Abstract
It has previously been shown that high dietary salt impairs vascular function independent of changes in blood pressure. Rodent studies suggest that NADPH-derived reactive oxygen species mediate the deleterious effect of high salt on the vasculature, and here we translate these findings to humans. Twenty-nine healthy adults (34 ± 2 yr) participated in a controlled feeding study. Participants completed 7 days of a low-sodium diet (LS; 20 mmol sodium/day) and 7 days of a high-sodium diet (HS; 300 mmol sodium/day) in random order. All participants were salt resistant, defined as a ≤5-mmHg change in 24-h mean BP determined while on the LS and HS diets. Laser Doppler flowmetry was used to assess cutaneous vasodilation in response to local heating (42°C) during local delivery of Ringer’s (n = 29), 20 mM ascorbic acid (AA; n = 29), 10 µM Tempol (n = 22), and 100 µM apocynin (n = 22). Additionally, endothelial cells were obtained in a subset of participants from an antecubital vein and stained for nitrotyrosine (n = 14). Cutaneous vasodilation was attenuated by the HS diet compared with LS [LS 93.0 ± 2.2 vs. HS 86.8 ± 2.0 percentage of maximal cutaneous vascular conductance (%CVCmax); P < 0.05] and was restored by AA during the HS diet (AA 90.7 ± 1.2 %CVCmax; P < 0.05 vs. HS). Cutaneous vasodilation was also restored with the local infusion of both apocynin (P < 0.01) and Tempol (P < 0.05) on the HS diet. Nitrotyrosine expression was increased on the HS diet compared with LS (P < 0.05). These findings provide direct evidence of dietary sodium-induced endothelial cell oxidative stress and suggest that NADPH-derived reactive oxygen species contribute to sodium-induced declines in microvascular function.
NEW & NOTEWORTHY High-sodium diets have deleterious effects on vascular function, likely mediating, in part, the increased cardiovascular risk associated with a high sodium intake. Local infusion of apocynin and Tempol improved microvascular function in salt-resistant adults on a high-salt diet, providing evidence that reactive oxygen species contribute to impairments in microvascular function from high salt. This study provides insight into the blood pressure-independent mechanisms by which dietary sodium impairs vascular function.
Listen to this article’s corresponding podcast at https://ajpheart.podbean.com/e/dietary-sodium-oxidative-stress-and-microvascular-function/.
Keywords: cutaneous vasodilation; nitric oxide; oxidative stress, sodium
INTRODUCTION
Cardiovascular disease (CVD) remains the number one cause of death in the United States (45). Excess sodium consumption has been linked to the development of hypertension (1) and other forms of CVD (42). Endothelial dysfunction, characterized by a decreased bioavailability and/or production of nitric oxide (NO), is predictive of future cardiovascular events (29, 41, 44) and may mediate, in part, the increased cardiovascular risk associated with increased sodium intake. NO is important for vascular homeostasis, as it promotes vasodilation and has antiatherosclerotic properties, such as prevention of smooth muscle cell proliferation, platelet aggregation, and leukocyte adhesion (16).
A deleterious effect of high salt on endothelial function has been demonstrated in a number of human studies (10, 22); however, blood pressure (BP) is altered as well, suggesting that endothelial impairments may be mediated by BP changes. Rodent studies have demonstrated that endothelial function is impaired by high salt intake in the absence of changes in BP (26, 27, 34, 46), suggesting a direct effect of salt on the vasculature. Recent research in humans has provided additional evidence for BP-independent effects of high salt on the vasculature (13, 15, 20). A modest reduction in dietary sodium in normotensive, overweight/obese individuals resulted in improved brachial artery flow-mediated dilation independent of BP (9). Additionally, dietary sodium restriction in middle-aged and older adults with elevated systolic BP was shown to improve both conduit and resistance vessel endothelial function that occurred beyond the effect on BP (23). We have observed that a high-sodium diet impairs both cutaneous microvascular (20) and conduit artery endothelial function (15) in normotensive subjects with salt-resistant BP. Taken together, these data provide evidence for a BP-independent effect of dietary salt on the vasculature.
One mechanism that appears to play a role in salt-induced impairments of endothelial function is an increase in reactive oxygen species (ROS) production, which can reduce NO bioavailability by oxidizing critical cofactors essential for NO synthesis and/or by reacting directly with NO itself, forming potent oxidants (3, 18). We (20) and others (23) have demonstrated a role for ROS in salt-induced reductions in endothelial function. Evidence from rodent studies suggests that the ROS-mediating effect of salt is superoxide (34, 46), likely via NADPH oxidase (NOX); however, this has yet to be investigated in humans.
Here we present results from a study investigating the role of salt-induced oxidative stress on cutaneous vascular function in normotensive adults with salt-resistant BP during dietary sodium loading. To translate the previous findings in rodents described above to humans, we assessed cutaneous vasodilation in the presence of Tempol and apocynin to elucidate the potential source of ROS that may be upregulated during a high-sodium diet. We hypothesized that pharmacological inhibition of Tempol-sensitive ROS and NOX would improve cutaneous vasodilation in response to local heating following high dietary sodium, providing evidence for a role of NOX-derived superoxide in the sodium-induced impairments in microvascular function. We also directly assessed the expression of nitrotyrosine (NT), a marker of oxidative stress, in human vascular endothelial cells collected during dietary sodium loading. We hypothesized that NT expression in vascular endothelial cells would be increased in response to dietary sodium loading. Importantly, this study was performed in salt-resistant adults, allowing us to assess the BP-independent effects of sodium.
METHODS
Subjects
Twenty-nine healthy, salt-resistant adults participated in this study. Participants ranged in age from 23 to 59 yr. Experimental procedures were approved by the Institutional Review Board at the University of Delaware and conformed to the guidelines set forth by the Declaration of Helsinki. All subjects gave verbal and written consent before the study.
Participants reported to the laboratory for a screening visit after a 12-h fast. Participants provided a medical history, and a resting BP and 12-lead electrocardiogram (ECG) were performed. Height and weight were also measured, and a venous blood sample was obtained. As the focus of this study was healthy adults, subjects with a history of hypertension, CVD, malignancy, diabetes mellitus, or renal impairment were excluded. Obese subjects (body mass index ≥ 30 kg/m2) and those who used tobacco products were also excluded. Older adults were not included because of age-related alterations in the cutaneous vascular responses to local heating (2, 33). Postmenopausal women were also excluded from the study, as salt sensitivity of BP increases with menopause (38).
