Abstract
Background
People with diabetes are at high risk for shoulder pain, limited joint mobility, and adhesive capsulitis.
Objective
The objective of this study was to evaluate the effects of a shoulder movement intervention (ShoMo) compared to a wellness intervention on the primary outcomes of active shoulder flexion and reported Shoulder Pain and Disability Index (SPADI) measured after intervention and 9 months later.
Design
The design was a prospective, randomized, controlled clinical trial.
Setting
The setting was a research center at an academic medical center.
Participants
Fifty-two participants with type 2 diabetes and shoulder pain or limited motion were randomized to a group receiving ShoMo (N = 27; mean age = 59.3; SD = 7.0) or a group receiving wellness activities (N = 25; mean age = 57.9; SD = 7.7).
Intervention
The ShoMo group received instruction in a progressive, active shoulder movement program. The wellness group received instruction in diabetes management.
Measurements
Measurements were made at baseline, after 3 months of intervention, and at 6, 9, and 12 months after baseline.
Results
After intervention, the ShoMo group had a 7.2-degree increase in active shoulder flexion compared with the wellness group (95% CI = 0.9–13.5°), but there was no difference at subsequent follow-ups. The ShoMo group showed a 12.7-point improvement in the SPADI score compared to the wellness group after intervention (95% CI = 1.1–24.3), which remained better than the wellness group 9 months later.
Limitations
The number of participants and duration of follow-up were inadequate to determine if intervention can help to prevent future severe shoulder problems.
Conclusions
A progressive shoulder movement program can have meaningful effects on active motion and symptoms in people with type 2 diabetes and mild-to-moderate shoulder symptoms, with symptom improvement lasting at least 9 months.
Musculoskeletal shoulder problems leading to pain and disability are substantial, under-recognized, and tend to worsen over time in people with type 2 diabetes.1–4 Unlike shoulder problems in people without diabetes, shoulder pain and musculoskeletal problems in general may be part of a systemic problem. For example, musculoskeletal pain in persons with type 2 diabetes is reported to be 1.7–2.1 times the rate compared to those without diabetes.5 Sixty-three percent of patients attending an outpatient diabetes clinic reported shoulder pain or disability as measured with the Shoulder Pain and Disability Index (SPADI).6 In another survey of patients attending a diabetes clinic who had pre-existing shoulder pain or disability, 58% reported clinically significant worsening (10 percentage points of SPADI) in shoulder pain over 12 months.4 These mild-to-moderate shoulder symptoms can become severe; people with diabetes have a risk ratio of 5.0 to 5.9 (95% CI = 3.3–7.5, P < .001) for developing idiopathic frozen shoulder compared to those without diabetes.7 These reports indicate that people with diabetes have frequent shoulder complications and the symptoms tend to worsen over time.
The systemic nature of progressive musculoskeletal disease in people with diabetes indicates that shoulder disorders may have a distinct metabolic and movement-related mechanism in this group.1,2,8–15 Although the exact mechanism is not fully understood, long-term hyperglycemia, as seen in diabetes, can lead to high levels of advanced glycation end-products (AGEs) in collagen-rich structures such as tendons, ligaments, and joint capsules,9–11 resulting in tissues that are thicker, stiffer, and more susceptible to injury.11–13 High AGE levels have been directly associated with musculoskeletal shoulder complications2,14 and may have a direct effect on inflammation and pain.15 These metabolic factors likely contribute to the insidious and progressive development of limited shoulder joint mobility and subsequent pain and disability.8 Common indicators of long-term hyperglycemia used in the clinic and reported in this study include duration of diabetes and the percent of glycated hemoglobin (A1C value), a 3-month indicator of serum glucose (A1C > 6.5% consistent with diabetes).16
Shoulder movement is a modifiable factor that can also contribute to shoulder pain and disability. A recent publication indicated that in addition to having higher AGE levels, people with type 2 diabetes had 23% lower shoulder activity (measured with accelerometers over a 24-hour period) compared to matched controls without type 2 diabetes.17 Accelerometers are increasingly being used to monitor and quantify duration and magnitude of upper extremity activity in the home and community.18–20 Important for this population with diabetes, low tissue loading from low activity levels is known to cause atrophy and reduced tolerance to subsequent stresses in bone, muscle, tendon, and ligament.21
Because people with diabetes demonstrate decreased shoulder motion and strength,8,17,22,23 we propose that specific movement and exercise strategies targeting progressive stretching and strengthening will help to reverse these shoulder complications. Although there is some evidence that therapeutic exercise can have a positive effect on shoulder pain and function,24 the specific effect on people with diabetes, metabolic abnormalities, and shoulder complications is unknown. Treating patients with diabetes and mild-to-moderate shoulder symptoms is important to help manage the pain and disability associated with this complication. Because shoulder symptoms are known to worsen over time,4 intervention also may have implications for secondary prevention of future severe shoulder complications known to occur in this population.1–3,7
This study evaluated the effects of a 3-month shoulder movement intervention compared to a wellness program on the primary outcomes of active shoulder range of motion (ROM) and reported shoulder pain and disability (SPADI) in participants with type 2 diabetes with mild-to-moderate shoulder symptoms (decreased motion, pain) measured after intervention and 9 months after intervention. Secondary outcomes included magnitude and duration of shoulder activity, shoulder flexion strength, and passive shoulder flexion of the involved arm. We hypothesized that the group receiving the shoulder movement intervention would have improved outcomes at the end of intervention and at 1-year follow-up compared to the group who received the wellness program.
