Abstract
One of the main functions of the kidney is to excrete an acid load derived from both dietary and endogenous sources, thus maintaining the pH of other fluids in the body. Urine pH is also of particular interest in stone formers, since it determines the presence of either calcium phosphate or uric acid content in stones. Others have noted in epidemiological studies a rise in incidence of low pH-dependent uric acid stones with age, coinciding with a decrease in the incidence of high pH-dependent phosphate stones. Taken together, these trends are suggestive of a longitudinal decline in urine pH in stone-forming patients, and, if true, this could explain the observed trends in stone incidence. We studied 7,891 stone formers, all of whom collected a 24-h urine sample and matching serum. Multivariate modeling revealed that urine pH did indeed fall with age and particularly between the ages of 20 and 50 yr old in both men and women. We sought to explain this trend through the inclusion of traditionally understood determinants of urine pH such as urinary buffers, estimates of glomerular filtration, and dietary acid load, but these, taken together, accounted for but a small fraction of the pH fall. Gastrointestinal anion absorption was the strongest predictor of urine pH in all age groups, as we have previously reported in middle-aged normal men and women. However, we found that, despite a decreasing urine pH, gastrointestinal anion absorption increased monotonically with age. In fact, after adjustment for gastrointestinal anion absorption, urine pH declined more markedly, suggesting that bicarbonate-producing anion absorption is regulated in a manner that offsets the decline of urine pH.
Keywords: acid base, kidney stones, urine pH
INTRODUCTION
Systemic acid-base balance and renal acid excretion have a special importance among stone-forming patients. Urine pH governs whether the stone mineral will be calcium phosphate or uric acid as opposed to the most common calcium oxalate crystal, which forms independent of urine pH. Because both calcium phosphate and uric acid stone formers plug terminal nephrons more than calcium oxalate stone formers do, the stone mineral phase affects the potential for kidney tissue damage (5, 40).
Elsewhere, we have presented evidence for a higher urine pH in normal women versus men (42). With age, especially among women, the abundance of calcium phosphate stones falls while that of uric acid stones rises (4, 22). Given that alkaline urine pH fosters the former and acid pH the latter, these epidemiological data suggest that urine pH falls with age. Indeed, in a cohort of recurrent calcium oxalate stone formers, Otto et al. (28) found that urine pH fell with age, although this has not been studied in other groups.
Such a fall in pH could, in turn, reflect changes in systemic acid-base balance, renal acidification mechanisms, or both. It is known that urine pH falls with glomerular filtration rate (GFR); however, phosphate stone prevalence falls between early adulthood and young middle age (4, 22, 31) while GFR declines later in life (31), suggesting that the mechanism for falling urine pH is not a decline in renal function. Obesity and diabetes also lower urine pH (3, 21), the prevalence of both rise with age, and both are higher among uric acid stone formers than other stone formers (39). Although diabetes and obesity certainly occur even in early middle age, one might have expected more effects from both a decade later. In other words, the mechanism for this presumed urine pH change is not apparent.
Therefore, we undertook an analysis of urine pH, renal acidification, and dietary acid-base contribution with age using cross-sectional 24-h urine data from a large population of stone formers ranging from 18 to >70 yr of age. In particular, we sought to determine the effects of body mass index, GFR, ammonia production, and gastrointestinal anion absorption (27).
We found that urine pH fell with age and, indeed, most rapidly in the years in which stone phosphate content falls most steeply. As one might expect given their higher prevalence of phosphate stones, women produced urine of higher pH than men at all ages. Straightforward multivariable analysis revealed that age itself, separate from body mass index, renal function, and gastrointestinal anion absorption, had a large and independent association with urine pH. Gastrointestinal anion absorption had a strong positive correlation with urine pH, but gastrointestinal anion excretion increased with age as if in compensation as opposed to cause.
METHODS
Subjects and Data
We used a large United States national data set of stone former pretreatment collections (Litholink, a Division of LabCorp). Each patient contributed one set of serum and 24-h urine data. The data set included 12,839 patients (5,586 women and 7,253 men) from which we excluded 4,948 (1,981 women and 2,967 men) because of missing height, weight, or serum data, ammonia excretion > 100 mmol/day, citrate excretion < 30 mg/day, or body surface area < 1 m2. The remaining 7,891 cases were divided into hexiles by age (Table 1). They were reasonably balanced with regard to sex ratio, age, weight, and body mass index.
Table 1.
Characteristics of age hexiles
| Age Hexiles, yr | ||||||
|---|---|---|---|---|---|---|
| <33 | 33–42 | 42–49 | 49–56 | 56–64 | >64 | |
| Number | ||||||
| Women | 821 | 681 | 559 | 568 | 525 | 452 |
| Men | 413 | 644 | 688 | 779 | 881 | 880 |
| Age, yr | ||||||
| Women | 26.0 ± 0.2 | 37.0 ± 0.1 | 45.2 ± 0.1 | 51.9 ± 0.1 | 59.2 ± 0.1 | 70.4 ± 0.3 |
| Men | 26.2 ± 0.2 | 37.3 ± 0.1 | 45.2 ± 0.1 | 52.1 ± 0.1 | 59.3 ± 0.1 | 70.2 ± 0.2 |
| Weight, kg | ||||||
| Women | 69.3 ± 0.6 | 74.6 ± 0.8 | 77.4 ± 0.9 | 80.1 ± 1.0 | 78.9 ± 0.9 | 77.6 ± 0.9 |
| Men | 83.9 ± 0.8 | 91.8 ± 0.8 | 93.6 ± 0.7 | 93.8 ± 0.7 | 94.6 ± 0.7 | 88.9 ± 0.6 |
| Body mass index, kg/m2 | ||||||
| Women* | 25.9 ± 0.2 | 27.9 ± 0.3 | 28.7 ± 0.3 | 30.1 ± 0.3 | 29.8 ± 0.3 | 29.8 ± 0.3 |
| Men* | 26.4 ± 0.2 | 29.1 ± 0.8 | 29.2 ± 0.2 | 29.5 ± 0.2 | 29.7 ± 0.2 | 28.2 ± 0.2 |
| Serum potassium | ||||||
| Women* | 4.21 ± 0.02 | 4.24 ± 0.02 | 4.22 ± 0.02 | 4.27 ± 0.02 | 4.32 ± 0.02 | 4.26 ± 0.02 |
| Men* | 4.29 ± 0.02 | 4.30 ± 0.02 | 4.30 ± 0.02 | 4.34 ± 0.02 | 4.36 ± 0.02 | 4.41 ± 0.02 |
| Serum total CO2 | ||||||
| Women* | 24.5 ± 0.1 | 24.8 ± 0.1 | 25.0 ± 0.1 | 25.5 ± 0.1† | 25.7 ± 0.1 | 25.7 ± 0.1 |
| Men | 25.9 ± 0.1 | 25.7 ± 0.1 | 25.7 ± 0.1 | 25.6 ± 0.1 | 25.7 ± 0.1 | 25.5 ± 0.1 |
Values are means ± SE. Trend analysis was undertaken for body mass index, potassium, and total CO2.