Dietary Salt Perturbation
This was a controlled feeding study with all food prepared by a registered dietitian. Participants first completed a 7-day run in diet of 100 mmol sodium/day to normalize baseline sodium intake. All subjects were then assigned to receive 7 days of a low-sodium (LS) diet (20 mmol sodium/day) and 7 days of a high-sodium (HS) diet (300 mmol sodium/day) in random rder. These sodium intakes were selected for us to properly classify adults with salt-resistant BP and are consistent with previously published studies (13, 20). The Mifflin-St. Jeor equation was used to adjust the energy content of the diet for subjects to maintain a constant body weight throughout the study (19). The diet consisted of 50% carbohydrates, 30% fat, and 20% protein. Potassium intake was held constant during all conditions and averaged ~2,700 mg/day. Subjects were instructed to maintain their normal activity levels throughout the study. Body mass was recorded at the beginning of each experimental visit.
During the last 24 h of the LS and HS conditions, a 24-h urine collection was obtained and analyzed for total volume, urinary electrolytes (Easy-Electrolyte Analyzer; Medica Corporation), and urine osmolality (Advanced 3D3 Osmometer; Advanced Instruments). Free water clearance was calculated using standard equations. During the same 24-h period, subjects wore an ambulatory BP monitor (Spacelabs Medical) on their nondominant arm. The monitor measured BP every 20 min during waking hours and every 30 min during sleep. Laboratory BP was also measured using an automated oscillometric sphygmomanometer (Dinamap Dash 2000; GE Medical Systems) during the LS and HS experimental visits to the laboratory.
Hemoglobin (HB201+ model; HemoCue), hematocrit (Clay Adams Brand, Readacrit Centrifuge; Becton Dickinson), serum electrolytes (EasyElectrolyte Analyzer; Medica), and plasma osmolality (Advanced 3D3 Osmometer; Advanced Instruments) were assessed from a venous blood sample collected at screening and during both experimental visits.
Salt-Resistant Status Classification
A total of 34 subjects completed the protocol, and 29 were determined to be salt resistant and included in the present study. Salt resistance was defined as a ≤5 mmHg change in 24-h mean arterial pressure (MAP) when going from the LS to HS diet (37), an assessment that is reproducible within subjects (43). The classification of salt resistance was determined on an individual basis after completion of the full dietary sodium perturbation as has been previously reported (15, 20). Subjects classified as salt sensitive (i.e., a >5 mmHg change in 24-h MAP) were not included in the analysis because they could not be used to test the a priori study hypothesis regarding the role of oxidative stress in the BP-independent effects of dietary sodium loading.
Study Procedures
Subjects reported to the laboratory on the last day of both the LS and HS diets for assessment of endothelial cell protein expression and cutaneous microvascular function.
Assessment of cutaneous microvascular function.
With the use of a sterile technique, subjects were instrumented with four microdialysis fibers (10-mm, 30-kDa cut-off membrane, MD 2000; Bioanalytical Systems) in the ventral side of the left forearm for the local delivery of pharmacological agents as previously described (13, 14, 16, 20). Briefly, the forearm was temporarily anaesthetized with ice for 10 min before fiber insertion (21). Next, a 25-gauge needle was inserted into the skin with entry and exit points ~2.5 cm apart, and microdialysis fibers were threaded through the lumen of the needle, which was removed upon placement of the semipermeable membrane of the fiber in the skin. All fibers were then perfused with lactated Ringer’s solution for 60–90 min to allow for local hyperemia attributable to needle insertion to subside.
Skin blood flow was measured as cutaneous red blood cell (RBC) flux from 1.5 mm2 of skin with a multifiber laser Doppler probe placed in a local heater (MoorLAB, Temperature Monitor SH02; Moor Instruments). Each local heater was placed directly on the skin above each microdialysis site. BP was measured on the contralateral arm every 20 min during the protocol.
Local heating protocol.
A standard, nonpainful local heating protocol was employed to induce NO-dependent vasodilation (32). Once the local hyperemia subsided, the microdialysis sites were randomly assigned to receive either Ringer’s solution (control site) or 20 mM ascorbic acid [AA, consistent with our previous work (20)] in all 29 subjects. In 22 subjects, two additional sites were assigned 10 µM Tempol (to scavenge superoxide; Calbiochem) or 100 µM apocynin (NOX inhibition; Sigma Aldrich) (Fig. 1). All pharmacological solutions were mixed immediately before usage and filtered using syringe microfilters (Whatman Puradisc 13-mm Syringe Filters). All solutions were perfused at a rate of 2 μl/min. Concentrations of 20 mM AA were chosen based on previous studies from our laboratory (14, 20). Apocynin (100 µM) and 10 µM Tempol were chosen based on previous work in patients with kidney disease (16) and healthy adults (30).
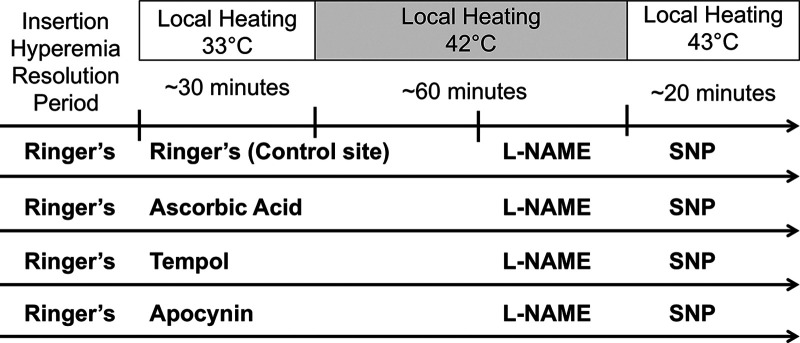
Fig. 1.
