Abstract
Iatrogenic injury to the healthy ureter during ureteroscope-guided ablation of malignant or nonmalignant disease can result in ureteral stricture. Transforming growth factor (TGF)-β1-mediated scar formation is considered to underlie ureteral stricture, but the cellular sources of this cytokine and the sequelae preceding iatrogenic stricture formation are unknown. Using a swine model of ureteral injury with irreversible electroporation (IRE), we evaluated the cellular sources of TGF-β1 and scar formation at the site of injury and examined in vitro whether the effects of TGF-β1 could be attenuated by pirfenidone. We observed that proliferation and α-smooth muscle actin expression by fibroblasts were restricted to injured tissue and coincided with proliferation of macrophages. Collagen deposition and scarring of the ureter were associated with increased TGF-β1 expression in both fibroblasts and macrophages. Using in vitro experiments, we demonstrated that macrophages stimulated by cells that were killed with IRE, but not LPS, secreted TGF-β1, consistent with a wound healing phenotype. Furthermore, using 3T3 fibroblasts, we demonstrated that stimulation with paracrine TGF-β1 is necessary and sufficient to promote differentiation of fibroblasts and increase collagen secretion. In vitro, we also showed that treatment with pirfenidone, which modulates TGF-β1 activity, limits proliferation and TGF-β1 secretion in macrophages and scar formation-related activity by fibroblasts. In conclusion, we identified wound healing-related macrophages to be an important source of TGF-β1 in the injured ureter, which may be a paracrine source of TGF-β1 driving scar formation by fibroblasts, resulting in stricture formation.
Keywords: fibroblast, iatrogenic injury, macrophage, obstructive uropathy, transforming growth factor-β1, ureteral stricture
INTRODUCTION
Trauma or iatrogenic injury to the ureter can occur during surgery, pelvic irradiation (20), or ureteroscope-guided ablation (2) and is associated with an increased risk of obstructive uropathy. Injury to the ureter can reduce peristalsis at the site of insult (22, 25), stimulate ureteral hypertrophy, and initiate scarring in the muscularis layer of the ureteral wall, resulting in stricture formation (2). Strictures can impede normal urine flow, leading to hydronephrosis, scarring of the kidney parenchyma, and reduction of renal function (5, 15). Ureteral strictures thus create a negative feedback loop that can progressively worsen the symptoms of obstructive uropathy and induce permanent loss of renal function in some patients (16, 31). While ureteral strictures are usually treated with dilatation of the lumen, reconstructive anastomosis, or the placement of grafts, treatment outcomes for many patients remain poor (1); moreover, additional surgery may be necessary to relieve recurrent strictures (33). The propensity for, and the severity of, ureteral strictures cannot be easily predicted, underscoring the importance of developing strategies to prevent this condition when possible.
The key factors underlying nonmalignant ureteral stricture formation are an exuberant wound healing response to trauma and the subsequent remodeling of the ureteral wall (1, 9). Several inflammatory (e.g., cyclooxygenase-2, IL-1β, IL-6, and TNF-α) and wound healing cytokines [e.g., transforming growth factor (TGF)-β1] have been implicated in this process, playing a key role in initiating and inducing the progressive production of collagen by fibroblasts (1, 6, 42). To suppress the inflammation due to cytokines, several authors have demonstrated that drugs such as steroids, cyclooxygenase-2 inhibitors (7), and atorvastatin (8) are effective in reducing collagen deposition and levels of inflammatory cytokines in ureters that were ligated to induce obstructive renal uropathy.
TGF-β1 is a wound healing cytokine and has been associated with stricture formation (6–8, 42) and is considered a mediator of fibroblast activation and collagen production. Wound healing cytokines are typically secreted by multiple cell types, including macrophages, endothelial cells, and platelets, initiating the cascade leading to scar formation. However, the paracrine or cellular source of TGF-β1 (21) that contributes to fibroblast activation in the specific context of unilateral ureteral obstruction (UUO) has not been defined. A clinically relevant model that can be used to study the biological conditions underlying stricture, including those related to TGF-β1, was provided by Srimathveeravalli et al. (34, 35), who previously showed in their model that injury to normal porcine ureter with irreversible electroporation (IRE) results in reproducible and progressive stricture formation. IRE is a nonthermal ablation technique (38) using ultrashort electric pulses to permeabilize the cell membrane, resulting in cell death from the loss of homeostasis. This nonthermal cell killing effect of IRE allows the ureter to be injured to induce stricture without the risk of other complications such as perforation, urinoma, or fistula formation (1, 2). Thus, the objective of the present study was to use longitudinal samples of healthy porcine ureters injured with IRE to determine the main cellular sources of TGF-β1 and its role in stricture induction and to validate a pharmacologic adjuvant in vitro to abrogate the effects of TGF-β1 on fibroblast differentiation and function. We hypothesized that TGF-β1 secreted by wound healing-related macrophages activates fibroblasts, stimulating collagen production that induces ureteral stricture, and that this process can be mitigated by treatment with pirfenidone (PFD), an antifibrotic drug targeting the TGF-β1 pathway.
MATERIALS AND METHODS
Animal model and ureteral ablation experiments.
All animal experiments were performed following protocols approved by the Institutional Animal Care and Use Committee. After inhaled isoflurane anesthesia, a nephrostomy was performed to introduce a catheter-mounted electrode into the ureter of healthy female swine (n = 8, weight: 35–50 kg). Strictures were induced by performing transmural, circumferential injury of the ureteral wall (2-cm segment) using IRE at two different locations in the ureter of each animal (n = 4 per time point). IRE was performed by delivering square wave electric pulses (100-μs pulse width, 90 pulses, 1,500 V/cm electric field strength) between the electrode mounted on the catheter and a grounding pad attached to the flank of the animal. All animals were recovered and kept under observation with weekly computed tomographic imaging until the time of designated euthanization. Two animals each were euthanized at 1, 7, 14, or 28 days post-IRE, and the urinary tract along with the bilateral kidney and bladder cuff was harvested and processed for histology. Tissue samples from the contralateral untreated ureter were used as controls for histology analysis. Complete details of the IRE procedure, progression of the ureteral injury to stricture, and information on animal care can be found in previous work (34, 35).
Histopathological analysis.
Samples containing the entire length of the injured ureteral wall along with the surrounding uninjured ureter were processed for histology using techniques described in Histopathological sample processing and Cell culture conditions and in vitro IRE. In each animal, the ureter was injured at two locations, and there were two animals per time point. This yielded sufficient/replicate (n = 4) tissue samples per time point for evaluation with immunohistochemistry. Briefly, we used Masson’s trichrome stain to determine tissue levels of collagen and TUNEL staining for cell death, and antibodies were used to stain cell markers for fibroblasts (vimentin, M0725, Dako, Glostrup, Denmark), myofibroblasts [α-smooth muscle actin (α-SMA), no. 14-9760-82, ThermoFisher Scientific, Waltham, MA], macrophages (Iba-1, no. 019-19741, Wako Chemicals, Richmond, VA), Ki-67 (Orb378204, Biorbyt), and TGF-β1 (ab25121, Abcam, Cambridge, MA).
Quantitative immunohistochemistry.
Immunohistochemistry (IHC)-stained slides were scanned with a microscope (TCS SP5, Leica, Wetzlar, Germany) at high resolution, and 20 regions from each slide were acquired at high magnification (×40 or ×100). We performed quantification by either measuring the percentage area of tissue staining positive in each sample (Masson’s trichrome, vimentin, α-SMA, and Iba-1) or counting the specific cell types in the field of view staining positive for a marker (TGF-β1). ImageJ software (National Institutes of Health) was used (28) to perform the quantification using the built-in “Color Deconvolution” tool to extract blue (Masson’s trichrome) or brown color channels (vimentin, α-SMA, Iba-1, and TGF-β1). Nonspecific staining of filaments and vascular smooth muscle was censored from measurements during quantification of vimentin and α-SMA. Cell morphology (spindle shape for fibroblasts) or comparative evaluation (Iba-1 to distinguish macrophages) was used to identify cells of interest (fibroblasts and macrophages) expressing TGF-β1 on ×100 magnification images.
Cell culture.
The BALB 3T3 mouse fibroblast cell line and RAW 264.7 mouse macrophage cell line were obtained from the American Type Culture Collection (Manassas, VA). Both cells were maintained in DMEM containing 10% FBS and 100 U/ml penicillin-streptomycin at 37°C with 5% CO2 in T-175 flasks. PFD (5 mM, Selleck Chemicals, Houston, TX) was dissolved in DMSO (Millipore Sigma, St. Louis, MO) to a concentration of 40 mg/ml and then diluted with DMEM to a 5 mM concentration for in vitro experiments. Recombinant human TGF-β1 (Peprotech, Rocky Hill, NJ) at 5 ng/ml was used for cell stimulation in all experiments. Cells were harvested after treatment or a control condition for viability analysis, immunoblot analysis, collagen quantification, quantitative PCR, and ELISA measurements. Specific details for seeding, treatment, and assessment conditions for each assay were as below.