Significant linear trend, P < 0.001. Adjacent difference analysis was undertaken only for serum analytes.
Significant difference (P < 0.001) from the previous hexile.
For each patient, we had available serum calcium, phosphorus, magnesium, sodium, potassium, total CO2 content, chloride, uric acid, and creatinine as well as the corresponding 24-h urine values for volume, pH, calcium, phosphorus, magnesium, sodium, potassium, uric acid, creatinine, oxalate, citrate, chloride, ammonium, and sulfate. Analytes were measured using techniques previously described elsewhere (29, 30).
Calculations
Urine excretions were calculated per 24 h per 1.73 m2 of body surface.
We calculated titratable acidity (TA) as previously described (9). Briefly, TA was calculated with the following equations derived from the Henderson-Hasselbalch relation, referring to the physiologically active pKa of the phosphate anion:
| (1) |
| (2) |
| (3) |
| (4) |
where antiph is the antilog of pH, uph is urine pH, and p24M is molality of phosphate in the 24-h urine collection. TA was expressed in millimoles per liter.
The value calculated from Eq. 4 is the concentration of titrated protons, and multiplying by volume adjusted per 1.73 m2 body surface area returned the daily excretion of TA.
Gastrointestinal anion levels were calculated as follows: ∑Na+ + K+ + Ca2+ + Mg2+ − ∑Cl− + 1.8 × P, where all solutes were in milliequivalents per liter except for P, which was in millimoles per liter; multiplying by 1.8 converts P to milliequivalents per liter at blood pH. Gastrointestinal anion levels in milliequivalents per liter were then multiplied by the 24-h urine volume adjusted for body surface area. This composite variable represents gastrointestinal alkali absorption and has been shown to correlate closely with anion absorptions measured in a total body balance study (27).
In the absence of urine total CO2 (TCO2), we were unable to calculate net acid excretion (8). However, we performed some analyses using the sum of ammonia and TA, which we termed “acid excretion” (AE).
Creatinine clearance (CCr) was calculated as follows:
| (5) |
where UC = urine creatinine (mg/dl) × body surface area-adjusted volume [ml(1.73 m2) × body surface area].
Statistical Analysis
Comparisons across age hexiles by sex used fixed-effects multivariable ANOVA, since each person had only one 24-h urine sample and paired serum. The significance of age effects was gauged using post hoc polynomial contrast testing of the ANOVA models (Systat 13.2, San Jose, CA).
