Abstract
Context
Plasma betaine correlates with insulin sensitivity in humans. Betaine supplementation improves metabolic effects in mice fed a high-fat diet.
Objective
To assess metabolic effects of oral betaine in obese participants with prediabetes.
Design
A 12-week, parallel arm, randomized, double-masked, placebo-controlled trial.
Setting
University-affiliated hospital.
Participants and Interventions
Persons with obesity and prediabetes (N = 27) were randomly assigned to receive betaine 3300 mg orally twice daily for 10 days, then 4950 mg twice daily for 12 weeks, or placebo.
Main Outcome Measures
Changes from baseline in insulin sensitivity, glycemia, hepatic fat, and endothelial function.
Results
There was a 16.5-fold increase in plasma dimethylglycine [dimethylglycine (DMG); P < 0.0001] levels, but modest 1.3- and 1.5-fold increases in downstream serine and methionine levels, respectively, in the betaine vs placebo arm. Betaine tended to reduce fasting glucose levels (P = 0.08 vs placebo) but had no other effect on glycemia. Insulin area under curve after oral glucose was reduced for betaine treatment compared with placebo (P = 0.038). Insulin sensitivity, assessed by euglycemic hyperinsulinemic clamp, was not improved. Serum total cholesterol levels increased after betaine treatment compared with placebo (P = 0.032). There were no differences in change in intrahepatic triglyceride or endothelial function between groups.
Conclusion
DMG accumulation supports DMG dehydrogenase as rate limiting for betaine metabolism in persons with prediabetes. Betaine had little metabolic effect. Additional studies may elucidate mechanisms contributing to differences between preclinical and human responses to betaine, and whether supplementation of metabolites downstream of DMG improves metabolism.
Betaine improves insulin sensitivity during glucose tolerance testing but has little other metabolic effect. Dimethylglycine dehydrogenase limits betaine metabolism in prediabetes.
Identifying novel biomarkers of type 2 diabetes (T2D) risk may reveal new pathways relevant to diabetes pathophysiology and new therapeutic targets. Insulin resistance precedes and predicts incident T2D (1). Metabolomic profiling has revealed low plasma concentrations of betaine (also known as glycine betaine or trimethylglycine) correlate with insulin resistance in humans without T2D, with plasma betaine levels about 14% lower in insulin-resistant compared with insulin-sensitive persons (2). Consistently, lower systemic and higher urinary concentrations of betaine are also associated with risk of incident T2D (3). The hazard ratio for incident T2D is 16% lower with increasing plasma betaine level (per SD log metabolite level) within the Diabetes Prevention Program cohort, even after adjustment for other risk variables (4). Furthermore, plasma betaine level increases with lifestyle intervention, which is the most effective diabetes prevention approach. Low plasma betaine level also is associated with obesity, higher non–high density lipoprotein cholesterol and triglycerides, blood pressure, and risk for myocardial infarction and heart failure in patients with established disease (5–7).
Betaine has three key physiologic functions: It acts as a substrate for glutathione synthesis and is a methyl donor and an organic osmolyte. Animal models demonstrate a direct role for betaine in modulating metabolic health, suggesting a potential therapeutic role in humans. High-fat diets reduce plasma betaine despite preserved dietary intake, likely attributable to increased expression of genes involved in betaine metabolism, including betaine homocysteine methyl transferase (BHMT), dimethylglycine dehydrogenase (DMGDH), methionine adenosyltransferase I α (MAT1A), and phosphatidylethanolamine N-methyltransferase (PEMT) (2). Dietary betaine supplementation reduces weight gain, levels of insulin under fasted and fed conditions, and hepatic fat, and increases whole-body glucose homeostasis and energy expenditure in rodents receiving a high-fat diet, with similar improvements in metabolism in cells (2). In vivo effects are fibroblast growth factor (FGF)-21 dependent.
Betaine is generated by oxidation of choline and is a dietary constituent of grains and leafy green vegetables. It is particularly abundant in Goji berries, which have been used for centuries in Chinese culture with multiple purported health benefits. Oral betaine is rapidly absorbed and distributed, and is eliminated by metabolism and urinary excretion (8). Anhydrous betaine is approved by the Food and Drug Administration to reduce plasma homocysteine levels in patients with rare inborn errors in metabolism involving cystathionine β-synthase, 5,10-methylenetetrahydrofolate reductase, or cobalamin cofactor metabolism.
In the setting of epidemiologic data supporting that low betaine level is associated with cardiometabolic risk, preclinical studies demonstrating efficacy of dietary betaine to improve multiple metabolic measures, and prior human exposure to oral betaine, we tested the hypothesis that betaine supplementation would improve glycemia, insulin sensitivity, liver fat, or endothelial function when administered over 12 weeks to persons with obesity and prediabetes (ClinicalTrials.gov identifier: NCT01950039).
Methods
Participants
The Partners Human Research Committee Institutional Review Board approved the study. All participants provided written informed consent. Between January 2014 and December 2016, 85 participants were recruited via advertisements and patient outreach. Eligible participants were 21 to 70 years old; prediabetes was defined as impaired fasting glucose (≥100 mg/dL), impaired glucose tolerance (2-hour glucose level 140 to 200 mg/dL after ingesting 75 g of oral glucose) and/or HbA1C 5.7% to 6.5% (9), and overweight or obese with body mass index range of 25 to 45 kg/m2. Key exclusion criteria included cystathionine β-synthase deficiency; uncontrolled hypertension, heart, renal, or liver disease other than nonalcoholic fatty liver disease (NAFLD); use of weight loss medications or those causing insulin resistance or steatosis; or alcohol consumption of at least two drinks daily. Detailed exclusion criteria are provided in Supplemental Methods.
Study design and interventions
This study was a single-center, parallel arm, randomized, placebo-controlled, investigator- and participant-masked, proof-of-concept study. Eligible participants were randomly assigned (1:1) to receive betaine or placebo for 12 weeks, with allocation and masking performed by the Investigational Drug Service at Brigham and Women’s Hospital. Assessments included fasting endothelial function, hepatic MRI for intrahepatic triglyceride, 75-g oral glucose tolerance test (OGTT), and two-step euglycemic-hyperinsulinemic clamp performed the following day at baseline and 12 weeks of treatment. Follow-up visits occurred at 2 and 6 weeks, and at a postdosing safety visit 2 weeks after discontinuation of the study drug.
Study drug
Betaine (Letco Medical, Decatur, AL), 10 g, or microcrystalline cellulose (Fagron, St. Paul, MN) for placebo, was mixed with Crystal Light (Kraft Foods, Northfield, IL) and Ora-blend SF (Perrigo, Minneapolis, MN) for final betaine concentration of 330 mg/mL (Food and Drug Administration Investigational New Drug no. 117735). Participants were instructed to take betaine 3300 mg (10 mL) orally twice daily for 10 days, then 4950 mg (15 mL) orally twice daily through 12 weeks, or identical appearance placebo.
Physiologic assessments
Physiologic assessments were performed at baseline and following 12 weeks of betaine or placebo. Intrahepatic triglyceride levels were assessed by MRI and magnetic resonance spectroscopy (Siemens 3T TIM Skyra, software version VD13; Siemens, Erlangen, Germany) (10–12). Endothelial function was measured in accordance with published guidelines (13) by high-resolution B-mode ultrasonography of the brachial artery using edge detection software (Brachial Analyzer for Research, version 5.8.9 SP-2; Medical Imaging Applications, Coralville, IA) in response to ischemic flow and nitroglycerin stimulus.