Macrophage stimulation.
RAW 264.7 cells were stimulated with lipopolysaccharide (LPS) or IRE-treated 3T3 or RAW 264.7 cells with or without additional treatment with PFD. RAW 264.7 cells were plated in DMEM containing 10% FBS at an initial seeding density of 5 × 10−4 cells/well in 24-well plates and, after attachment, underwent serum deprivation for 48 h in DMEM containing 1% FBS. Stimulation with LPS (1 μg/ml) was performed after serum deprivation. IRE-treated 3T3 or RAW 264.7 cells were prepared by performing IRE (70-μs pulse width, 90 pulses, 2,000 V/cm) on 250 μl of 3T3 cell suspension (1.6 × 10−6 cells/ml) using an electroporation cuvette (Electroporation Cuvettes Plus, ThermoFisher Scientific). IRE-treated 3T3 or RAW 264.7 cells (~1 × 10−5 cells) were used per well for stimulation of RAW 264.7 cells. PFD (5 mM) was added to the culture immediately after stimulation with LPS or IRE-treated 3T3 cells. RAW 264.7 cells were incubated until assessment time points at 24 and 48 h poststimulation with LPS or IRE-treated 3T3 cells.
Cell viability measurements.
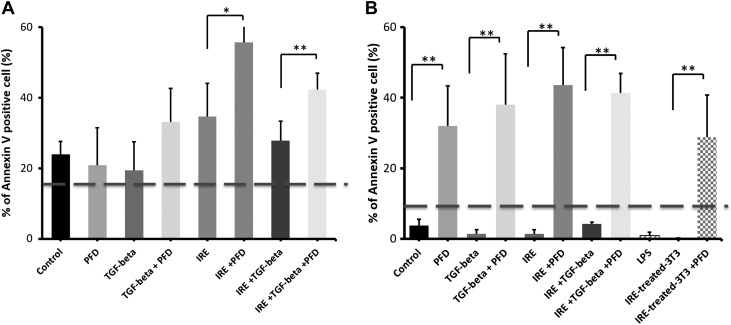
Preliminary cell viability after in vitro IRE or other treatment was assessed by performing trypan blue staining and then counting stained/unstained nuclei using a hemocytometer. To assess the cytotoxicity of IRE, RAW 264.7 and 3T3 cells were grown in 24-well plates (5 × 10−4 cell seeding density) with DMEM supplemented with 10% or 1% FBS for 48 h and then treated with or without IRE, with or without PFD (5 mM), and with or without human TGF-β1 (5 ng/ml). IRE treatment was by delivering square wave electric pulses into the cell culture using pin electrodes (70-μs pulse width, 90 pulses, 1,500 V/cm). Apoptosis of cells at 48 h posttreatment was assessed with annexinV staining and quantified using automated cell counting with a Countess II FL Automated Cell Counter (ThermoFisher Scientific) using DAPI as a counterstain. Cell proliferation and viability were further evaluated under the same experimental conditions with Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan).
ELISA.
ELISA was used to quantify the secretion of TGF-β1 by 3T3 fibroblasts and RAW 264.7 macrophage cells after activation. 3T3 and RAW 264.7 cells were plated in 24-well plates (5 × 10−4 cells/well) and grown with DMEM containing 10% FBS. After adhesion, cells underwent serum deprivation for 48 h (DMEM with 1% FBS), after which they were assigned to different treatment conditions (with or without IRE, with or without PFD, and with or without human TGF-β1). Concentrations of PFD and human TGF-β1 used for treating cells were 5 mM and 5 ng/ml, respectively. IRE treatment was performed using the protocol described in cell viability assessment experiments. Cell culture supernatant for ELISA was collected before, immediately after serum deprivation, and then again at 24 and 48 h after treatment with IRE/PFD/human TGF-β1. A mouse TGF-β1 ELISA kit (Quantikine ELISA kit, R&D Systems, Minneapolis, MN) was used for ELISA with optical density measurements performed at 450 nm in a microplate reader (infinite M1000Pro, TECAN, Männedorf, Switzerland) to determine TGF-β1 levels.
Immunoblot assay.
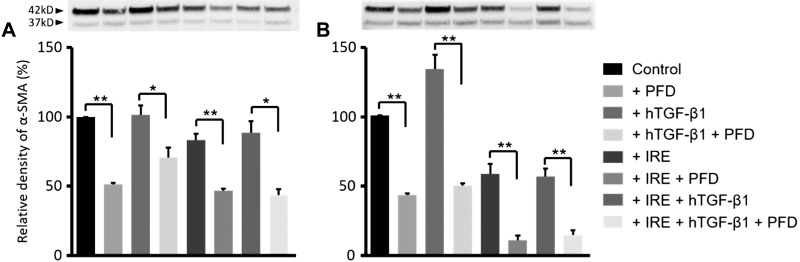
Immunoblot analysis were performed for the following eight conditions: control, PFD, human TGF-β1, PFD + human TGF-β1, IRE, IRE + PFD, IRE + human TGF-β1, and IRE + human TGF-β1 + PFD. 3T3 cells were grown in six-well plates (4 × 10−5 cell seeding density). Two hours after seeding, cells underwent serum deprivation for 48 h (5 ml DMEM with 1% FBS), after which cells underwent treatment with IRE/PFD and/or human TGF-β1 using protocols and techniques described for cell viability and ELISA measurement experiments. Cells were harvested for protein analysis after 24 and 48 h of incubation. We followed techniques previously reported by Chuang et al. (6) for immunoblot analysis. Proteins were extracted with Pierce RIPA buffer (ThermoFisher Scientific) and protease/phosphatase inhibitor cocktail (halt protease and phosphatase inhibitor cocktail, ThermoFisher Scientific) and isolated. The protein concentration of each sample was analyzed with the Pierce BCA protein assay kit (ThermoFisher Scientific). Proteins were separated on 4–12% bis-Tris Plus gels (ThermoFisher Scientific) in MES-SDS running buffer and transferred nitorocellulose membrane using the iBlot 2 Dry Blotting System (ThermoFisher Scientific). The following primary and secondary antibodies were used: α-SMA (ab5694, Abcam), GAPDH (ab9485, Abcam), and VeriBlot for IP Detection Reagent (ab131366, Abcam). The membrane was incubated with primary and secondary antibodies using iBind Flex Western Device 3 h in room temperature (ThermoFisher Scientific). Enhanced chemiluminescent solution (SupreSignal West Pico PLUS Chemiluminescent Substance, ThermoFisher Scientific) was then added onto the membrane for 1 min of reaction at room temperature. Chemiluminescent signals were detected by the ChemiDoc XRS+ system (Bio-Rad Laboratories, Hercules, CA), and quantification of immunoblots was performed by ImageJ software (version 1.5, National Institutes of Health). The relative density of α-SMA was as follows: relative density (%) = 100 × (density of α-SMA)/(density of GAPDH).
Quantitative PCR.
