 mg/dL or higher [to convert to millimoles per liter, multiply by 0.0259] or not documented, anticoagulants for atrial fibrillation or flutter, and smoking cessation education) in the first 48 hours and at discharge as indicated based on the patient diagnosis (AIS or TIA). This outcome consisted of an opportunity score22 defined as the sum of evidence-based therapies used among the patients’ total eligible opportunities. Secondary outcomes included the proportion of prescription of evidence-based strategies in the first 48 hours and at discharge in an all-or-none approach,22 individual components of the primary end point, the rt-PA rate among patients with stroke admitted within 24 hours of symptoms, the proportion of use of antihypertensives at discharge, the proportion of patients treated with thrombolysis within a door-to-needle time of no more than 45 minutes, and clinical outcomes at 90 days after admission (stroke recurrence, death, and disability measured by the modified Rankin scale).
Statistical Analysis
Considering a control group composite adherence score of 75% and an intracluster correlation coefficient (ICC) of 0.25 (both based on our baseline survey), we needed to randomize at least 36 clusters and 1440 patients (mean of 40 patients per cluster) to detect a 12.5% absolute improvement in the score with 80% power, 2-tailed α = .05, and an ICC of 0.25. Main analysis followed the intention-to-treat principle. The primary outcome was analyzed using a mixed-effects regression model with random effects to account for the correlation of observations within clusters. Components of the primary outcome were individually evaluated using mixed-effects general linear models considering binomial distribution (logistic regression with random effects at the intercept per cluster). All models were adjusted for the cluster baseline values (obtained during the observational phase). Treatment effects are expressed as absolute mean difference for the composite outcome and odds ratio (OR) for binary outcomes with their respective 95% CIs. We also compared the effects of our intervention in the following subgroups: teaching vs nonteaching hospitals, hospitals with or without stroke units, hospitals with or without a neurologist in the emergency department, final diagnosis (AIS vs TIA), and country. Clinical events were compared using frailty Cox proportional hazards regression models with health care center as the random effect. We performed the following sensitivity analyses: including only patients with AIS diagnosis, including only patients with TIA diagnosis, and an adjusted analysis for hospital status and presence of a stroke unit. The significance level was set at 5% as 2-sided P < .05. Analyses were conducted using R software, version 3.5.1 (R Foundation for Statistical Computing).
Results
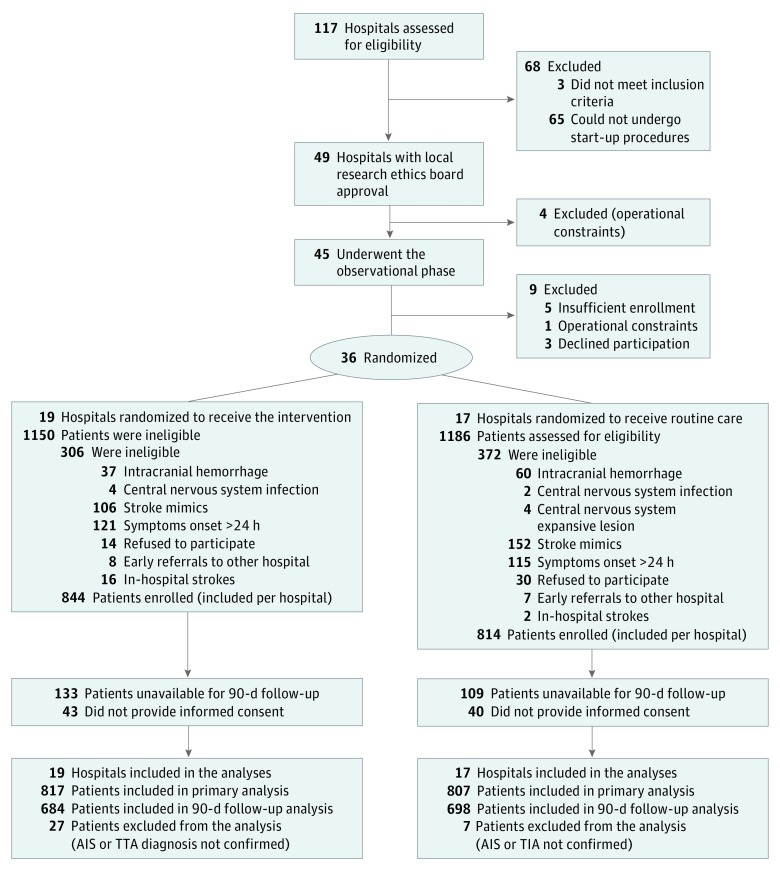
Of 117 potentially eligible hospitals that were invited, 72 were excluded (65 could not undergo start-up procedures, 4 were excluded owing to operational constraints, and 3 did not meet inclusion criteria). From the remaining 45 hospitals that confirmed interest and completed the baseline survey, 9 were excluded before the randomization phase (3 declined participation, 5 had insufficient enrollment, and 1 had operational constraints), leaving 36 sites. From these randomized hospitals that completed the trial, a total of 1624 patients were enrolled prospectively (Figure 1).
