ABSTRACT
Background
Some dietary factors have been linked to outcomes of infertility treatment with assisted reproductive technology (ART), but the role of intake of meats and other protein-rich foods remains unclear.
Objective
The aim of this manuscript was to study the relation between preconception intake of meat and other protein-rich foods and outcomes of infertility treatment with ART.
Design
A total of 351 women enrolled in a prospective cohort at the Massachusetts General Hospital Fertility Center and underwent 598 ART cycles for infertility treatment. Meat intake was assessed with a validated food-frequency questionnaire, and ART outcomes were abstracted from electronic medical records. We estimated the associations between intake of protein-rich foods (meats, eggs, beans, nuts, and soy) and the outcome of live birth per initiated cycle using generalized linear mixed models.
Results
The average total meat intake was 1.2 servings/d, with most coming from poultry (35%), fish (25%), processed meat (22%), and red meat (17%). Fish intake was positively related to the proportion of cycles resulting in live birth. The multivariable-adjusted probabilities of live birth for women in increasing quartiles of fish intake were 34.2% (95% CI: 26.5%, 42.9%), 38.4% (95% CI: 30.3%, 47.3%), 44.7% (95% CI: 36.3%, 53.4%), and 47.7% (95% CI: 38.3%, 57.3%), respectively (P-trend = 0.04). In the estimated substitution analyses, the ORs of live birth associated with increasing fish intake by 2 servings/wk were 1.54 (95% CI: 1.14, 2.07) when fish replaced any other meat, 1.50 (95% CI: 1.13, 1.98) when fish replaced any other protein-rich food, and 1.64 (95% CI: 1.14, 2.35) when fish replaced processed meat.
Conclusions
Fish consumption is related to a higher probability of live birth following infertility treatment with ART. This trial was registered at clinicaltrials.gov as NCT00011713.
Keywords: infertility, women, assisted reproductive technologies, meat, fish, diet, protein, food and nutrition
INTRODUCTION
Evidence pointing to the importance of nutrition on human fertility continues to grow (1). Previous work has shown that closer adherence to a Mediterranean dietary pattern (2) and a dietary pattern favoring intake of protein from vegetable sources, low glycemic carbohydrates, unsaturated fats, and supplemental folic acid and iron was related to lower risk of infertility (3). Conversely, adherence to a “Western” diet was related to increased risk for polycystic ovarian syndrome (4). Recent work has also linked intake of specific dietary factors to treatment outcomes of women undergoing treatment with assisted reproductive technology (ART) (5, 6), including intakes of folic acid and vitamin B-12 (7), dairy foods (8), soy (9, 10), low-pesticide-residue fruits and vegetables (11), and whole grains (12).
Despite this increasing knowledge, little is known about the contribution of meat and other protein-rich foods to infertility treatment outcomes. Protein sources vary significantly in nutrient composition owing to their different biology, food processing, and cooking practices (13). Not surprisingly, the relation between intakes of protein with risk of chronic conditions differs by source. For example, intake of red and processed meats has been consistently related to higher risk of cardiovascular disease (14), whereas fish and vegetable protein sources have been related to lower risk of cardiovascular disease (15–17). In prior studies evaluating the relation between meat intake and semen quality, a proxy for male fertility potential, we found processed meat intake to be related to lower semen quality, whereas fish intake was related to higher semen quality among men in couples presenting at a fertility clinic (18). However, these differences did not translate into differences in treatment outcomes (19). Few studies have examined how women's meat consumption impacts fertility or infertility treatment outcomes (20–22). Furthermore, although some studies have related dietary patterns to outcomes of infertility treatment with in vitro fertilization (5, 6), it is not clear how individual food components of these patterns, including the specific protein sources, influence the overall effect of the pattern.
Based on these data, we hypothesized that higher intake of red and processed meats would be related to lower success rates, whereas higher intake of fish would be related to higher success rates and intake of other protein-rich foods would not be related to success rates. To test this hypothesis, we evaluated the association between intakes of meats and other protein-rich foods (eggs, soy-derived products, beans, and nuts) and outcomes of infertility treatment among women participating in the Environment and Reproductive Health (EARTH) Study.
METHODS
Study design
The EARTH Study is an ongoing prospective cohort study that started in 2004, aimed at identifying determinants of fertility among couples presenting to the Massachusetts General Hospital (MGH) Fertility Center in Boston, MA (23). The study was registered prospectively at clinicaltrials.gov as NCT00011713. The Institutional Review Boards of Harvard TH Chan School of Public Health and MGH approved the study. Written informed consent was obtained from every participant.
Women presenting at the MGH Fertility Center for treatment who met the eligibility criteria (age: 18–45 y) were invited to participate in the study. Research nurses contact eligible women and ∼55% of those referred by physicians (78% of those who can be contacted by research staff) ultimately enroll in the study. Women who participated in the EARTH Study and later returned to the MGH Fertility Center for assistance in becoming pregnant again were eligible to re-enroll in the study. All the baseline and diet information of the women who re-enrolled was updated. Of the 752 women who had enrolled since April 2007 (when diet assessment was introduced), 420 had completed ≥1 treatment cycle with ART by August 2017. Of these, 351 (90%) had completed a dietary assessment and undergone 598 treatment cycles with ART (Supplemental Figure 1).
Assessment of baseline characteristics and diet
A brief staff-administered questionnaire was used to collect data on demographics and medical history upon enrollment. Participants’ height and weight were measured by trained research study staff, and were used to calculate BMI (kg/m2). Participants also completed a detailed, take-home questionnaire with information on lifestyle factors, physical activity, reproductive health, and medical history. Moderate-to-vigorous activity (hours per week) was assessed using a validated questionnaire (24), and was calculated by summing the time spent in all physical activities, including weight and aerobic exercises and sports, as described previously in this cohort (25).