Glycemia was assessed by HbA1c and a 75-g oral glucose load with glucose, insulin, and C-peptide levels obtained at 0, 30, 60, and 120 minutes. β-cell function was estimated using the corrected incremental insulin response (14, 15), and the oral composite insulin sensitivity index was calculated (14, 16).
Insulin sensitivity was also evaluated by euglycemic, two-step hyperinsulinemic clamp (17,18), with administration of primed, continuous insulin (Humulin Regular; Eli Lilly Inc., Indianapolis, IN) at 25 and 180 mIU/m2/min, for two hours each step, targeting glucose of 90 mg/dl, with infusion of 6,6-D2 glucose (Cambridge Isotope Laboratories, Inc., Tewksbury, MA) for assessment of endogenous glucose production(19) and indirect calorimetry (VMAX Encore 29N; Carefusion, Yorba Linda, CA) to quantify oxidative vs nonoxidative glucose disposal (20, 21). To assess change in insulin sensitivity before and after betaine administration, glucose infusion rates and corrected M-values of glucose utilization were compared.
Laboratory assessments
Clinical laboratory assessments were made at Laboratory Corporation of America (Burlington, NC). Commercial immunoassays were used according to instructions, including serum insulin and C-peptide (electrochemiluminescent immunoassay; Roche Diagnostics, Indianapolis, IN), plasma caspase-cleaved cytokeratin-18 by enzyme-linked immunosorbent assay (ELISA) (M30 Apoptosense PEVIVA kit, Bromma, Sweden), and plasma human FGF-21 and FGF-19 (both Quantikine ELISA kits, Minneapolis, MN). Betaine and other metabolites were quantified using liquid chromatography-mass spectrometry (LC/MS) (laboratory of co-author RSG) as described (22).
Statistical analyses
Coprimary outcomes were changes from baseline in insulin sensitivity, glycemia, hepatic fat, and endothelial function after 12 weeks of treatment, not corrected for multiple comparisons in this proof-of-concept study. Secondary outcomes were safety measures and changes from baseline in betaine pathways. A priori estimates found 24 completers would provide adequate power to detect a difference in clamp glucose utilization of 0.96 mg/kg/min between groups (25% difference in change from baseline between groups). Power estimates for additional coprimary outcomes are provided in Supplemental Materials.
Data were analyzed using the intention-to-treat principle; all participants with data at baseline and up to the point of dropout (if dropout occurred) were included in analyses. All participants completed visits before data analysis. Differences in baseline characteristics were compared between treatment groups using Student t test for normally distributed continuous traits and χ2 for categorical traits. Natural logarithm transformations were used for variables with log-normal distributions. Baseline characteristics are provided as mean (SD) or median (interquartile range), and changes from baseline are provided with 95% CIs or SE, as indicated. Statistical tests report 2-sided P values, with P < 0.05 considered significant. Change from baseline and difference between groups in change from baseline were assessed using linear mixed-effects models with unstructured covariance matrices to account for correlation within subjects over time. Analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC).
Results
Baseline characteristics
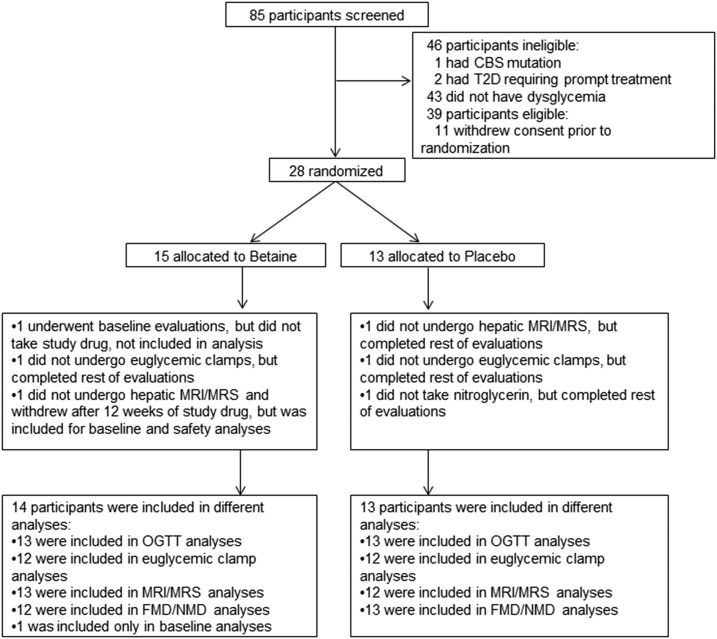
We assessed 85 persons for eligibility in the study. The primary reason for exclusion was normal glucose tolerance (n = 43; Fig. 1). A total of 28 people underwent baseline evaluation and were randomly assigned to receive betaine or placebo. One participant in the betaine group experienced a vasovagal reaction after the first clamp, was withdrawn prior to dispensing study drug, and not included in analysis. Baseline clinical characteristics were generally similar between groups (Table 1).
Figure 1.
Consort diagram. Disposition of study participants is shown. A total of 85 persons were screened for eligibility. Twenty-eight eligible participants were randomly assigned to receive either betaine or placebo for 12 wk. One participant in each group did not undergo hepatic MRI and MRS, due to metal implants, but were included in analyses of other end points. One participant in each group did not undergo the euglycemic-hyperinsulinemic clamps due to intravenous difficulties, but they were included in other end point analyses. One participant from the active drug group withdrew after taking the study drug for 12 wk, reporting inconvenience of the last visit scheduled, but was included in analyses through the duration of participation. One participant in the placebo group was included in the FMD analyses but could not be administered nitroglycerin and so was not included in NMD analyses. The study was completed by 13 participants in the betaine group and 13 in the placebo group. CBS, cystathionine β synthase; FMD, flow-mediated dilation, MRS, magnetic resonance spectroscopy; NMD, nitroglycerine-mediated dilation.
Table 1.