Methods
Study Design and Randomization
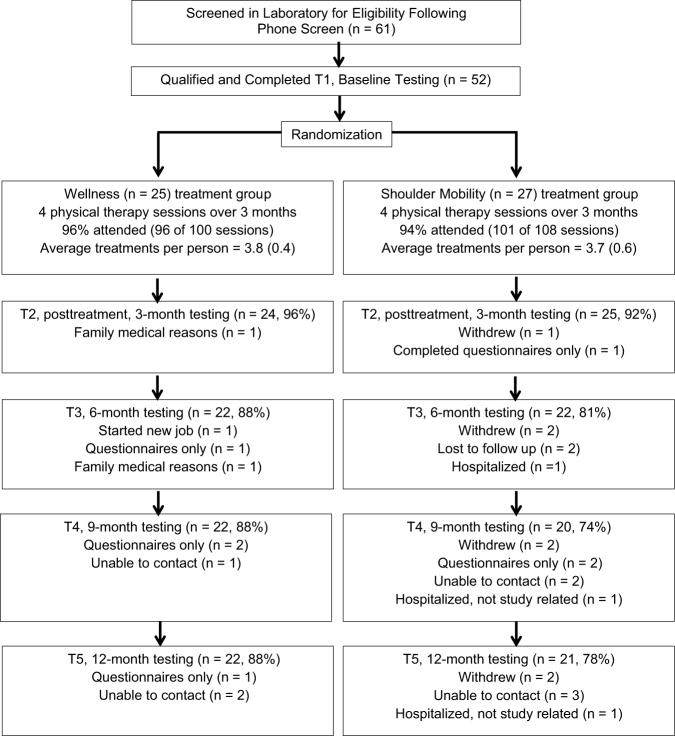
This prospective randomized controlled clinical trial contrasted a 3-month progressive shoulder movement intervention (ShoMo group) with a wellness intervention (wellness group). Participants who met the inclusion criteria, provided written informed consent, and completed their baseline testing were allocated into 2 groups using a computer-generated block randomization sequence provided by the statistician, with a block size of 4 and stratification according to sex. The study coordinator used this computer-generated sequence for group assignment, and the sequence was concealed from study personnel enrolling participants. Participants were tested 4 more times over 1 year for a total of 5 testing sessions (Fig. 1, T1–T5).
Figure 1.
CONSORT flow diagram including the number of people who attended each follow-up session and reasons people missed the follow-up session. Note that a participant could miss 1 follow-up session but remain in the study and attend subsequent follow-up sessions. T1–T5 = testing sessions 1 through 5.
Sample Size and Recruitment
Recruitment began in September 2014 and all data acquisition was complete by November 2016. A priori power analyses were based on 2-sided statistical tests at a significance level of .05 and 80% power, using 2-sample t tests with anticipated effect sizes of mean difference between groups over time for an increase of 10 (SD = 11) degrees in shoulder flexion and a 15-point (SD = 17) decrease in the SPADI scores25 of the ShoMo group versus the wellness group. Allowing for an 18% dropout rate over the 1 year would require a total of 52 participants (26 in each group). The change in shoulder flexion was based on our clinical impression of a feasible and meaningful change, and SDs were taken from our pilot work.6,14,23 Participants were recruited through the Washington University Diabetes Center and 2 community-based, university-operated recruitment services (Tab. 1).
Table 1.
Participant Characteristics
| Participant Characteristic | Group | |
|---|---|---|
| Wellness (n = 25) | ShoMo (n = 27) | |
| Sex: female, male | 14, 11 | 15, 12 |
| Race: African American, white, more than 1 | 10, 22, 3 | 12, 14, 1 |
| Age, y (SD) | 57.9 (7.7) | 59.3 (7.0) |
| Body mass index, kg/m2 (SD) | 35.8 (9.8) | 33.9 (5.2) |
| A1C % (SD) | 7.41 (1.27) | 7.57 (1.85) |
| A1C mmol/mol (SD) | 57.57 (13.82) | 59.19 (20.20) |
| Duration of diabetes, y (SD) | 13.90 (5.85) | 12.72 (5.69) |
| Positive prayer sign: no, yes | 18, 7 | 18, 9 |
Inclusion criteria
The aim of our inclusion criteria was to identify people with diabetes with mild-to-moderate shoulder symptoms who were at high risk for developing severe shoulder problems.4,26–32 We specifically included: individuals with type 2 diabetes between the ages of 40 and 70 years and with a duration of diagnosed type 2 diabetes of more than 10 years OR presence of a “positive prayer sign”1,3,6 (indicative of limited joint mobility in the hand), OR shoulder flexion < 150 degrees; OR a SPADI score greater than 20 but less than 70.