Schematic representation of local heating protocol. Lactated Ringer’s solution was infused through all fibers for ~60 min to let the hyperemia subside. Sites were then randomly assigned to receive either lactated Ringer’s solution or 20 mM ascorbic acid. In 22 subjects, 2 additional sites were randomized to receive 100 µM apocynin or 10 µM Tempol. Baseline measurements were made at 33°C. After ~30 min, the temperature of the local heaters was increased to 42°C. After a postheating plateau was achieved in all sites, 10 mM NG-nitro-l-arginine methyl ester (l-NAME) was infused through sites. After a post-l-NAME plateau was achieved in all sites, 28 mM sodium nitroprusside (SNP) was infused through all fibers, and the temperature was increased to 43°C to achieve maximal dilation.
Local heaters were set to 33°C for baseline measurements (Fig. 1). Following at least a 30-min baseline period, local temperature was increased from 33°C to 42°C at a rate of 1°C/s and remained at 42°C for the duration of the local heating protocol. Local heating of the skin results in an initial peak occurring within the first 10 min of heating, which is primarily mediated by an axon reflex with a small NO contribution (32). This was followed by a secondary plateau that has been shown to be primarily NO mediated (25, 32). After RBC flux reached a stable plateau (~40–45 min), 10 mM NG-nitro-l-arginine methyl ester (l-NAME; Sigma-Aldrich) was perfused at 4 μl/min through all microdialysis fibers to quantify the NO contribution to vasodilation at all four sites. Following a new l-NAME plateau in RBC flux, 28 mM sodium nitroprusside (SNP; Nitropress; Hospira) was perfused at 4 μl/min through all sites, and local heaters were increased to 43°C to induce maximal cutaneous vasodilation (32). Doses of 10 mM l-NAME and 28 mM SNP were chosen, as these dosages have been shown to sufficiently inhibit NO synthase and elicit maximal vasodilation in the skin, respectively (32).
Endothelial cell protein expression.
NT expression was evaluated in endothelial cells collected from a subset of participants (n = 14). The procedures used for collection of endothelial cells and assessment of endothelial cell protein expression were adapted from Seals et al. (12). Briefly, an 18-gauge catheter was inserted into the antecubital vein, and a J-wire was advanced ~4 cm beyond the tip of the catheter. The J-wire was then withdrawn, and cells were washed and fixed with 4.0% paraformaldehyde. Cells were then washed twice with PBS, plated on coverslips coated with poly-l-lysine (Neuvitro), and then dried down at 60°C. Coverslips were stored at −80°C until analysis.
After being blocked with normal goat serum, cells were then incubated with monoclonal antibodies for VE Cadherin (1:200; Abcam cat. no. ab7047, RRID:AB_2077943) and NT (1:100; Molecular Probes cat. no. A-21285, RRID:AB_221457), then incubated with Alexa Fluor secondary antibodies (1:500; Abcam anti-mouse IgG, cat. no. ab150117, RRID:AB_2688012 and anti-rabbit IgG, cat. no. ab150083, RRID:AB_2714032), and then mounted on slides with mounting media containing DAPI to confirm nuclear integrity (Vectashield Hard Set; Vector Laboratories).
Images were captured via Zeiss Axio Imager A2 and analyzed using Zen Software to quantify fluorescence intensity. Values are reported as a ratio of endothelial cell protein expression:human umbilical vein endothelial cell (HUVECs) controls to minimize the variability in fluorescence intensity between staining sessions. One technician analyzed all slides and was blinded to the diet during staining and analysis procedures.
Data and Statistical Analyses
RBC flux data were collected at 40 Hz using the PL3516 PowerLab data acquisition system and LabChart software (ADInstruments). Cutaneous vascular conductance (CVC) was calculated as RBC flux divided by MAP. CVC data were normalized to a percentage of maximal CVC (%CVCmax) that was obtained during SNP perfusion. Baseline, plateau, and l-NAME plateau values were obtained over a stable 10-min period. The contribution of NO to the plateau phase was calculated as the difference between the plateau and the l-NAME plateau.
Student’s paired t-tests were used to compare hemodynamic and biochemical measurements between the LS and HS diets, as well as the ratio of endothelial cell NT expression:HUVEC obtained during the LS and HS diets. A two-way repeated-measures ANOVA was used to determine differences during the LS and HS diets on baseline, plateau, and l-NAME plateau, as well as the effect of pharmacological treatments. One-way ANOVA with Bonferroni corrected post hoc comparisons was used when appropriate to determine specific differences within diets. The level of significance was set at P < 0.05, and values are reported as means ± SE.
RESULTS
Subject Characteristics
Baseline subject characteristics are presented in Table 1. All subjects were normotensive with normal liver and kidney function. All subjects completed the standardized run-in diet followed by the 2-wk randomized dietary sodium perturbation.
Table 1.
Baseline characteristics
| Characteristics | Value |
|---|---|
| Demographic data | |
| Subject, n (men/women) | 29 (18/11) |
| Age, yr | 34 ± 2 |
| Height, cm | 174 ± 2 |
| Mass, kg | 74 ± 2 |
| BMI, kg/m2 | 24.1 ± 0.4 |
| Systolic BP, mmHg | 122 ± 2 |
| Diastolic BP, mmHg | 77 ± 1 |
| Heart rate, beats/min | 68 ± 1 |
| Biochemical parameters | |
| Hemoglobin, g/dl | 14.2 ± 0.2 |
| Hematocrit, % | 42.8 ± 0.7 |
| Serum sodium, mmol/l | 139.6 ± 0.3 |
| Serum potassium, mmol/l | 4.5 ± 0.1 |
| Serum chloride, mmol/l | 104.4 ± 0.4 |
| Plasma osmolality, mOsm/kg H2O | 288.6 ± 0.9 |
| Serum creatinine, mg/dl | 0.9 ± 0.0 |
| Blood urea nitrogen, mg/dl | 14.0 ± 0.8 |
| Fasting glucose, mg/dl | 89.6 ± 1.1 |
| Fasting total cholesterol, mg/dl | 177.2 ± 8.2 |
| Fasting HDL, mg/dl | 59.3 ± 3.6 |
| Fasting LDL, mg/dl | 100.2 ± 5.7 |
| Fasting triglycerides, mg/dl | 88.8 ± 7.2 |
Values are means ± SE. BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Dietary Sodium Perturbation
Hemodynamic, biochemical, and renal function measurements during the HS and LS perturbations are presented in Table 2. By design, 24-h MAP did not differ between diets. The HS diet increased 24-h urinary sodium excretion, urine and plasma osmolality, flow rate, and free water clearance. Twenty-four hour urinary potassium excretion and serum potassium levels were unchanged between the LS and HS diets.