Contrast testing assesses for the existence of a relationship across multiple levels of a variable with a specified polynomial form (linear, quadratic, cubic, etc.) and returns an estimate of the coefficient of the highest-order polynomial of the equation defining the relationship between these levels. The null hypothesis is that the coefficient is equal to zero, and a significant result demonstrates that the coefficient is nonzero. When adding adjusted variables to the ANOVA model, it is instructive to compare the magnitude of linear coefficients in particular, since these are a multivariate proxy of the slope of the effect.
In our analysis, hexiles of age were ordered from youngest to oldest, and variables of interest were tested with linear (order 1) polynomials with the exception of CCr, which was additionally tested with a quadratic (order 2) polynomial.
Figures 1–6, displaying age hexiles, use dashed lines connecting adjacent hexile values for visual clarity.
RESULTS
Urine pH With Age
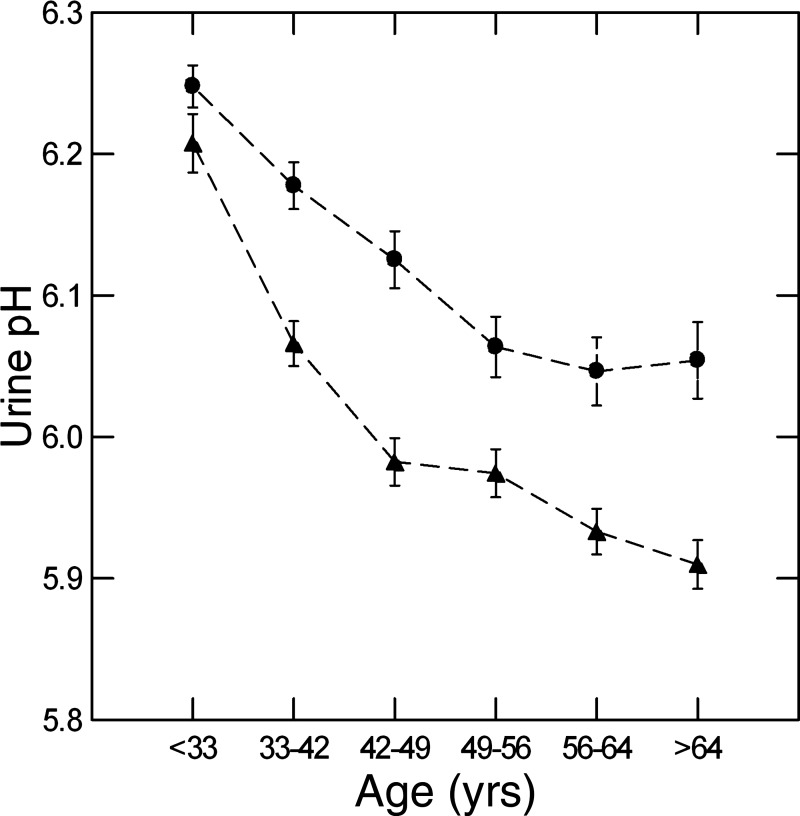
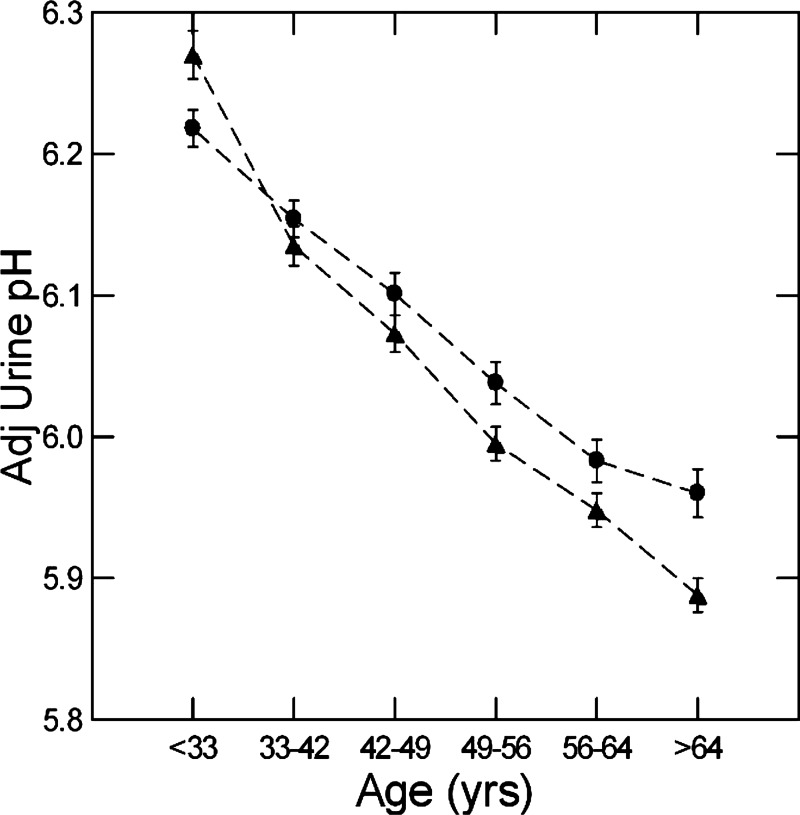
In men and women, urine pH fell with increasing age hexile (Fig. 1). pH decrements by hexile were significant (P < 0.01) for all but the transitions between hexiles 3 and 4 and hexiles 5 and 6 (men) as well as hexiles 4 and 5 and hexiles 5 and 6 (women). In other words, the older age hexile transitions were not generally significant for either sex. The linear contrast for pH versus age was highly significant (F = 145 and 71, men and women, respectively, and 208 for both sexes combined, P < 0.001 for both; linear slope = −0.227 and −0.170, men and women, respectively, and −0.198 for both sexes combined).
Fig. 1.
Urine pH versus age hexiles in men and women. Triangles, men; circles, women. Values are means ± SE. pH fell progressively in both sexes (linear contrast for pH over age, F = 144 and 71, men and women, respectively, P < 0.001 for both). Dashed lines are for visual clarity only and imply no interposed information.
Effect of Rising Body Mass Index
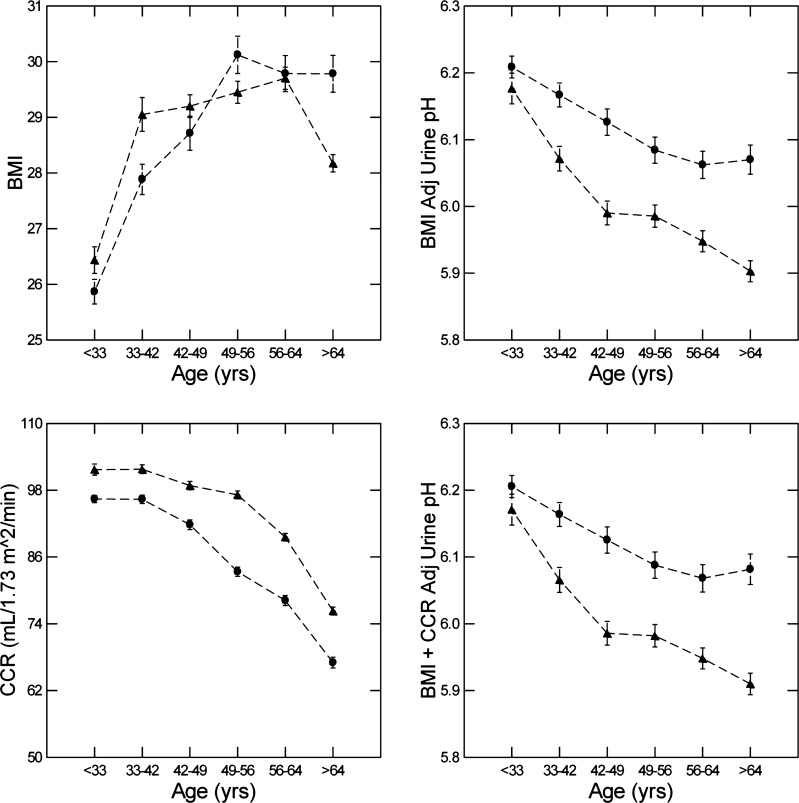
We (24) have previously shown the urine pH of stone-forming patients falls with weight adjusted for urine creatinine, a proxy for body mass index when height is not recorded. In addition, Taylor and Curhan (38) have shown an inverse association between urine pH and body mass index itself in non-stone-forming patients. Therefore, increasing body mass index with age could account for the fall in urine pH. Body mass index rose with age (Fig. 2, top left, and Table 1) in both men and women (linear contrast F = 146, both sexes combined, P < 0.001). In ANOVA containing body mass index, sex, and age hexile, all three factors were significant, but adjusted urine pH fell with age with little change in the contrast slope (Fig. 2, top right, and Table 2) compared with the unadjusted slope for both sexes combined (Table 2).
Fig. 2.