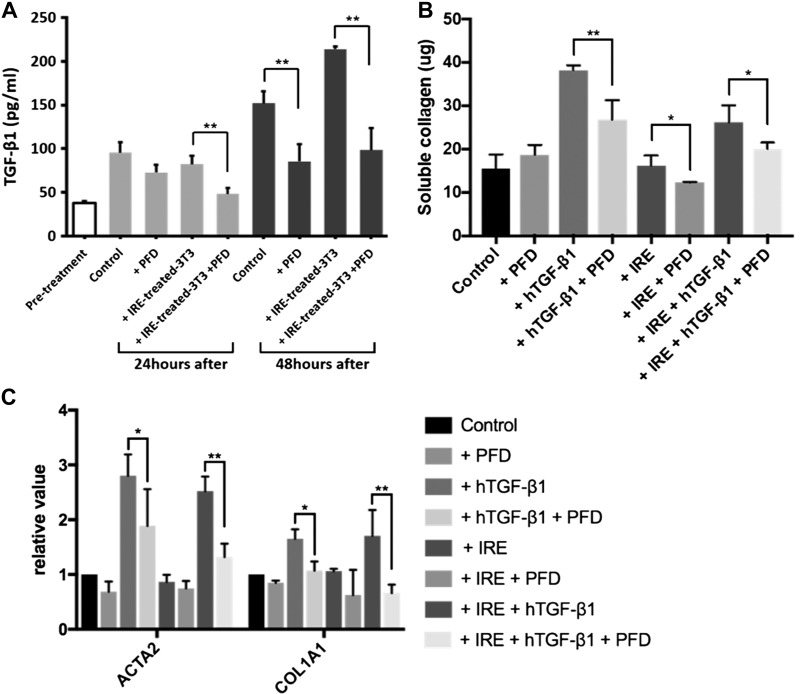
Cell samples were prepared the following protocols described for Western blot experiments to generate samples for quantitative PCR used to determine changes in RNA expression of α-SMA (ACTA2), collagen [collagen type I-α1 (COL1A1)], TGF-β1, and GAPDH (control) in 3T3 cells after treatment with IRE, TGF-β1, and/or PFD. Assessment was performed at 48 h after treatment conditions. RNA extraction and processing were performed using techniques previously reported by Chuang et al. (6). Briefly, total RNA was isolated from cultured cells using TRIzol reagent (Invitrogen), and mRNA was purified using the Oligotex extraction kit (Qiagen, Valencia, CA). Platinum SYBR Green Quantitative PCR SuperMix was used to perform quantitative PCR (ThermoFisher Scientific) using the following protocol: room temperature for 2 min at 50°C, initial activation step for 2 min at 95°C, denaturation for 15 s at 95°C, annealing for 30 s at 60°C, and extension for 30 s at 72°C, with 35 rounds of amplification. Threshold cycle (CT) values were acquired using the DNA Engine Opticon Continuous Fluorescence Detection System (Bio-Rad). The specificity of the desired products was determined using high-resolution gel electrophoresis. Quantification for real-time data was determined using the method. The primer sequences were used as follows: mouse ACTA2, forward 5′-ATGAAGCCCAGAGCAAGAGA-3′ and reverse 5′-CTTTTCCATGTCGTCCCAGT-3′; COL1A1, forward 5′-GATGCTAACGTGGTTCGTGA-3′ and reverse 5′-GCTGCGGATGTTCTCAATCT-3′; TGF-β1, forward 5′-ATCTACTGCCTCTGCCCTGA-3′ and reverse 5′-ACACATACGAGGCGGAAATC-3′; and mouse-specific GAPDH, forward 5′- TGTTCCTACCCCCAATGTGTC-3′ and reverse 5′-TGCTTCACCACCTTCTTGATGT-3′. cDNA was amplified by SYBR Green Master Mix to a final volume of 20 μl. cDNA was denatured for 5 min at 94°C followed by 40 cycles of heating (95°C for 30 s, 52°C for 30 s, and 72°C for 45 s). All test CT values were normalized to that of GAPDH.
Quantification of collagen protein.
The amount of soluble collagen in 3T3 culture supernatants was quantified using a colorimetric assay (Sircol Collagen Assay Kit, Belfast, Northern Ireland). Cells were prepared following protocols described for Western blot experiments to generate samples for the different treatment conditions. Assessment was performed at 48 h after treatment conditions. Samples for collagen measurement were prepared by washing with PBS, treated with 0.5 M acetic acid, and digested with pepsin overnight at 4°C. Samples were centrifuged to remove insoluble debris and then incubated with Sircol dye reagent for 30 min at room temperature on a shaker. Alkali reagent was then added and mixed to dissolve bound dye, and samples were assayed at 540 nm in a microplate reader.
Histopathological sample processing.
All tissue samples were fixed in 10% neutral buffered formalin, processed routinely in ethanol and xylene, embedded in paraffin, sectioned at 5 μm thickness, and mounted on slides for staining. Masson’s trichrome staining was performed by incubating samples in Bouin’s picric-formalin and staining with solutions of Weigert’s iron hematoxylin, Biebrich scarlet/acid fuschin, phosphomolybdic/phosphotungstic acid, and aniline blue, with the latter staining collagen blue. For antibody staining, sections were incubated with antigen retrieval solution at 37°C for 30 min overnight at 4°C, after which they were washed twice in PBS and incubated with biotinylated secondary antibody (BA-2001 for α-SMA and CD68 and BA-1000 for TGF-β1, Vector Laboratories, Burlingame, CA) at 37°C for 30 min. Sections were washed again, incubated with Avidin-Biotin Complex Elite (catalog no. PK-6100, Vectastain ABC Elite Kit, Vector Laboratories), followed by DAB substrate, and counterstained with hematoxylin.
Cell culture conditions and in vitro IRE.
All cell lines were cultured in DMEM supplemented with 10% FCS, 200 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C with 5% CO2. Cells in exponential phase were used for in vitro experiments. In vitro IRE was performed on cells in 24-well plates using two cylindrical electrodes (25-mm length, 0.8-mm diameter, and 10-mm spacing between electrodes) suspended into the well using three-dimensional printed electrode holders or using electroporation cuvettes having plate electrodes at 4-mm gap (Bio-Rad). Electric pulses were generated using a square wave generator (ECM830, Harvard Apparatus, Holliston, MA).
Statistical analysis.
All in vitro experiments were repeated in triplicate, and data were aggregated and expressed as means ± SD unless otherwise indicated. Statistical analyses were performed using SPSS software (version 22, IBM, Armonk, New York, NY). Statistical data comparisons of histology quantification on the ablated ureter and periablated normal ureter were performed by one-way ANOVA. Correlations between each group of staining were evaluated by within-subject correlation coefficients. For all in vitro experiments, a standard two-tailed unpaired Student’s t-test was used. All test results were considered significant at P < 0.05.
RESULTS
Proliferation and α-SMA expression by fibroblasts in the IRE-treated ureter coincides with macrophage proliferation.
Endoluminal IRE of the ureter was performed in swine. Ureters were removed between 1 and 28 days after treatment. We examined both the IRE-treated ureter and healthy ureter surrounding the IRE-treated area.
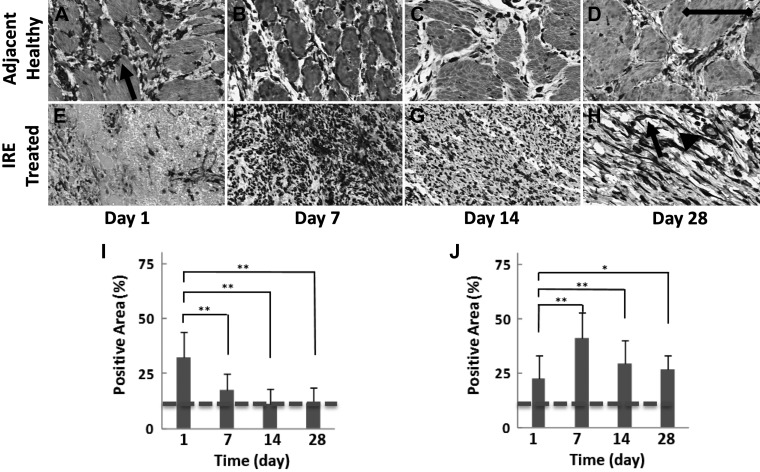
After treatment with IRE, there was an increase in the population of fibroblasts (spindle-shaped cells staining positive for vimentin) in the healthy ureter surrounding the treatment region at day 1 (32.1 ± 11.9% positive staining, P < 0.01; Fig. 1, A and I), but by day 7, the population of fibroblasts returned to levels comparable to that of the untreated control (17.5 ±7.3% positive staining, not significant; Fig. 1, B and I). On the other hand, the fibroblast population in the IRE-treated ureter increased starting at day 7 (41.4 ± 11.3% positive staining, P < 0.01; Fig. 1, F and J), and while there was a slight decline at later time points, the population of fibroblasts remained at higher levels than in the untreated control (28.7 ± 7.5% positive staining, P < 0.01; Fig. 1, H and J) at day 28. A comparison of samples from the IRE-treated ureter and adjacent healthy ureter at day 1 (Fig. 1, A and E) and day 7 (Fig. 1, B and F) suggested that fibroblasts migrated from the healthy ureter into the region of injury.
Fig. 1.
Vimentin staining on immunohistochemistry used to identify and quantify fibroblasts in the ureter. The healthy ureter adjacent to the treatment site (A–D and I) and the irreversible electroporation (IRE)-treated ureter (E–H and J) were collected at 1, 7, 14, and 28 days after treatment. An increased presence of fibroblasts (arrow) was observed in the healthy tissue adjacent to the treatment site only in day 1 samples (A and I). In comparison, the peak population of fibroblasts was observed at day 7 in the IRE-treated ureter (F and J) and remained greater than that of the surrounding healthy tissue (B–D and I) at all subsequent time points (G, H, and J). Other than fibroblasts (arrow), vimentin staining was also observed in the blood vessel wall within the treated ureter in day 28 samples (H, arrowhead) and was censored from measurements. Samples were collected from two treatment sites in two animals at each indicated time point. Scale bar = 50 μm. *P < 0.05; **P < 0.01. Staining levels in untreated control samples are denoted with a dashed line.