Diet was assessed at study entry using a food-frequency questionnaire (FFQ) designed and previously validated as a self-administered instrument (26, 27). This FFQ has been used extensively in multiple epidemiologic studies. The FFQ asked participants to report how often, on average, they consumed 131 foods and supplements during the previous year. The questionnaire included 23 items regarding intake of meats, 3 items regarding eggs, 4 items regarding nuts, and 4 items regarding beans. Soy intake was assessed using a supplemental questionnaire that asked about the frequency of intake of 15 soy-based foods (tofu, tempeh, soy sausages, soy burgers, soy packages, miso soup, soy milk, soy cheese, soy yogurt, tofu cream, soy beans, soy nuts, soy drinks, soy protein, and soy bars), a method previously found to produce valid estimates of intake (28). There were 9 possible frequencies of intake for each food, ranging from “never or less than once per month” to “twice or more per day.” Total meat intake was defined as the sum of the intakes of all the meat items on the questionnaire, including intake of processed meats (hamburger, sausage, bacon, salami, or bologna), unprocessed red meat (beef, pork, or lamb as a main dish or mixed dish), organ meat (liver from cow, calf, pig, chicken, or turkey), poultry (chicken or turkey), and fish and seafood (canned tuna; dark meat fish, e.g., salmon; other fish, e.g., cod, haddock; and shellfish, e.g., shrimp, scallops). Egg intake was defined as the sum of regular eggs, eggs fortified with omega-3 fatty acids, and egg beaters or egg whites. Nut intake included peanut butter, peanuts, walnuts, and other nuts. Beans included peas or lima beans (fresh, frozen, or canned), beans or lentils (baked, dried, or soup), and string beans. Intake of soy and soy-derived foods included tofu, tempeh, soy sausages, soy burgers, soy packages, miso soup, soy milk, soy cheese, soy yogurt, tofu cream, soy beans, soy nuts, soy drinks, soy protein, and soy bars. The intakes of soy-based foods, beans, and nuts were combined into a single variable becauseof the low intake of these foods individually. In a validation study (29), the deattenuated correlation coefficient comparing FFQ estimates with prospectively collected diet records ranged from 0.56 for poultry to 0.83 for processed red meat. The nutrient contents and portion size were calculated based on the nutrient database of the US Department of Agriculture (30).
To address the possibility of residual confounding owing to overall dietary behavior, we identified 2 data-derived dietary patterns using principal component analysis based on the intake of all foods in the questionnaire excluding meats and other protein-rich foods (31). The patterns identified were similar to those previously identified in this population using the full food intake data: the “prudent pattern” (characterized by intakes of fruits, cruciferous vegetables, yellow vegetables, tomatoes, wine, and leafy green vegetables) and the “Western pattern” (characterized by high intakes of liquor and beer, high-fat dairy, fries, refined grains, sweets, pizza, and mayonnaise). Prudent scores ranged between −1.8 and 4.0, and Western scores between −2.6 and 4.7, with higher scores reflecting closer adherence to each pattern (32).
Clinical management and assessment of outcomes
Women underwent a pretreatment cycle of oral contraceptives for 2–5 wk to suppress ovulation before their ART cycles, unless contraindicated. On day 3 of induced menses, patients began controlled ovarian stimulation. Women were monitored during gonadotropin stimulation for serum estradiol and endometrial thickness until 2 d before egg retrieval. Human chorionic gonadotrophin was administered 35 h before the scheduled egg retrieval procedure to induce ovulation.
Embryologists classified oocytes as germinal vesicle, metaphase I, metaphase II, or degenerated. Following egg retrieval, couples underwent conventional insemination or intracytoplasmic sperm injection for fertilization, as clinically indicated. Embryologists determined the fertilization rate 17–20 h after insemination as the number of oocytes with 2 pronuclei divided by the number of metaphase II oocytes inseminated or injected. We defined successful implantation as a serum β-human chorionic gonadotrophin concentration ≥6 mIU/mL, typically measured 17 d (range 15–20 d) after egg retrieval; clinical pregnancy as the presence of an intrauterine gestational sac confirmed by ultrasound; and live birth as the birth of a neonate at or after 24 wk of gestation. All clinical information was abstracted from electronic medical records.
Statistical analysis
Women were divided into quartiles according to their intake of each type of protein source. Because intake of organ meats was infrequent in our sample, we compared women with no intake against those with any intake of organ meats. Descriptive statistics were calculated for demographic, dietary, and reproductive characteristics according to the quartiles of pretreatment total meat intake. In order to assess differences in demographic, lifestyle, and dietary characteristics across meat intake categories, we used the chi-square or Fisher's exact test for discrete variables and the Kruskal-Wallis test for continuous variables.
We used multivariable generalized linear mixed models to evaluate the associations between pretreatment protein source intakes and ART outcomes, with random intercepts to account for multiple treatment cycles per woman. The specified distributions and link functions for the outcomes were: Poisson distribution and log link function for oocyte and embryos counts, and binomial distribution with logit link function for fertilization rates and clinical outcomes. We accounted for the overdispersion in the Poisson models when appropriate. Population marginal means were used to present results adjusted for the covariates at their average levels for continuous variables and weighted average level of categorical variable in the model (33). We considered the covariates in the model based on the literature and on a statistical basis. We examined all the linear terms of each continuous covariate using penalized splines. Based on these analyses, we modeled all of the covariates as linear terms. The final models included terms for age (years, continuous), BMI (continuous), ethnicity (white compared with other), smoking history (never, ever), total energy intake (calories per day, continuous), 2 data-derived dietary patterns scores (Western and prudent patterns, continuous), and supplemental intakes of folate (micrograms per day, continuous), vitamin B-12 (micrograms per day, continuous), iron (milligrams per day, continuous), and use of supplemental long-chain ω-3 fatty acids (yes/no). We accounted for the possibility that intake of a specific protein source may have confounded the effect of intake of other protein sources by adjusting our models for all the other types of protein simultaneously as continuous variables. We conducted tests for linear trend using a continuous variable for the median values of intake in each quartile.