Figure 1. Study Flow Diagram.
AIS indicates acute ischemic stroke; TIA, transient ischemic attack.
Hospital and Patient Characteristics
Baseline hospital and patient characteristics were generally similar in each group (Table 1). From the included clusters, 17 (47.2%) had stroke units, 32 (88.9%) had intra-arterial thrombolysis capabilities available 24 hours per day, 28 (77.8%) were teaching hospitals, and the median volume of patients seen in the ED was about 1500 patients per month (interquartile range, 500-3700). Among enrolled patients, the mean (SD) age was 69.4 (13.5) years, 913 (56.2%) were men, 456 (28.1%) had prior stroke, 1219 (75.1%) had hypertension, and 484 (29.8%) had diabetes. From the included patients, 1434 (88.3%) had a final diagnosis of AIS and 190 (11.7%) of TIA. The mean number of patients in each hospital was 43 (range, 9-85).
Table 1. Baseline Characteristics of Clusters (Hospitals) and Patients.
| Characteristic | Study Groupa | |
|---|---|---|
| Intervention | Control | |
| Patients | ||
| Men | 441/817 (54.0) | 472/807 (58.5) |
| Age, mean (SD), y | 70.3 (13.6) | 68.4 (13.4) |
| Diabetes | 252/817 (30.8) | 232/806 (28.8) |
| Hypertension | 627/817 (76.7) | 592/807 (73.4) |
| Dyslipidemia | 224/817 (27.4) | 150/807 (18.6) |
| Current smoking | 129/817 (15.8) | 169/807 (20.9) |
| Family history of stroke | 62/817 (7.6) | 116/807 (14.4) |
| Family history of CAD | 53/817 (6.5) | 121/807 (15.0) |
| Prior stroke | 243/817 (29.7) | 213/807 (26.4) |
| CAD | 113/817 (13.8) | 156/807 (19.3) |
| Atrial fibrillation | 120/817 (14.7) | 74/807 (9.2) |
| Renal failure | 25/817 (3.1) | 32/807 (4.0) |
| Use of aspirin in the past month | 211/817 (25.8) | 201/807 (24.9) |
| Use of anticoagulants in the past month | 77/817 (9.4) | 68/807 (8.4) |
| Use of statins in the past month | 207/817 (25.3) | 190/807 (23.5) |
| Final diagnosis | ||
| AIS | 711/817 (87.0) | 723/807 (89.6) |
| TIA | 106/817 (13.0) | 84/807 (10.4) |
| Clusters | ||
| Neurologist available at ED | 13/19 (68.4) | 9/17 (52.9) |
| Mechanical thrombectomy capabilities | 17/19 (89.5) | 15/17 (88.2) |
| Stroke unit | 10/19 (52.6) | 7/17 (41.2) |
| Stroke protocol available at ED | 17/19 (89.5) | 17/17 (100) |
| Stroke protocol available at the hospital | 19/19 (100) | 17/17 (100) |
| JCI accreditation | 1/19 (5.3) | 3/17 (17.6) |
| Teaching hospital | 13/19 (68.4) | 15/17 (88.2) |
| Prior participation in multicenter clinical trial | 17/19 (89.5) | 15/17 (88.2) |
| Patients seen in ED per mo, median (IQR), No. | 1600 (425-3000) | 1400 (800-4000) |
| Baseline rate of composite adherence score, median (IQR)b | 77.1 (67.7-82.5) | 75.3 (66.2-79.8) |
Abbreviations: AIS, acute ischemic stroke; CAD, coronary artery disease; ED, emergency department; IQR, interquartile range, JCI, Joint Commission International; TIA, transient ischemic attack.