Body mass index (BMI) versus age hexile, BMI-adjusted (Adj) urine pH, creatinine clearance (CCr) versus age hexile, and BMI + CCr-adjusted urine pH. Triangles, men; circles, women. Dashed lines are for visual clarity only and imply no interposed information. Values are means ± SE. Linear contrast F = 146, both sexes combined, P < 0.001.
Table 2.
ANOVA models
|
Model 1 |
Model 2 |
Model 3 |
Model 4 |
|||||
|---|---|---|---|---|---|---|---|---|
| F | Effect | F | Effect | F | Effect | F | Effect | |
| Constant | 6.47 | 6.42 | 6.55 | 5.9 | ||||
| Body mass index, kg/m2 | 295* | −0.14 | 294* | −0.14 | 364* | −0.15 | 170* | −0.08 |
| Sex | 99* | Men = −0.054 | 102* | Men = −0.056 | 73* | Men = −0.045 | 8* | Men = −0.012 |
| Age, yr | 32* | −0.167 | 25* | −0.186 | 25* | −0.159 | 113* | −0.266 |
| Creatinine clearance | 4 | 186* | 0.04 | 19* | 0.01 | |||
| Urine ammonia | 659* | −0.12 | ||||||
| Urine sulfate | 839* | −0.1 | ||||||
| Gastrointestinal anion | 6,299* | 0.16 | ||||||
| Serum total CO2 | 25* | 0.09 | ||||||
| Age × sex | 3 | † | 3 | † | 4 | † | 4* | † |
| Multiple R2 | 0.084 | 0.085 | 0.156 | 0.498 | ||||
Significance at P < 0.01; all effects except age were per 10 units of the variable; effects for age were the linear contrast slope.
Many cross products use sex, not shown for lack of important contribution to the model.
Effect of Falling Glomerular Filtration
As expected, CCr fell with age (Fig. 2, bottom left). Unlike urine pH, decrements for both sexes were not significant between the youngest three hexiles, in which urine pH most markedly fell. Even so, the contrast trend was significant (F = 114 and 58, men and women, respectively, order 2, and F = 925 and 585, men and women, respectively, order 1, all P < 0.001). When added to body mass index (Table 2), CCr had no significant effect on the fall of urine pH with age. The adjusted urine pH values were therefore practically identical with those from model 1 in Table 2 and the unadjusted value (compare Fig. 2, right, top and bottom, vs. Fig. 1).
Estimated GFR (eGFR) falls consistently with age (data not shown; changes between each hexile P < 0.001) because the CKD-Epi eGFR algorithm has 0.993age as a multiplier (20). Likewise, by design, eGFR was nearly identical for the sexes within a given hexile, as the equation demands. For this reason, we do not offer a formal trend analysis.
Renal Acid Excretion With Age
Ammonia.
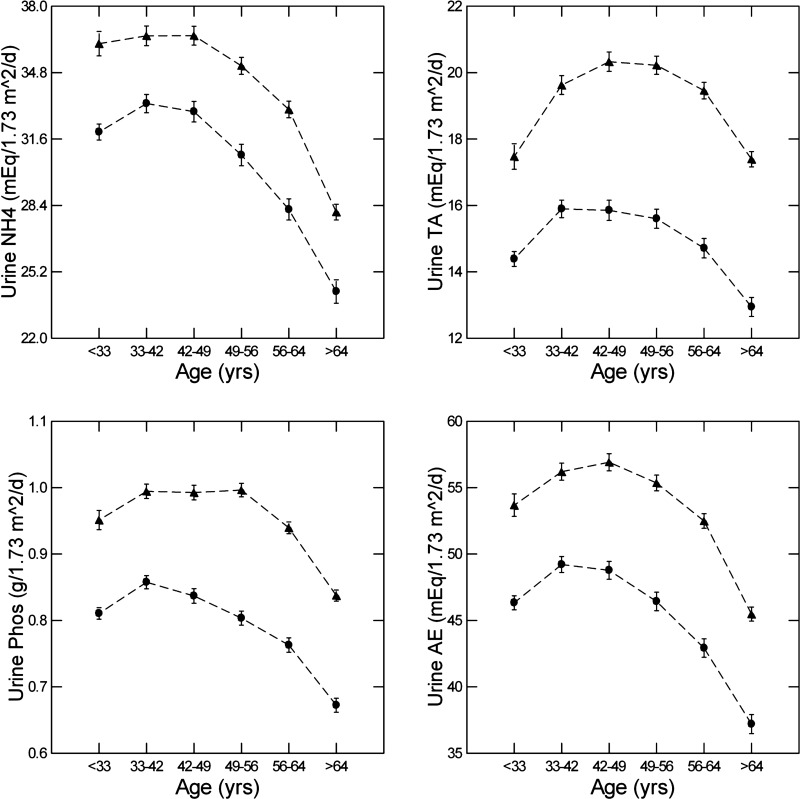
Urine ammonia excretion rose (in women) or was stable (in men) over the first three hexiles of age (Fig. 3, top left) and then declined over the latter three hexiles. In both sexes, linear contrast tests showed a highly significant relationship between ammonia excretion and age (F = 191 and 173, men and women, respectively, order 1, all P < 0.001). When added to the ANOVA (Table 2), urine ammonia excretion had a large effect, and its addition improved the significance of the model (multiple R2 = 0.156). Urine pH still fell significantly with age, albeit with a reduced contrast slope (Table 2).
Fig. 3.
Urine ammonia (NH4), urine titratable acidity (TA), urine phosphate (Phos), and urine acid excretion (AE), all versus age hexile. Triangles, men; circles, women. Dashed lines are for visual clarity only and imply no interposed information.
Titratable acidity and acid excretion.
TA rose and then fell (Fig. 3, top right). The behavior of TA with age is complex because falling urine pH would increase it, whereas falling urine phosphate excretion (Fig. 3, bottom left) would decrease it. Being the sum of ammonia and TA, AE (Fig. 3, bottom right) rose and then fell. We do not offer a formal analysis of urine pH as a function of TA or AE, since TA was calculated using urine pH as an exponent (methods).