Exclusion criteria
Because we were targeting people with relatively early shoulder involvement, participants with severe shoulder problems such as a currently diagnosed adhesive capsulitis, rotator cuff tears, recent (within the past 6 months) upper extremity injury and/or fractures, surgery in the upper extremity or thorax, cervical radiculopathy, thoracic outlet syndrome, carpal tunnel syndrome, stroke with residual upper extremity involvement, severe skin allergies in area to be tested, rheumatic conditions, use of a cane or other mobility assistance, and known connective tissue diseases were excluded. We also excluded people who engaged in heavy upper extremity/overhead use (eg, painters, tennis players) because they likely have a different mechanism of injury than those with diabetes and low shoulder activity. These criteria helped us capture the shoulder impairments early in the disease process before the onset of severe pain, disability, or reduction of ROM and strength.
Masking
All outcome measures were collected by trained investigators who were masked with regard to group assignment. We were unable to mask for exercise intervention. Therefore, participants and the physical therapists providing treatment knew group assignment.
Interventions
Shoulder mobility program (ShoMo)
The experimental intervention was an optimized shoulder movement program, standardized and described in detail in the eAppendix (available at https://academic.oup.com/ptj). Participants were seen by a trained physical therapist for 4 visits (30–60 minutes’ duration each visit) during the 3-month intervention period. The visits consisted of the therapist teaching the participant the exercises included in the shoulder movement program to enable the participant to become independent in a home exercise program. We believed the progressive nature of the end-range active shoulder mobility exercises would be the most important aspect (ie, the active ingredient) of the intervention program leading to improvements. Briefly, participants started with passive stretching of end-range shoulder flexion and rotation (internal, external) that progressed to active and then resisted shoulder motions tailored to their ability level. Participants were instructed to perform 3 assigned stretching motions for a minimum of 2 sets of 10 repetitions every day. Participants also were instructed in active shoulder movement that could be incorporated into daily activities with a dose based on the participant's measured activity count at baseline using accelerometers.17 Anticipated duration of home exercise was 10 to 15 minutes, 2 times per day. The upper extremity movements are based on results of our previous research in this population,14,23 conclusions from other clinical trials,24,33 and the clinical and research experience of the authors who have successfully managed upper extremity problems and who have documented the need to achieve sufficient “dose” of activity to make meaningful change.34
Wellness program
The intent of the wellness program was to control for time and personal interactions with physical therapists (participants seen 4 times over 3 months) and investigators, and to provide useful information for disease management, but not provide intervention that directly targeted shoulder joint motion. This wellness program was based on American Diabetes Association guidelines35 and included instruction in blood sugar control (goal is HbA1c < 7.0%), physical activity (150 min/wk of moderate intensity aerobic activity), foot care (examine feet daily, monofilament testing), and blood pressure control (goal is < 130/80 mm Hg). Additional details are provided in the eAppendix.
Participants in both groups were given a logbook to record the amount of prescribed activity completed each day during the 3-month intervention period (a visual appraisal scale with 0 indicating no prescribed exercises were performed and 100% indicating all prescribed exercises were performed). At the final testing session (Fig. 1, T5), participants were given the following 1-question survey to record self-reported adherence to home exercises during the previous 9 months of unsupervised participation in the study: “Over the past 9 months, what percentage of your activities or exercises as instructed by your physical therapist did you actually perform?” They were given a row of options ranging from 0% to 100% in 10% increments. Participants in both groups were given 10 dollars cash at the completion of every treatment visit to cover travel expenses and serve as an incentive for attendance.
Measures
Testing of all measures occurred 5 times: at baseline immediately before intervention started, after the 3-month intervention period, and again at 6, 9, and 12 months after baseline, as illustrated in Figure 1.
Primary Outcomes and End Points
The primary outcomes were active shoulder flexion ROM and the SPADI. The key end points were immediately after intervention (3 months after baseline) to determine effect of intervention and 9 months later (12 months after baseline) to determine if the intervention effect persisted.
The involved arm was selected and recorded for each participant based on the following criteria: a) the more painful side as reported by the participant, b) if there was not a more painful side, the side with less passive shoulder flexion ROM, and c) if passive shoulder flexion ROM was equal, the side with less external ROM. Active shoulder flexion ROM of the involved shoulder was measured as the angle between humerus and thorax with a digital goniometer (JAMAR, Patterson Medical, Warrenville, Illinois) while the participant was seated.36,37
The SPADI is a standardized 13-item self- report questionnaire with 5 items specific to shoulder pain and 8 items specific to shoulder disability.38,39 The SPADI score can range from 0%, indicating no pain or disability, to 100%, indicating severe pain and total disability.39 SPADI measurement properties have been studied extensively, with reported excellent reliability and a minimal clinically important difference (MCID) between 8 and 13.4,25,40,41 Another recent publication reported a 43% or more change in score from baseline to be clinically relevant.42
Secondary Outcomes and End Points
Secondary outcome measures were 1) magnitude and duration of shoulder activity, 2) shoulder flexion strength of the involved arm, and 3) passive shoulder flexion of the involved arm. Shoulder activity was measured using triaxial accelerometers (GT3X + Activity Monitor, ActiGraph, Pensacola, Florida) and previously established methods17–20 except that the accelerometers were placed proximal to both elbow joints rather than the wrists to help isolate shoulder activity. Accelerometers were placed on both arms after each of the 5 laboratory testing sessions. Participants were given a prepaid, preaddressed shipping envelope to return the devices after wearing them for 24 hours, a time consistent with previous studies and long enough to obtain representative data, but not so long as to be burdensome or reduce wearing adherence. Because there were no differences in any measures of shoulder activity between arms, only data from the right arm are reported. Shoulder flexion strength was measured using a handheld digital strain-gauge dynamometer (Microfet, Hoggan Health Industries Inc, West Jordan, Utah) with the participant in a supine position and the shoulder in 90 degrees of flexion using standardized methods and stabilization.43 Passive shoulder flexion ROM of the involved shoulder was measured as the angle between humerus and thorax with a digital goniometer while the participant was supine.36,37 Two measures were obtained and averaged for the shoulder ROM variable and shoulder strength. All outcomes were collected at the secondary end points of 6, 9, and 12 months after baseline measures to determine how long any effect lasted.