Unless otherwise indicated, data are expressed as number/total number (percentage) of patients or clusters.
Indicates primary outcome.
Adherence to the Quality Improvement Intervention
In the intervention group, health care professionals acted as case managers in 18 of 19 hospitals (94.7%), the audit and feedback system was adhered to by 18 of 19 clusters (94.7%), and adherence to a therapeutic plan roadmap occurred during the first 48 hours in 539 of 817 patients (66.0%) and at discharge in 519 of 817 (63.5%) (eTable 2 in Supplement 2). One hospital did not adhere to any of the quality improvement tools from our intervention.
Effects on Evidence-Based Therapies
The effects of the quality improvement intervention on prescription rates of evidence-based therapies are shown in Table 2. The overall mean (SD) composite adherence score was not significantly higher in the intervention than in the control groups (85.3% [20.1%] vs 77.8% [18.4%]; mean difference, 4.2%; 95% CI, −3.8% to 12.2%; ICC, 0.332; P = .29). As a prespecified secondary end point, patients in the intervention hospitals were more likely to receive all acute therapies during hospitalization than those in control hospitals (402 of 817 [49.2%] vs 203 of 807 [25.2%]; OR, 2.59; 95% CI, 1.22-5.53; ICC, 0.251; P = .01). These results were mainly driven by the following individual components that were more likely to be provided to the intervention group patients: intravenous rt-PA within the therapeutic window (122 of 222 [55.0%] vs 107 of 268 [39.9%]; OR, 2.77; 95% CI, 1.31-5.82; ICC, 0.169; P = .01) and smoking cessation education (93 of 129 [72.1%] vs 82 of 169 [48.5%]; OR, 3.22; 95% CI, 1.05-9.88; ICC, 0.278; P = .04) (Table 2).
Table 2. Effect of a Quality Improvement Intervention on Adherence to Evidence-Based Therapies for Patients With AIS and TIA (Intention-to-Treat Analysis)a.
| End Point | Intervention Groupb | Control Groupc | OR (95% CI) | P Value | ICCd |
|---|---|---|---|---|---|
| Composite adherence score, mean (SD), %e | 85.3 (20.1) | 77.8 (18.4) | 4.2 (−3.8 to 12.2)f | .29 | 0.332 |
| Secondary | |||||
| Acute therapies during first 48 h | |||||
| Intravenous rt-PAg | 122/222 (55.0) | 107/268 (39.9) | 2.77 (1.31 to 5.82) | .01 | 0.169 |
| Global rt-PAh | 145/538 (27.0) | 121/602 (20.1) | 2.07 (1.05 to 4.09) | .04 | 0.173 |
| Rt-PA within 3 hi | 69/123 (56.1) | 47/143 (32.9) | 3.31 (1.33 to 8.23) | .01 | 0.194 |
| Door-to-needle time <60 min | 84/145 (57.9) | 59/121 (48.8) | 2.47 (0.97 to 6.28) | .06 | 0.158 |
| Door-to-needle time <45 min | 59/145 (40.7) | 35/121 (28.9) | 1.86 (0.85 to 4.09) | .12 | 0.037 |
| Early antithrombotics | 759/811 (93.6) | 756/803 (94.1) | 0.59 (0.26 to 1.38) | .22 | 0.183 |
| Prophylaxis for DVT | 326/450 (72.4) | 234/466 (50.2) | 2.56 (0.92 to 7.11) | .07 | 0.375 |
| Dysphagia screening | 577/711 (81.2) | 460/723 (63.6) | 2.82 (0.71 to 11.24) | .14 | 0.547 |
| Discharge therapies | |||||
| Antithrombotics | 751/811 (92.6) | 759/806 (94.2) | 0.59 (0.25 to 1.35) | .21 | 0.211 |
| Oral anticoagulants for atrial fibrillation or flutter | 111/146 (76.0) | 77/97 (79.4) | 1.02 (0.41 to 2.50) | .97 | 0.044 |
| LDL-C level ≥100 mg/dL or not documented | 613/675 (90.8) | 631/701 (90.0) | 0.87 (0.50 to 1.51) | .61 | 0.08 |
| Smoking cessation | 93/129 (72.1) | 82/169 (48.5) | 3.22 (1.05 to 9.88) | .04 | 0.278 |
| Assessed for rehabilitation | 620/711 (87.2) | 574/723 (79.4) | 1.92 (0.58 to 6.33) | .28 | 0.457 |
| Antihypertensives | 479/622 (77.0) | 499/586 (85.2) | 0.69 (0.33 to 1.47) | .34 | 0.213 |
| Complete adherence to all eligible acute and discharge therapiesj | 402/817 (49.2) | 203/807 (25.2) | 2.59 (1.22 to 5.53) | .01 | 0.251 |
Abbreviations: AIS, acute ischemic stroke; DVT, deep venous thrombosis; ICC, intracluster correlation coefficient; LDL-C, low-density lipoprotein cholesterol; OR, odds ratio; rt-PA, recombinant plasminogen activator; TIA, transient ischemic attack.