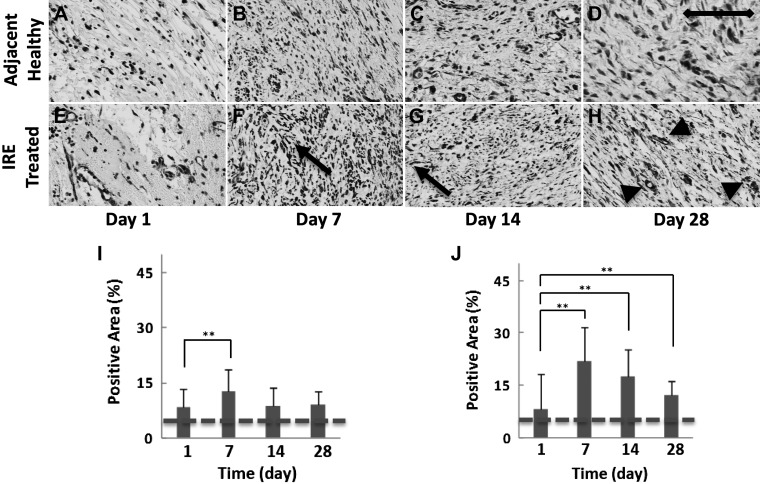
Correspondingly, there was an increase in the presence of α-SMA-expressing cells (Fig. 2, F and J) in the IRE-treated ureter at day 7 that exhibited a strong correlation with vimentin staining (r = 0.288, within-subject correlation coefficient, P < 0.01). Such a correlation was not observed in the healthy ureter adjacent to the IRE-treated area. When considering vimentin staining, this observation indicated that despite the initial migration of fibroblasts from the healthy to injured ureter, differentiation, as denoted by α-SMA expression, seemed to be largely restricted to fibroblasts in the injured tissue at day 1 (Fig. 2, A and E) and day 7 (Fig. 2, B and F).
Fig. 2.
α-Smooth muscle actin (α-SMA) staining with immunohistochemistry used to identify and quantify myofibroblasts in the ureter. The healthy ureter adjacent to the treatment site (A–D and I) and the irreversible electroporation (IRE)-treated ureter (E–H and J) were collected at 1, 7, 14, and 28 days after treatment. The myofibroblast population (F and G, arrow) in the healthy ureter surrounding the treatment site appeared similar at all time points (A–D and I), whereas the population peaked at day 7 (F and J) in the IRE-treated ureter and remained greater than the surrounding healthy tissue (B–D and I) at all subsequent time points (G, H, and J). Apart from fibroblasts, α-SMA staining was also observed in smooth muscle cells found in blood vessels and regenerating smooth muscle in the treated ureter in day 28 samples (H, arrowhead) and was censored from measurements. Samples were collected from two treatment sites in two animals at each indicated time point. Scale bar = 50 μm. **P < 0.01. Staining levels in untreated control samples are denoted with a dashed line.
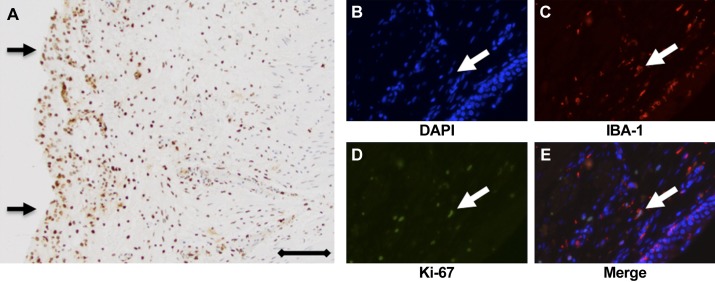
TUNEL staining indicated that injury with IRE caused substantial cell death in the ureteral wall at day 1 posttreatment (Fig. 3A). The presence of necrotic cells in the ureteral wall was expected to serve as a chemoattractant to monocytic cells. Double staining performed for Iba-1 and Ki-67 on IRE-treated ureter samples from day 7 suggested dividing or proliferating macrophages in the ureteral wall (Fig. 3, B–E). Iba-1 staining (Fig. 4) indicated that the peak population of macrophages occurred in the IRE-treated ureter at day 7 (37.2 ± 18.3% positive staining, P < 0.01; Fig. 4, F and J), with staining at day 28 (20.0 ± 21.5% positive staining) remaining significantly higher than the surrounding healthy ureter and untreated control (Fig. 4J). There was a strong correlation between Iba-1 and α-SMA expression in the IRE-treated ureter (r = 0.384, P < 0.01) but not in the healthy ureter adjacent to the treatment site. Both macrophages and fibroblasts seemed widely spread in the IRE-treated ureter at days 7 and 14 (Fig. 2, F and G, and Fig. 4, F and G). Furthermore, general spatial overlap was observed for both cell types at all time points evaluated in the study.
Fig. 3.
Evaluation of macrophage chemoattractant and proliferation in the irreversible electroporation (IRE)-treated ureter. The nuclei of cells in the IRE-treated ureter wall (brown; A) stained strongly positive for TUNEL at day 1 posttreatment, suggesting ongoing cell death. Arrows indicate the ureteral lumen and basal membrane, with the notable absence of the urothelium, which is expected to slough as a consequence of IRE. B−E: multiplex immunofluorescence with DAPI (blue; B) for the nucleus, Iba-1 (red; C) for macrophages, Ki-67 (green; D) for cell proliferation, and merged image (E) showing the collocation of Ki-67 and Iba-1 staining (arrow), suggesting the presence of proliferating macrophages in the ureteral wall at day 7 post-IRE.
Fig. 4.
Iba-1 staining on immunohistochemistry used to identify macrophages in the ureter. The healthy ureter adjacent to the treatment site (A–D and I) and the irreversible electroporation (IRE)-treated ureter (E–H and J) were collected at 1, 7, 14, and 28 days after treatment. The macrophage population in the healthy ureter surrounding the treatment site was briefly elevated in day 1 samples (A and I) but reverted to reduced levels at subsequent time points (B–D and I). The peak macrophage population in the IRE-treated ureter (G and H, arrow) was observed at day 7 (F and J) and remained greater than the surrounding healthy tissue (B–D and I) at all subsequent time points (G, H, and J). Samples were collected from two treatment sites in two animals at each indicated time point. Scale bar = 50 μm. **P < 0.01. Staining levels in untreated control samples are denoted with a dashed line.
Scarring of the ureteral wall was associated with increased TGF-β1 expression in fibroblasts and macrophages.
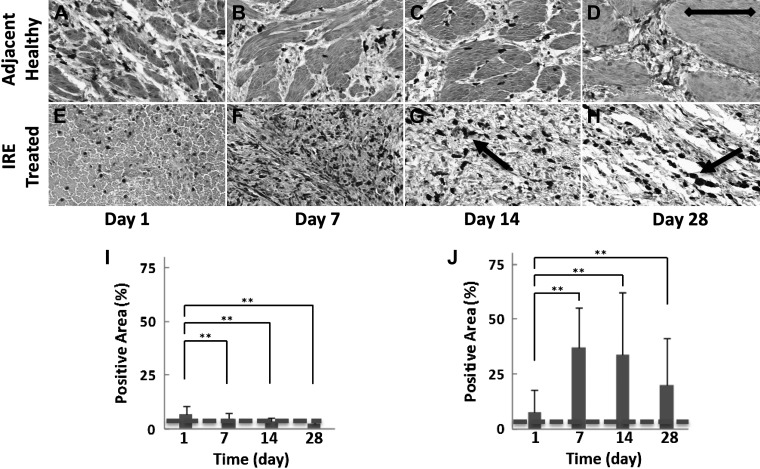
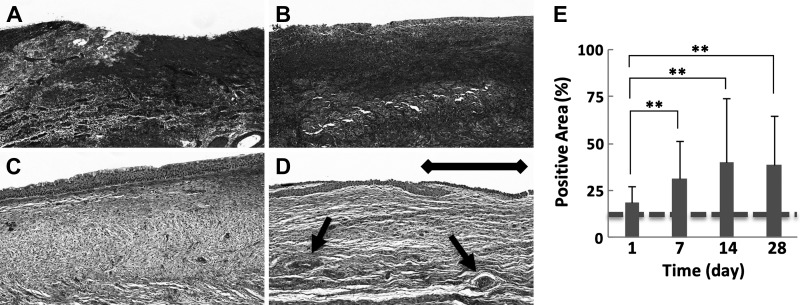
Scarring of the IRE-treated ureteral wall was characterized by a steady increase in the percent area of the ureteral wall staining positive for collagen, which plateaued at elevated levels by day 28 (Fig. 5, A–D). Collagen-stained areas in the IRE-treated ureter were twofold greater (38.9 ± 25.6% positive staining, P < 0.01; Fig. 5A) at day 28 compared with day 1 (18.6 ±8.7% positive staining; Fig. 5D) and were more than twofold greater compared with the healthy ureteral wall adjacent to the site of treatment (16.3 ± 9.5% positive staining) and untreated control (12.5 ± 2.6%; Fig. 5E). Collagen staining of the healthy ureter adjacent to the site of IRE did not demonstrate any remarkable changes at any of the time points evaluated, and the cellular architecture of the muscularis appeared intact, suggesting an absence of scar formation in the region (Supplemental Fig. S1, E−H; https://doi.org/10.5281/zenodo.2538189).
Fig. 5.