Dietary Acid-Base Contribution
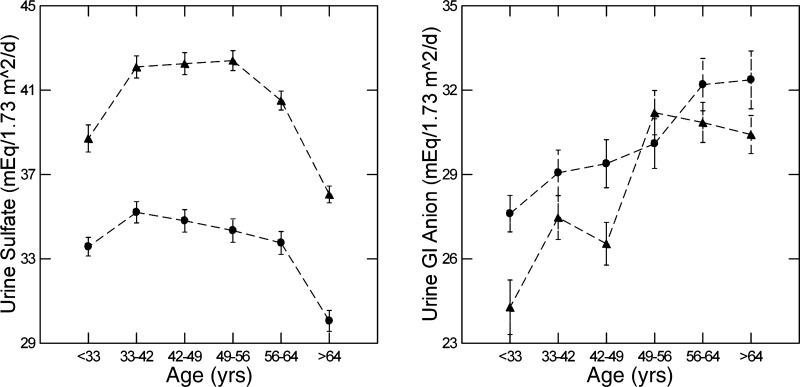
Urine sulfate.
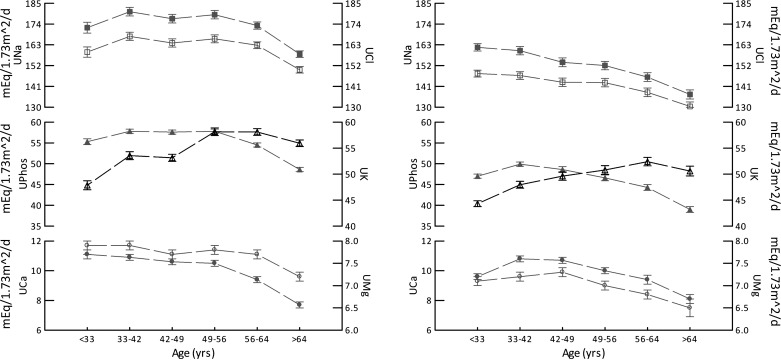
Urine sulfate, taken as the dietary acid load (8), rose in men and women between the first two hexiles (Fig. 4, left) and then remained more or less stable until the last hexile, when it decreased significantly. Despite the modestness of changes between hexiles, there was a significant linear relationship between age and sulfate excretion in both sexes (F = 16 and 27, men and women, respectively, P < 0.001), mainly because of the decline in the terminal hexiles. At all ages, values for men far exceed women despite normalization for body surface area. This is well known within the United States population (28a).
Fig. 4.
Urine sulfate and urine gastrointestinal (GI) anion levels versus age hexile. Triangles, men; circles, women. Dashed lines are for visual clarity only and imply no interposed information.
Gastrointestinal anion levels.
Gastrointestinal anion levels (methods) rose with age (linear contrast, F = 42 and 23, men and women, respectively, P < 0.001; Fig. 4, right). Notably, whereas the overall trend of increase was significant in both sexes, in women there were no differences between adjacent hexiles, whereas in men there was a significant increase (P < 0.001) between hexiles 3 and 4. Mean gastrointestinal anion values for women exceeded those for men in the youngest hexile (P = 0.007), but the difference disappeared with age such that the sex difference in the second hexile was of borderline significance (P = 0.015). In all other hexiles, the sexes did not differ.
Role of urine potassium.
Notably, all of the constituents of gastrointestinal anions (Fig. 5) were constant or decreased with age with the exception of potassium, which increased with age (linear contrast, F = 84 and 53, contrast slope = 7.06 and 5.49, men and women, respectively, P < 0.001 for all comparisons). In men, there were increases between hexiles 1 and 2 and hexiles 3 and 4, whereas in women, there was an increase between hexiles 1 and 2 (all P < 0.001). It is this increase in urine potassium that drives the increase in calculated gastrointestinal anion values.
Fig. 5.
Urine excretion of the components of gastrointestinal anion levels versus age hexile. Triangles, men; circles, women. Filled symbols are urinary sodium (UNa), urinary phosphate (UPhos) × 1.8, and urinary calcium (UCa); open symbols are urinary chloride (UCl), urinary potassium (UK), and urinary magnesium (UMg). All values are in meq·day−1·1.73 m−2 body surface area. Dashed lines are for visual clarity only and imply no interposed information.
Serum Measurements
Serum potassium.
In addition to the notable increases in urinary potassium excretion, serum potassium also increased with age (linear contrast, F = 28 and 17, men and women, respectively, P < 0.001; Table 1). There were no significant differences between adjacent hexiles in either sex despite highly significant overall trends.
Serum TCO2.
Serum TCO2 also displayed age-related changes (Table 1). In women, there was a significant upward trend (linear contrast, F = 128, P < 0.001) and an increase between hexiles 3 and 4 (P < 0.001). In men, no such changes were seen (linear contrast, F = 6, P = not significant).
Final ANOVA Model of Urine pH
The addition of gastrointestinal anions, urine sulfate, and serum TCO2 (Table 2) almost fully accounted for the sex difference (Fig. 6). The decline of urine pH with age remained highly significant. Indeed, after full adjustment, the fall in urine pH with age was monotonic, and, except for the final hexile in women, every hexile was significantly lower than the previous, and the contrast slope was higher than the unadjusted value (Table 2, compare model 4 with the unadjusted value of −0.198, both sexes combined). The overall regression now accounted for a highly significant fraction of the total variation of urine pH with age (multiple R2 = 0.498). The effect size of gastrointestinal anions was particularly notable.
Fig. 6.
Fully adjusted urine pH versus age hexiles from model 4. Triangles, men; circles, women. Dashed lines are for visual clarity only and imply no interposed information.
Interactions Between CCr and Urine Ammonia
Ammonia was not included in model 4 because of its close correlation with CCr. Inclusion of ammonia drastically reduced the value of the F statistic of CCr (19–0.1) and did not greatly improve the variance explained by the model (multiple R2 = 0.498 vs. 0.505). Taken together, this implies that ammonia and CCr covary and thus are not independent predictors of urine pH.