SI conversion factor: To convert LDL-C to millimoles per liter, multiply by 0.0259.
Unless otherwise indicated, data are presented as number/total number eligible for therapy (percentage).
Includes 19 clusters (hospitals) and 817 patients.
Includes 17 clusters (hospitals) and 807 patients.
Estimated from mixed logistic regression model considering group (intervention and control) adjusted by clusters’ observational phase mean end point.
Indicates primary end point, including early use of antithrombotics, rt-PA within therapeutic window, door-to-needle time of less than 60 minutes, DVT prophylaxis, dysphagia screening, antithrombotics at discharge, anticoagulants for atrial fibrillation or flutter assessment for rehabilitation, statins in patients with LDL-C level of at least 100 mg/dL or not documented, smoking cessation education, and assessment for rehabilitation.
Data shown are mean difference (95% CI).
Indicates within therapeutic window (patients who arrived within 3.5 hours of symptoms onset and were treated within 4.5 hours).
Indicates delivered in patients who arrived within 24 hours of symptoms and had no contraindications.
Indicates patients who arrived within 2 hours of symptoms and were treated within 3 hours.
Includes patients who received all eligible therapies in an all-or-none model.
Effects on Clinical Events
The effects of our intervention on clinical events are shown in Table 3. Total mortality rates in 90 days were 12.6% (103 of 817 patients) in the intervention group and 11.8% (95 of 807 patients) in the control group (hazard ratio, 1.16; 95% CI, 0.68-2.01; P = .58). The rates of in-hospital hemorrhagic transformation were 5.1% (42 of 817 patients) in the intervention group and 2.5% (20 of 804 patients) in the control group (OR, 2.11; 95% CI, 1.13-3.94; P = .02).
Table 3. Results of Quality Improvement Intervention for Clinical Cardiovascular Events, in Hospital and at 90 Days.
| Event | No./Total No. (%) | OR (95% CI) | P Value | |
|---|---|---|---|---|
| Intervention | Control | |||
| Prespecified clinical events (within 90 d) | ||||
| Stroke recurrence | 11/817 (1.3) | 5/807 (0.6) | 2.26 (0.78-6.49)a | .13 |
| Total mortality | 103/817 (12.6) | 95/807 (11.8) | 1.16 (0.68-2.01)a | .58 |
| Cardiovascular mortality | 17/817 (2.1) | 14/807 (1.7) | 1.53 (0.54-4.27)a | .42 |
| Disability (modified Rankin score <2)b | 274/662 (41.4) | 255/670 (38.1) | 1.11 (0.74-1.68) | .61 |
| Additional clinical events (in hospital) | ||||
| Hemorrhagic transformation | 42/817 (5.1) | 20/804 (2.5) | 2.11 (1.13-3.94) | .02 |
| Nonfatal cardiac arrest | 4/817 (0.5) | 3/805 (0.4) | 1.24 (0.17-8.90) | .83 |
| Major bleedingc | 12/817 (1.5) | 3/805 (0.4) | 5.15 (0.81-32.55) | .08 |
| Acute coronary syndrome | 17/817 (2.1) | 11/805 (1.4) | 1.51 (0.43-5.35) | .52 |
| Stroke recurrence | 7/817 (0.9) | 0/807 | NA | .93 |
| Total mortality | 22/817 (2.7) | 17/807 (2.1) | 1.67 (0.66-4.22) | .28 |
| Cardiovascular mortality | 17/817 (2.1) | 14/807 (1.7) | 1.64 (0.55-4.94) | .38 |
Abbreviations: NA, not applicable; OR, odds ratio.