Baseline Characteristics of Study Participants
| Characteristic | Placebo (n = 13) | Betaine (n = 14) | P Value a |
|---|---|---|---|
| Women, no.%b | 4 (31) | 4 (29) | >0.999 |
| Age, y | 61 ± 7 | 57 ± 8 | 0.171 |
| Race or ethnicity no., %b | |||
| Hispanic/Latino | 0 (0) | 2 (14) | 0.482 |
| Asian | 0 (0) | 2 (14) | 0.482 |
| Black/African American | 2 (15) | 3 (21) | >0.999 |
| White | 11 (85) | 7 (50) | 0.114 |
| Weight, kg | 97 ± 20 | 91 ± 13 | 0.646 |
| BMI, kg/m2 | 32.1 ± 3.8 | 31.0 ± 2.7 | 0.724 |
| Waist circumference, cm | 112 ± 13 | 105 ± 10 | 0.607 |
| Systolic BP, mm Hg | 132 ± 13 | 132 ± 13 | 0.597 |
| Diastolic BP, mm Hg | 79 ± 13 | 80 ± 10 | 0.941 |
| Laboratory | |||
| HbA1C %c | 6.1 (0.5) | 6.1 (0.5) | 0.766 |
| Fasting glucose, mg/dLd | 107 ± 18 | 111 ± 16 | 0.552 |
| 2-Hour glucose, mg/dL | 187 ± 66 | 180 ± 60 | 0.764 |
| ALT, mg/dL | 22 ± 8 | 27 ± 13 | 0.193 |
| AST, mg/dL | 23 ± 4 | 23 ± 8 | 0.712 |
| Cholesterol, mg/dL | 165 ± 31 | 168 ± 31 | 0.803 |
| HDL, mg/dLe | 50 ± 13 | 44 ± 10 | 0.249 |
| LDL, mg/dLe | 103 ± 30 | 111 ± 35 | 0.560 |
| Triglycerides, mg/dL | 131 ± 65 | 141 ± 70 | 0.713 |
| Antihypertensive drugs, no. (%) | |||
| ACE inhibitor | 5 (38) | 3 (21) | 0.420 |
| ARB | 3 (23) | 0 (0) | 0.098 |
| β-Blockers | 3 (23) | 1 (7) | 0.326 |
| Calcium channel blockers | 4 (31) | 1 (7) | 0.165 |
| Diuretics | 3 (23) | 3 (21) | >0.999 |
| Lipid-lowering drugs | |||
| HMG-CoA reductase inhibitor (statin) | 9 (69) | 6 (43) | 0.252 |
| Antiplatelet drugs | |||
| Low-dose aspirin | 6 (46) | 3 (21) | 0.236 |
All values are presented as mean ± SD unless otherwise noted.
Abbreviations: ACE, angiotensin-converting enzyme; ALT, alanine aminotransferase; ARB, angiotensin II receptor blocker; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; HMG-CoA, hydroxy methyl glutamyl coenzyme A; LDL, low-density lipoprotein.
Variables were analyzed using two-sample equal variance t test unless noted.
For sex, ethnicity, and medications, differences were calculated using Fisher’s exact test.
Calculation of HbA1C per NGSP (%) = [0.09148 × IFCC (mmol/mol)] + 2.152).
Fasting glucose obtained as means of two values drawn at baseline during OGTT.
HDL and LDL conversion calculation: mg/dL × 0.0259 = mmol/L.
Betaine metabolism
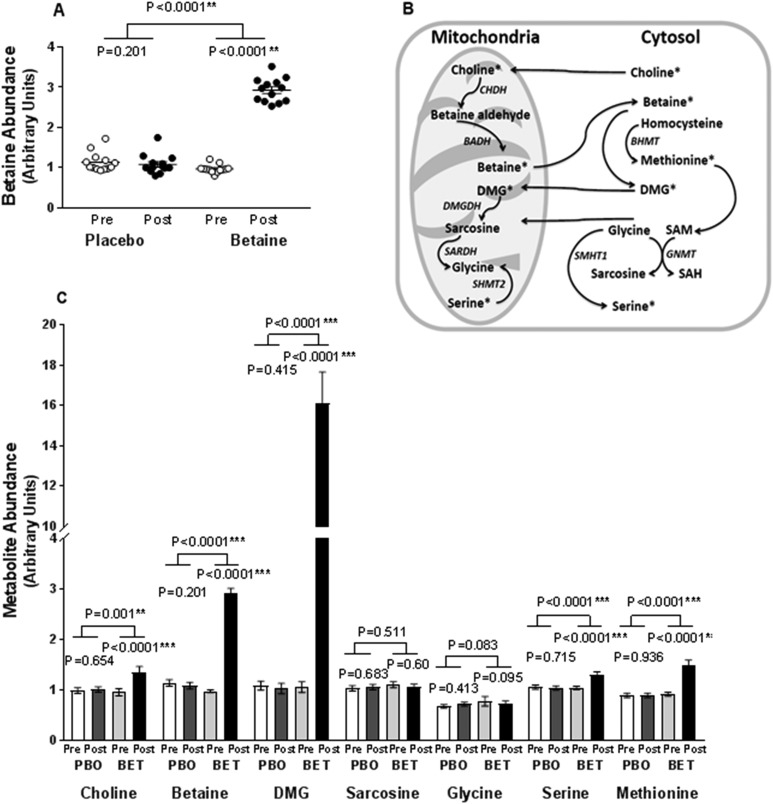
A 3.2-fold increase in plasma betaine, assessed by liquid chromatograph–mass spectroscopy, was seen within the betaine-assigned group compared with placebo, demonstrating betaine absorption (Fig. 2A). Betaine metabolism was documented by a 16.5-fold increase in plasma dimethylglycine (DMG), and 1.3 and 1.5-fold increases in downstream serine and methionine, respectively. Choline levels also differed between groups, increasing only in the betaine-treated group (Fig. 2C). There were no changes in concentrations of plasma betaine or betaine metabolites within the placebo group.
Figure 2.
Betaine metabolism. (A) Plasma betaine concentration assessed by liquid chromatography–mass spectrometry (LC/MS) is shown. Concentrations are shown for baseline (○) and posttreatment (●). (B) Key aspects of the betaine metabolic pathway. (C) Betaine metabolic pathway intermediates by LC/MS: placebo baseline (white column), placebo posttreatment (gray column), betaine baseline (light gray column), betaine posttreatment (black column). BADH, betaine-aldehyde dehydrogenase; BET, betaine; CHDH, choline dehydrogenase; GNMT, glycine-N-methyltransferase; PBO, placebo; post, after; pre, before; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; SARDH, sarcosine dehydrogenase; SHMT, serine hydroxymethyltransferase. *P < 0.05, **P < 0.01, ***P < 0.001.
Glycemia
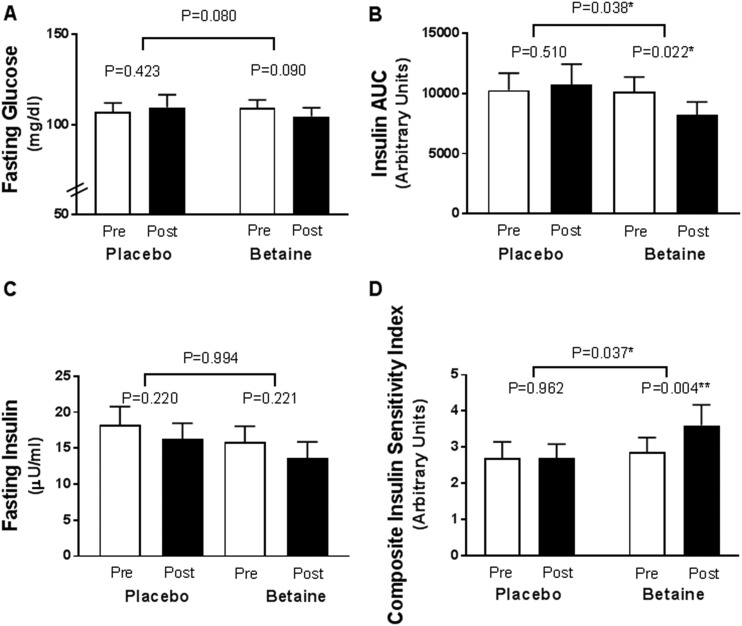
Fasting glucose levels trended lower after 12 weeks in the betaine group vs the placebo group (−8 mg/dL; 95% CI, −17 to 1; P = 0.080) and trended lower within the betaine group (−5 mg/dL; 95% CI, −12 to 1; P = 0.090), but did not change within the placebo group (3 mg/dL; 95% CI, −4 to 9; P = 0.423; Fig. 3A). There was no difference in change for HbA1c (Table 2) or in glucose area under the curve (AUC) during OGTT at 12 weeks between or within groups.