Table 2.
Biochemical and renal responses to dietary sodium perturbation
| LS | HS | |
|---|---|---|
| Mass, kg | 71 ± 2 | 73 ± 2* |
| Hemoglobin, g/dl | 13.8 ± 0.3 | 12.9 ± 0.3* |
| Hematocrit, % | 41.8 ± 0.7 | 39.9 ± 0.9* |
| Serum sodium, mmol/l | 138.6 ± 0.4 | 140.2 ± 0.4* |
| Serum potassium, mmol/l | 3.9 ± 0.1 | 4.0 ± 0.08 |
| Serum chloride, mmol/l | 102.0 ± 0.3 | 105.4 ± 0.5* |
| Plasma osmolality, mOsm/kg H2O | 286.4 ± 0.5 | 288.5 ± 0.7* |
| Urine osmolality, mOsm/kg H2O | 348.7 ± 31.7 | 440.5 ± 35.3* |
| Urine flow rate, ml/min | 1.3 ± 0.1 | 1.8 ± 0.1* |
| 24-h Sodium excretion, mmol/24 h | 32.1 ± 4.0 | 257.5 ± 17.3* |
| 24-h Potassium excretion, mmol/24 h | 30.3 ± 2.6 | 42.3 ± 9.0 |
| Free water clearance, ml/min | 0.0 ± 0.1 | −0.5 ± 0.1* |
| 24-h Systolic BP, mmHg | 117 ± 2 | 118 ± 2 |
| 24-h Diastolic BP, mmHg | 72 ± 1 | 70 ± 2* |
| 24-h MAP, mmHg | 87 ± 1 | 86 ± 1 |
| 24-h Pulse pressure, mmHg | 45 ± 1 | 48 ± 1* |
| 24-h Heart rate, beats/min | 70 ± 2 | 65 ± 1* |
Values are means ± SE. BP, blood pressure; HS, high sodium; LS, low sodium; MAP, mean arterial pressure.
P < 0.05 vs. LS.
Microvascular Function
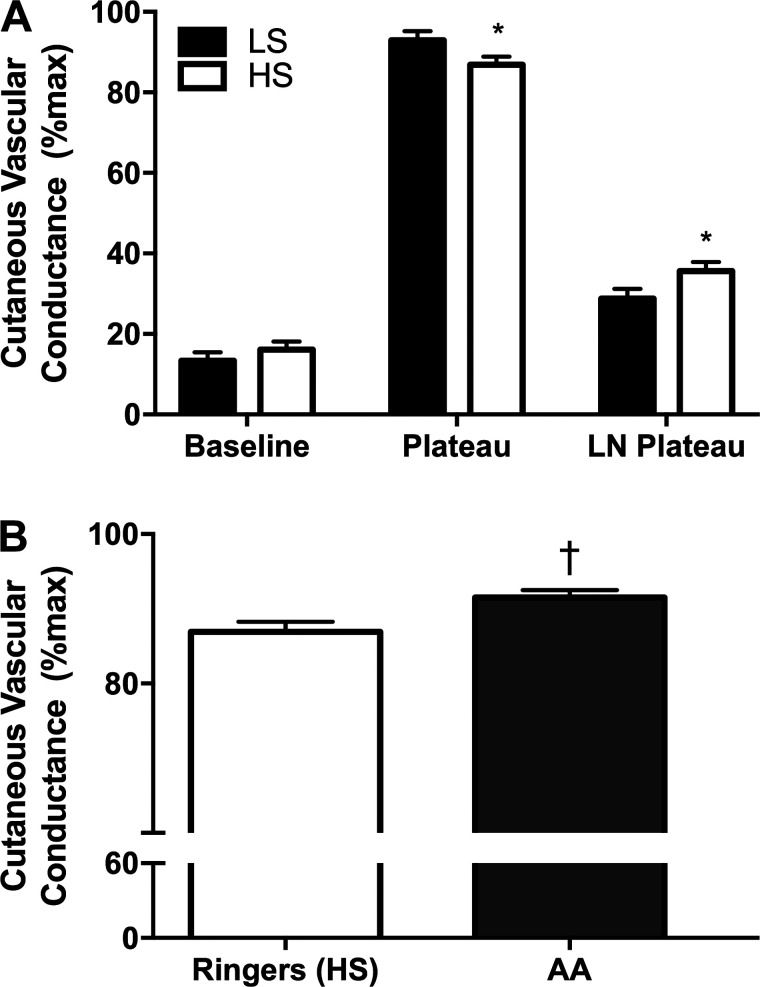
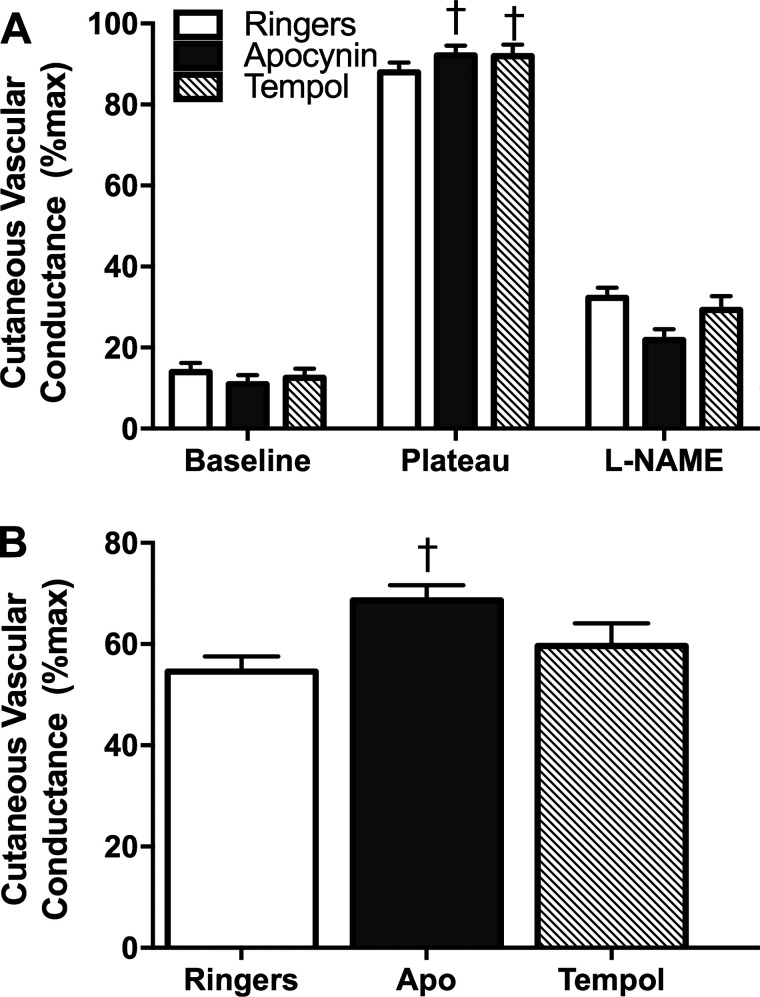
Consistent with our previous work (20), baseline %CVCmax did not differ between HS and LS diets (Fig. 2A) or between sites (Fig. 3). The HS diet attenuated the plateau at the Ringer’s site (Fig. 2A; LS 93.0 ± 2.2 vs. HS 86.8 ± 2.0 %CVCmax; P < 0.05). The l-NAME plateau was significantly lower on the LS diet compared with the HS diet (Fig. 2A). Therefore, the HS diet decreased the NO contribution to the plateau at the Ringer’s site (63.3 ± 3.0 vs. 56.7 ± 2.5 %CVCmax; P < 0.05). During the HS diet, AA improved the plateau %CVCmax compared with the Ringer’s site (Fig. 2B; P < 0.05). As described in methods, we infused Tempol and apocynin in 22 subjects to specifically scavenge superoxide and inhibit NOX, respectively. During the LS diet, there was no effect of apocynin or Tempol on the plateau (Ringer’s: 92.8 ± 1.0, apocynin: 90.4 ± 1.4, Tempol: 92.1 ± 1.9 %CVCmax; P = 0.39, n = 22). The infusion of apocynin and Tempol improved the plateau %CVCmax on the HS diet compared with the Ringer’s site (Fig. 3; P < 0.05, n = 22). Infusion of l-NAME attenuated the plateau similarly in the Ringer’s, apocynin, and Tempol sites (Fig. 3); however, only apocynin was able to improve the NO contribution to the plateau %CVCmax (Fig. 3; P < 0.05, n = 22). The NO contribution was unaltered by Tempol or apocynin during the LS diet (not shown, P = 0.65).