Data are shown as hazard ratios (95% CI).
A modified Rankin score <2 indicates that the patient is independent.
Excludes central nervous system bleeding.
Subgroup Analysis
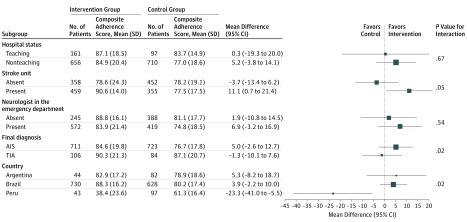
Subgroup analysis is shown in Figure 2. The effect of our intervention on the primary outcome was greater in patients with AIS (mean [SD] composite adherence score, 84.6% [19.8%] vs 76.7% [17.8%]; mean difference, 5.0%; 95% CI, −2.6% to 12.7%) compared with patients with TIA (mean [SD], 90.3% [21.3%] vs 87.1% [20.7%]; mean difference, −1.3%; 95% CI, −10.1% to 7.6%; P = .02 for interaction) and in hospitals with a stroke unit (mean [SD] score, 90.6% [14.0%] vs 77.5% [17.5%]; mean difference, 11.1%; 95% CI, 0.7%-21.4%) compared with clusters without a stroke unit (mean [SD] score, 78.6% [24.3%] vs 78.2% [19.1%]; mean difference, −3.7%; 95% CI, −13.4% to 6.2%; P = .05 for interaction).
Figure 2. Primary End Point According to Prespecified Subgroups.
Primary end point consisted of a composite adherence score of evidence-based therapies in patients without contraindications. AIS indicates acute ischemic stroke; TIA, transient ischemic attack.
Sensitivity Analysis
A sensitivity analysis including only patients with AIS diagnosis is shown in eTable 3 in Supplement 2. Overall composite adherence scores were higher in the intervention group than in the control group (mean [SD], 84.6% [19.8%] vs 76.7% [17.8%]; mean difference, 5.2%; 95% CI, −2.9% to 13.4%; ICC, 0.363; P = .20). An analysis including only patients with TIA diagnosis (eTable 4 in Supplement 2) and an analysis adjusted for hospital status and presence of a stroke unit (eTable 5 in Supplement 2) showed similar results.
Discussion
In this cluster randomized trial, a quality improvement intervention that included case management, reminders, a therapeutic plan and checklist, educational materials, and audit and feedback was not effective in the care of patients with AIS and TIA as assessed by a composite adherence score. However, as assessed by a dichotomous all-or-none approach, the study presents hypothesis-generating findings of improved uptake of in-hospital evidence-based therapies. These findings were driven by increased prescription rates of rt-PA within a therapeutic window in intervention hospitals. The results were consistent among different subgroups, but with greater effect in hospitals with stroke units and in patients with AIS.
Previous nonrandomized quality improvement initiatives have demonstrated improvement in stroke quality of care. A study on the Get With the Guidelines–Stroke program23 showed a 40.3% absolute increase in evidence-based therapies (all-or-none measures) and a 10.72% absolute increase in the composite adherence score associated with use of the program. In our trial, we observed a 25% absolute increase in all-or-none measures adherence and an 8% increase in the composite adherence score. In another study,24 use of a quality improvement strategy was associated with increased uptake of rt-PA, which is also similar to our results.
Previous quality improvement cluster randomized clinical trials were predominantly conducted in high-income countries. Trials testing the use of critical pathways and therapeutic plans demonstrated increased use of evidence-based management procedures such as early aspirin prescription and dysphagia screening.17,25 In addition, cluster trials testing multidimensional implementation strategies conducted in the Netherlands26 and in the United Kingdom27 found small to moderate improvements in the proportion of patients with AIS treated with thrombolysis within 4 hours from onset, which is in line with our findings. Conversely, the Project for the Improvement of Stroke Care Management in Minnesota (PRISMM) failed to identify an intervention effect on 10 quality measures of stroke care. The neutral results may be related to suboptimal adherence to quality improvement tools (stroke care order sets, protocols, and patient educational materials) and to large secular trends.18
We believe that our trial adds complementary information to studies conducted in higher-resource settings. To our knowledge, the BRIDGE-Stroke study constitutes the first randomized clinical trial aimed at improving stroke care conducted in Latin America. In this region, additional barriers to implementing evidence-based care include overcrowding, heavier individual clinical workloads, and fewer personnel devoted to continuing educational activities. Despite the neutral finding in our primary outcome, our results suggest that quality improvement interventions might be feasible in these settings, and we generated the hypothesis that the interventions might improve some performance measures. Therefore, we consider that the BRIDGE-Stroke findings represent an initial step to improve stroke care in the region, and the quality improvement tools developed for our trial need to be further improved and tested in future larger projects.