Figure 3.
OGTT-derived measures before and after 12 wk of treatment with betaine or placebo. (A) Fasting glucose. (B) AUC for insulin during the OGTT. (C) Fasting insulin. (D) The Matsuda Composite Insulin Sensitivity Index calculated as 10,000 divided by the square root of [(glucose × insulin) (glucosemean × insulinmean)]. Baseline pretreatment (white column); posttreatment (black column). *P < 0.05, **P < 0.01. AUC, area under the curve; post, after; pre, before.
Table 2.
Key Outcomes Related to Safety and Efficacy
| Measurement | Placebo | Betaine | Placebo | Betaine | Difference in Change | P Value |
|---|---|---|---|---|---|---|
| Baseline, Mean ± SE | Baseline, Mean ± SE | Mean Change From Baseline (95% CI) | Mean Change From Baseline (95% CI) | |||
| Vital signs | ||||||
| Weight, kg | 97.0 ± 4.7 | 91.0 ± 4.5 | 0.6 (−0.4 to 1.6) | 0.3 (–0.7 to 1.3) | −0.3 (–1.7 to 1.1) | 0.639 |
| BMI, kg/m2 | 32.1 ± 1.0 | 31.0 ± 1.0 | 0.2 (–0.1 to 0.6) | 0.1 (–0.2 to 0.5) | −0.1 (–0.5 to 0.4) | 0.790 |
| Waist, cm | 111.8 ± 3.1 | 105.0 ± 3.0 | 0.9 (–1.1 to 2.9) | 1.5 (–0.4 to 3.5) | 0.6 (–2.1 to 3.4) | 0.633 |
| SBP, mm Hg | 132 ± 4 | 132 ± 3 | 0.8 (–8.4 to 10.0) | 4.0 (–5.1 to 13.1) | 3.2 (–9.7 to 16.2) | 0.612 |
| DBP, mm Hg | 79 ± 3 | 80 ± 3 | 0.1 (–4.4 to 4.5) | 0.5 (–4.0 to 5.0) | 0.4 (–6.0 to 6.6) | 0.901 |
| Endocrine | ||||||
| HbA1C, %a | 6.1 ± 0.1 | 6.1 ± 0.1 | 0.1 (–0.1 to 0.3) | 0.1 (–0.1 to 0.3) | 0.0 (–0.2 to 0.3) | 0.925 |
| Fasting glucose, mg/dL | 107 ± 5 | 111 ± 5 | 3 (–4 to 9) | −5 (–12 to 1) | −8 (–17 to 1) | 0.080 |
| 2-Hour glucose, mg/dL | 187 ± 17 | 180 ± 17 | −4 (–22 to 13) | 7 (–10 to 25) | 12 (–12 to 36) | 0.324 |
| AUC glucose | 21,662 ± 1402 | 22,263 ± 1351 | −413 (–1749 to 923) | 340 (–993 to 1672) | 752 (–1134 to 2640) | 0.419 |
| Fasting insulin, μU/mL | 18 ± 2 | 16 ± 2 | −2 (–5 to 1) | −2 (–5 to 1) | 0 (–5 to 5) | 0.994 |
| 2-Hour insulin, μU/mL | 109 ± 18 | 123 ± 18 | 10 (–19 to 38) | −17 (–45 to 12) | −26 (–67 to 14) | 0.193 |
| AUC insulin | 10,281 ± 1360 | 10,116 ± 1310 | 427 (–887 to 1740) | −1553 (–2866 to –240) | −1980 (–3837 to –123) | 0.038b |
| C-peptide, ng/mL | 3.8 ± 0.3 | 3.3 ± 0.3 | −0.2 (–0.6 to 0.3) | −0.2 (–0.7 to 0.3) | −0.0 (–0.7 to 0.6) | 0.906 |
| AUC C-peptide | 1208 ± 88 | 1118 ± 85 | −56 (–127 to 15) | −69 (–142 to 5) | −12 (–115 to 90) | 0.808 |
| TSH, μU/mL | 2.4 ± 0.3 | 1.7 ± 0.3 | −0.4 (–0.8 to 0.1) | −0.1 (–0.6 to 0.3) | 0.2 (–0.4 to 0.9) | 0.438 |
| Cortisol, μg/dL | 10.5 ± 1.5 | 11.6 ± 1.4 | −2.0 (–5.1 to 1.1) | −3.0 (–6.0 to 0.0) | 1.0 (–5.3 to 3.3) | 0.640 |
| IGF-1, ng/mL | 101 ± 13 | 114 ± 12 | −8.2 (–16 to –0.3) | −4.7 (–12.5 to 3.0) | 3.4 (–7.6 to 14.5) | 0.528 |
| Calcium, mg/dL | 9.2 ± 0.1 | 9.1 ± 0.2 | −0.2 (–0.3 to –0.0) | −0.2 (–0.3 to 0.0) | 0.0 (–0.2 to 0.2) | 0.775 |
| Lipids, mg/dL | ||||||
| Total cholesterol | 165 ± 9 | 168 ± 8 | −7 (–20 to 7) | 15 (1 to 28) | 21 (3 to 40) | 0.032b |
| HDL cholesterolc | 50 ± 3 | 44 ± 3 | −1 (–4 to 2) | −1 (–4 to 2) | 0 (–4 to 4) | 0.845 |
| LDL cholesterolc | 130 ± 9 | 111 ± 9 | −7 (–16 to 2) | 2 (–6 to 11) | 9 (–3 to 22) | 0.106 |
| Triglycerides | 131 ± 19 | 141 ± 19 | −13 (–57 to 31) | 54 (11 to 97) | 67 (5 to 129) | 0.069 |
| Renal | ||||||
| Creatinine, mg/dL | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.0 (–0.0 to 0.1) | −0.1 (–0.1 to –0.0) | −0.1 (–0.2 to –0.0) | 0.030b |
| eGFR, mL/min per 1.73 m2 | 93 ± 4 | 97 ± 4 | −0.2 (–4 to 3) | 3 (–1 to 7) | 3 (–2 to 8) | 0.365 |
| Uric acid | 6 ± 0.3 | 6 ± 0.3 | 0.1 (–0.4 to 0.5) | −0.2 (–0.6 to 0.2) | −0.3 (–1 to 0.3) | 0.543 |
| BUN, mg/dL | 14 ± 1 | 16 ± 1 | 2 (–1 to 4) | 0 (–3 to 2) | −2 (–6 to 2) | 0.198 |
| BUN–creatinine ratio, mL/min per 1.73 m2 | 17.5 ± 1.7 | 19.4 ± 1.6 | 1.4 (–1.7 to 4.5) | 1.5 (–1.6 to 4.5) | 0.1 (–4.3 to 4.5) | 0.414 |
| Sodium, mmol/L | 141 ± 0.5 | 141.1 ± 0.5 | 0.2 (–1.5 to 1.6) | 0.2 (–1.2 to 1.5) | 0.0 (–2.0 to 2.0) | 0.999 |