Fig. 2.
A: percentage of maximal cutaneous vascular conductance (%CVCmax) in the Ringer’s site during the low-sodium (LS, solid bars) and high-sodium (HS, open bars) diets during baseline, plateau, and NG-nitro-l-arginine methyl ester (l-NAME) plateau phases. The HS diet attenuated the plateau compared with the LS diet. The l-NAME plateau was greater on the HS diet compared with LS. B: %CVCmax during the plateau phase on the HS diet in the Ringer’s site (open bars) and in the ascorbic acid site (AA, solid bars). The infusion of AA restored the plateau compared with the Ringer’s site during the HS diet. Values are means ± SE, n = 29 (18 M/11 F). Data were analyzed by 2-way ANOVA. *P < 0.05 vs. LS, †P < 0.05 vs. Ringer’s HS.
Fig. 3.
A: percentage of maximal cutaneous vascular conductance (%CVCmax) in the Ringer’s (open bars), apocynin (shaded bars), and Tempol (hashed bars) sites during the high-sodium (HS) diet during baseline, plateau, and post-NG-nitro-l-arginine methyl ester (l-NAME) plateau. During the HS diet, apocynin and Tempol augmented the plateau compared with the Ringer’s site. There was no significant difference between sites for the l-NAME plateau. n = 22 (14 M/8 F). Data were analyzed by 1-way ANOVA with Bonferroni corrected post hoc comparisons. Values are means ± SE, †P < 0.05 vs. Ringer’s HS. B: nitric oxide (NO) contribution to the plateau during the HS diet in the Ringer’s (open bar), apocynin (Apo) (solid bar), and Tempol (hashed bar) sites. Apocynin, but not Tempol, augmented the NO contribution to the plateau during the HS diet. n = 22 (14 M/8 F). Data were analyzed by 1-way ANOVA with Bonferroni corrected post hoc comparisons. Values are means ± SE, †P < 0.05 vs. Ringer’s HS.
Endothelial Cell NT Expression
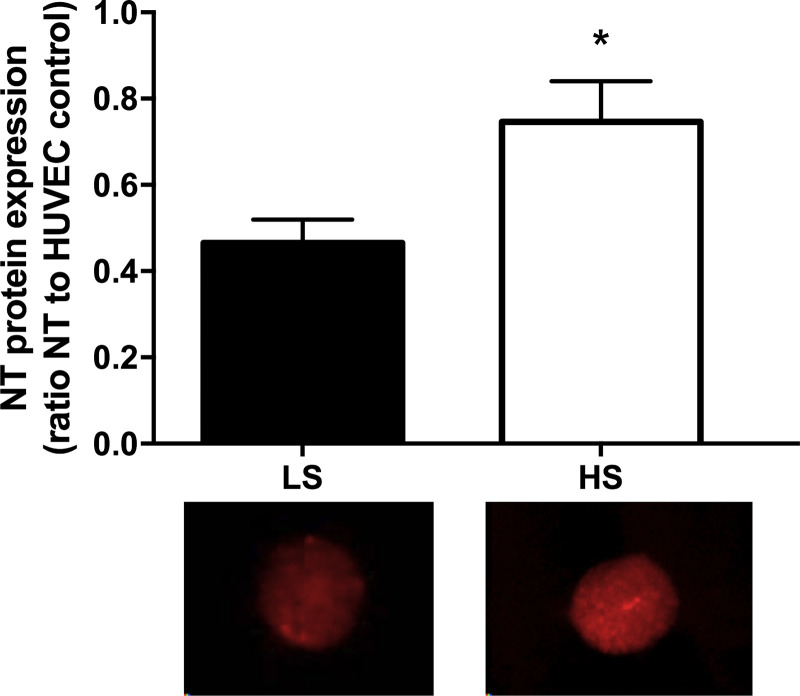
NT expression was significantly increased after 7 days of HS diet compared with 7 days of LS diet (Fig. 4; 0.47 ± 0.05 vs. 0.75 ± 0.09; P < 0.01, n = 14).