Masson’s trichrome staining for collagen for the quantification of ureteral wall scarring after irreversible electroporation (IRE). The IRE-treated ureter showed a progressive loss of normal ureteral wall architecture with increased collagen content, with a steady increase in levels on day 1 (A) compared with subsequent time points at day 7 (B), day 14 (C), and day 28 (D). Sparse regenerating blood vessels and smooth muscle (arrow) can be seen at day 28 (D), but the overall ureteral wall architecture was lost to scarring. E: percentage of the positive area at the indicated time points. See Supplemental Fig. S1 (https://doi.org/10.5281/zenodo.2538189) for comparative images of collagen staining in the healthy ureteral wall adjacent to the treatment site. Samples were collected from two treatment sites in two animals at each indicated time point. Scale bar = 200 μm. **P < 0.01. Staining levels in untreated control samples are denoted with a dashed line.
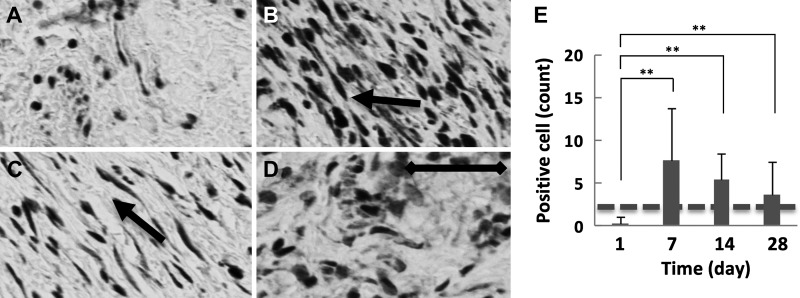
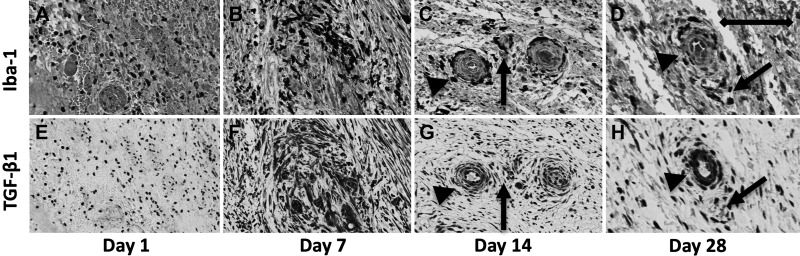
We performed IHC for TGF-β1 and counted the population of TGF-β1-positive fibroblasts based on their spindle-shaped morphology (Fig. 6). Increased collagen levels in the IRE-treated ureter were strongly correlated with the population of fibroblasts staining positive for TGF-β1 (r = 0.347, P < 0.01). The population of TGF-β1-positive fibroblasts (Fig. 6, A–D) within the IRE-treated ureter peaked at day 7 after treatment. At day 28, the TGF-β1-positive population in the IRE-treated ureter remained greater than that of the untreated control. Scattered TGF-β1-positive fibroblasts could be observed in the healthy ureter adjacent to the site of IRE treatment, but cell numbers were no different than the untreated control ureteral wall (Supplemental Fig. S1, A–D; https://doi.org/10.5281/zenodo.2538189). Changes in the population of TGF-β1-positive fibroblasts (Fig. 6E) correlated closely with α-SMA staining at all time points (r = 0.221, P < 0.05). Comparative evaluation of Iba-1 (Fig. 7, A–D) and TGF-β1 (Fig. 7, E–H) suggested that the expression of TGF-β1 by macrophages in the IRE-treated ureter overlapped with staining in fibroblasts for all time points. Besides macrophages and fibroblasts, comparison of TGF-β1 staining with hematoxylin and eosin-stained slides suggested that TGF-β1 staining was also present in smooth muscle and endothelial cells (Fig. 7, G and H), identified based on their morphological appearance.
Fig. 6.
Transforming growth factor (TGF)-β1 staining and quantification of expression in fibroblasts (spindle-shaped cells) in the irreversible electroporation (IRE)-treated ureter. A−D: TGF-β1-positive cells (arrow) in the swine ureter treated with IRE at day 1 (A) day 7 (B), day 14 (C), and day 28 (D). E: quantification of immunohistochemical staining indicating that the population of fibroblasts staining positive for TGF-β1 peaked at day 7 (B and E), with a gradual decrease at subsequent time points (C–E). See Supplemental Fig S1 (https://doi.org/10.5281/zenodo.2538189) for comparative images of TGF-β1-positive cells in the healthy ureteral wall adjacent to the treatment site. Samples were collected from two treatment sites in two animals at each indicated time point (n = 4). Scale bar = 50 μm. **P < 0.01. Staining levels in untreated control samples are denoted with a dashed line.
Fig. 7.
Macrophage (top) and transforming growth factor (TGF)-β1 (bottom) staining in the irreversible electroporation (IRE)-treated ureter at days 1, 7, 14, and 28 from matched tissue sections. Comparison of macrophage (Iba-1; A–D) and (TGF-β1; E–H) immunohistochemical stains suggested TGF-β1 staining in macrophages (arrows) on days 1–14, and staining could also be observed in vascular cells at day 14 and 28 (arrowheads). Scale bar = 100 μm. Samples were collected from two treatment sites in two animals at each indicated time point.
Macrophages stimulated using cells killed with IRE promotes TGF-β1 production.
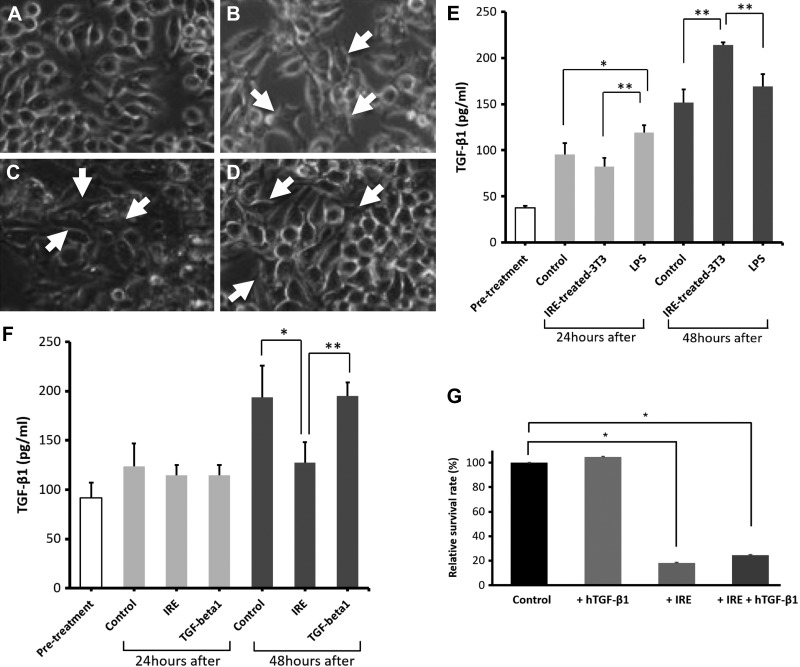
To further assess the implication of concurrent TGF-β1 staining and colocation of macrophages and α-SMA-positive fibroblasts in the IRE-treated ureter, we performed correlative in vitro experiments to understand the conditions under which macrophages and fibroblasts secrete TGF-β1. RAW 264.7 macrophages were incubated alone, with LPS, or with either RAW 264.7 or 3T3 cells killed with IRE. Control cells exhibited rounded macrophage morphology (Fig. 8A), whereas macrophages activated by either LPS (Fig. 8B) or cells killed with IRE (Fig. 8, C and D) exhibited increased adhesion and a dendritic morphology. Compared with pretreatment levels, control, or IRE-treated 3T3 cells, LPS stimulation increased TGF-β1 secretion by macrophages after 24 h of incubation (control: 95.6 ± 12.0 pg/ml, IRE-killed 3T3: 82.3 ±9.4 pg/ml, and LPS: 119.1 ± 7.9 pg/ml, P < 0.05). However, TGF-β1 secretion was greatest in macrophages stimulated with IRE-treated 3T3 cells at 48 h, exceeding comparative cytokine levels in LPS or control samples (control: 151.9 ± 13.9 pg/ml, IRE killed 3T3: 213.9 ± 3.1 pg/ml, and LPS: 169.2 ± 13.3 pg/ml, P < 0.02; Fig. 8E).
Fig. 8.
Transforming growth factor (TGF)-β1 secretion by macrophages and fibroblasts in vitro. Macrophages were incubated with vehicle (A), LPS (B), irreversible electroporation (IRE)-treated RAW 264.7 cells (C), or IRE-treated 3T3 fibroblasts (D). All treatments resulted in activation as observed by a morphological dendritic shape (white arrows, B–D). RAW 264.7 macrophages demonstrated increased secretion of TGF-β1 upon activation with IRE-treated 3T3 cells killed with IRE (E). Compared with control or IRE treatment, 3T3 cells stimulated with TGF-β1 after IRE demonstrated increased autocrine secretion of TGF-β1 at 48 h post-IRE (F). IRE treatment reduced the population and proliferation of 3T3 cells in vitro, the effects of which were not overcome with recombinant human (h)TGF-β1 stimulation (G). *P < 0.05; **P < 0.02.