In another set of models, we estimated the effect of substituting specific protein sources for one another on the odds of live birth. To do this, we included all protein food sources in the same model as continuous variables [scaled to 2 servings/wk in accordance to Environmental Protection Agency and the Food and Drug Administration recommendations on fish intake for women who are pregnant or may become pregnant (34)] and estimated the effect of substitution as the difference between their regression coefficients and the corresponding 95% CIs by using the estimated covariance matrix for the regression coefficients (35). Because of the infrequent intake of organ meats in this population, we could not estimate the association with organ meats. We conducted sensitivity analyses to minimize the impact of measurement error and of multiple treatment cycles per woman, including analysis restricted to cycles that ended within 1 y of the diet assessment and another analysis restricted to the first treatment cycle for each woman. All the analyses were conducted using the statistical analysis system software package SAS 9.4 (SAS Institute Inc.) and we considered 2-sided Pvalues <0.05 to be statistically significant.
RESULTS
Participants were 351 women who collectively underwent 598 ART cycles between 2007 and 2017. Most women were Caucasian (83%), and had a mean age of 35.0 y and a BMI of 23.1. The average total meat intake was 1.20 servings/d (range: 0–4.12 servings/d), most of which was poultry (35%, 0.44 servings/d), followed by fish (25%, 0.25 serving/d), processed meats (22%, 0.28 serving/d), unprocessed red meats (17%, 0.21 servings/d), and organ meats (1%, 0.02 servings/d) (Supplemental Figure 2). The average egg intake was 0.44 servings/d (range: 0–4.0 servings/d), and the average intake of vegetable sources of protein (beans, nuts, and soy) was 1.29 servings/d (range: 0.04–7.56 servings/d). At study entry, women with a higher total meat intake had a higher BMI, higher total energy intake, greater adherence to the Western and prudent dietary patterns, higher total protein, and higher total fat intakes (Table 1).
TABLE 1.
Baseline characteristics of 351 women (369 unique FFQs) from the EARTH Study by quartiles of pretreatment total meat intake1
| Quartiles | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Total | Q1 (lowest) | Q2 | Q3 | Q4 (highest) | Pvalue2 |
| Servings/d | 1.15 (0, 4.12) | 0.46 (0, 0.71) | 0.94 (0.72, 1.14) | 1.35 (1.15, 1.57) | 1.93 (1.58, 4.12) | <0.0001 |
| Women/FFQs, n/n | 351/369 | 83/91 | 89/93 | 91/93 | 88/92 | |
| Cycles, n (%) | 598 (100) | 144 (24) | 144 (24) | 156 (26) | 154 (26) | 0.84 |
| Maternal characteristics | ||||||
| Age, y | 35.0 (32.0, 38.0) | 35.0 (32.0, 38.0) | 35.0 (32.0, 39.0) | 35.0 (32.0, 38.0) | 35.5 (32.0, 38.9) | 0.93 |
| BMI, kg/m2 | 23.1 (21.2, 25.8) | 21.8 (20.5, 24.8) | 23.0 (21.1, 25.6) | 23.7 (21.7, 26.1) | 24.0 (22.1, 27.0) | 0.004 |
| Caucasian, n (%) | 290 (83) | 69 (81) | 79 (90) | 73 (90) | 69 (78) | 0.21 |
| Ever smoker, n (%) | 93 (27) | 18 (21.2) | 23 (26) | 28 (31) | 24 (27) | 0.52 |
| Graduate degree, n (%) | 205 (58) | 51 (61) | 56 (63) | 51 (56) | 47 (53) | 0.54 |
| Baseline reproductive characteristics | ||||||
| Initial infertility diagnosis, n (%) | 0.32 | |||||
| Female factors | 101 (29) | 24 (28) | 24 (27) | 25 (28) | 28 (32) | |
| Diminished ovarian reserve | 30 (9) | 12 (14) | 6 (7) | 6 (7) | 6 (7) | |
| Endometriosis | 14 (4) | 2 (2) | 4 (5) | 5 (6) | 3 (3) | |
| Ovulatory factor | 27 (8) | 4 (5) | 8 (9) | 8 (9) | 7 (8) | |
| Tubal factor | 23 (7) | 2 (2) | 6 (6) | 6 (7) | 9 (10) | |
| Uterine factor | 6 (2) | 2 (2) | 1 (1) | 0 (0) | 3 (3) | |
| Male factors | 112 (32) | 26 (29) | 21 (25) | 35 (38) | 30 (34) | |
| Unexplained | 139 (40) | 35 (42) | 43 (48) | 31 (34) | 30 (34) | |
| Stimulation protocol, n (%) | 0.28 | |||||
| Antagonist | 48 (13) | 15 (16) | 9 (10) | 7 (7.53) | 17 (18) | |
| Flare3 | 38 (10) | 10 (11) | 7 (7) | 13 (14) | 8 (9) | |
| Luteal phase agonist4 | 248 (67) | 56 (62) | 66 (71) | 67 (72) | 59 (64) | |
| Endometrial preparation5 | 35 (10) | 10 (11) | 11 (12) | 6 (6) | 8 (9) | |
| Day 3 FSH concentrations, mIU/L | 6.85 (5.90, 8.30) | 7.00 (6.00, 8.30) | 6.60 (5.90, 8.20) | 6.70 (5.90, 8.30) | 7.00 (5.90, 8.40) | 0.97 |