Fig. 4.
Nitrotyrosine (NT) protein expression on the low-sodium (LS, solid bars) and high-sodium (HS, open bars) diets. Data are expressed as the ratio of NT:human umbilical vein endothelial cell (HUVEC) control. 7 days of an HS diet significantly increased NT protein expression compared with 7 days of an LS diet. n = 14 (8 M/6 F). Data were analyzed by paired t-test. Values are means ± SE; *P < 0.05 vs. LS.
DISCUSSION
There are two novel findings of this study. First, we demonstrate that local infusion of Tempol and apocynin improved the plateau phase of local heating-induced cutaneous vasodilation, suggesting that superoxide contributes to the dietary sodium-induced impairments in microvascular function and that NOX is the likely source of this superoxide, respectively. Second, we provide direct evidence of oxidative stress in venous endothelial cells from normotensive, salt-resistant adults on a high-sodium diet. Additionally, our results confirm our previous findings that NO-mediated cutaneous vasodilation in response to local heating is impaired in normotensive, salt-resistant adults on a high-sodium diet and that local infusion of AA normalizes this response (20). Together, these findings suggest that high dietary sodium impairs microvascular function via increased production of ROS and further that NOX is a source of the sodium-induced increase in ROS. The deleterious effects of a high-sodium diet on microvascular function observed in the present study are independent of BP, as our study participants were salt resistant.
Endothelial dysfunction as a result of high sodium intake may lead to increased risk of CVD, and this may occur in the absence of any effect on BP. A number of studies utilizing rodent models have demonstrated that increased dietary sodium consumption impairs endothelial function independent of BP (4, 34, 46). We have previously demonstrated that a high-salt diet impairs both micro- and macrovascular endothelial function in normotensive salt-resistant adults (15, 20). Additionally, dietary sodium restriction has been shown to improve endothelial function in middle-aged and older adults with elevated systolic BP, and these findings were also independent of change in systolic BP (23). Our present findings are consistent with a BP-independent effect of high dietary sodium on endothelial function.
Oxidative stress has been shown to be a key mediator of sodium-induced declines in endothelial function in rodent models. Endothelium-dependent dilation (EDD) in response to acetylcholine was found to be attenuated in Sprague-Dawley rats after 4–5 wk of HS diet, but, when EDD was assessed in the presence of ROS scavengers, dilation was restored to a level that was not different from rats that consumed an LS diet (27). This suggests that the sodium-induced impairments in endothelial function are at least partially due to oxidative stress. Our laboratory has previously demonstrated similar findings in humans by showing that microvascular function is impaired in normotensive, salt-resistant adults on an HS diet, and local infusion of AA, a nonspecific ROS scavenger, restores this function (20). The results of the present study confirm those findings and also provide direct evidence of endothelial oxidative stress, as demonstrated by the increase in endothelial NT levels observed on the high-sodium diet.
Although AA can be used to identify oxidative stress as an underlying mechanism, it cannot detect the specific source of ROS generation. Superoxide has been shown to be a major ROS species increased in rodents during a high-sodium diet, and there is evidence that NOX is the source (27, 46). Superoxide limits NO availability via its reaction with NO to form peroxynitrite (ONOO−), another potent oxidant that can further prevent NO production by oxidizing tetrahydrobiopterin, an essential cofactor for NO production. Oxidation of tetrahydrobiopterin leads to the uncoupling of endothelial NO synthase, which can become an additional source of ROS generation.
In high-salt-fed male Sprague-Dawley rats, basal levels of superoxide generation in mesenteric arteries were increased, and both methacholine-induced NO release and relaxation were decreased compared with LS animals (46). However, when vessels were incubated with either the superoxide dismutase mimetic Tempol or the NOX inhibitor apocynin, a significant reduction in superoxide levels in vessels from the HS animals was observed, as well as increased methacholine-induced NO release and relaxation (46). In the present study, we translated these findings from rodents to humans by locally infusing Tempol to scavenge superoxide and apocynin to inhibit NOX while assessing cutaneous microvascular function. Although the plateau phase of the skin blood flow response to local heating was reduced in the control site in salt-resistant adults on the HS diet, the local infusion of either Tempol or apocynin restored this response. Additionally, the local infusion of apocynin increased the NO contribution to this response, suggesting that NOX is the likely source of increased superoxide in salt-resistant adults on an HS diet and may be at least partially responsible for the microvascular impairments observed during high dietary sodium consumption. It is not clear why Tempol did not result in an increase in the NO contribution to dilation similar to apocynin. The variability in the Tempol response was greater; thus we may have been underpowered. Alternatively, in addition to inhibiting NOX production of superoxide, apocynin may have altered the NO contribution in other ways.
NOX has been shown to be a source of ROS in hypertension (36), atherosclerosis (39), and coronary artery disease (40). Apocynin inhibits NOX assembly by preventing the association of p47phox with the membrane-bound heterodimer; it does not indicate which NOX isoform(s) is upregulated, contributing to increases in ROS. Thus a limitation of using apocynin is that it does not provide information on isoform specificity (8). However, it is likely that increased NOX2 activity is a source of superoxide because NOX2-derived superoxide contributes to endothelial injury and reductions in NO signaling that are characteristic of vascular disease (5, 31). Of note, apocynin is also a scavenger of hydrogen peroxide (40, 41), and its inhibitory effect on NADPH appears to require activation by myeloperoxidase (40). The NOX4 isoform has been shown to produce hydrogen peroxide instead of superoxide (11). Thus hydrogen peroxide may be increased and contribute to reductions in NO bioavailability during a high-sodium diet; however, there is mounting evidence for the role of hydrogen peroxide as a vasodilator in the vasculature (24).