Data Analysis
Primary outcomes of active shoulder flexion ROM and SPADI score were analyzed using repeated measures analysis of variance (ANOVA) and tested for between-group over-time changes (interactions) between baseline and 3-month testing (following treatment) as the primary end point to determine effects following the intervention; and between baseline and at 12 months after baseline to determine if effects continued. All primary and secondary outcome variables also were analyzed using repeated measures ANOVA implemented with a linear mixed-effects model to test for between-group over-time changes (interactions) at all end points (3, 6, 9, and 12 months after baseline). Between-group differences over time and 95% CI were estimated from the linear mixed-effects models. All analyses were performed in SAS version 9.4 (SAS Institute, Cary, North Carolina).
Role of the Funding Source
This research was funded by NIH Grant R21 DK100793 (M.J.M.), and the Diabetes Research Center at Washington University School of Medicine (NIH P30 DK020579). The funder played no role in the design, conduct, or reporting of this study.
Results
Participant Characteristics
Participant characteristics are summarized in Table 1; there were 27 participants in the ShoMo group and 25 participants in the wellness group. All characteristics were similar between groups. Figure 1 shows the CONsolidated Standards of Reporting Trials (CONSORT) diagram. Mean and standard deviation of duration of diabetes and A1C of total sample were 13.3 (5.7) years and 7.5% (1.6), respectively, indicative of longstanding elevated hyperglycemia consistent with recruitment aims.16 Ninety-four percent (101 of 108) of the treatment sessions for the ShoMo group were completed, and 96% (96 of 100) of the treatment sessions for the wellness group were completed (Fig. 1). Forty-three of 52 (82.7%) participants completed all testing at the 1-year follow-up session, consistent with our anticipated dropout rate (18%). One participant completed the SPADI by mail but did not complete shoulder ROM and strength measures. At 1-year follow-up, participants in the ShoMo group self-reported that they were 55.2% (SD = 22.1) compliant with their home exercise program, whereas the wellness group self-reported 62.1% (SD = 27.6) compliance with their assigned daily guidelines since completing supervised treatments at 3 months.
Adverse Events
There were no adverse events directly related to treatment in either group.
Primary Outcomes
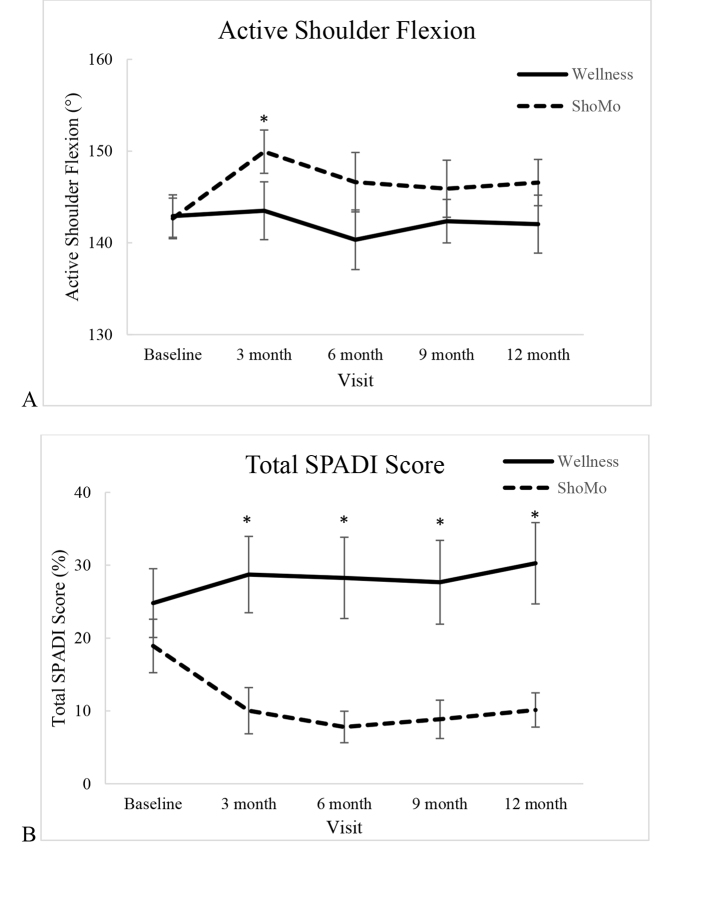
Group means and standard deviations for time points at baseline, after 3 months of intervention, and 12 months after baseline are provided along with mean within- and between-group differences over time after 3 months of intervention, and 12 months after baseline for all outcome variables in Table 2. Group means and standard errors for baseline and all follow-up time points are illustrated in Figure 2 to help visualize the change in scores over time.
Table 2.