| Dietary characteristics | ||||||
| Total energy intake, kcal/d | 1696 (1378, 2146) | 1323 (1101, 1645) | 1595 (1378, 1857) | 1799 (1476, 2144) | 2101 (1745, 2535) | <0.0001 |
| Caffeine intake, mg/d | 108 (43.9, 176) | 103 (42.3, 172) | 106 (45.1, 166) | 108 (28.5, 214) | 117 (64.4, 208) | 0.32 |
| Alcohol intake, g/d | 5.46 (1.55, 13.2) | 3.57 (0.61, 8.62) | 4.91 (1.39, 9.76) | 6.46 (1.80, 14.8) | 9.64 (3.07, 16.1) | 0.0001 |
| Carbohydrate intake, % of total energy | 49.0 (44.1, 54.5) | 54.2 (48.5, 58.3) | 50.7 (45.7, 54.7) | 47.8 (42.1, 51.6) | 46.0 (41.5, 49.3) | <0.0001 |
| Total fat intake, % of total energy | 32.3 (29.0, 36.2) | 31.0 (27.3, 34.9) | 32.0 (28.3, 35.7) | 33.0 (30.5, 37.8) | 33.2 (30.0, 37.8) | 0.003 |
| Protein intake, % of total energy | 16.5 (14.9, 18.3) | 14.8 (13.5, 16.1) | 16.5 (15.1, 17.9) | 17.0 (15.9, 18.6) | 17.9 (16.7, 19.7) | <0.0001 |
| Prudent pattern score | −0.17 (−0.68, 0.46) | −0.51 (−0.92, 0.11) | −0.32 (−0.74, 0.09) | −0.17 (−0.72, 0.22) | 0.40 (−0.10, 0.95) | <0.0001 |
| Western pattern score | −0.10 (−0.67, 0.46) | −0.76 (−1.07, −0.44) | −0.16 (−0.72, 0.31) | 0.05 (−0.32, 0.61) | 0.51 (−0.08, 1.31) | <0.0001 |
| Supplemental folate, µg/d | 543 (400, 800) | 800 (400, 800) | 406 (400, 800) | 457 (400, 800) | 800 (400, 800) | 0.37 |
| Supplemental vitamin B-12, µg/d | 6.00 (4.57, 12.0) | 8.00 (4.57, 12.0) | 6.00 (4.57, 8.00) | 6.00 (3.43, 12.0) | 8.00 (5.29, 12.0) | 0.28 |
| Supplemental iron, mg/d | 18.0 (0, 28.0) | 18.0 (0, 28.0) | 18 (0, 28.0) | 16.4 (0, 28.0) | 18.0 (0, 28.0) | 0.84 |
| Supplemental zinc, mg/d | 15.0 (0, 25.0) | 15.0 (0, 25.0) | 15 (0, 25.0) | 15.0 (0.08, 25.0) | 14.3 (0, 25.0) | 0.99 |
| Use of fish oil supplements, n (%) | 88 (24.0) | 22 (24.0) | 24 (26.0) | 18 (19.0) | 24 (16.0) | 0.68 |
| Physical activity | ||||||
| Moderate to vigorous physical activity,6h/wk | 2.48 (0.49, 5.24) | 1.99 (0.49, 3.88) | 2.50 (0.64, 5.68) | 1.49 (0.40, 5.00) | 3.13 (1.14, 6.62) | 0.05 |
1Values are n (%) for the categorical variables and medians (IQRs) for the continuous variables. EARTH Study, Environment and Reproductive Health Study; FFQ, food-frequency questionnaire; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; Q, quartile.
2From chi-square test (or Fisher's exact test when appropriate) for discrete variables and Kruskal-Wallis test for continuous variables.
3Follicular-phase GnRH agonist/flare protocol.
4Luteal-phase GnRH agonist protocol.
5Includes endometrial preparation protocols for donor recipient cycles and autologous cryo-thaw cycles.
6Includes weight and aerobic exercise and sports.
There were no consistent associations between intake of protein-rich foods and the intermediate ART outcomes examined (Supplemental Table 1). Processed meat intake was associated with lower fertilization rate (P-trend = 0.005). However, this did not translate into significant differences in the number of transferable embryos. Similarly, whereas fish intake was associated with lower peak estradiol concentrations (P-trend = 0.01), there were no associations with number of total or mature oocytes retrieved.
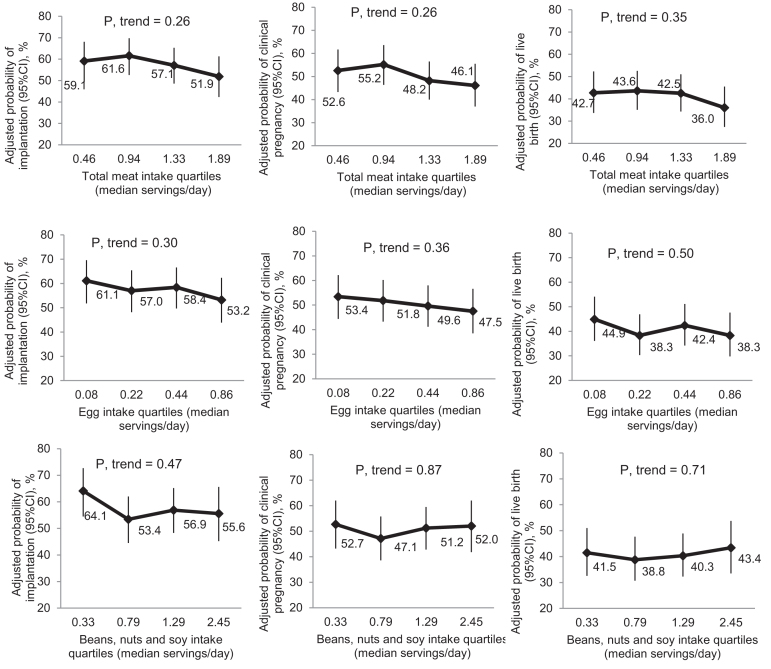
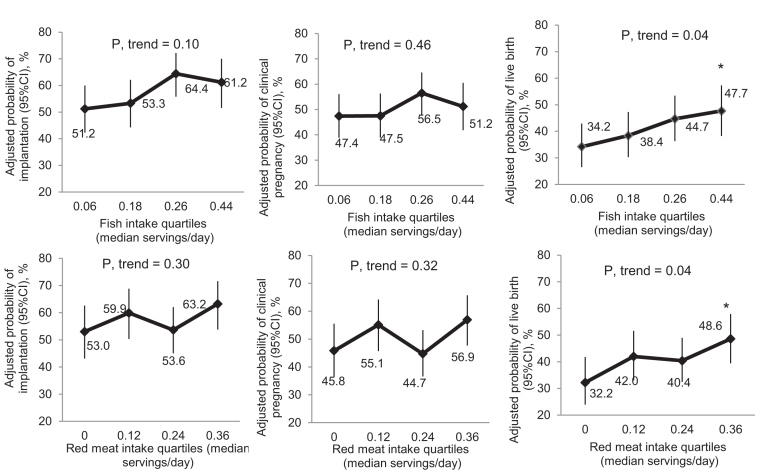
Total meat intake, as well as intake of eggs and vegetable sources of protein (beans, nuts, and soy) were not associated with ART outcomes (Figure 1 and Supplemental Table 2). However, when specific meats were separately assessed, higher fish intake was significantly associated with higher odds of live birth (Figure 2 and Supplemental Table 2). The adjusted probabilities (95% CI) of live birth for women in increasing quartiles of fish intake were 34.2% (26.5%, 42.9%), 38.4% (30.3%, 47.3%), 44.7% (36.3%, 53.4%), and 47.7% (38.3%, 57.3%), respectively (P-value for trend = 0.04), and the OR (95% CI) of live birth associated with increasing fish intake by 1 serving/d was 3.36 (1.27, 8.91) (Supplemental Table 3). This association was similar when intake of the 3 types of fish (i.e., dark meat fish, white fish, and shellfish) were separately modeled (data not shown). In addition, fish oil supplement use was not related to live birth rates in this population [41.2% (36.3%, 46.3%) compared with 41.1% (32.4%, 50.4%), P = 0.98].