| Potassium, mmol/L | 4.3 ± 0.1 | 4.3 ± 0.1 | 0.0 (–0.2 to 0.1) | 0.1 (–0.1 to 0.2) | 0.1 (–0.1 to 0.3) | 0.146 |
| Chloride, mmol/L | 102 ± 0.6 | 102 ± 0.6 | 0.6 (–0.8 to 2.1) | −0.3 (–1.7 to 1.2) | −0.9 (–2.9 to 1.2) | 0.384 |
| Hepatic | ||||||
| ALT, IU/L | 22 ± 3 | 27 ± 3 | −1.4 (–5.8 to 3.1) | 0.7 (–3.7 to 5.1) | 2.1 (–4.2 to 8.4) | 0.534 |
| AST, IU/L | 23 ± 2 | 23 ± 2 | −0.6 (–3.8 to 2.6) | 1.0 (–2.2 to 4.2) | 1.6 (–2.9 to 6.1) | 0.253 |
| GGT, IU/L | 31 ± 11 | 44 ± 10 | −1 (–21 to 19) | 11 (–9 to 30) | 11 (–39 to 17) | 0.649 |
| ALP, IU/L | 68 ± 4 | 60 ± 4 | −1 (–5to 2) | −9 (–13 to –6) | −8 (–13 to –3) | 0.005d |
| FGF-21, pg/mL | 456 ± 73 | 317 ± 70 | −56 (–135 to 23) | −34 (–113 to 45) | 22 (–90 to 134) | 0.683 |
| CK-18, U/L | 181 ± 18 | 163 ± 17 | 12 (–14 to 38) | 23 (–2 to 49) | 11 (–25 to 48) | 0.519 |
| Other | ||||||
| hsCRP, mg/L | 3.3 ± 1.0 | 1.5 ± 1.0 | 0.1 (–1.2 to 1.5) | −0.1 (–1.4 to 1.3) | −0.2 (–2.1 to 1.7) | 0.828 |
| Hematocrit, % | 40.1 ± 0.9 | 41.2 ± 0.8 | −1.0 (–2.0 to 0.0) | −0.9 (–1.9 to 0.1) | 0.1 (–1.3 to 1.5) | 0.378 |
| Leukocyte count, × 109 cells/L | 6.2 ± 0.3 | 5.9 ± 0.3 | −0.1 (–0.7 to 0.4) | −0.2 (–0.8 to 0.4) | −0.1 (–0.8 to 0.7) | 0.632 |
| Vitamin B12, pg/mL | 547 ± 71 | 564 ± 68 | −16 (–93 to 61) | 77 (–0 to 154) | 93 (–16 to 202) | 0.092 |
| Folate, mg/mL | 16 ± 1 | 17 ± 1 | 0.6 (–0.4 to 1.5) | −0.6 (–1.6 to 0.3) | −1.2 (–2.6 to 0.2) | 0.083 |
| FGF-19, pg/mL | 141 ± 29 | 140 ± 28 | 0.1 (–83 to 83) | 2.9 (–78 to 84) | 2.8 (–114 to 119) | 0.960 |
Outcome measures at baseline and after 12 wk of treatment with betaine or placebo are reported.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUC, area under curve; BMI, body mass index; BUN, blood urea nitrogen; CK-18, cytokeratin 18; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; GGT, γ-glutamyl transferase; HDL, high-density lipoprotein; hsCRP, high-sensitive C-reactive protein; LDL, low-density lipoprotein; SBP, systolic blood pressure.
Calculation of HbA1C, per NGSP (%) = [0.09148 × IFCC (mmol/mol)] + 2.152).
P < 0.05.
HDL and LDL conversion calculation: mg/dL × 0.0259 = mmol/L.
P < 0.01.
Insulin response to oral glucose was reduced more after betaine treatment vs placebo (AUC, −1980 μU/mL/min; 95% CI, −3837 to −123; P = 0.038), with reductions within the betaine group but not the placebo group (Fig. 3B). Change in fasting insulin did not differ between the groups after 12 weeks (0 μU/mL; 95% CI −5 to 5; P = 0.994; Fig. 3C). Change in C-peptide (fasting or AUC) did not differ between or within groups (Table 2).
Insulin sensitivity
Insulin sensitivity, calculated using the Matsuda composite insulin sensitivity index (14) during the OGTT, improved more from baseline after betaine treatment than after receiving placebo (P = 0.037), with 24% improvement within the betaine group (P = 0.004) and no change within the placebo group (P = 0.962; Fig. 3D). The corrected insulin response during the OGTT did not differ from baseline to 12 weeks between or within groups (Supplemental Table 2).
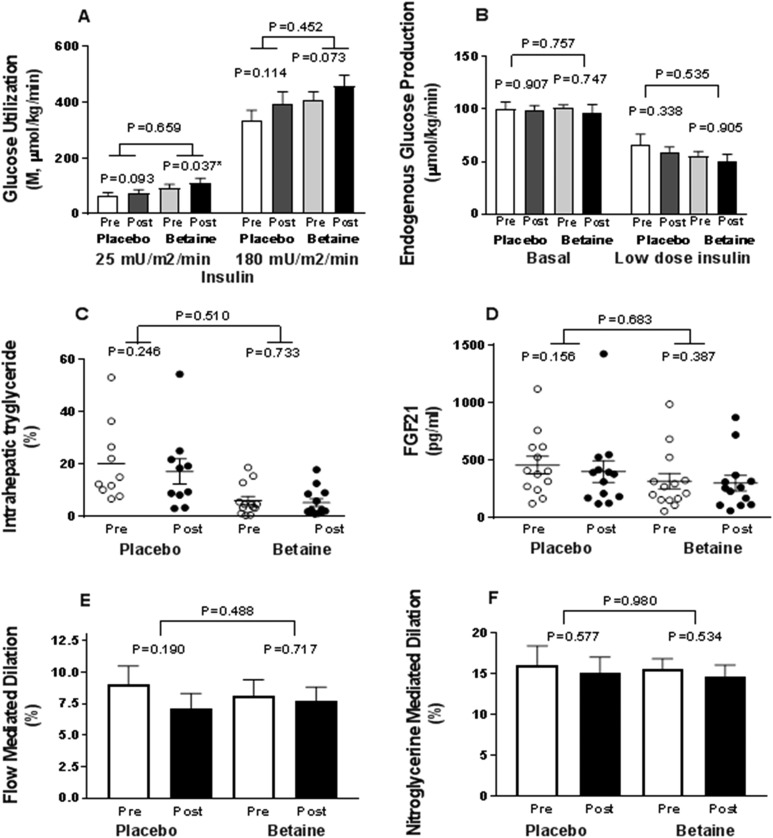
In contrast, change in insulin sensitivity did not differ during the two-step euglycemic-hyperinsulinemic clamp at either low-dose (25 mIU/m2/min; P = 0.659) or high-dose (180 mIU/m2/min; P = 0.452) insulin between or within the betaine and placebo groups (Fig. 4A). Clamp glucose and insulin concentrations were similar between groups, permitting comparison (Supplemental Fig. 1A and 1B). There was no difference between groups in insulin clearance or endogenous glucose production rates during fasting or low-dose insulin administration, with full suppression at higher dose under all conditions (Fig. 4B). Likewise, there were no differences in change from baseline in oxidative or nonoxidative glucose disposal under basal or insulin-stimulated conditions between or within groups (Supplemental Fig. 1C and 1D).