We recognize several limitations of the present study. The 42°C heating protocol used in this study is less dependent on NO for vasodilation and more susceptible to a ceiling effect than the more moderate 39°C protocol proposed by Choi et al. (7). As such, it is possible that a lower temperature may have revealed a greater effect of the antioxidant treatments on NO-mediated cutaneous vasodilation during the HS condition. Additionally, we did not include a washout period between dietary conditions, and thus a carryover effect of HS-induced ROS may have occurred in those who consumed the HS diet before the LS diet. Despite this potential shortcoming of the design, control site %CVCmax was greater, and endothelial cell NT expression was lower during the LS condition, suggesting that any residual effect is minimal. The present study included six subjects aged 45–59 yr. Age-related impairments in cutaneous dilation have been observed as early as middle age (6), thus potentially confounding our results. Notably, the crossover design of the present study minimizes any potential age effects and allows us to attribute our findings to the level of dietary sodium consumption. Lastly, although this study suggests that HS diets result in increased ROS produced via NOX, we are unable to determine whether this is due to reduced endogenous antioxidant activity secondary to HS-induced reductions in angiotensin II (17, 35) or via increased activation of NOX through a non-angiotensin II mechanism, such as increased endothelin-1 (28).
In summary, the present study demonstrates that dietary salt loading impairs microvascular function via oxidative stress. Apocynin and Tempol improved cutaneous microvascular function on a high-salt diet, providing evidence that NADPH-derived ROS may impair microvascular function as a result of high salt. The effects of high salt are independent of BP, as we studied normotensive, salt-resistant individuals, providing evidence that lower salt intake has beneficial BP-independent effects. Despite multiple organizations recommending ≤2,300 mg/day of dietary sodium, Americans’ dietary sodium intake remains high. Our results provide additional rationale for limiting sodium intake and/or strategies to limit the effects of dietary sodium.
GRANTS
This work was supported by National Institutes of Health Grant HL-104106.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.B.F. and D.G.E. conceived and designed research; M.G.R., M.S.B., and E.L.M. performed experiments; M.G.R. analyzed data; M.G.R., J.C.P., S.L.L., W.B.F., and D.G.E. interpreted results of experiments; M.G.R. prepared figures; M.G.R. and D.G.E. drafted manuscript; M.G.R., M.S.B., E.L.M., J.C.P., D.R.S., S.L.L., W.B.F., and D.G.E. edited and revised manuscript; M.G.R., M.S.B., E.L.M., J.C.P., D.R.S., S.L.L., W.B.F., and D.G.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank research volunteers who participated in these studies.
REFERENCES
- 1.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N, Simons-Morton D, McCullough M, Swain J, Steele P, Evans MA, Miller ER, Harsha DW; DASH Collaborative Research Group . A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 336: 1117–1124, 1997. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 2.Black MA, Green DJ, Cable NT. Exercise prevents age-related decline in nitric-oxide-mediated vasodilator function in cutaneous microvessels. J Physiol 586: 3511–3524, 2008. doi: 10.1113/jphysiol.2008.153742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boegehold MA. The effect of high salt intake on endothelial function: reduced vascular nitric oxide in the absence of hypertension. J Vasc Res 50: 458–467, 2013. doi: 10.1159/000355270. [DOI] [PubMed] [Google Scholar]
- 4.Boegehold MA. Effect of dietary salt on arteriolar nitric oxide in striated muscle of normotensive rats. Am J Physiol Heart Circ Physiol 264: H1810–H1816, 1993. doi: 10.1152/ajpheart.1993.264.6.H1810. [DOI] [PubMed] [Google Scholar]
- 5.Brandes RP, Schröder K. Composition and functions of vascular nicotinamide adenine dinucleotide phosphate oxidases. Trends Cardiovasc Med 18: 15–19, 2008. doi: 10.1016/j.tcm.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Bruning RS, Santhanam L, Stanhewicz AE, Smith CJ, Berkowitz DE, Kenney WL, Holowatz LA. Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. J Appl Physiol (1985) 112: 2019–2026, 2012. doi: 10.1152/japplphysiol.01354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi PJ, Brunt VE, Fujii N, Minson CT. New approach to measure cutaneous microvascular function: an improved test of NO-mediated vasodilation by thermal hyperemia. J Appl Physiol (1985) 117: 277–283, 2014. doi: 10.1152/japplphysiol.01397.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cifuentes-Pagano E, Meijles DN, Pagano PJ. The quest for selective NOX inhibitors and therapeutics: challenges, triumphs and pitfalls. Antioxid Redox Signal 20: 2741–2754, 2014. doi: 10.1089/ars.2013.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson KM, Clifton PM, Keogh JB. A reduction of 3 g/day from a usual 9 g/day salt diet improves endothelial function and decreases endothelin-1 in a randomised cross-over study in normotensive overweight and obese subjects. Atherosclerosis 233: 32–38, 2014. doi: 10.1016/j.atherosclerosis.2013.11.078. [DOI] [PubMed] [Google Scholar]
- 10.Dickinson KM, Keogh JB, Clifton PM. Effects of a low-salt diet on flow-mediated dilatation in humans. Am J Clin Nutr 89: 485–490, 2009. doi: 10.3945/ajcn.2008.26856. [DOI] [PubMed] [Google Scholar]
- 11.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 45: 1340–1351, 2008. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 7: 805–812, 2008. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DuPont JJ, Farquhar WB, Edwards DG. Intradermal microdialysis of hypertonic saline attenuates cutaneous vasodilatation in response to local heating. Exp Physiol 96: 674–680, 2011. doi: 10.1113/expphysiol.2011.058404. [DOI] [PubMed] [Google Scholar]