DISCUSSION
Urine pH Falls With Age
In this study of 7,891 stone-forming individuals, urine pH fell with age, most dramatically between the ages of 18 and 55 yr old. The decline echoes that seen in calcium oxalate stone formers in a cohort that includes calcium oxalate, calcium phosphate, and urine acid stone formers (28). This finding likely explains why phosphate content in kidney stones decreases with age while uric acid content rises (4, 13). Indeed, the fall in kidney stone phosphate content occurred most between the ages of 18 and 50 yr old, coinciding with the decline in urine pH we observed. Similarly, the higher urine pH of women throughout all age groups probably explains their higher kidney stone phosphate content versus men (22).
Our Data Exclude Certain Potential Mechanisms
Body mass index, CCr, urine ammonia.
While consistent with our understanding of kidney stone formation, the physiological mechanisms of the age decline in urine pH are less clear. We (24) have previously shown that, in two separate stone centers, urine pH fell with body weight adjusted for urine creatinine. In the present study, body mass index indeed correlated inversely with urine pH, but this explains only a small fraction of the pH decline with age. Although CCr and urine ammonia excretion strongly correlate with urine pH, these measures accounted for only a small fraction of the age effect.
Diabetes lowers urine pH (10), and its prevalence rises with age. Most of our pH decline occurred between the ages of 30 and 50 yr old, whereas one would expect diabetes prevalence to rise continuously after 50 yr old, as well. We cannot explore this possibility further with the information available except to mention that a defect of renal ammonia production has been identified as an important contributor to low urine pH in diabetes (35) and was not important as an explanation for what we found here.
Urine sulfate and gastrointestinal anions.
Although urine sulfate excretion correlated negatively with urine pH, it rose very little between the ages of 30 and 50 yr old and actually fell thereafter. In our models, its addition accounted for little of the fall in pH. Gastrointestinal anion levels had a preponderant and powerful positive correlation with urine pH. However, gastrointestinal anion levels rose with age, and, as currently understood, this increase would tend to offset a fall in urine pH. In fact, adjusting for gastrointestinal anions and urine sulfate caused pH to fall more steeply and consistently.
Sex differences.
Adjusting for gastrointestinal anion and sulfate removed the majority of the urine pH difference between sexes. A diet consisting of greater amounts of fruits and vegetables could contribute to the higher urine pH in women. However, the fraction of diet gastrointestinal alkali absorbed might also differ between the sexes. We have previously shown in nonstone formers that higher urine pH in women is entirely related to their higher urine gastrointestinal anion excretion even though both sexes ate identical diets (42). Here, the adjustment for sex differences arose from both the higher gastrointestinal anion levels in women and higher sulfate in men.
Urine Potassium Rises With Age
This is the immediate arithmetic cause of the increase in gastrointestinal anion levels. All of the other constituents of the urine charge difference from which gastrointestinal anion level is calculated (Na+, Mg2+, Ca2+, Cl−, and ) either remained steady or declined with age. Although declines in the excretion of either Cl− or could account for the change in calculated gastrointestinal anion levels, in our data set changes in urinary potassium excretion accounted for >90% of the changes in gastrointestinal anion levels. In addition to the increased urinary excretion of potassium, we also observed an increase in serum potassium. Taken together, these phenomena suggest that potassium intake, gastrointestinal potassium handling, or perhaps both are subject to aging effects.
Diet.
The increasing urine potassium could perhaps be diet driven, caused by a move away from meats and toward fruits and vegetables, favoring increased potassium alkali absorption. However, while some dietary changes occur through adulthood, it is unlikely that shifts in diet could cause the monotonic and isolated increase of potassium (12a). Our data cannot allow us to pursue this matter in more detail.
Transport selectivity.
Selective net absorption of potassium, perhaps with food anions, rather than age-driven changes in food intake might play a major role in raising gastrointestinal anion absorption with age. Absorption of potassium has long been known to occur primarily in the small intestine (1), and the colon is the principal site of potassium secretion (36). A large number of potassium channels have been identified in the gut (12); however, regulation of the transmembrane potassium transport events leading to gut net potassium absorption is not well elucidated, nor are any potential effects of age. This represents an important area for future study.
Anion Absorption
Gastrointestinal potassium and alkali absorption could be coupled or could occur independent of each other. Although our data cannot test the possibility, gastrointestinal anion absorption might increase in compensation for the factors reflected by a falling urine pH. In other words, gastrointestinal alkali absorption might be regulated in response to rising systemic acid retention, for example. Such potential renal-gastrointestinal cross talk could act to offset increasing acid loads and prevent metabolic acidosis. Such regulation could be because of increased gastrointestinal transporter in situ activity or abundance, as is seen with organic anion transporters (19). Alternatively, age-related changes in the microbiome could affect gastrointestinal alkali absorption, as has been proposed by Maalouf et al. (11, 23).
The Fall of Urine pH With Age Is Not Fully Accounted For
Multiple potential mechanisms exist that may explain the remaining decline in urine pH with age. For instance, endothelin-1 levels rise with age (37), and Wesson and Dolson (41) have demonstrated that endothelin-1 stimulates proton secretion in the collecting duct. Alternatively, the fall of urine pH may represent a consequence of the previously described increase in steady-state acid serum H+ in older individuals (6). A detailed examination of bicarbonate balance across multiple data sets is necessary to explain this finding and is worthy of further study. Experiments targeted at explaining this phenomenon will need to include urine collections to preserve TCO2 and likewise direct measurement of total urine organic ions (33, 34).
Potential Limitations of This Study
The study sample comes from a testing laboratory population, so it is possible that uric acid stone formers are selectively enriched in our data set. Their urine pH would be particularly low and would downweight the average urine pH with age. However, in a similar laboratory testing population (22), uric acid stones were only ~8% in women and 12% in men from the ages of 50–59 yr old, by which point most of the decline in urine pH has occurred.
The decrease on average from a urine pH of 6.2–5.9 appears a clinically modest one, since the median pH of 24-h urine collections is ~6. However, this decrease of 0.3 pH units represents approximately two times the concentration of free protons in urine, which likely arises from a combination of decreased urinary buffers and increased proton excretion, since AE only falls by 25% over the same time period. Although not of apparent clinical significance, these observations independently could account for the clinical observations of decreasing calcium phosphate stone content in aging (2).