Figure 4.
Key metabolic end points at baseline and after 12 wk of treatment with betaine or placebo. (A) Insulin sensitivity is shown as M-values of glucose utilization during euglycemic-hyperinsulinemic clamp averaged over 90 to 120 mins and 210 to 240 mins, respectively, for each of two doses of insulin. (B) Endogenous glucose production: basal and clamp at low-dose insulin (25 mIU/m2/min). Placebo baseline (white column); placebo posttreatment (gray column); betaine baseline (light gray column); betaine posttreatment (black column). (B) Intrahepatic triglyceride measured by MRI and magnetic resonance spectroscopy is shown. (C) FGF21 concentrations are shown at baseline (○) and posttreatment (●). (D) Brachial artery flow-meditated dilation. (E) Brachial artery nitroglycerine-mediated dilation at baseline, pretreatment (white column), and posttreatment (black column). Post, after; pre, before.
Hepatic fat
Participants randomly assigned to the betaine group had 30% lower intrahepatic triglyceride levels compared with those assigned to placebo (P = 0.048) at study entry, although the two groups were well matched for all other baseline variables. However, there was no difference in change in intrahepatic triglyceride levels between the betaine and placebo groups (P = 0.510) or within either group at 12 weeks (Fig. 4C).
Endothelial function
There were no effects of betaine on change in endothelial-dependent flow-mediated dilation (P = 0.488) or endothelial-independent nitroglycerin-mediated dilation (P = 0.980) compared with placebo between or within the treatment groups (Fig. 4E and 4F; Supplemental Table 3).
Outcomes related to safety and efficacy
Total cholesterol level increased after betaine treatment (21 mg/dL, 95% CI, 3 to 40; P = 0.032) vs placebo, with no changes in triglyceride, high density lipoprotein– or low density lipoprotein–cholesterol levels (Table 2). Serum creatinine concentration was reduced more in the betaine group than in the placebo group (−0.1 mg/dL; 95% 95% CI, −0.2 to −0.0; P = 0.030). Serum alkaline phosphatase (ALP) level was reduced more after betaine than placebo treatment (−8.0 mg/dL; 95% 95% CI, −13 to −3; P = 0.005). There were no changes in bilirubin, alanine aminotransferase, aspartate aminotransferase, or γ-glutamyl transferase levels between groups. There were no changes in Hb, hematocrit, or white blood cell counts, indirectly suggesting nonhepatic sources of ALP. FGF-21 (Fig. 4D), FGF-19, and caspase-cleaved cytokeratin-18 values did not differ between or within groups (Table 2).
Multiple long-chain plasma fatty acids belonging to sphingomyelin (namely, C18:2 and C24:1), phosphatidylcholine (namely, C38:3), phosphatidylinositol (namely, C34:2 and C34:3), and triacylglycerol (namely, C48:4) families were significantly increased from baseline in the betaine vs placebo group. Levels of asparagine, creatine, fumaric acid, glyceric acid, N-carbamoyl–β-aminoisobutyric acid, proline, pyroglutamic acid, and taurine were also significantly increased after betaine treatment compared with placebo. Levels of arginine, ornithine, 3-hydroxyanthranilic acid, C3-carnitine, C4-butyryl-carnitines, inosine, nicotinamide-N-oxide, phosphoethanolamine, xanthosine, and xanthurenic acid declined after betaine treatment compared with placebo. Metabolites are shown in Supplemental Table 1.
The palmitic acid–linoleic acid ratio index of de novo lipogenesis was evaluated. Change from baseline did not differ between the betaine and placebo groups (P = 0.716) or within the betaine-treated group (baseline to week 12: 1.08 ± 0.23 to 1.06 ± 0.26; P = 0.602) or the placebo group (baseline to week 12: 1.12 ± 0.36 to 1.12 ± 0.34; P = 0.979).
Adverse events
There was no change in body weight or blood pressure (Table 2) within or between the two groups. There were no serious adverse events, and nonserious adverse events were sparse and balanced between groups (Supplemental Table 4).
Discussion
Betaine has emerged as a novel metabolite inversely associated with insulin resistance, dyslipidemia, T2D, and cardiovascular risk (2–5, 7, 22). Dietary supplementation improves multiple metabolic phenotypes in preclinical models (2), mediated at least in part via FGF-21 signaling. Oral betaine has been administered safely in healthy volunteers (Orphan Therapeutics, Celine Plisson and Pascale Adam, personal communication, March 8, 2013) (23) and patients with liver disease (24, 25), and is marketed for disorders of cysteine metabolism. Thus, we sought to examine effects of oral betaine on glycemia, insulin sensitivity, intrahepatic triglyceride levels, and endothelial function.
Daily betaine intake varies from 30 to 400 mg/d (26). We administered approximately 2.5 times the typical consumption, achieving 3.2-fold higher plasma levels. The magnitude of increase is greater than the 20% reduction in plasma betaine levels seen in obese rodents or the difference between insulin resistant and sensitive persons (2). Thus, the dose of betaine administered in our study should be sufficient to elucidate metabolic effects of restoration of betaine plasma levels.
The 16.5-fold increase in DMG and lesser increase in downstream metabolites support DMGDH as rate limiting for betaine metabolism in persons with prediabetes. Allele variants at the DMGDH locus are associated with DMG levels, with the major allele associated with lower DMG, and higher plasma insulin levels, homeostatic model assessment insulin resistance, and incident diabetes (27). Variation in DMGDH levels or activity could contribute to insulin resistance and diabetes or cardiovascular risk by reducing important downstream metabolites, even in the setting of the major allele. However, our findings provide limited support for therapeutic replacement of betaine in the general population to correct these metabolic defects. Our participants were not genotyped; findings may differ in subsets of individuals selected for genetic variants.
Other pathways of betaine metabolism were also affected by supplementation. Levels of the PEMT substrate phosphoethanolamine were reduced after betaine treatment (Supplemental Table 1), supporting a possible increase in PEMT enzymatic activity. Betaine can be synthesized by oxidation of choline or carnitine; it is an electron acceptor in a reduction-oxidation reaction catalyzed to produce trimethylamine and acetate (28). Interestingly, concentrations of plasma carnitine and related C3-carnitine and C4-butyryl-carnitine were all reduced after betaine treatment, suggesting substrate (i.e., betaine) availability is not rate limiting. β-aminoisobutyric acid, which induces expression of genes associated with fatty acid oxidation (29), was increased in the betaine group vs the placebo group, which is consistent with potential betaine-mediated increased fatty acid oxidation. Arginine and ornithine were reduced in the betaine-treated group, suggesting possible changes in the malate shuttle and NADH transport into mitochondria. Finally, betaine plays a key role in the choline and methionine pathways for glutathione synthesis and conversion of homocysteine to methionine. Although we did not measure the direct products of the betaine pathway enzymes BHMT and MAT1A, the pathway intermediate S-adenosyl homocysteine did not change after betaine treatment.
