Abstract
Myocardial inflammation can lead to lethal acute or chronic heart failure (HF). T lymphocytes (T cells), have been reported in the inflamed heart in different etiologies of HF, and more recent studies support that different T-cell subsets play distinct roles in the heart depending on the inflammation-triggering event. T cells follow sequential steps to extravasate into tissues, but their specific recruitment to the heart is determined by several factors. These include differences in T-cell responsiveness to specific chemokines in the heart environment, as well as differences in the expression of adhesion molecules in response to distinct stimuli, which regulate T-cell recruitment to the heart and have consequences in cardiac remodeling and function. This review focuses on recent advances in our understanding of the role T cells play in the heart, including its critical role for host defense to virus and myocardial healing postischemia, and its pathogenic role in chronic ischemic and nonischemic HF. We discuss a variety of mechanisms that contribute to the inflammatory damage to the heart, as well as regulatory mechanisms that limit the magnitude of T-cell-mediated inflammation. We also highlight areas in which further research is needed to understand the role T cells play in the heart and distinguish the findings reported in experimental animal models and how they may translate to clinical observations in the human heart.
Keywords: heart failure, T cells
INTRODUCTION
T cells represent the major component of the adaptive immune response. It has long been established that T cells respond to myocardial antigens in specific inflammatory states: infection of heart cells; conditions such as autoimmune myocarditis, in which self-tolerance to cardiac antigens becomes disturbed; and cardiac allograft rejection as a response to foreign antigens (48). In these cases, the T-cell immune response results in acute and chronic inflammatory processes that impair cardiac function by direct cytotoxicity or by enhancing the inflammatory functions of other cells. This results in permanent damage to the cardiac tissue that is replaced by fibrosis and often leads to dilated cardiomyopathy with congestive heart failure (HF). Thus, under the above primary inflammatory conditions, T cells can drive the pathogenesis of HF.
It has become evident in the past decade, however, that T-cell immune responses also play central roles in the much more prevalent pathophysiological stresses that contribute to human HF, such as cardiac ischemia, hypertension, and pressure overload. T-cell infiltration into the heart has now been reported in patients with both ischemic and nonischemic HF (1, 61), in the absence of the overt primary inflammatory conditions described above. Furthermore, systemic regulation of T-cell subsets differs between these patients and healthy controls. Several experimental animal models have reported specific mechanisms involved in T-cell regulation of immune cell tolerance to cardiac antigens, in T-cell activation and cytokine production, and in T-cell recruitment to the heart in response to ischemic or nonischemic sterile inflammation. These findings identify central roles for adaptive immunity, and T cells in particular, in modulating cardiac remodeling in response to the most common pathophysiologic stimuli, thus highlighting the need to better understand these processes.
Here, we review the current knowledge on T-cell development and thymic selection, a process critical to preserve cardiac health by inducing tolerance to cardiac antigens. We also review the mechanisms of T-cell activation and peripheral tolerance in secondary lymphoid organs in response to different stimuli in the heart and how this response results in T-cell recruitment to the heart. Each of these tightly regulated steps protects the heart from T-cell recruitment during homeostasis, as well as from the initiation and perpetuation of cardiac inflammation and resultant HF. We detail the emerging concept in which diverse pathologic stimuli may promote stimulus-specific presentation of as yet unknown antigens, thus inducing differential T-cell responses and cytokine production patterns and effects on resident cardiac cells. Ultimately, however, these processes lead to a shared final end point of adverse cardiac remodeling and HF, with features of cardiac fibrosis, cardiac hypertrophy, cardiac inflammation, and both systolic and diastolic dysfunction. While reviewing the literature on T-cell immune responses in the heart using experimental models and human heart and T-cell samples, we also discuss similarities and differences reported in heart inflammation induced by different T-cell triggers and how this understanding could inform strategies for immunomodulation in HF.