FIGURE 1.
Total meat, eggs, and vegetable sources of protein-rich foods (beans, nuts, and soy) and clinical outcomes analysis in the EARTH Study (n = 351 women; 598 cycles). Data are presented as predicted marginal means with 95% CIs adjusted for total daily calories, age, BMI, race, smoking status, daily supplemental dietary folate, supplemental vitamin B-12, supplemental iron intake, supplemental ω-3 (binary, yes/no), and prudent and Western dietary patterns (excluding meats), with all continuous variables at their mean level and all categorical variables at their weighted average level. All analyses were adjusted for all the other protein-rich foods. All outcomes were analyzed using generalized linear mixed models with random intercepts, binary distribution, and logit link function. Test for trend was performed using a continuous variable for the median number of servings per day for each quartile of the corresponding meat intake in the regression models. EARTH Study, Environment and Reproductive Health Study.
FIGURE 2.
Fish and red meat intake and clinical outcomes analysis in the EARTH Study (n = 351 women; 598 cycles). Data are presented as predicted marginal means with 95% CIs adjusted for total daily calories, age, BMI, race, smoking status, daily supplemental dietary folate, supplemental vitamin B-12, supplemental iron intake, supplemental ω-3 (binary, yes/no), and prudent and Western dietary patterns (excluding meats). All analyses were adjusted for all the other protein-rich foods. All outcomes were analyzed using generalized linear mixed models with random intercepts, binary distribution and logit link function. Test for trend was performed using a continuous variable for the median number of servings per day for each quartile of the corresponding meat intake in the regression models. *P < 0.05 when compared with the lowest category of intake. EARTH Study, Environment and Reproductive Health Study.
There was also a positive relation between unprocessed red meat intake and live birth when intake was modeled in categories (Figure 1); however, unprocessed red meat intake was unrelated to live birth when modeled as a continuous variable (Supplemental Table 3).
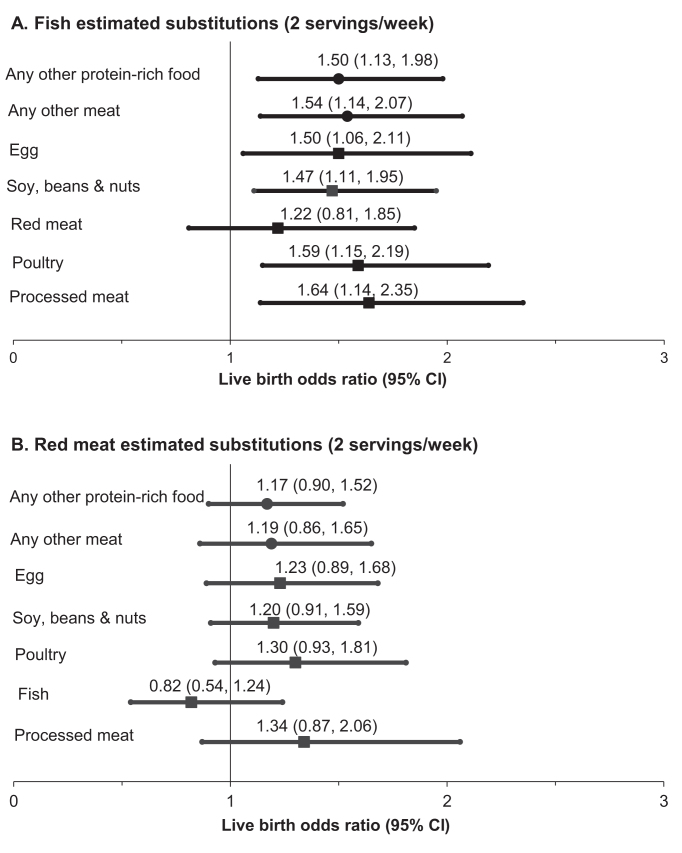
We next estimated the effect of substituting one source of dietary protein for another on the probability of live birth. Consuming fish instead of any other protein-rich food (i.e., all other meats, eggs, and beans, soy, and nuts) was consistently related to greater odds of live birth (Figure 3A). The contrast was greatest when fish was consumed instead of processed meats. On the other hand, there was no apparent benefit of consuming unprocessed red meat instead of other protein-rich foods (Figure 3B). Other food-by-food estimated substitutions were unrelated to live birth (Supplemental Table 3). When analyses were restricted to cycles ending within 1 y of diet assessment and to the first treatment cycle for each woman, the associations were similar but weaker owing to the smaller sample size (data not shown).