In our study, the gastrointestinal anion level was estimated via Oh’s formula (27), which has been confirmed to match that determined in total body balance studies. It is possible that Oh’s relationship does not hold with aging, since the original paper introducing the concept is based on a review of Lemann and Relman’s research performed largely on young men (7, 14–18, 27, 32). However, Oh’s method is based on physical chemistry, molar balance, and conservation, which are unlikely to vary with age.
Although our work does not elucidate the mechanisms for the fall in urine pH with age, even in this selected population, it does falsify the leading hypotheses concerning these mechanisms based on present knowledge of acid-base physiology. This was our intent, since it offers to other investigators opportunities for experiments concerning alternatives such as abnormal conversion of diet anion to bicarbonate or perhaps inadequacies of contemporary calculations of gastrointestinal anions.
Finally, and importantly, this data set is limited to a stone-forming population. Therefore, it is unclear whether this phenomenon is limited to stone formers or is more generalizable to a larger population.
Summary
In conclusion, this work provides an explanation for the decreasing prevalence of phosphate-based kidney stones seen in aging by demonstrating a progressive decline in urine pH in stone-forming individuals. This decline seems ameliorated by an increase in gastrointestinal alkali absorption with age. Both of these findings need further investigation in the form of direct experiments to fully elucidate the underlying mechanisms.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.J.M., E.M.W., F.L.C., K.J.B., and B.K. analyzed data; C.J.M., E.M.W., F.L.C., and B.K. interpreted results of experiments; C.J.M., E.M.W., F.L.C., and K.J.B. prepared figures; C.J.M., E.M.W., F.L.C., and B.K. drafted manuscript; C.J.M., E.M.W., F.L.C., J.A., and B.K. edited and revised manuscript; C.J.M., E.M.W., F.L.C., J.A., K.J.B., and B.K. approved final version of manuscript; E.M.W. and F.L.C. conceived and designed research.
REFERENCES
- 1.Agarwal R, Afzalpurkar R, Fordtran JS. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology 107: 548–571, 1994. doi: 10.1016/0016-5085(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 2.Bergsland KJ, Coe FL, Parks JH, Asplin JR, Worcester EM. Evidence for a role of PDZ domain-containing proteins to mediate hypophosphatemia in calcium stone formers. Nephrol Dial Transplant 33: 759–770, 2018. doi: 10.1093/ndt/gfx284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carbone A, Al Salhi Y, Tasca A, Palleschi G, Fuschi A, De Nunzio C, Bozzini G, Mazzaferro S, Pastore AL. Obesity and kidney stone disease: a systematic review. Minerva Urol Nefrol 70: 393–400, 2018. doi: 10.23736/S0393-2249.18.03113-2. [DOI] [PubMed] [Google Scholar]
- 4.Daudon M, Doré JC, Jungers P, Lacour B. Changes in stone composition according to age and gender of patients: a multivariate epidemiological approach. Urol Res 32: 241–247, 2004. doi: 10.1007/s00240-004-0421-y. [DOI] [PubMed] [Google Scholar]
- 5.Evan AP, Lingeman JE, Worcester EM, Sommer AJ, Phillips CL, Williams JC, Coe FL. Contrasting histopathology and crystal deposits in kidneys of idiopathic stone formers who produce hydroxy apatite, brushite, or calcium oxalate stones. Anat Rec (Hoboken) 297: 731–748, 2014. doi: 10.1002/ar.22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frassetto L, Sebastian A. Age and systemic acid-base equilibrium: analysis of published data. J Gerontol A Biol Sci Med Sci 51: B91–B99, 1996. doi: 10.1093/gerona/51A.1.B91. [DOI] [PubMed] [Google Scholar]
- 7.Goodman AD, Lemann J Jr, Lennon EJ, Relman AS. Production, excretion, and net balance of fixed acid in patients with renal acidosis. J Clin Invest 44: 495–506, 1965. doi: 10.1172/JCI105163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamm LL, Nakhoul N, Hering-Smith KS. Acid-base homeostasis. Clin J Am Soc Nephrol 10: 2232–2242, 2015. doi: 10.2215/CJN.07400715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamm LL, Simon EE. Roles and mechanisms of urinary buffer excretion. Am J Physiol 253: F595–F605, 1987. doi: 10.1152/ajprenal.1987.253.4.F595. [DOI] [PubMed] [Google Scholar]
- 10.Hartman C, Friedlander JI, Moreira DM, Elsamra SE, Smith AD, Okeke Z. Differences in 24-h urine composition between nephrolithiasis patients with and without diabetes mellitus. BJU Int 115: 619–624, 2015. doi: 10.1111/bju.12807. [DOI] [PubMed] [Google Scholar]
- 11.Heintz C, Mair W. You are what you host: microbiome modulation of the aging process. Cell 156: 408–411, 2014. doi: 10.1016/j.cell.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heitzmann D, Warth R. Physiology and pathophysiology of potassium channels in gastrointestinal epithelia. Physiol Rev 88: 1119–1182, 2008. doi: 10.1152/physrev.00020.2007. [DOI] [PubMed] [Google Scholar]
- 12a.Hoy MK, Goldman JD. Potassium Intake of the U.S. Population. What We Eat in America, NHANES 2009–2010. Beltsville, MD: United States Department of Agriculture, 2012. [PubMed] [Google Scholar]
- 13.Knoll T, Schubert AB, Fahlenkamp D, Leusmann DB, Wendt-Nordahl G, Schubert G. Urolithiasis through the ages: data on more than 200,000 urinary stone analyses. J Urol 185: 1304–1311, 2011. doi: 10.1016/j.juro.2010.11.073. [DOI] [PubMed] [Google Scholar]