Compared with control or stimulation with exogenous TGF-β1, treatment with IRE resulted in a substantial drop in the proliferation of 3T3 cells (Fig. 8G). The reduction in the population of 3T3 cells from IRE could not be rescued by further stimulation with TGF-β1 (Fig. 8G). Autocrine TGF-β1 secretion was modest in 3T3 cells and was not different when control, IRE, and IRE+ TGF-β1 conditions were compared. By 48 h, 3T3 cells treated with IRE had reduced secretion of TGF-β1, but levels were no different between control and 3T3 cells stimulated with exogenous TGF-β1 after IRE, suggesting an exogenous source improved autocrine secretion even when cell population levels were reduced (Fig. 8F).
External source of TGF-β1 promotes differentiation of fibroblasts and collagen secretion.
To assess the impact of extracellular TGF-β1 on 3T3 cells, we treated 3T3 cells with either human TGF-β1 or IRE alone or with a combination of human TGF-β1 and IRE. We then studied changes in the expression of scar-related mRNA or proteins, collagen, and proliferation in vitro.
Western blot analysis showed that stimulation with human TGF-β1 alone was sufficient to significantly increase α-SMA protein synthesis in 3T3 cells, whereas neither IRE alone nor IRE in the presence of human TGF-β1 affected α-SMA expression (Fig. 9). Increased expression of α-SMA manifested within 24 h after stimulation with human TGF-β1 (Fig. 9A) and persisted at the 48-h assessment time point (Fig. 9B). Similar results were found when cells were examined for collagen content (Fig. 10B). Quantitative PCR results showed that genes related to fibroblast activation and differentiation (ACTA2) and collagen production (COL1A1) demonstrated significantly higher expression only when cells were stimulated with human TGF-β1 (Fig. 10C), mimicking results from α-SMA and collagen measurements.
Fig. 9.
Effect of transforming growth factor (TGF)-β1, irreversible electroporation (IRE), or TGF-β1 + IRE on α-smooth muscle actin (α-SMA) synthesis in 3T3 fibroblasts. Compared with control or IRE treatment alone, stimulation of 3T3 cells with recombinant human (h)TGF-β1 was necessary and sufficient to promote α-SMA synthesis as early as 24 h post-IRE (A), the effects of which persisted through 48 h post-IRE (B). Treatment with pirfenidone (PFD) attenuated α-SMA at all time points. *P < 0.01; **P < 0.001.
Fig. 10.
Effect of transforming growth factor (TGF)-β1 stimulation on collagen production, profibrotic mRNA expression in 3T3 fibroblasts, and TGF-β1 production in macrophages in combination with irreversible electroporation (IRE) and pirfenidone (PFD). Treatment with PFD reduced TGF-β1 secretion by RAW 264.7 macrophages activated with IRE-treated 3T3 cells (A). In 3T3 cells stimulated with recombinant human (h)TGF-β1, there was increased collagen production (B) and mRNA expression of both α-smooth muscle actin (ACTA2) and collagen [collagen type I-α1 (COL1A1); C]. Both collagen production and mRNA expression were reduced with PFD treatment. *P < 0.05; **P < 0.01.
PFD limits proliferation and TGF-β1 secretion in macrophages and curtails profibrotic activity of fibroblasts.
We investigated the use of PFD, an antifibrotic drug acting through the TGF-β1 pathway, to suppress the profibrotic effects of TGF-β1 in 3T3 fibroblasts. Treatment with IRE or PFD and the combination of both agents increased the number of apoptotic macrophages and 3T3 cells, with macrophages being more likely to undergo cell death treatment with one or both agents (Fig. 11). Stimulation with exogenous TGF-β1 substantially reduced annexin expression in 3T3 cells, especially after treatment with either PFD or IRE, compared with stimulation with the cytokine under the sham condition (Fig. 11). Overall, macrophages were more susceptible to PFD compared with 3T3 fibroblasts (Fig. 11B), and PFD treatment seemed to promote cell death in 3T3 cells even when stimulated with exogenous TGF-β1 (Fig. 11A).
Fig. 11.
Effect of pirfenidone (PFD) and irreversible electroporation (IRE) treatment of macrophages and fibroblasts. Fibroblasts (A) and macrophages (B) demonstrated increased annexin V expression after treatment with IRE, PFD, or a combination of the two. Stimulation with transforming growth factor (TGF)-β1 somewhat reduced the number of apoptotic cells after IRE, but this effect was overcome with the addition of PFD to the treatment regimen. *P < 0.02; **P < 0.01.
ELISA demonstrated that PFD significantly suppressed TGF-β1 secretion in RAW 264.7 cells activated with IRE-killed cells but not in unactivated cells or control conditions (P < 0.05; Fig. 10A). Simultaneously, PFD treatment also dampened TGF-β1-mediated profibrotic changes in 3T3 fibroblasts. Sircol collagen assay also demonstrated that treatment with PFD reduced soluble collagen type I synthesis of fibroblasts stimulated with TGF-β1 compared with controls, and these results were independent of IRE treatment (P < 0.05; Fig. 10B). PFD treatment also suppressed both ACTA2 and COL1A1 expression assessed by quantitative PCR (Fig. 10C) and α-SMA protein synthesis as measured using Western blot analysis (Fig. 9, A and B).
DISCUSSION
Ureterscopic (20) and laparascopic (2) guided procedures are increasingly used for the treatment of malignant and nonmalignant urological diseases. With the use of such image guidance, ablation is performed with a variety of energy modalities, such as laser, ultrasound, shockwave, and electrocautery, for the removal of stones in the upper urinary tract and for the treatment of early stage urothelial cancers. These procedures can result in injury to the healthy ureter adjacent to the treatment site, which can lead to UUO. The current literature predominantly focuses on mitigating the deleterious effects of UUO, but there is limited knowledge on the prevention of iatrogenic UUO. TGF-β1 is an important mediator of the normal wound healing response and contributes to aberrant wound healing observed in pulmonary and renal fibrosis (3, 19, 27, 40). TGF-β1 is necessary and essential for the differentiation of fibroblasts (26), and it promotes the collagen production (10) and contractile function (11) of these cells. We used IRE as a relatively noninvasive model of ureteral injury to study the cellular interactions underlying stricture formation.
Our in vivo results using IRE in the porcine ureter suggest that TGF-β1 activity during normal wound healing after ureteral injury with IRE may underlie stricture formation. Furthermore, our in vitro results demonstrated that PFD, a TGF-β1-targeted antifibrotic, was able to act on both macrophages and fibroblasts in vitro, ameliorating the effects of TGF-β1 and collagen secretion. These results support the investigation of PFD as an adjuvant to ureteroscope-guided ablation or other procedures to prevent or ameliorate ureteral stricture formation.
In the in vivo experiments, we performed IRE in the ureters of swine and followed the animals for up to 28 days. Scar formation in the ureter was marked by the proliferation of fibroblasts and macrophages, differentiation of fibroblasts into the myofibroblast phenotype, and increased staining for TGF-β1. Increased collagen deposition in the ureteral wall was associated with the loss of normal cellular architecture and muscularis, which may lead to progressive UUO in a negative feedback loop. Scarring of the ureter was largely complete by day 14 post-IRE, with a gradual reduction in the population of fibroblasts and macrophages, trending toward baseline levels by day 28. While 3T3 fibroblasts produce modest quantities of TGF-β1 in vitro under control conditions, exposure to an external source of TGF-β1 seems to significantly stimulate collagen production and α-SMA expression and promote proliferation of fibroblasts.
Our in vivo and in vitro findings, taken together, suggest wound healing-related macrophages can be an important paracrine source of TGF-β1, leading to activation and proliferation of fibroblasts in the wounded ureter. Macrophages are central to normal wound healing and secrete a variety of cytokines to coordinate the healing and regeneration process. Unlike LPS activation, which is known to induce an inflammatory phenotype in macrophages, our work shows that exposure of macrophages to cells killed with IRE results in exuberant TGF-β1 secretion by macrophages, consistent with a wound healing phenotype. TGF-β1 is secreted by a number of cell types, including the urothelium (41) and vascular smooth muscle cells (4, 18, 27), and serves important cytostatic functions under normal conditions (13, 27, 32). Analysis of IHC slides of the healthy untreated ureter did not reveal an increased presence of fibroblasts or macrophages positive for TGF-β1. However, after injury to the ureter with IRE, we observed proliferation of macrophages only within the wounded ureter by day 7. This was matched by proliferation and migration of fibroblasts from the peripheral healthy ureter adjacent to the treatment site but with an increased population of α-SMA-expressing fibroblasts only within the wounded ureter. The dynamics of proliferation and migration of fibroblasts in wounded ureters mirrored corresponding autocrine TGF-β1 expression, which was not observed in the healthy ureter adjacent to the site of ablation, reinforcing the importance of TGF-β1-secreting macrophages, but not other cellular sources, as central to fibroblast differentiation and activity. These findings underscore the important role of macrophages in scar formation and identify an important early target that can be modulated for preventing stricture formation and subsequent UUO.