FIGURE 3.
ORs (95% CIs) of live birth per treatment cycle initiated associated with substituting 1) fish or 2) red meat for other food sources (2 servings/wk) in the EARTH Study (n = 351 women; 598 cycles). (A) Fish estimated substitutions (2 servings/wk). (B) Red meat estimated substitutions (2 servings/wk). All outcomes were analyzed using generalized linear mixed models with random intercepts, binary distribution, and logit link function. All analyses were adjusted for total daily calories, age, BMI, race, smoking status, daily supplemental dietary folate, supplemental vitamin B-12, supplemental iron intake, supplemental ω-3 (binary, yes/no), and prudent and Western dietary patterns (excluding meats). All analyses were adjusted for all the other protein-rich foods (i.e., processed meat, red meat, organ meat, fish, poultry and vegetarian; servings per day). All outcomes were analyzed using generalized linear mixed models with random intercepts, binary distribution, and logit link function. “Any other protein-rich food” includes all other meats, eggs, and beans, soy, and nuts. EARTH Study, Environment and Reproductive Health Study.
DISCUSSION
We evaluated the associations between intake of protein-rich foods and outcomes of infertility treatment with ART in a prospective cohort study. In agreement with our prespecified hypothesis, intake of fish was related to a higher probability of live birth when consumed instead of any other specific meat and when replacing other protein-rich foods. Contrary to our hypothesis, however, unprocessed red meat intake was not related to less favorable ART outcomes. In fact, it was related to greater reproductive success when intake was modeled as a categorical variable but was not related to live births when modeled as a continuous outcome. These findings provide additional evidence that dietary choices may influence fertility and outcomes of infertility treatment (1) and, more specifically, add to the growing literature suggesting that intake of seafood and long-chain ω-3 fatty acids may improve fertility (22, 36–39).
In agreement with our findings, Braga et al. (22) reported that fish consumption was associated with increased likelihood of blastocyst formation among women undergoing infertility treatment. Similarly, we have previously reported that, in a subpopulation of this cohort, higher pretreatment serum concentrations of long-chain ω-3 fatty acids, and in particular eicosapentaenoic acid, were related to a higher probability of live birth (36). Furthermore, in a prospective cohort study of pregnancy planners in the United States who did not use fish oil supplements, intake of ω-3 fatty acids was associated with shorter time to pregnancy (37). In a separate study of couples planning pregnancy, fish intake by both men and women was associated with shorter time to pregnancy (39). Similar to these previous studies conducted in the United States, in our population, fish intake was positively related to fertility; however, fish oil supplement use was not related to live birth. In aggregate, these findings are consistent with a beneficial effect of fish intake on fertility that may or may not be mediated through its ω-3 fatty acid content. Multiple mechanisms may be at play in this regard. A study in aging mice found that dietary ω-3 fatty acids were linked to prolonged female reproductive lifespan and improved egg quality (40). In addition, PUFAs are precursors for prostaglandins, which are important in aspects of reproductive physiology such as successful implantation (41, 42), and have anti-inflammatory properties (41). It is also possible that PUFAs in fish mediate intracellular signaling pathways in embryo implantation (43).
A common concern about recommending the consumption of fish to women who are pregnant or may become pregnant is that seafood is the primary source of exposure to important environmental contaminants, including mercury, organochlorines, dioxins, and polychlorinated biphenyls. This concern has resulted in guidance from the Food and Drug Administration and the Environmental Protection Agency recommending that women who are pregnant or may become pregnant eat no more than 3 servings of seafood/wk (44), with the goal of limiting fetal exposure to methyl-mercury. The balance of risk and benefits in terms of fertility is not clear at the moment. Buck et al. (21) suggested that maternal consumption of fish contaminated with polychlorinated biphenyls may delay pregnancy among couples attempting to achieve it (45). On the other hand, we have previously reported that hair mercury concentrations were not related to in vitro fertilization outcomes among women in this cohort (46), suggesting that the fertility benefits of fish intake may outweigh the harms of some environmental contaminants of these foods. Additional work is needed to clarify the balance for couples attempting pregnancy.
Contrary to our hypothesis, we found that a higher intake of red meat was related to a higher probability of live birth when the intake was modeled as a categorical variable. Data on the potential role of red meat intake on reproduction is sparse. Braga and colleagues (22) found that among women undergoing infertility treatment with ART and using intracytoplasmic sperm injection as the only insemination procedure, red meat consumption was inversely related to implantation and pregnancy rates. A large prospective cohort reported a positive association between intake of protein from animal sources and risk of infertility owing to anovulation, which was driven by intakes of red meat and poultry (20). However, total protein intake and intake of protein from animal sources were higher among women in that study than among women in our study population, which may explain the diverging results. Clearly, more research is needed to clarify the role that unprocessed red meat intake plays among women undergoing infertility treatment.
Our study is not without limitations. First, dietary assessment is subject to measurement error. However, since diet assessment preceded outcome ascertainment, measurement error is likely nondifferential with respect to the outcome, resulting in attenuation of the observed association toward the null. Second, we cannot exclude the possibility of residual confounding. However, our analyses took into consideration a large number of potential confounders and known predictors of treatment outcomes, guarding against this risk to a large extent. A strength of our study is that women in our cohort had comparable intake ranges for meat, eggs, and beans, soy, and nuts to women in the general US population (47, 48). Additional strengths include the prospective design, the use of a validated diet assessment tool, and the analysis of intermediate endpoints that cannot be observed in couples attempting conception naturally.
In summary, women with higher pretreatment intakes of fish had a higher probability of live birth following an ART cycle. This study provides additional evidence to the growing literature suggesting that intake of fish may result in improved fertility in humans. Additional research, and specifically randomized trials among couples trying to conceive either unassisted or in the setting of infertility treatment, are needed.
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the contributions of all members of the EARTH Study team, specifically research nurse Myra G Keller, senior research staff Ramace Dadd and Patricia Morey, and the physicians and staff at the Massachusetts General Hospital Fertility Center. A special thank you is due to all of the study participants.