Insulin concentrations after oral glucose were reduced; however, the glucose AUC and C-peptide did not change. The difference between insulin and C-peptide response to oral glucose warrants discussion. Insulin and C-peptide have equimolar secretion by pancreatic β-cells. Circulating insulin is preferentially degraded in the liver, whereas C-peptide excretion mainly occurs in kidneys. It is possible betaine increases hepatic insulin clearance, although whole-body insulin clearance measured during insulin-clamp studies was not increased after betaine treatment, as compared with placebo, and was modestly decreased in each group. There was no evidence in this study that C-peptide clearance is delayed: There was no evidence of impaired renal function, and creatinine concentrations were modestly lower after betaine treatment. C-peptide clearance was not directly measured.
Lower insulin levels after oral glucose load led to a difference between insulin sensitivity estimated by the Matsuda oral index as compared with the euglycemic hyperinsulinemic clamp. These two methods are typically strongly correlated (16). The lack of difference in glucose and C-peptide levels after oral glucose load supports no difference in insulin sensitivity, in agreement with the absence of difference in glucose utilization by the gold standard euglycemic clamp. Additional studies would be required to evaluate potential gut-derived hormone or gut microbiome contributions to betaine effects on insulin metabolism after oral load. Alternatively, differences in carbohydrate and fatty acid oxidation may differ between oral and clamp conditions, contributing to observed differences between the two model estimates of insulin sensitivity.
Betaine improves NAFLD in obese mice by reducing liver fat and inflammatory gene expression and normalizing postreceptor insulin signaling to improve gluconeogenesis and glycogen synthesis (2, 30). Similarly, a pilot study in 10 human patients with nonalcoholic steatohepatitis suggested some reduction in aminotransferases and steatosis, inflammation, and fibrosis (24). By contrast, neither the findings of a larger study in nonalcoholic steatohepatitis (25) nor those of our study in prediabetes support any effect of betaine in NAFLD. There was no difference in change in intrahepatic triglyceride content, transaminase concentrations, the fibrosis biomarker CK-18, or FGF-21. Baseline intrahepatic triglyceride content was randomly lower in the betaine group vs the placebo group. One might speculate a high hepatic fat content would be required to observe a reduction and thus it is possible the effect was missed. ALP concentration was reduced in the betaine group compared with the placebo group. Fractionation was not performed. No other liver biomarker changes were observed. Betaine has been reported to promote differentiation of human osteoblasts in culture (31). Potential betaine effects on bone in vivo may warrant additional investigation.
In mice, betaine increases FGF-21 concentrations (2). There is no effect of betaine on Fgf21 expression in primary hepatocytes, suggesting no direct transcriptional effects. FGF-21 has multiple metabolic effects in preclinical and human studies (32). However, Fgf21-null mice do not manifest the metabolic improvements to betaine seen in wild-type mice, demonstrating the important role of Fgf21 in mediating betaine effects on weight loss, energy expenditure, glucose and insulin tolerance, and liver fat in mice (2). It is possible the absence of change in FGF21 in humans after betaine treatment underlies the lack of translation of preclinical findings to humans.
Although epidemiologic studies link low betaine levels to cardiovascular risk (7), there are few data on endothelial function. Associations between homocysteine metabolism and cardiovascular risk support the hypothesis that betaine supplementation would enhance vascular function. However, single-dose oral betaine (2.5 g) did not alter plasma nitrate or cardiovascular responses to exercise in healthy young adults (23). Likewise, we do not find any effect of larger doses of betaine administered over 12 weeks to alter flow-mediated endothelial dilation in prediabetes. Levels of arginine, a precursor of nitric oxide, were lower after betaine treatment, potentially attenuating beneficial effects.
Furthermore, betaine increased total cholesterol level, with a similar trend for triglycerides, as previously described (33, 34), which also may attenuate any potential benefit on vascular function. High-fat diets reduce plasma betaine concentration despite preserved dietary intake (2). Reductions in levels of plasma carnitines and increases in multiple long-chain fatty acids after betaine administration support reciprocal regulation of lipid metabolism. However, static plasma metabolomics data cannot be used to predict magnitude or directionality of betaine-mediated impact on hepatic lipid metabolic flux. Due to the deleterious effects seen on lipid profiles and because of the absence of clear metabolic benefit, we must recommend against use of high doses of betaine supplementation outside of medical use for inborn errors of metabolism.
Although we provide a comprehensive assessment of metabolic effects of betaine in a population of individuals with prediabetes, our findings must be considered in the context of the small study population, relatively short duration of betaine administration, and multiple end points assessed within the trial. Plasma metabolite concentrations do not directly measure potentially relevant tissue metabolites or pathway flux.
In summary, low betaine concentration is associated with multiple metabolic disease risk markers, and betaine supplementation in preclinical models improves many disease-linked phenotypes. Our present study shows that DMGDH may be rate limiting for betaine metabolism in prediabetic persons. Although betaine affects the choline-betaine pathway, the effects on downstream metabolites are more modest. In parallel, the modest metabolic effects suggest obesity and prediabetes may be associated with betaine resistance. We found deleterious effects on serum cholesterol profiles. Our current data do not support additional studies of betaine supplementation in prediabetes or insulin resistance to treat or reduce cardiometabolic risk. Additional studies may be warranted to understand the mechanisms contributing to differences between preclinical and human responses to betaine supplementation, and to determine whether supplementation of metabolites downstream of DMGDH may be beneficial to persons with insulin resistance and T2D.
Supplementary Material
Acknowledgments
We thank the Joslin Clinical Research Center’s philanthropic donors. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
Financial Support: This work was supported by American Diabetes Association Grant 7-13-CE-17 (Bedside to Bench and Back: Cardiometabolic Effects of Betaine Supplementation, to A.B.G.); American Diabetes Association Mentor-Based Minority Postdoctoral Fellowship (Grant 1-15-MI-05 to A.B.G.); National Institutes of Health (NIH; Grants P30 DK036836 and R01 DK 029953 to R.B. and R01DK1081572, DK108159, and DK08157209 to R.E.G.); Sunstar Foundation Postdoctoral Fellowship; The Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH Award UL1 TR001102); and financial contributions from Harvard University and its affiliated academic health care centers. We also acknowledge support of the Joslin Clinical Research Center.
Clinical Trial Information: ClinicalTrials.gov no. NCT01950039 (registered 25 September 2013).