Summary of Results of Primary and Secondary Outcomes
| Variable | Group | Baseline Mean (SD) | 3 Mo Mean (SD) | Mean Within-Group Difference (95% CI) Baseline to 3 Mo | Mean Between-Group Difference, Change Over Time (95% CI) Baseline to 3 Mo | 12 Mo Mean (SD) | Mean Within-Group Difference (95% CI) Baseline to 12 Months | Mean Between-Group Difference, Change Over Time (95% CI) Baseline to 12 Months |
|---|---|---|---|---|---|---|---|---|
| Primary outcomes | ||||||||
| Involved active shoulder flexion (°) | Wellness | 142.9 (11.6) | 143.5 (15.4) | 0.5 (–5.1 to 6.1) | 142.0 (14.8) | –0.4 (–5.9 to 5.1) | ||
| ShoMo | 142.7 (11.5) | 149.9 (11.8) | 7.7 (4.3 to 11.1) | –7.2 (–13.5 to –0.9)a | 146.6 (11.6) | 5.1 (0.8 to 9.4) | –5.5 (–12.3 to 1.3) | |
| Total SPADI (%) | Wellness | 24.8 (23.6) | 28.7 (25.7) | 3.1 (–5.3 to 11.6) | 30.3 (26.8) | 6.8 (–1.7 to 15.4) | ||
| ShoMo | 18.9 (19.1) | 10.0 (16.1) | –9.5 (–17.9 to –1.1) | 12.7 (1.1 to 24.3)a | 10.1 (10.8) | –6.3 (–13.1 to 0.5) | 13.1 (2.4 to 23.9)a | |
| Secondary outcomes | ||||||||
| Involved passive shoulder flexion (°) | Wellness | 154.8 (14.9) | 152.7 (17.6) | –2.0 (–7.3 to 3.2) | 149.5 (17.7) | –5.0 (–11.2 to 1.2) | ||
| ShoMo | 158.5 (12.6) | 160.3 (11.4) | 2.3 (–0.7 to 5.3) | –4.3 (–10.1 to 1.5) | 159.2 (11.8) | 2.1 (–1.2 to 5.5) | –7.1 (–14.0 to –0.2) | |
| Involved shoulder flexion strength | Wellness | 32.8 (11.0) | 33.9 (11.0) | 0.8 (–2.0 to 3.7) | 38.5 (9.1) | 5.4 (1.3 to 9.4) | ||
| ShoMo | 34.6 (11.7) | 37.2 (12.4) | 3.4 (0.3 to 6.6) | –2.6 (–6.7 to 1.5) | 39.4 (11.3) | 4.5 (0.0 to 8.9) | 0.9 (–4.9 to 6.7) | |
| Right shoulder magnitude (g) of activity | Wellness | 2298.8 (870.0) | 2104.9 (962.0) | –195.0 (–533.1 to 143.0) | 2036.4 (882.0) | –187.7 (–511.7 to 136.3) | ||
| ShoMo | 1930.0 (620.5) | 1763.2 (472.5) | –84.6 (–269.7 to 100.5) | –110.4 (–488.1 to 267.3) | 1969.7 (823.3) | 140.9 (–130.2 to 412.1) | –328.6 (–745.1 to 77.8) | |
| Right shoulder duration of activity (hours) | Wellness | 6.7 (2.0) | 6.1 (2.5) | –0.5 (–1.3 to 0.4) | 6.0 (2.1) | –0.6 (–1.3 to 0.2) | ||
| ShoMo | 5.6 (1.4) | 5.2 (1.2) | –0.2 (–0.7 to 0.3) | –0.3 (–1.2 to 0.7) | 5.8 (2.1) | 0.4 (–0.4 to 1.2) | –1.9 (–2.0 to 0.1) | |
a P < .05.
Figure 2.
Means and standard error of (A) active shoulder flexion of the involved arm and (B) Shoulder Pain and Disability Index (SPADI) at baseline, immediately following treatment (3 months) and 6, 9, and 12 months after baseline. * = P < .05.
Involved shoulder active ROM
The ShoMo group had a 7.2-degree increase in active shoulder flexion compared to the wellness group after 3 months of intervention (95% CI = 0.9–13.5). However, the difference did not persist past 3 months, as indicated by insignificant group by time interactions at the 6-, 9-, and 12-month testing sessions (Fig. 2A, Tab. 2).
SPADI
The ShoMo group showed a 12.7-point improvement in the total SPADI score compared to the wellness group following 3 months of intervention (95% CI = 1.1–24.3). The significant difference between groups over time persisted throughout the 6-, 9-, and 12-month follow-up time points (Fig. 2B, Tab. 2). At 1-year follow-up, the ShoMo group had a 13.1-point (95% CI = 2.4–23.9) between-groups over-time improvement in SPADI score compared with the wellness group.
Secondary Outcomes
There were no significant group by time interactions for magnitude and duration of shoulder activity, shoulder strength of the involved arm, or passive shoulder flexion of the involved arm at any of the time points (Tab. 2).