PFD is antifibrotic drug that is approved to treat patients with idiopathic pulmonary fibrosis. While the exact mechanism of action of the drug is not fully understood, PFD has been described to exert its antifibrotic effect by modulating the TGF-β1 pathway (30). Our results suggest that under in vitro conditions, PFD acts against both macrophages (14, 29) and fibroblasts (24, 36), ameliorating TGF-β1 and collagen secretion that underlies stricture formation in vivo. While TGF-β1 signaling is not associated with the regulation of cell apoptosis or death, our results indicate that PFD by itself, or in combination with IRE and/or TGF-β1 stimulation, substantially increases apoptosis in macrophages (Fig. 11) and, to a lesser extent, in fibroblasts. Kozono et al. (23) and Sun et al. (37) have reported PFD to have antiproliferative effect on pancreatic stellate cells and human intestinal fibroblastsm respectively. Furthermore, Sun et al. (37) reported both necrotic cell death and apoptosis to underlie the reduction in proliferation of fibroblasts in vitro, where the effect of PFD was accentuated with TGF-β1 stimulation. Walter et al. (39) reported similar synergistic toxicity in fibroblasts when PFD was combined with sirolimus. While a number of studies have evaluated the antifibrotic effect of PFD on macrophages, the toxicity of PFD on macrophages and related cell death have not been reported.
We have previously shown that administration of PFD after cryoablation injury to healthy tissue attenuates macrophage populations during wound healing with an attendant decrease in the secretion of TGF-β1 and other cytokines (17). PFD has also been demonstrated to reduce fibroblast proliferation, inhibit TGF-β-stimulated collagen production, and improve fibrosis (12) in preclinical models of chronic lung, liver, and kidney fibrosis. Our data support the use of PFD to modulate TGF-β1 signaling during a typical wound healing response to potentially reduce the risk of stricture formation. Our in vitro experiments suggest that while PFD reduces the expression of profibrotic gene signatures and reduces collagen secretion, the overall levels remain elevated compared with its control. It may therefore be important to administer the drug prophylactically in patients to further reduce the risk of UUO. A significant proportion of patients undergoing surgery or treatment in the urinary tract have oncological disease, and the impact of PFD on wound healing and oncological control is unknown and will require further investigation before translation to clinical use.
Iatrogenic injuries to the urinary tract occur during ureteroscopic or surgical procedures. Our work suggests that macrophages attracted by cells killed by IRE at the site of injury initiate a cascade of events leading to stricture formation and urinary obstruction. Here, we describe the cellular mediators and timeline of such stricture formation and demonstrate in vitro that clinically used pharmacological agents (PFD) can arrest cellular activity related to scar formation. In present clinical practice, placement of a urinary stent is the primary option available for the prevention of postprocedure stricture formation in the urinary tract. This approach is not feasible in all patients, and in a considerable number of patients, injury to the ureter may not be evident until clinical manifestation of ureteral obstruction. Our findings suggest that TGF-β1 modulators such as PFD can be considered as a prophylactic option to prevent this common treatment-related complication.
GRANTS
The work reported here was supported by the Thompson Family Foundation (to G. Srimathveeravalli and J. A. Coleman), a Society of Interventional Radiology Ernest Ring Award (to G. Srimathveeravalli), and National Cancer Institute (NCI) Grants U54-CA-137788/U54-CA-132378 (to G. Srimathveeravalli). We acknowledge the support of NCI Grant P30-CA-008748 for core laboratory services that were used for the reported work. Additional support was from the Frederick J. and Theresa Dow Wallace Fund of the New York Community Trust (to D. Felsen).
DISCLOSURES
S. B. Solomon is a consultant to BTG, Johnson & Johnson, XACT, Adegro, and Medtronic. S. B. Solomon has funding support from GE Healthcare and Angiodynamics and holds stock in Aperture Medical. J. C. Durack is on the scientific advisory board and holds stock in Adient Medical. G. Srimathveeravalli holds stock options in Aperture Medical. The other authors do not have conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
E.U., D.F., and G.S. conceived and designed research; E.U., M.F., H.K., J.C., J.C.D., and G.S. performed experiments (large animal experiments: E.U, H.K, J.C.D, S.B.S and J.A.C; histology, image analysis, and quantification: E.U, H.K, and G.S; in vitro experiments: M.F., E.U, D.F, and J.C); E.U., M.F., H.K., D.F., J.C., and G.S. analyzed data; E.U., M.F., D.F., J.C., and G.S. interpreted results of experiments; E.U., M.F., J.C., and G.S. prepared figures; E.U., D.F., and G.S. drafted manuscript; E.U., M.F., H.K., D.F., J.C.D., S.B.S., J.A.C., and G.S. edited and revised manuscript; E.U., M.F., H.K., D.F., J.C., J.C.D., S.B.S., J.A.C., and G.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the contribution of Sebastien Monette and Maria Jiao (Laboratory of Comparative Pathology and Memorial Sloan Kettering Cancer Center) for assistance with histological preparation and immunohistochemical staining.
REFERENCES
- 1.Andreoni CR, Lin HK, Olweny E, Landman J, Lee D, Bostwick D, Clayman RV. Comprehensive evaluation of ureteral healing after electrosurgical endopyelotomy in a porcine model: original report and review of the literature. J Urol 171: 859–869, 2004. doi: 10.1097/01.ju.0000108383.18165.f5. [DOI] [PubMed] [Google Scholar]
- 2.Anidjar M, Mongiat-Artus P, Brouland JP, Meria P, Teillac P, Le Duc A, Berthon P, Cussenot O. Thermal radiofrequency induced porcine ureteral stricture: a convenient endourologic model. J Urol 161: 298–303, 1999. doi: 10.1016/S0022-5347(01)62135-9. [DOI] [PubMed] [Google Scholar]
- 3.Ask K, Bonniaud P, Maass K, Eickelberg O, Margetts PJ, Warburton D, Groffen J, Gauldie J, Kolb M. Progressive pulmonary fibrosis is mediated by TGF-beta isoform 1 but not TGF-beta3. Int J Biochem Cell Biol 40: 484–495, 2008. doi: 10.1016/j.biocel.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belmadani S, Zerfaoui M, Boulares HA, Palen DI, Matrougui K. Microvessel vascular smooth muscle cells contribute to collagen type I deposition through ERK1/2 MAP kinase, αvβ3-integrin, and TGF-β1 in response to ANG II and high glucose. Am J Physiol Heart Circ Physiol 295: H69–H76, 2008. doi: 10.1152/ajpheart.00341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchi D, Vespasiani G, Bove P. Acute kidney injury due to bilateral ureteral obstruction in children. World J Nephrol 3: 182–192, 2014. doi: 10.5527/wjn.v3.i4.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuang YH, Chuang WL, Chen SS, Huang CH. Expression of transforming growth factor-β1 and its receptors related to the ureteric fibrosis in a rat model of obstructive uropathy. J Urol 163: 1298–1303, 2000. doi: 10.1016/S0022-5347(05)67767-1. [DOI] [PubMed] [Google Scholar]
- 7.Chuang YH, Chuang WL, Huang SP, Huang CH. Cyclooxygenase-2 inhibitor ameliorates ureteric damage in rats with obstructed uropathy. Eur J Pharmacol 569: 126–137, 2007. doi: 10.1016/j.ejphar.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 8.Chuang YH, Chuang WL, Huang SP, Liu CK, Huang CH. Atorvastatin ameliorates tissue damage of obstructed ureter in rats. Life Sci 89: 795–805, 2011. doi: 10.1016/j.lfs.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Chuang YH, Chuang WL, Liu KM, Chen SS, Huang CH. Tissue damage and regeneration of ureteric smooth muscle in rats with obstructive uropathy. Br J Urol 82: 261–266, 1998. doi: 10.1046/j.1464-410X.1998.00725.x. [DOI] [PubMed] [Google Scholar]
- 10.Creely JJ, DiMari SJ, Howe AM, Haralson MA. Effects of transforming growth factor-beta on collagen synthesis by normal rat kidney epithelial cells. Am J Pathol 140: 45–55, 1992. [PMC free article] [PubMed] [Google Scholar]