The authors’ contributions were as follows—FLN: performed the statistical analysis and wrote the manuscript; Y-HC and JCV: technically reviewed the analysis; Y-HC, JCV, AJG, PLW, JBF, JA, and RH: reviewed and edited the manuscript; RH, PLW, and JEC: designed research; FLN and JEC: had primary responsibility for final content; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Notes
Supported by NIH grants R01-ES009718, P30ES000002, P30DK46200 and K99ES026648.
Supplemental Figures 1 and 2 and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: ART, assisted reproductive technology; EARTH Study, Environment and Reproductive Health Study; FFQ, food-frequency questionnaire; MGH, Massachusetts General Hospital.
REFERENCES
- 1. Gaskins AJ, Chavarro JE. Diet and fertility: a review. Am J Obstet Gynecol. 2018;218(4):379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Toledo E, Lopez-del Burgo C, Ruiz-Zambrana A, Donazar M, Navarro-Blasco I, Martinez-Gonzalez MA, de Irala J. Dietary patterns and difficulty conceiving: a nested case-control study. Fertil Steril. 2011;96(5):1149–53. [DOI] [PubMed] [Google Scholar]
- 3. Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstet Gynecol. 2007;110(5):1050–8. [DOI] [PubMed] [Google Scholar]
- 4. Tsai YH, Wang TW, Wei HJ, Hsu CY, Ho HJ, Chen WH, Young R, Liaw CM, Chao JC. Dietary intake, glucose metabolism and sex hormones in women with polycystic ovary syndrome (PCOS) compared with women with non-PCOS-related infertility. Br J Nutr. 2013;109(12):2190–8. [DOI] [PubMed] [Google Scholar]
- 5. Vujkovic M, de Vries JH, Lindemans J, Macklon NS, van der Spek PJ, Steegers EA, Steegers-Theunissen RP. The preconception Mediterranean dietary pattern in couples undergoing in vitro fertilization/intracytoplasmic sperm injection treatment increases the chance of pregnancy. Fertil Steril. 2010;94(6):2096–101. [DOI] [PubMed] [Google Scholar]
- 6. Karayiannis D, Kontogianni MD, Mendorou C, Douka L, Mastrominas M, Yiannakouris N. Association between adherence to the Mediterranean diet and semen quality parameters in male partners of couples attempting fertility. Hum Reprod. 2017;32(1):215–22. [DOI] [PubMed] [Google Scholar]
- 7. Gaskins AJ, Afeiche MC, Wright DL, Toth TL, Williams PL, Gillman MW, Hauser R, Chavarro JE. Dietary folate and reproductive success among women undergoing assisted reproduction. Obstet Gynecol. 2014;124(4):801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Afeiche MC, Chiu YH, Gaskins AJ, Williams PL, Souter I, Wright DL, Hauser R, Chavarro JE. Dairy intake in relation to in vitro fertilization outcomes among women from a fertility clinic. Hum Reprod. 2016;31(3):563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vanegas JC, Afeiche MC, Gaskins AJ, Minguez-Alarcon L, Williams PL, Wright DL, Toth TL, Hauser R, Chavarro JE. Soy food intake and treatment outcomes of women undergoing assisted reproductive technology. Fertil Steril. 2015;103(3):749–55. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Unfer V, Casini ML, Gerli S, Costabile L, Mignosa M, Di Renzo GC. Phytoestrogens may improve the pregnancy rate in in vitro fertilization-embryo transfer cycles: a prospective, controlled, randomized trial. Fertil Steril. 2004;82(6):1509–13. [DOI] [PubMed] [Google Scholar]
- 11. Chiu YH, Williams PL, Gillman MW, Gaskins AJ, Minguez-Alarcon L, Souter I, Toth TL, Ford JB, Hauser R, Chavarro JE. Association between pesticide residue intake from consumption of fruits and vegetables and pregnancy outcomes among women undergoing infertility treatment with assisted reproductive technology. JAMA Intern Med. 2018;178(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gaskins AJ, Chiu YH, Williams PL, Keller MG, Toth TL, Hauser R, Chavarro JE. Maternal whole grain intake and outcomes of in vitro fertilization. Fertil Steril. 2016;105(6):1503–10. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fein SB, Lando AM, Levy AS, Teisl MF, Noblet C. Trends in U.S. consumers' safe handling and consumption of food and their risk perceptions, 1988 through 2010. J Food Prot. 2011;74(9):1513–23. [DOI] [PubMed] [Google Scholar]
- 14. Kaluza J, Akesson A, Wolk A. Long-term processed and unprocessed red meat consumption and risk of heart failure: a prospective cohort study of women. Int J Cardiol. 2015;193:42–6. [DOI] [PubMed] [Google Scholar]
- 15. Bryant J, Hanson M, Peebles C, Davies L, Inskip H, Robinson S, Calder PC, Cooper C, Godfrey KM. Higher oily fish consumption in late pregnancy is associated with reduced aortic stiffness in the child at age 9 years. Circ Res. 2015;116(7):1202–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tani S, Takahashi A, Nagao K, Hirayama A. Association of fish consumption-derived ratio of serum n–3 to n–6 polyunsaturated fatty acids and cardiovascular risk with the prevalence of coronary artery disease. Int Heart J. 2015;56(3):260–8. [DOI] [PubMed] [Google Scholar]
- 17. Shridhar K, Dhillon PK, Bowen L, Kinra S, Bharathi AV, Prabhakaran D, Reddy KS, Ebrahim S. The association between a vegetarian diet and cardiovascular disease (CVD) risk factors in India: the Indian Migration Study. PLoS One. 2014;9(10):e110586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Afeiche MC, Gaskins AJ, Williams PL, Toth TL, Wright DL, Tanrikut C, Hauser R, Chavarro JE. Processed meat intake is unfavorably and fish intake favorably associated with semen quality indicators among men attending a fertility clinic. J Nutr. 2014;144(7):1091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xia W, Chiu YH, Williams PL, Gaskins AJ, Toth TL, Tanrikut C, Hauser R, Chavarro JE. Men's meat intake and treatment outcomes among couples undergoing assisted reproduction. Fertil Steril. 2015;104(4):972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Protein intake and ovulatory infertility. Am J Obstet Gynecol. 2008;198(2):210.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buck GM, Vena JE, Schisterman EF, Dmochowski J, Mendola P, Sever LE, Fitzgerald E, Kostyniak P, Greizerstein H, Olson J. Parental consumption of contaminated sport fish from Lake Ontario and predicted fecundability. Epidemiology. 2000;11(4):388–93. [DOI] [PubMed] [Google Scholar]