T CELLS: DEVELOPMENT, ACTIVATION, AND RECIRCULATION IN HOMEOSTASIS AND HEART INFLAMMATION
T-Cell Development, Thymic Selection, and Peripheral Tolerance in Secondary Lymphoid Organs
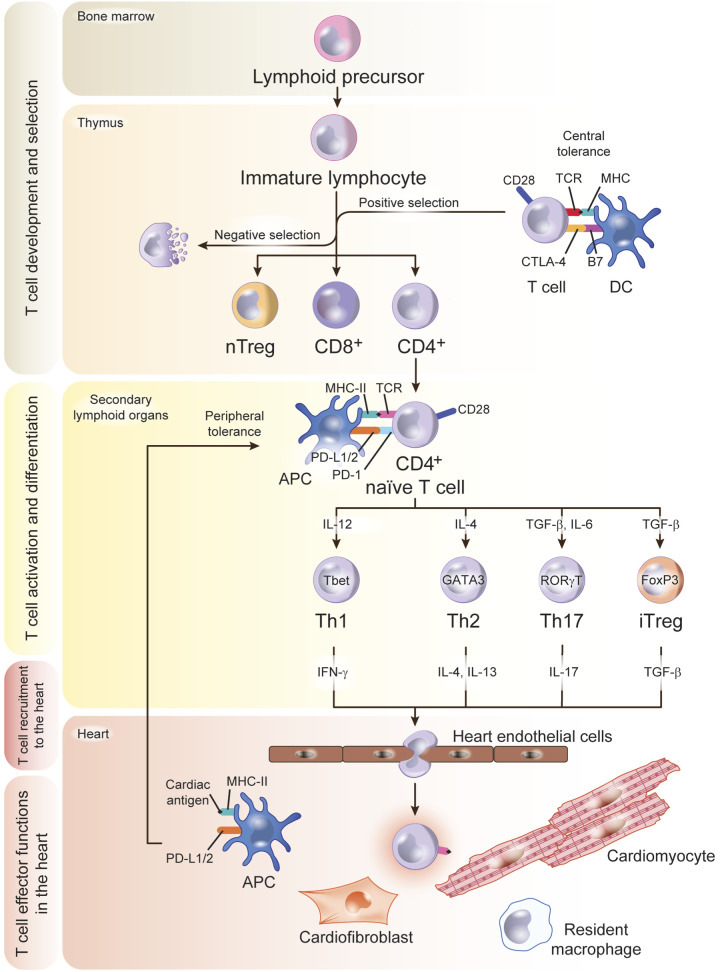
Lymphoid progenitors arise from the bone marrow and populate the thymus, where immature T cells proliferate and create a wide repertoire of T-cell receptors (TCRs). A selection process follows to eliminate self-reactive T cells and to retain nonself-reactive T cells, which mature and migrate to secondary lymphoid organs (the spleen and the lymph nodes), where they respond to specific antigens presented by antigen-presenting cells. The TCRs on CD8+ T cells and CD4+ T cells recognize antigens presented by major histocompatibility complex (MHC) class I and class II molecules, respectively, on the surface of dendritic cells (DCs). These interactions in the thymic environment, as well as in the periphery, once T cells have circulated to secondary lymph node organs, are critical to maintain T-cell tolerance to self-antigens, and to induce an effective immune response to unknown antigens for which T cells have not negatively been selected against during development. In addition to TCR recognition of antigens presented by MHC molecules, T cells require additional signals during antigen presentation. These signals are induced by CD8 and CD4 engagement of MHC-I and MHC-II, respectively, which facilitate signaling during TCR recognition of antigens. Additionally, costimulatory as well as coinhibitory signals are part of T-cell antigen recognition to either activate or shut down the T-cell immune response. The T-cell coreceptor CD28 interacts with B7 ligands in DCs to activate naïve cells and promote optimal T-cell stimulation together with the TCR. T cells also express coinhibitory molecules such as program death 1 (PD-1) and cytotoxic T-lymphocyte antigen- 4 (CTLA-4), which bind to their ligands PD-L1 and PD-L2, and B7.1 and B7.2, respectively, as a mechanism to terminate T-cell responses (11, 90). While CTLA-4 functions exclusively during the initial phase of T-cell activation in the thymus, PD-1 functions predominantly within peripheral tissues during effector T-cell activation. These immune checkpoints serve as negative regulators of T-cell immune function and are critical to induce central and peripheral T-cell tolerance to avoid undesired T-cell activation by self-antigens that escape the thymic selection and lead to autoimmunity, which, in the heart, can induce potentially lethal dilated cardiomyopathy and HF (48, 67) (Fig. 1).
Fig. 1.