Recently, the Intervention to Bridge the Evidence-Based Gap in Stroke Care Quality (Golden-Bridge) trial, which included 40 clusters in China,20 showed a statistically significant improvement in the composite adherence score of 3.5% and a nonsignificant improvement of 6.7% with an all-or-none approach. These results are directionally similar to those seen in the BRIDGE-Stroke study, in which we observed an 8% nonsignificant improvement in the composite adherence score and a significant 25% improvement in the all-or-none measure. The Golden-Bridge study included only patients with AIS, whereas the BRIDGE-Stroke study included patients with AIS and TIA, and we found evidence of the interaction favoring patients with stroke; second, the BRIDGE-Stroke study assessed an international cluster assembly that expresses a stronger cluster effect as denoted by the larger ICC. Also, the BRIDGE-Stroke study assessed quality measures referring to the multidisciplinary stroke care pathway and was not restricted to medical prescriptions.
Owing to the nonpowered sample size for clinical outcomes and the observed low number of events with wide CIs, our results on clinical outcomes are exploratory. Nevertheless, we observed an increased incidence of hemorrhagic transformation events in clusters randomized to the intervention, probably owing to the increased use of rt-PA in patients with AIS. This finding is consistent with the higher hemorrhagic transformation incidence observed in large-scale trials of thrombolysis.5 Despite this finding, major bleeding and all-cause mortality (in-hospital and at 90 days) were similar between groups.
Strengths and Limitations
Our trial had specific strengths. The cluster-randomized design using the hospitals as the unit of randomization reduced the possibility of contamination. We prevented bias by using concealed allocation and blinded adjudication of outcomes. We analyzed data according to the intention-to-treat principle and took the cluster randomized trial design into account. We monitored screening logs from all sites to guarantee that clusters randomized a consecutive sample. Data were collected by independent research coordinators at each site, minimizing the risk of selective reporting of outcomes. Independent data collection was complemented by on-site monitoring and central statistical checks. The multifaceted intervention targeted 10 different quality indicators comprehending not only medical prescriptions but also assessment for rehabilitation and smoking cessation education.
Our trial has several limitations. First, for the intervention we had a hospital that did not adhere to the intervention because of dramatic changes in the leadership and management of the hospital, which could have influenced the general results of the study and certainly influenced the subgroup analysis per country as observed in Figure 2. Second, we found evidence of interaction in the subgroup analysis for clusters with vs without stroke units. Thus, our intervention may be best suited for hospitals that are more motivated and that are better organized to provide stroke care. Third, the observed effect size was lower and the ICC for our primary outcome was greater than the variables used to calculate our sample size, which may have limited our power to detect a positive finding on the primary outcome. Fourth, our study is underpowered to detect meaningful differences in clinical outcomes, and the losses observed in the 90-day follow-up might also limit the interpretation of these findings. Fifth, cluster randomized clinical trials are prone to additional limitations, such as lesser statistical power and the variation within or between clusters, compared with trials with randomization at the individual level. Nevertheless, clustering was considered in all reported analyses using appropriate methods.
Conclusions
A multifaceted quality improvement intervention did not result in a significantly increased composite adherence score for evidence-based therapies in patients with AIS or TIA. These findings do not support the routine use of our intervention among unselected institutions. However, our results generate the hypothesis that the intervention might improve adherence to evidence-based therapies using an all-or-none approach and might improve the use of thrombolysis in eligible patients. Therefore, our intervention might be particularly useful for patients with AIS and for hospitals that have stroke units and in which staff is motivated to adhere to the quality improvement tools. Further trials conducted in lower-resource settings and adequately powered to assess the impact of quality improvement interventions on clinical outcomes are warranted.