Discussion
The results of this prospective, randomized clinical trial indicate that participants with type 2 diabetes and mild-to-moderate shoulder symptoms had improved active shoulder motion and reported reduced pain and disability after a progressive, active shoulder motion intervention compared with a group who received wellness training. The shoulder mobility program demonstrated high attendance (94%), low dropout (8%), and no adverse events directly related to treatment, indicating that this intervention can be performed safely and without injury (Fig. 1). The results are important because they show that a relatively simple progression of shoulder movement exercises can reduce reported shoulder pain and disability in a patient population that normally worsens over time.4 The improvement in active shoulder ROM seen following intervention did not persist at subsequent testing (ie, between-group difference was no longer statistically significant at 6-, 9-, and 12-month follow-up testing) (Fig. 2); however, improvements in shoulder pain and disability did persist with the ShoMo group reporting SPADI scores of only about one-third that of the wellness group (Tab. 2) at 1 year.
The self-reported shoulder pain and disability score (SPADI) showed a sustained and clinically meaningful between-group difference of 13 points after treatment and a within-group drop of 47% (18.9–10.0) that persisted throughout follow-up at 1 year.4,42 These improvements surpass previously documented MCID values for the SPADI (8–13 points, or 43% of baseline value).25,42 We believe the most important component for change (the “active ingredient”) of the ShoMo program was the progressive nature of the end-range active shoulder motions encouraged as long as movements did not increase pain or symptoms. The wellness group received the same number and duration of treatment sessions and had attendance and dropout rates similar to those of the ShoMo group; therefore, we do not believe the improvements seen in the ShoMo group can be attributed to personal attention or interactions.
Furthermore, the improvements were seen with a small number of visits, 4, and a relatively easy-to-implement progressive exercise program taught by physical therapists (eAppendix). Although as many as 46% to 63% of patients with diabetes report shoulder pain and disability,4,6 it is our observation that few are referred for physical therapy or other treatment. This shoulder mobility program has the potential to have an impact on those with diabetes known to have decreased shoulder ROM,8,22,23 decreased shoulder activity, higher reports of shoulder pain and disability,17 and high AGE levels2,14 that typically worsen over time.4 Although long-term hyperglycemia and high AGE levels historically have been implicated as the primary cause of diabetic shoulder pain and disability,2,3,9,10,14,15,44 limited shoulder movement may be the more modifiable and clinically meaningful target for treatment to implement improvements.17
The improvement in active shoulder flexion following treatment (between-group differences of 7.2°) was modest, and the between-group difference at 1-year follow-up (5.5°) was not statistically significant. One reason for this modest improvement is that initial active shoulder flexion (143°; SD = 12) of participants in this study was only minimally impaired compared to the active shoulder flexion (150°; SD = 10) of age-matched controls without diabetes or shoulder impairments reported in another study.14 We could find no other studies investigating the effect of an exercise or mobility program on early onset diabetic shoulder complications. A meta-analysis on the effectiveness of therapeutic exercise for painful shoulder conditions, however, did conclude that the rapeutic exercise has a positive effect on pain and function above all other interventions, whereas the effect on ROM was inconclusive, consistent with the findings in this study.24 Additional research is needed to help determine optimal intensity of and adherence to the intervention including the subsequent home exercise program. Future work also should determine if this type of movement program can help to prevent the high rate and progression of severe shoulder complications (eg, adhesive capsulitis or “frozen shoulder”) seen in people with diabetes.2,4,7 Better understanding of the etiology and systemic nature of musculoskeletal problems in people with diabetes may help drive pharmaceutical, nutritional, and movement interventions to reduce these complications.
There are limitations of this study that should be considered when interpreting these data. First, this study was an early phase trial; a relatively small number of participants were enrolled to explore if a movement program could affect shoulder pain and disability. The study needs to be replicated in a larger trial that follows participants for a longer duration of time. An important outcome for future work would be to determine if a shoulder mobility program could help to prevent severe shoulder complications in people with type 2 diabetes. Purposefully, the participants in this study had only mild-to-moderate losses in shoulder mobility and symptoms of pain and disability (Tab. 1). Given the stated inclusion criteria, some participants had relatively little room for improvement. Those patients who develop severe shoulder symptoms (eg, acute adhesive capsulitis or rotator cuff tear) likely require a more extensive evaluation and treatment management than described in this study.
In summary, people with type 2 diabetes commonly have a loss of shoulder ROM and pain, likely caused by metabolic and movement factors. These results indicate that a fairly simple progressive shoulder movement program can have an effect on active motion and symptoms, with benefits of symptom improvement lasting at least a year. Additional research is needed to determine feasible implementation, and importantly, to determine if active shoulder exercise could produce sustainable improvements in shoulder motion and reduce the incidence of adhesive capsulitis in this population.
Supplementary Material
Author Contributions and Acknowledgments
Concept/idea/research design: M.J. Mueller, C.J. Sorensen, J.B. McGill, B.R. Clark, C.E. Lang, L. Chen, K.L. Bohnert, M.K. Hastings
Writing: M.J. Mueller, C.J. Sorensen
Data collection: K.L. Bohnert, M.K. Hastings
Data analysis: L. Chen
Fund procurement: M.J. Mueller
Providing participants: J.B. McGill
Consultation (including review of manuscript before submitting): M.J. Mueller, C.J. Sorensen, J.B. McGill, B.R. Clark, C.E. Lang, L. Chen, K.L. Bohnert, M.K. Hastings
Ellen Frye, Whitney Korgan, Jessica Luth, Rose Reida, and Darrah Snozek provided valuable assistance with participant interventions, data entry, data analyses, and participant recruitment.