Current Affiliation: A. B. Goldfine’s current affiliation is Novartis Institutes of Biomedical Research, Cambridge, Massachusetts 02139.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ALP
alkaline phosphatase
- DMG
dimethylglycine
- DMGDH
dimethylglycine dehydrogenase
- FGF
fibroblast growth factor
- NAFLD
nonalcoholic fatty liver disease
- OGTT
oral glucose tolerance test
- PEMT
phosphatidylethanolamine N-methyltransferase
- T2D
type 2 diabetes
References
- 1. Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992;340(8825):925–929. [DOI] [PubMed] [Google Scholar]
- 2. Ejaz A, Martinez-Guino L, Goldfine AB, Ribas-Aulinas F, De Nigris V, Ribó S, Gonzalez-Franquesa A, Garcia-Roves PM, Li E, Dreyfuss JM, Gall W, Kim JK, Bottiglieri T, Villarroya F, Gerszten RE, Patti ME, Lerin C. Dietary betaine supplementation increases Fgf21 levels to improve glucose homeostasis and reduce hepatic lipid accumulation in mice. Diabetes. 2016;65(4):902–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Svingen GF, Schartum-Hansen H, Pedersen ER, Ueland PM, Tell GS, Mellgren G, Njølstad PR, Seifert R, Strand E, Karlsson T, Nygård O. Prospective associations of systemic and urinary choline metabolites with incident type 2 diabetes. Clin Chem. 2016;62(5):755–765. [DOI] [PubMed] [Google Scholar]
- 4. Walford GA, Ma Y, Clish C, Florez JC, Wang TJ, Gerszten RE; Diabetes Prevention Program Research Group . Metabolite profiles of diabetes incidence and intervention response in the diabetes prevention program. Diabetes. 2016;65(5):1424–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lever M, George PM, Atkinson W, Molyneux SL, Elmslie JL, Slow S, Richards AM, Chambers ST. Plasma lipids and betaine are related in an acute coronary syndrome cohort. PLoS One. 2011;6(7):e21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Konstantinova SV, Tell GS, Vollset SE, Nygård O, Bleie Ø, Ueland PM. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J Nutr. 2008;138(5):914–920. [DOI] [PubMed] [Google Scholar]
- 7. Lever M, George PM, Elmslie JL, Atkinson W, Slow S, Molyneux SL, Troughton RW, Richards AM, Frampton CM, Chambers ST. Betaine and secondary events in an acute coronary syndrome cohort. PLoS One. 2012;7(5):e37883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwahn BC, Hafner D, Hohlfeld T, Balkenhol N, Laryea MD, Wendel U. Pharmacokinetics of oral betaine in healthy subjects and patients with homocystinuria. Br J Clin Pharmacol. 2003;55(1):6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Diabetes Association 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(Suppl 1):S11–S24. [DOI] [PubMed] [Google Scholar]
- 10. Ortiz-Lopez C, Lomonaco R, Orsak B, Finch J, Chang Z, Kochunov VG, Hardies J, Cusi K. Prevalence of prediabetes and diabetes and metabolic profile of patients with nonalcoholic fatty liver disease (NAFLD). Diabetes Care. 2012;35(4):873–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355(22):2297–2307. [DOI] [PubMed] [Google Scholar]
- 12. Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288(2):E462–E468. [DOI] [PubMed] [Google Scholar]
- 13. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R; International Brachial Artery Reactivity Task Force . Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–265. [DOI] [PubMed] [Google Scholar]
- 14. Hanson RL, Pratley RE, Bogardus C, Narayan KM, Roumain JM, Imperatore G, Fagot-Campagna A, Pettitt DJ, Bennett PH, Knowler WC. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol. 2000;151(2):190–198. [DOI] [PubMed] [Google Scholar]
- 15. Lyssenko V, Jonsson A, Almgren P, Pulizzi N, Isomaa B, Tuomi T, Berglund G, Altshuler D, Nilsson P, Groop L. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med. 2008;359(21):2220–2232. [DOI] [PubMed] [Google Scholar]
- 16. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. [DOI] [PubMed] [Google Scholar]
- 17. DeFronzo RA, Ferrannini E, Simonson DC. Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism. 1989;38(4):387–395. [DOI] [PubMed] [Google Scholar]
- 18. Goldfine AB, Patti ME, Zuberi L, Goldstein BJ, LeBlanc R, Landaker EJ, Jiang ZY, Willsky GR, Kahn CR. Metabolic effects of vanadyl sulfate in humans with non-insulin-dependent diabetes mellitus: in vivo and in vitro studies. Metabolism. 2000;49(3):400–410. [DOI] [PubMed] [Google Scholar]
- 19. Ferrannini EDS, DeFronzo RA. Glucose kinetics and tracer methods In: Clarke WL, Larner J, and Pohl SL, eds. Methods in Diabetics Research. Volume II Hoboken, NJ: Wiley Interscience; 1986:107–142. [Google Scholar]
- 20. Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988;37(3):287–301. [DOI] [PubMed] [Google Scholar]
- 21. Simonson DC, DeFronzo RA. Indirect calorimetry: methodological and interpretative problems. Am J Physiol. 1990;258(3 Pt 1):E399–E412. [DOI] [PubMed] [Google Scholar]
- 22. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O’Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pryor JL, Wolf ST, Sforzo G, Swensen T. The effect of betaine on nitrate and cardiovascular response to exercise. Int J Exerc Sci. 2017;10(4):550–559. [PMC free article] [PubMed] [Google Scholar]
- 24. Abdelmalek MF, Angulo P, Jorgensen RA, Sylvestre PB, Lindor KD. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: results of a pilot study. Am J Gastroenterol. 2001;96(9):2711–2717. [DOI] [PubMed] [Google Scholar]
- 25. Abdelmalek MF, Sanderson SO, Angulo P, Soldevila-Pico C, Liu C, Peter J, Keach J, Cave M, Chen T, McClain CJ, Lindor KD. Betaine for nonalcoholic fatty liver disease: results of a randomized placebo-controlled trial. Hepatology. 2009;50(6):1818–1826. [DOI] [PubMed] [Google Scholar]
- 26. Obeid R. The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients. 2013;5(9):3481–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Magnusson M, Wang TJ, Clish C, Engström G, Nilsson P, Gerszten RE, Melander O. Dimethylglycine deficiency and the development of diabetes. Diabetes. 2015;64(8):3010–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Naumann E, Hippe H, Gottschalk G. Betaine: new oxidant in the Stickland reaction and methanogenesis from betaine and l-alanine by a Clostridium sporogenes-Methanosarcina barkeri coculture. Appl Environ Microbiol. 1983;45(2):474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jung TW, Hwang HJ, Hong HC, Yoo HJ, Baik SH, Choi KM. BAIBA attenuates insulin resistance and inflammation induced by palmitate or a high fat diet via an AMPK-PPARδ-dependent pathway in mice. Diabetologia. 2015;58(9):2096–2105. [DOI] [PubMed] [Google Scholar]
- 30. Kathirvel E, Morgan K, Nandgiri G, Sandoval BC, Caudill MA, Bottiglieri T, French SW, Morgan TR. Betaine improves nonalcoholic fatty liver and associated hepatic insulin resistance: a potential mechanism for hepatoprotection by betaine. Am J Physiol Gastrointest Liver Physiol. 2010;299(5):G1068–G1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Villa I, Senesi P, Montesano A, Ferraretto A, Vacante F, Spinello A, Bottani M, Bolamperti S, Rubinacci A, Luzi L, Terruzzi I. Betaine promotes cell differentiation of human osteoblasts in primary culture. J Transl Med. 2017;15(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fisher FM, Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol. 2016;78(1):223–241. [DOI] [PubMed] [Google Scholar]
- 33. Rajaie S, Esmaillzadeh A. Dietary choline and betaine intakes and risk of cardiovascular diseases: review of epidemiological evidence. ARYA Atheroscler. 2011;7(2):78–86. [PMC free article] [PubMed] [Google Scholar]
- 34. Olthof MR, van Vliet T, Verhoef P, Zock PL, Katan MB. Effect of homocysteine-lowering nutrients on blood lipids: results from four randomised, placebo-controlled studies in healthy humans. PLoS Med. 2005;2(5):e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.