Trial Protocol
eMethods 1. Details of the Baseline Observational Phase
eMethods 2. Details of the Intervention
eTable 1. Use of Evidence-Based Therapies During the Observational Phase
eTable 2. Adherence to the Multifaceted Quality Improvement Intervention
eTable 3. Results of Quality Improvement Intervention on Use of Evidence-Based Therapies for Patients With Acute Ischemic Stroke
eTable 4. Results of Quality Improvement Intervention on Use of Evidence-Based Therapies for Patients with Transient Ischemic Attack
eTable 5. Results of Quality Improvement Intervention on Use of Evidence-Based Therapies
Data Sharing Statement
References
- 1.GBD 2016 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1260-1344. doi: 10.1016/S0140-6736(17)32130-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.França EB, Passos VMA, Malta DC, et al. . Cause-specific mortality for 249 causes in Brazil and states during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Popul Health Metr. 2017;15(1):39. doi: 10.1186/s12963-017-0156-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lotufo PA, Goulart AC, Passos VMA, et al. . Cerebrovascular disease in Brazil from 1990 to 2015: Global Burden of Disease 2015. Rev Bras Epidemiol. 2017;(20)(suppl 01):129-141. doi: 10.1590/1980-5497201700050011 [DOI] [PubMed] [Google Scholar]
- 4.Lees KR, Bluhmki E, von Kummer R, et al. ; ECASS, ATLANTIS, NINDS and EPITHET rt-PA Study Group . Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695-1703. doi: 10.1016/S0140-6736(10)60491-6 [DOI] [PubMed] [Google Scholar]
- 5.Emberson J, Lees KR, Lyden P, et al. ; Stroke Thrombolysis Trialists’ Collaborative Group . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929-1935. doi: 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Stroke Trial Collaborative Group The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. Lancet. 1997;349(9065):1569-1581. doi: 10.1016/S0140-6736(97)04011-7 [DOI] [PubMed] [Google Scholar]
- 7.Pahan K, Sheikh FG, Namboodiri AM, Singh I. Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J Clin Invest. 1997;100(11):2671-2679. doi: 10.1172/JCI119812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Wang Y, Zhao X, et al. ; CHANCE Investigators . Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369(1):11-19. doi: 10.1056/NEJMoa1215340 [DOI] [PubMed] [Google Scholar]
- 9.Ruff CT, Giugliano RP, Braunwald E, et al. . Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi: 10.1016/S0140-6736(13)62343-0 [DOI] [PubMed] [Google Scholar]
- 10.Alves MB, Silva GS, Miranda RCA, et al. . Patterns of care and temporal trends in ischemic stroke management: a Brazilian perspective. J Stroke Cerebrovasc Dis. 2017;26(10):2256-2263. doi: 10.1016/j.jstrokecerebrovasdis.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 11.Lange MC, Braga GP, Nóvak EM, et al. . Key performance indicators for stroke from the Ministry of Health of Brazil: benchmarking and indicator parameters. Arq Neuropsiquiatr. 2017;75(6):354-358. doi: 10.1590/0004-282x20170051 [DOI] [PubMed] [Google Scholar]
- 12.de Carvalho JJ, Alves MB, Viana GA, et al. . Stroke epidemiology, patterns of management, and outcomes in Fortaleza, Brazil: a hospital-based multicenter prospective study. Stroke. 2011;42(12):3341-3346. doi: 10.1161/STROKEAHA.111.626523 [DOI] [PubMed] [Google Scholar]
- 13.Sposato LA, Esnaola MM, Zamora R, Zurrú MC, Fustinoni O, Saposnik G; ReNACer Investigators; Argentinian Neurological Society . Quality of ischemic stroke care in emerging countries: the Argentinian National Stroke Registry (ReNACer). Stroke. 2008;39(11):3036-3041. doi: 10.1161/STROKEAHA.108.521062 [DOI] [PubMed] [Google Scholar]
- 14.Conforto AB, Paulo RB, Patroclo CB, et al. . Stroke management in a university hospital in the largest South American city. Arq Neuropsiquiatr. 2008;66(2B):308-311. doi: 10.1590/S0004-282X2008000300004 [DOI] [PubMed] [Google Scholar]