Ethics Approval
The study was approved by the Washington University Human Research Protection Office and registered at clinicaltrials.gov before recruitment of any subjects (ref. no. NCT02162212). The authors acknowledge a discrepancy between the use of SPADI, as reported here, and the use of the Disabilities of the Arm, Shoulder, and Hand (DASH)25 as reported a priori at clinicaltrials.gov. The reason for this discrepancy is that during final review for external funding, 1 reviewer expressed concern that the SPADI may be more responsive than the DASH for this shoulder-specific application. Therefore, we collected data on both the DASH and SPADI but neglected to update clinicaltrials.gov. The SPADI did prove to be more responsive to change than the DASH in this study, so SPADI is reported here.
Funding
This research was funded by a National Institutes of Health (NIH) grant (R21 DK100793) (M.J.M.) and the Diabetes Research Center at Washington University School of Medicine in St Louis (NIH P30 DK020579).
Clinical Trial Registration
This clinical trial was registered at ClinicalTrials.gov (ref. no. NCT02162212).
Disclosures and Presentations
The authors completed the ICJME Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
Parts of this study were presented in abstract form at the American Physical Therapy Association Combined Section Meeting, San Antonio, Texas, February 15–18, 2017, and as a poster at the 77th Scientific Session of the American Diabetes Association, San Diego, California, June 9–13, 2017.
References
- 1. Mueller MJ. Musculoskeletal impairments are often unrecognized and underappreciated complications from diabetes. Phys Ther. 2016;96:1861–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Larkin ME, Barnie A, Braffett BH et al. . Musculoskeletal complications in type 1 diabetes. Diabetes Care. 2014;37:1863–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mustafa KN, Khader YS, Bsoul AK, Ajlouni K. Musculoskeletal disorders of the hand in type 2 diabetes mellitus: prevalence and its associated factors. Int J Rheum Dis. 2016;19:730–735. [DOI] [PubMed] [Google Scholar]
- 4. Laslett LL, Burnet SP, Redmond CL, McNeil JD. Predictors of shoulder pain and shoulder disability after one year in diabetic outpatients. Rheumatology (Oxford). 2008;47:1583–1586. [DOI] [PubMed] [Google Scholar]
- 5. Molsted S, Tribler J, Snorgaard O. Musculoskeletal pain in patients with type 2 diabetes. Diabetes Res Clin Pract. 2012;96:135–140. [DOI] [PubMed] [Google Scholar]
- 6. Shah KM, Clark BR, McGill JB, Mueller MJ. Upper extremity impairments, pain and disability in patients with diabetes mellitus. Physiotherapy. 2015;101:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Milgrom C, Novack V, Weil Y, Jaber S, Radeva-Petrova DR, Finestone A. Risk factors for idiopathic frozen shoulder. Isr Med Assoc J. 2008;10:361–364. [PubMed] [Google Scholar]
- 8. Abate M, Schiavone C, Pelotti P, Salini V. Limited joint mobility (LJM) in elderly subjects with type II diabetes mellitus. Arch Gerontol Geriatr. 2011;53:135–140. [DOI] [PubMed] [Google Scholar]
- 9. Brownlee M. Glycation products and the pathogenesis of diabetic complications. Diabetes Care. 1992;15:1835–1843. [DOI] [PubMed] [Google Scholar]
- 10. Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol (1985). 2007;103:2068–2076. [DOI] [PubMed] [Google Scholar]
- 11. Reddy GK. Cross-linking in collagen by nonenzymatic glycation increases the matrix stiffness in rabbit achilles tendon. Exp Diabesity Res. 2004;5:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klaesner JW, Hastings MK, Zou DQ, Lewis C, Mueller MJ. Plantar tissue stiffness in patients with diabetes mellitus and peripheral neuropathy. Arch Phys Med Rehabil. 2002;83:1796–1801. [DOI] [PubMed] [Google Scholar]
- 13. Tang SY, Vashishth D. Non-enzymatic glycation alters microdamage formation in human cancellous bone. Bone. 2010;46:148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shah KM, Clark BR, McGill JB, Lang CE, Maynard J, Mueller MJ. Relationship between skin intrinsic fluorescence—an indicator of advanced glycation end products—and upper extremity impairments in individuals with diabetes mellitus. Phys Ther. 2015;95:1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15:16R–28R. [DOI] [PubMed] [Google Scholar]
- 16. American Diabetes A. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes–2018. Diabetes Care. 2018;41:S13–S27. [DOI] [PubMed] [Google Scholar]
- 17. Sorensen CJ, Hastings MK, Lang CE et al. . Relationship of shoulder activity and skin intrinsic fluorescence with low level shoulder pain and disability in people with type 2 diabetes. J Diabetes Complications. 2017;31:983–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bailey RR, Klaesner JW, Lang CE. Quantifying real-world upper-limb activity in nondisabled adults and adults with chronic stroke. Neurorehabil Neural Repair. 2015;29:969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bailey RR, Klaesner JW, Lang CE. An accelerometry-based methodology for assessment of real-world bilateral upper extremity activity. PLoS One. 2014;9:e103135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bailey RR, Lang CE. Upper-limb activity in adults: referent values using accelerometry. J Rehabil Res Dev. 2013;50:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mueller MJ, Maluf KS. Tissue adaptation to physical stress: a proposed “Physical Stress Theory” to guide physical therapist practice, education, and research. Phys Ther. 2002;82:383–403. [PubMed] [Google Scholar]