- 14.DuPont JJ, Farquhar WB, Townsend RR, Edwards DG. Ascorbic acid or L-arginine improves cutaneous microvascular function in chronic kidney disease. J Appl Physiol (1985) 111: 1561–1567, 2011. doi: 10.1152/japplphysiol.00419.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DuPont JJ, Greaney JL, Wenner MM, Lennon-Edwards SL, Sanders PW, Farquhar WB, Edwards DG. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens 31: 530–536, 2013. doi: 10.1097/HJH.0b013e32835c6ca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DuPont JJ, Ramick MG, Farquhar WB, Townsend RR, Edwards DG. NADPH oxidase-derived reactive oxygen species contribute to impaired cutaneous microvascular function in chronic kidney disease. Am J Physiol Renal Physiol 306: F1499–F1506, 2014. doi: 10.1152/ajprenal.00058.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durand MJ, Lombard JH. Low-dose angiotensin II infusion restores vascular function in cerebral arteries of high salt-fed rats by increasing copper/zinc superoxide dismutase expression. Am J Hypertens 26: 739–747, 2013. doi: 10.1093/ajh/hpt015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards DG, Farquhar WB. Vascular effects of dietary salt. Curr Opin Nephrol Hypertens 24: 8–13, 2015. doi: 10.1097/MNH.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frankenfield D, Roth-Yousey L, Compher C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: a systematic review. J Am Diet Assoc 105: 775–789, 2005. doi: 10.1016/j.jada.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Greaney JL, DuPont JJ, Lennon-Edwards SL, Sanders PW, Edwards DG, Farquhar WB. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol 590: 5519–5528, 2012. doi: 10.1113/jphysiol.2012.236992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodges GJ, Chiu C, Kosiba WA, Zhao K, Johnson JM. The effect of microdialysis needle trauma on cutaneous vascular responses in humans. J Appl Physiol (1985) 10: 1112–1118, 2009. doi: 10.1152/japplphysiol.91508.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jablonski KL, Gates PE, Pierce GL, Seals DR. Low dietary sodium intake is associated with enhanced vascular endothelial function in middle-aged and older adults with elevated systolic blood pressure. Ther Adv Cardiovasc Dis 3: 347–356, 2009. doi: 10.1177/1753944709345790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol 61: 335–343, 2013. doi: 10.1016/j.jacc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadlec AO, Gutterman DD. The Yin and Yang of endothelium-derived vasodilator factors. Am J Physiol Heart Circ Physiol 314: H892–H894, 2018. doi: 10.1152/ajpheart.00019.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellogg DL Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol (1985) 86: 1185–1190, 1999. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- 26.Lenda DM, Boegehold MA. Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation. Am J Physiol Heart Circ Physiol 282: H395–H402, 2002. doi: 10.1152/ajpheart.0354.2001. [DOI] [PubMed] [Google Scholar]
- 27.Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am J Physiol Heart Circ Physiol 279: H7–H14, 2000. doi: 10.1152/ajpheart.2000.279.1.H7. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Fink GD, Watts SW, Northcott CA, Galligan JJ, Pagano PJ, Chen AF. Endothelin-1 increases vascular superoxide via endothelin(A)-NADPH oxidase pathway in low-renin hypertension. Circulation 107: 1053–1058, 2003. doi: 10.1161/01.CIR.0000051459.74466.46. [DOI] [PubMed] [Google Scholar]
- 29.Lüscher TF, Tanner FC, Tschudi MR, Noll G. Endothelial dysfunction in coronary artery disease. Annu Rev Med 44: 395–418, 1993. doi: 10.1146/annurev.me.44.020193.002143. [DOI] [PubMed] [Google Scholar]
- 30.Medow MS, Bamji N, Clarke D, Ocon AJ, Stewart JM. Reactive oxygen species (ROS) from NADPH and xanthine oxidase modulate the cutaneous local heating response in healthy humans. J Appl Physiol (1985) 111: 20–26, 2011. doi: 10.1152/japplphysiol.01448.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller AA, Drummond GR, Sobey CG. Novel isoforms of NADPH-oxidase in cerebral vascular control. Pharmacol Ther 111: 928–948, 2006. doi: 10.1016/j.pharmthera.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol (1985) 91: 1619–1626, 2001. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 33.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol (1985) 93: 1644–1649, 2002. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- 34.Nurkiewicz TR, Boegehold MA. High salt intake reduces endothelium-dependent dilation of mouse arterioles via superoxide anion generated from nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 292: R1550–R1556, 2007. doi: 10.1152/ajpregu.00703.2006. [DOI] [PubMed] [Google Scholar]
- 35.Priestley JR, Kautenburg KE, Casati MC, Endres BT, Geurts AM, Lombard JH. The NRF2 knockout rat: a new animal model to study endothelial dysfunction, oxidant stress, and microvascular rarefaction. Am J Physiol Heart Circ Physiol 310: H478–H487, 2016. doi: 10.1152/ajpheart.00586.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidlin O, Sebastian AF, Morris RC Jr. What initiates the pressor effect of salt in salt-sensitive humans? Observations in normotensive blacks. Hypertension 49: 1032–1039, 2007. doi: 10.1161/HYPERTENSIONAHA.106.084640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulman IH, Aranda P, Raij L, Veronesi M, Aranda FJ, Martin R. Surgical menopause increases salt sensitivity of blood pressure. Hypertension 47: 1168–1174, 2006. doi: 10.1161/01.HYP.0000218857.67880.75. [DOI] [PubMed] [Google Scholar]
- 39.Sorescu D, Weiss D, Lassègue B, Clempus RE, Szöcs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of NOX family proteins in human atherosclerosis. Circulation 105: 1429–1435, 2002. doi: 10.1161/01.CIR.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 40.Spiekermann S, Landmesser U, Dikalov S, Bredt M, Gamez G, Tatge H, Reepschläger N, Hornig B, Drexler H, Harrison DG. Electron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation 107: 1383–1389, 2003. doi: 10.1161/01.CIR.0000056762.69302.46. [DOI] [PubMed] [Google Scholar]
- 41.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 101: 948–954, 2000. doi: 10.1161/01.CIR.101.9.948. [DOI] [PubMed] [Google Scholar]
- 42.Tuomilehto J, Jousilahti P, Rastenyte D, Moltchanov V, Tanskanen A, Pietinen P, Nissinen A. Urinary sodium excretion and cardiovascular mortality in Finland: a prospective study. Lancet 357: 848–851, 2001. doi: 10.1016/S0140-6736(00)04199-4. [DOI] [PubMed] [Google Scholar]
- 43.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension 27, 3 Pt 2: 481–490, 1996. doi: 10.1161/01.HYP.27.3.481. [DOI] [PubMed] [Google Scholar]
- 44.Widlansky ME, Gokce N, Keaney JF Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 42: 1149–1160, 2003. doi: 10.1016/S0735-1097(03)00994-X. [DOI] [PubMed] [Google Scholar]
- 45.Writing Group Members; Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121: e46–e215, 2010. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 46.Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. J Vasc Res 44: 382–390, 2007. doi: 10.1159/000102955. [DOI] [PubMed] [Google Scholar]