- 15.de Carvalho FA, Schwamm LH, Kuster GW, Bueno Alves M, Cendoroglo Neto M, Sampaio Silva G. Get With the Guidelines stroke performance indicators in a Brazilian tertiary hospital. Cerebrovasc Dis Extra. 2012;2(1):26-35. doi: 10.1159/000339578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuster GW, Dutra LA, Brasil IP, et al. . Outcome determinants of stroke in a Brazilian primary stroke center. Stroke Res Treat. 2014;2014:194768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Middleton S, McElduff P, Ward J, et al. ; QASC Trialists Group . Implementation of evidence-based treatment protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke (QASC): a cluster randomised controlled trial. Lancet. 2011;378(9804):1699-1706. doi: 10.1016/S0140-6736(11)61485-2 [DOI] [PubMed] [Google Scholar]
- 18.Lakshminarayan K, Borbas C, McLaughlin B, et al. . A cluster-randomized trial to improve stroke care in hospitals. Neurology. 2010;74(20):1634-1642. doi: 10.1212/WNL.0b013e3181df096b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston SC, Sidney S, Hills NK, et al. . Standardized discharge orders after stroke: results of the Quality Improvement in Stroke Prevention (QUISP) cluster randomized trial. Ann Neurol. 2010;67(5):579-589. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Li Z, Zhao X, et al. ; Golden Bridge—AIS Investigators . Effect of a multifaceted quality improvement intervention on hospital personnel adherence to performance measures in patients with acute ischemic stroke in China: a randomized clinical trial. JAMA. 2018;320(3):245-254. doi: 10.1001/jama.2018.8802 [DOI] [PubMed] [Google Scholar]
- 21.Machline-Carrion MJ, Santucci EV, Damiani LP, et al. . An international cluster-randomized quality improvement trial to increase the adherence to evidence-based therapies for acute ischemic stroke and transient ischemic attack patients: rationale and design of the BRIDGEStroke trial. Am Heart J. 2019;207:49-57. doi: 10.1016/j.ahj.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 22.Peterson ED, DeLong ER, Masoudi FA, et al. . ACCF/AHA 2010 position statement on composite measures for healthcare performance assessment: a report of American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures (Writing Committee to Develop a Position Statement on Composite Measures). J Am Coll Cardiol. 2010;55(16):1755-1766. doi: 10.1016/j.jacc.2010.02.016 [DOI] [PubMed] [Google Scholar]
- 23.Fonarow GC, Reeves MJ, Smith EE, et al. ; GWTG-Stroke Steering Committee and Investigators . Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in Get With the Guidelines–Stroke. Circ Cardiovasc Qual Outcomes. 2010;3(3):291-302. doi: 10.1161/CIRCOUTCOMES.109.921858 [DOI] [PubMed] [Google Scholar]
- 24.Fonarow GC, Zhao X, Smith EE, et al. . Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA. 2014;311(16):1632-1640. doi: 10.1001/jama.2014.3203 [DOI] [PubMed] [Google Scholar]
- 25.Panella M, Marchisio S, Brambilla R, Vanhaecht K, Di Stanislao F. A cluster randomized trial to assess the effect of clinical pathways for patients with stroke: results of the Clinical Pathways for Effective and Appropriate Care study. BMC Med. 2012;10:71. doi: 10.1186/1741-7015-10-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dirks M, Niessen LW, van Wijngaarden JD, et al. ; PRomoting ACute Thrombolysis in Ischemic StrokE (PRACTISE) Investigators . Promoting thrombolysis in acute ischemic stroke. Stroke. 2011;42(5):1325-1330. doi: 10.1161/STROKEAHA.110.596940 [DOI] [PubMed] [Google Scholar]
- 27.Scott PA, Meurer WJ, Frederiksen SM, et al. ; INSTINCT Investigators . A multilevel intervention to increase community hospital use of alteplase for acute stroke (INSTINCT): a cluster-randomised controlled trial. Lancet Neurol. 2013;12(2):139-148. doi: 10.1016/S1474-4422(12)70311-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods 1. Details of the Baseline Observational Phase
eMethods 2. Details of the Intervention
eTable 1. Use of Evidence-Based Therapies During the Observational Phase
eTable 2. Adherence to the Multifaceted Quality Improvement Intervention
eTable 3. Results of Quality Improvement Intervention on Use of Evidence-Based Therapies for Patients With Acute Ischemic Stroke
eTable 4. Results of Quality Improvement Intervention on Use of Evidence-Based Therapies for Patients with Transient Ischemic Attack
eTable 5. Results of Quality Improvement Intervention on Use of Evidence-Based Therapies
Data Sharing Statement