- 14.Lemann J Jr, Lennon EJ, Goodman AD, Litzow JR, Relman AS. The net balance of acid in subjects given large loads of acid or alkali. J Clin Invest 44: 507–517, 1965. doi: 10.1172/JCI105164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemann J Jr, Litzow JR, Lennon EJ. The effects of chronic acid loads in normal man: further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest 45: 1608–1614, 1966. doi: 10.1172/JCI105467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemann J Jr, Relman AS. The relation of sulfur metabolism to acid-base balance and electrolyte excretion: the effects of dl-methionine in normal man. J Clin Invest 38: 2215–2223, 1959. doi: 10.1172/JCI104001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lennon EJ, Lemann J Jr, Litzow JR. The effects of diet and stool composition on the net external acid balance of normal subjects. J Clin Invest 45: 1601–1607, 1966. doi: 10.1172/JCI105466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lennon EJ, Lemann J Jr, Relman AS. The effects of phosphoproteins on acid balance in normal subjects. J Clin Invest 41: 637–645, 1962. doi: 10.1172/JCI104519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leuthold S, Hagenbuch B, Mohebbi N, Wagner CA, Meier PJ, Stieger B. Mechanisms of pH-gradient driven transport mediated by organic anion polypeptide transporters. Am J Physiol Cell Physiol 296: C570–C582, 2009. doi: 10.1152/ajpcell.00436.2008. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li WM, Chou YH, Li CC, Liu CC, Huang SP, Wu WJ, Chen CW, Su CY, Lee MH, Wei YC, Huang CH. Association of body mass index and urine pH in patients with urolithiasis. Urol Res 37: 193–196, 2009. doi: 10.1007/s00240-009-0194-4. [DOI] [PubMed] [Google Scholar]
- 22.Lieske JC, Rule AD, Krambeck AE, Williams JC, Bergstralh EJ, Mehta RA, Moyer TP. Stone composition as a function of age and sex. Clin J Am Soc Nephrol 9: 2141–2146, 2014. doi: 10.2215/CJN.05660614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maalouf NM, Cameron MA, Moe OW, Sakhaee K. Metabolic basis for low urine pH in type 2 diabetes. Clin J Am Soc Nephrol 5: 1277–1281, 2010. doi: 10.2215/CJN.08331109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maalouf NM, Sakhaee K, Parks JH, Coe FL, Adams-Huet B, Pak CY. Association of urinary pH with body weight in nephrolithiasis. Kidney Int 65: 1422–1425, 2004. doi: 10.1111/j.1523-1755.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 27.Oh MS. A new method for estimating G-I absorption of alkali. Kidney Int 36: 915–917, 1989. doi: 10.1038/ki.1989.280. [DOI] [PubMed] [Google Scholar]
- 28.Otto BJ, Bozorgmehri S, Kuo J, Canales M, Bird VG, Canales B. Age, body mass index, and gender predict 24-hour urine parameters in recurrent idiopathic calcium oxalate stone formers. J Endourol 31: 1335–1341, 2017. doi: 10.1089/end.2017.0352. [DOI] [PubMed] [Google Scholar]
- 28a.Panel on Dietary Reference Intakes for Electrolytes and Water; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; Food and Nutrition Board . Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC: National Academies, 2005. [Google Scholar]
- 29.Parks JH, Coe FL. A urinary calcium-citrate index for the evaluation of nephrolithiasis. Kidney Int 30: 85–90, 1986. doi: 10.1038/ki.1986.155. [DOI] [PubMed] [Google Scholar]
- 30.Parks JH, Goldfischer ER, Coe FL. Changes in urine volume accomplished by physicians treating nephrolithiasis. J Urol 169: 863–866, 2003. doi: 10.1097/01.ju.0000044922.22478.32. [DOI] [PubMed] [Google Scholar]
- 31.Poggio ED, Rule AD, Tanchanco R, Arrigain S, Butler RS, Srinivas T, Stephany BR, Meyer KH, Nurko S, Fatica RA, Shoskes DA, Krishnamurthi V, Goldfarb DA, Gill I, Schreiber MJ Jr. Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int 75: 1079–1087, 2009. doi: 10.1038/ki.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Relman AS, Lennon EJ, Lemann J Jr. Endogenous production of fixed acid and the measurement of the net balance of acid in normal subjects. J Clin Invest 40: 1621–1630, 1961. doi: 10.1172/JCI104384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ring T. Modeling amount of acid. Kidney Int 91: 1519–1520, 2017. doi: 10.1016/j.kint.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 34.Ring T, Nielsen S. Whole body acid-base modeling revisited. Am J Physiol Renal Physiol 312: F647–F653, 2017. doi: 10.1152/ajprenal.00560.2016. [DOI] [PubMed] [Google Scholar]
- 35.Sakhaee K, Adams-Huet B, Moe OW, Pak CY. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int 62: 971–979, 2002. doi: 10.1046/j.1523-1755.2002.00508.x. [DOI] [PubMed] [Google Scholar]
- 36.Sorensen MV, Matos JE, Praetorius HA, Leipziger J. Colonic potassium handling. Pflugers Arch 459: 645–656, 2010. doi: 10.1007/s00424-009-0781-9. [DOI] [PubMed] [Google Scholar]
- 37.Stauffer BL, Westby CM, DeSouza CA. Endothelin-1, aging and hypertension. Curr Opin Cardiol 23: 350–355, 2008. doi: 10.1097/HCO.0b013e328302f3c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor EN, Curhan GC. Body size and 24-hour urine composition. Am J Kidney Dis 48: 905–915, 2006. doi: 10.1053/j.ajkd.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Trinchieri A, Montanari E. Biochemical and dietary factors of uric acid stone formation. Urolithiasis 46: 167–172, 2018. doi: 10.1007/s00240-017-0965-2. [DOI] [PubMed] [Google Scholar]
- 40.Viers BR, Lieske JC, Vrtiska TJ, Herrera Hernandez LP, Vaughan LE, Mehta RA, Bergstralh EJ, Rule AD, Holmes DR III, Krambeck AE. Endoscopic and histologic findings in a cohort of uric acid and calcium oxalate stone formers. Urology 85: 771–776, 2015. doi: 10.1016/j.urology.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wesson DE, Dolson GM. Endothelin-1 increases rat distal tubule acidification in vivo. Am J Physiol 273: F586–F594, 1997. doi: 10.1152/ajprenal.1997.273.4.F586. [DOI] [PubMed] [Google Scholar]
- 42.Worcester EM, Bergsland KJ, Gillen DL, Coe FL. Mechanism for higher urine pH in normal women compared with men. Am J Physiol Renal Physiol 314: F623–F629, 2018. doi: 10.1152/ajprenal.00494.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]