- 11.Desmoulière A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen 13: 7–12, 2005. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 12.Di Sario A, Bendia E, Svegliati Baroni G, Ridolfi F, Casini A, Ceni E, Saccomanno S, Marzioni M, Trozzi L, Sterpetti P, Taffetani S, Benedetti A. Effect of pirfenidone on rat hepatic stellate cell proliferation and collagen production. J Hepatol 37: 584–591, 2002. doi: 10.1016/S0168-8278(02)00245-3. [DOI] [PubMed] [Google Scholar]
- 13.Dooley S, ten Dijke P. TGF-β in progression of liver disease. Cell Tissue Res 347: 245–256, 2012. doi: 10.1007/s00441-011-1246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du J, Paz K, Flynn R, Vulic A, Robinson TM, Lineburg KE, Alexander KA, Meng J, Roy S, Panoskaltsis-Mortari A, Loschi M, Hill GR, Serody JS, Maillard I, Miklos D, Koreth J, Cutler CS, Antin JH, Ritz J, MacDonald KP, Schacker TW, Luznik L, Blazar BR. Pirfenidone ameliorates murine chronic GVHD through inhibition of macrophage infiltration and TGF-β production. Blood 129: 2570–2580, 2017. doi: 10.1182/blood-2017-01-758854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiuk J, Bao Y, Calleary JG, Schwartz BF, Denstedt JD. The use of internal stents in chronic ureteral obstruction. J Urol 193: 1092–1100, 2015. doi: 10.1016/j.juro.2014.10.123. [DOI] [PubMed] [Google Scholar]
- 16.Goldfischer ER, Gerber GS. Endoscopic management of ureteral strictures. J Urol 157: 770–775, 1997. doi: 10.1016/S0022-5347(01)65037-7. [DOI] [PubMed] [Google Scholar]
- 17.Gu Y, Srimathveeravalli G, Cai L, Ueshima E, Maybody M, Yarmohammadi H, Zhu YS, Durack JC, Solomon SB, Coleman JA, Erinjeri JP. Pirfenidone inhibits cryoablation induced local macrophage infiltration along with its associated TGFb1 expression and serum cytokine level in a mouse model. Cryobiology 82: 106–111, 2018. doi: 10.1016/j.cryobiol.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ha DM, Carpenter LC, Koutakis P, Swanson SA, Zhu Z, Hanna M, DeSpiegelaere HK, Pipinos II, Casale GP. Transforming growth factor-beta 1 produced by vascular smooth muscle cells predicts fibrosis in the gastrocnemius of patients with peripheral artery disease. J Transl Med 14: 39, 2016. doi: 10.1186/s12967-016-0790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins SP, Tang Y, Higgins CE, Mian B, Zhang W, Czekay RP, Samarakoon R, Conti DJ, Higgins PJ. TGF-β1/p53 signaling in renal fibrogenesis. Cell Signal 43: 1–10, 2018. doi: 10.1016/j.cellsig.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarosek SL, Virnig BA, Chu H, Elliott SP. Propensity-weighted long-term risk of urinary adverse events after prostate cancer surgery, radiation, or both. Eur Urol 67: 273–280, 2015. doi: 10.1016/j.eururo.2014.08.061. [DOI] [PubMed] [Google Scholar]
- 21.Khalil N, Bereznay O, Sporn M, Greenberg AH. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med 170: 727–737, 1989. doi: 10.1084/jem.170.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinn AC, Lykkeskov-Andersen H. Impact on ureteral peristalsis in a stented ureter. An experimental study in the pig. Urol Res 30: 213–218, 2002. doi: 10.1007/s00240-002-0258-1. [DOI] [PubMed] [Google Scholar]
- 23.Kozono S, Ohuchida K, Eguchi D, Ikenaga N, Fujiwara K, Cui L, Mizumoto K, Tanaka M. Pirfenidone inhibits pancreatic cancer desmoplasia by regulating stellate cells. Cancer Res 73: 2345–2356, 2013. doi: 10.1158/0008-5472.CAN-12-3180. [DOI] [PubMed] [Google Scholar]
- 24.Kurita Y, Araya J, Minagawa S, Hara H, Ichikawa A, Saito N, Kadota T, Tsubouchi K, Sato N, Yoshida M, Kobayashi K, Ito S, Fujita Y, Utsumi H, Yanagisawa H, Hashimoto M, Wakui H, Yoshii Y, Ishikawa T, Numata T, Kaneko Y, Asano H, Yamashita M, Odaka M, Morikawa T, Nakayama K, Kuwano K. Pirfenidone inhibits myofibroblast differentiation and lung fibrosis development during insufficient mitophagy. Respir Res 18: 114, 2017. doi: 10.1186/s12931-017-0600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lange D, Bidnur S, Hoag N, Chew BH. Ureteral stent-associated complications–where we are and where we are going. Nat Rev Urol 12: 17–25, 2015. doi: 10.1038/nrurol.2014.340. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Li Y, Li N, Teng W, Wang M, Zhang Y, Xiao Z. TGF-β1 promotes scar fibroblasts proliferation and transdifferentiation via up-regulating microRNA-21. Sci Rep 6: 32231, 2016. doi: 10.1038/srep32231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng XM, Tang PM, Li J, Lan HY. TGF-β/Smad signaling in renal fibrosis. Front Physiol 6: 82, 2015. doi: 10.3389/fphys.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol 23: 291–299, 2001. [PubMed] [Google Scholar]
- 29.Saito Y, Azuma A, Matsuda K, Kamio K, Abe S, Gemma A. Pirfenidone exerts a suppressive effect on CCL18 expression in U937-derived macrophages partly by inhibiting STAT6 phosphorylation. Immunopharmacol Immunotoxicol 38: 464–471, 2016. doi: 10.1080/08923973.2016.1247852. [DOI] [PubMed] [Google Scholar]
- 30.Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev 20: 85–97, 2011. doi: 10.1183/09059180.00001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selzman AA, Spirnak JP. Iatrogenic ureteral injuries: a 20-year experience in treating 165 injuries. J Urol 155: 878–881, 1996. doi: 10.1016/S0022-5347(01)66332-8. [DOI] [PubMed] [Google Scholar]
- 32.Siegel PM, Massagué J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer 3: 807–820, 2003. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 33.Soria F, Sun F, Sánchez FM, Ezquerra LJ, Díaz-Güemes I, Usón J. Treatment of experimental ureteral strictures by endourological ureterotomy and implantation of stents in the porcine animal model. Res Vet Sci 76: 69–75, 2004. doi: 10.1016/S0034-5288(03)00141-3. [DOI] [PubMed] [Google Scholar]
- 34.Srimathveeravalli G, Cornelis F, Wimmer T, Monette S, Kimm SY, Maybody M, Solomon SB, Coleman JA, Durack JC. Normal porcine ureter retains lumen wall integrity but not patency following catheter-directed irreversible electroporation: imaging and histologic assessment over 28 days. J Vasc Interv Radiol 28: 913–919.e1, 2017. doi: 10.1016/j.jvir.2017.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srimathveeravalli G, Silk M, Wimmer T, Monette S, Kimm S, Maybody M, Solomon SB, Coleman J, Durack JC. Feasibility of catheter-directed intraluminal irreversible electroporation of porcine ureter and acute outcomes in response to increasing energy delivery. J Vasc Interv Radiol 26: 1059–1066, 2015. doi: 10.1016/j.jvir.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stahnke T, Kowtharapu BS, Stachs O, Schmitz KP, Wurm J, Wree A, Guthoff RF, Hovakimyan M. Suppression of TGF-β pathway by pirfenidone decreases extracellular matrix deposition in ocular fibroblasts in vitro. PLoS One 12: e0172592, 2017. doi: 10.1371/journal.pone.0172592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Y, Zhang Y, Chi P. Pirfenidone suppresses TGF–β1–induced human intestinal fibroblasts activities by regulating proliferation and apoptosis via the inhibition of the Smad and PI3K/AKT signaling pathway. Mol Med Rep 18: 3907–3913, 2018. doi: 10.3892/mmr.2018.9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vroomen LG, Petre EN, Cornelis FH, Solomon SB, Srimathveeravalli G. Irreversible electroporation and thermal ablation of tumors in the liver, lung, kidney and bone: What are the differences? Diagn Interv Imaging 98: 609–617, 2017. doi: 10.1016/j.diii.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Walter RF, Zarogoulidis P, Mairinger FD, Werner R, Darwiche K, Zarogoulidis K, Freitag L. Cell viability of fibroblasts to pifenidone and sirolimus: a future concept for drug eluting stents. Int J Pharm 466: 38–43, 2014. doi: 10.1016/j.ijpharm.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18: 1028–1040, 2012. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiong M, Jiang L, Zhou Y, Qiu W, Fang L, Tan R, Wen P, Yang J. The miR-200 family regulates TGF-β1-induced renal tubular epithelial to mesenchymal transition through Smad pathway by targeting ZEB1 and ZEB2 expression. Am J Physiol Renal Physiol 302: F369–F379, 2012. doi: 10.1152/ajprenal.00268.2011. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Zhou X, Gao H, Ji SJ, Wang C. The expression of epidermal growth factor and transforming growth factor-beta1 in the stenotic tissue of congenital pelvi-ureteric junction obstruction in children. J Pediatr Surg 38: 1656–1660, 2003. doi: 10.1016/S0022-3468(03)00577-3. [DOI] [PubMed] [Google Scholar]