- 22. Braga DP, Halpern G, Setti AS, Figueira RC, Iaconelli A Jr., Borges E Jr.. The impact of food intake and social habits on embryo quality and the likelihood of blastocyst formation. Reprod Biomed Online. 2015;31(1):30–8. [DOI] [PubMed] [Google Scholar]
- 23. Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17(6):682–91. [DOI] [PubMed] [Google Scholar]
- 24. Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–9. [DOI] [PubMed] [Google Scholar]
- 25. Gaskins AJ, Williams PL, Keller MG, Souter I, Hauser R, Chavarro JE. Maternal physical and sedentary activities in relation to reproductive outcomes following IVF. Reprod Biomed Online. 2016;33(4):513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185(7):570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Rood JC, Harnack LJ, Sampson LK et al.. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records compared with urinary recovery and plasma concentration biomarkers: findings for women. Am J Epidemiol. 2018;187(5):1051–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frankenfeld CL, Patterson RE, Horner NK, Neuhouser ML, Skor HE, Kalhorn TF, Howald WN, Lampe JW. Validation of a soy food-frequency questionnaire and evaluation of correlates of plasma isoflavone concentrations in postmenopausal women. Am J Clin Nutr. 2003;77(3):674–80. [DOI] [PubMed] [Google Scholar]
- 29. Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–6. [DOI] [PubMed] [Google Scholar]
- 30. United States Department of Agriculture (USDA), Agricultural Research Service, Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference, Release 28. September 2015 ed. 2015. [Google Scholar]
- 31. Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod. 2012;27(10):2899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bryant FB, Yarnold PR. Principal components, and exploratory and confirmatory factor analysis. In: Yarnold PR, Grimm LG, editors. Reading and understanding multivariate statistics. Washington, DC: American Psychological Association; 1995. p. 99–136. [Google Scholar]
- 33. Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least squares means. The American Statistician. 1980;34(4):216–21. [Google Scholar]
- 34. Environmental Protection Agency and the Food and Drug Administration. 2017 EPA-FDA Advice about eating fish and shellfish [Internet].[cited 2018 January 15 ] Available from: https://www.epa.gov/fish-tech/2017-epa-fda-advice-about-eating-fish-and-shellfish. [Google Scholar]
- 35. Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. [DOI] [PubMed] [Google Scholar]
- 36. Chiu YH, Karmon AE, Gaskins AJ, Arvizu M, Williams PL, Souter I, Rueda BR, Hauser R, Chavarro JE. Serum omega-3 fatty acids and treatment outcomes among women undergoing assisted reproduction. Hum Reprod. 2018;33(1):156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wise LA, Wesselink AK, Tucker KL, Saklani S, Mikkelsen EM, Cueto H, Riis AH, Trolle E, McKinnon CJ, Hahn KA et al.. Dietary fat intake and fecundability in 2 preconception cohort studies. Am J Epidemiol. 2018;187(1):60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Al-Safi ZA, Liu H, Carlson NE, Chosich J, Harris M, Bradford AP, Robledo C, Eckel RH, Polotsky AJ. Omega-3 fatty acid supplementation lowers serum FSH in normal weight but not obese women. J Clin Endocrinol Metab. 2016;101(1):324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gaskins AJ, Sundaram R, Buck Louis GM, Chavarro JE. Seafood intake, sexual activity, and time to pregnancy. J Clin Endocrinol Metab. 2018:103(7):2680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nehra D, Le HD, Fallon EM, Carlson SJ, Woods D, White YA, Pan AH, Guo L, Rodig SJ, Tilly JL et al.. Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell. 2012;11(6):1046–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod. 2007;77(2):190–201. [DOI] [PubMed] [Google Scholar]
- 42. Salleh N. Diverse roles of prostaglandins in blastocyst implantation. TheScientificWorldJournal. 2014;2014:968141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ruan YC, Guo JH, Liu X, Zhang R, Tsang LL, Dong JD, Chen H, Yu MK, Jiang X, Zhang XH et al.. Activation of the epithelial Na+ channel triggers prostaglandin E(2) release and production required for embryo implantation. Nat Med. 2012;18(7):1112–7. [DOI] [PubMed] [Google Scholar]
- 44. US FDA, Eating fish: what pregnant women and parents should know [Internet]. [cited 2017 August 8]. Available from: https://www.fda.gov/Food/FoodborneIllnessContaminants/Metals/ucm393070.htm. [DOI] [PubMed] [Google Scholar]
- 45. Buck GM, Tee GP, Fitzgerald EF, Vena JE, Weiner JM, Swanson M, Msall ME. Maternal fish consumption and infant birth size and gestation: New York state angler cohort study. Environ Health. 2003;2(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wright DL, Afeiche MC, Ehrlich S, Smith K, Williams PL, Chavarro JE, Batsis M, Toth TL, Hauser R. Hair mercury concentrations and in vitro fertilization (IVF) outcomes among women from a fertility clinic. Reprod Toxicol. 2015;51C:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Daniel CR, Cross AJ, Koebnick C, Sinha R. Trends in meat consumption in the USA. Public Health Nutr. 2011;14(4):575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cifelli CJ, Houchins JA, Demmer E, Fulgoni VL. Increasing plant based foods or dairy foods differentially affects nutrient intakes: dietary scenarios using NHANES 2007–2010. Nutrients. 2016;8(7):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.