- 22. Schulte L, Roberts MS, Zimmerman C, Ketler J, Simon LS. A quantitative assessment of limited joint mobility in patients with diabetes. Goniometric analysis of upper extremity passive range of motion. Arthritis Rheum. 1993;36:1429–1443. [DOI] [PubMed] [Google Scholar]
- 23. Shah KM, Clark BR, McGill JB, Lang CE, Mueller MJ. Shoulder limited joint mobility in people with diabetes mellitus. Clin Biomech. 2015;30:308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marinko LN, Chacko JM, Dalton D, Chacko CC. The effectiveness of therapeutic exercise for painful shoulder conditions: a meta-analysis. J Shoulder Elbow Surg. 2011;20:1351–1359. [DOI] [PubMed] [Google Scholar]
- 25. Roy JS, MacDermid JC, Woodhouse LJ. Measuring shoulder function: a systematic review of four questionnaires. Arthritis Rheum. 2009;61:623–632. [DOI] [PubMed] [Google Scholar]
- 26. Cagliero E, Apruzzese W, Perlmutter GS, Nathan DM. Musculoskeletal disorders of the hand and shoulder in patients with diabetes mellitus. Am J Med. 2002;112:487–490. [DOI] [PubMed] [Google Scholar]
- 27. Pal B, Anderson J, Dick WC, Griffiths ID. Limitation of joint mobility and shoulder capsulitis in insulin- and non-insulin-dependent diabetes mellitus. Br J Rheumatol. 1986;25:147–151. [DOI] [PubMed] [Google Scholar]
- 28. Balci N, Balci MK, Tuzuner S. Shoulder adhesive capsulitis and shoulder range of motion in type II diabetes mellitus: association with diabetic complications. J Diabetes Complications. 1999;13:135–140. [DOI] [PubMed] [Google Scholar]
- 29. Smith LL, Burnet SP, McNeil JD. Musculoskeletal manifestations of diabetes mellitus. Br J Sports Med. 2003;37:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ramchurn N, Mashamba C, Leitch E et al. . Upper limb musculoskeletal abnormalities and poor metabolic control in diabetes. Eur J Intern Med. 2009;20:718–721. [DOI] [PubMed] [Google Scholar]
- 31. Thomas SJ, McDougall C, Brown ID et al. . Prevalence of symptoms and signs of shoulder problems in people with diabetes mellitus. J Shoulder Elbow Surg. 2007;16:748–751. [DOI] [PubMed] [Google Scholar]
- 32. Rosenbloom AL, Silverstein JH, Lezotte DC, Richardson K, McCallum M. Limited joint mobility in childhood diabetes mellitus indicates increased risk for microvascular disease. N Engl J Med. 1981;305:191–194. [DOI] [PubMed] [Google Scholar]
- 33. Griggs SM, Ahn A, Green A. Idiopathic adhesive capsulitis. A prospective functional outcome study of nonoperative treatment. J Bone Joint Surg Am. 2000;82-A:1398–1407. [PubMed] [Google Scholar]
- 34. Lang CE, Macdonald JR, Reisman DS et al. . Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil. 2009;90:1692–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. American Diabetes A. Standards of medical care in diabetes–2012. Diabetes Care. 2012;35:S11–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sabari JS, Maltzev I, Lubarsky D, Liszkay E, Homel P. Goniometric assessment of shoulder range of motion: comparison of testing in supine and sitting positions. Arch Phys Med Rehabil. 1998;79:647–651. [DOI] [PubMed] [Google Scholar]
- 37. Riddle DL, Rothstein JM, Lamb RL. Goniometric reliability in a clinical setting. Shoulder measurements. Phys Ther. 1987;67:668–673. [DOI] [PubMed] [Google Scholar]
- 38. MacDermid JC, Solomon P, Prkachin K. The Shoulder Pain and Disability Index demonstrates factor, construct and longitudinal validity. BMC Musculoskelet Disord. 2006;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a Shoulder Pain and Disability Index. Arthritis Care Res. 1991;4:143–149. [PubMed] [Google Scholar]
- 40. Paul A, Lewis M, Shadforth MF, Croft PR, Van Der Windt DA, Hay EM. A comparison of four shoulder-specific questionnaires in primary care. Ann Rheum Dis. 2004;63:1293–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schmitt JS, Di Fabio RP. Reliable change and minimum important difference (MID) proportions facilitated group responsiveness comparisons using individual threshold criteria. J Clin Epidemiol. 2004;57:1008–1018. [DOI] [PubMed] [Google Scholar]
- 42. Thoomes-de Graaf M, Scholten-Peeters W, Duijn E et al. . The responsiveness and interpretability of the Shoulder Pain and Disability Index. J Orthop Sports Phys Ther. 2017;47:278–286. [DOI] [PubMed] [Google Scholar]
- 43. Bohannon RW. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch Phys Med Rehabil. 1997;78:26–32. [DOI] [PubMed] [Google Scholar]
- 44. Abate M, Schiavone C, Salini V, Andia I. Management of limited joint mobility in diabetic patients. Diabetes Metab Syndr Obes. 2013;6:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.