T-cell development, activation, and trafficking to the heart. Lymphoid precursors originate in the bone marrow and traffic to the thymus, where they develop into CD4+ or CD8+ T cells and undergo a complex selection process to discard autoreactive and dysfunctional immature T cells. This crucial step in antigen recognition “learning” is the base of the concept of central tolerance, regulated by signals that involve major histocompatibility complex (MHC) and B7 in the antigen-presenting cell (APC), T-cell receptor (TCR), CD28, and CTLA-4 in the T cell. Positively selected T-cell clones traffic to the secondary lymphoid organs as naïve T cells. If heart inflammation is triggered, dendritic cells (DCs) process cardiac antigens and traffic to secondary lymphoid organs for antigen presentation to naïve T cells that differentiate into effector cell subsets upon antigen recognition. T-cell PD-1 and CD28 and DC PD-L1/2 and B7 signals are an additional mechanism of tolerance in the periphery to antigens that escaped negative selection in the thymus. Effector T cells acquire a migratory profile during differentiation and will be recruited to the heart in response to signals in the heart local environment. TGF-β, transforming growth factor-β; Treg, T regulatory cell.
T-Cell Activation, Differentiation, and Expansion at the Secondary Lymphoid Organs
CD8+ cytotoxic T cells respond rapidly to antigens by massive population expansion and differentiation into cytotoxic effector cells that effectively migrate to clear infection, and thus their cytotoxicity in the heart can be lethal. CD4+ helper T cells, on the other hand, cooperate with innate cells and CD8+ T cells and augment their immune functions. Upon recognition of antigens in secondary lymphoid organs, naïve T cells differentiate into several subsets of T-helper cells characterized by expression of distinct signature transcription factors, production of different cytokines, and different effector functions. Th type 1 (Th1) cells express the transcription factor T-bet, produce IFN-γ, and activate macrophages and other cells. Th type 17 (Th17) cells express the transcription factor RORγT; produce IL-17, IL-21, and IL-22; and promote neutrophil functions. T-helper 2 (Th2) cells express GATA-3; produce IL-4, IL-5, and IL-13; and promote antibody production in B lymphocytes. A subset of CD4+ T cells, T regulatory cells (Tregs), express the transcription factor FoxP3, produce IL-10 and transforming growth factor-β (TGF-β), and suppress the effector functions of Th cells (43). The transcription factors that characterize the T-cell subset lineages are master regulators that cross inhibit each other through protein-protein interactions. For instance, T-bet inhibits GATA-3 expression and function and also represses RORγT, thus preventing Th2 and Th17 differentiation (32, 45, 106). In addition to this, the cytokines produced by each T-cell lineage stabilize the signature transcription factor, thus working on a feedback signaling loop that further promotes specific T-cell subset proliferation and activation. Examples of this are IL-2- and TGF-β-stabilizing Foxp3 and the Treg lineage (43). Because of these well-controlled programs, T-cell differentiation is mutually exclusive. However, functional heterogeneity of T-cell subsets is observed in the setting of tissue inflammation, and this includes the heart, as discussed later in this review with cell plasticity happening in, for instance, the ischemic environment. These evolutionary mechanisms in place to protect hosts from several infectious agents and undesired immune responses to self turn out to be detrimental to the heart. This review touches on T-cell immune responses and recruitment to the heart and its consequences in heart function (Fig. 1).
Trafficking of Effector T Cells to the Heart
Effective T-cell immune surveillance and immune responses in the heart rely on continuous trafficking of T cells through blood, lymphoid organs, and the heart and involve interactions of T cells with the myocardial endothelium that lead to extravasation (97). Upon cardiac inflammation, endothelial cells (ECs) upregulate adhesion molecules that allow T-cell interactions and extravasation. The initial stage of T-cell trafficking is mostly mediated by endothelial selectins, which are responsible for T-cell rolling on the endothelium, an initial contact that also facilitates T-cell exposure to chemokines displayed on the EC surface, required for T-cell integrin activation. Activated T-cell integrins bind to their endothelial ligands intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), resulting in the firm arrest of T cells on the endothelium, followed by transendothelial migration into the tissue. Priming of naïve T cells in the secondary lymphoid organs and differentiation into specific effector T-cell subsets induce T cells to express unique sets of adhesion molecules and chemokine receptors. For example, the chemokine receptors CXCR3 and CCR5 are preferentially expressed on Th1 cells, whereas CCR4 is expressed on Th2 cells and CCR6 in Th17 cells (80). Thus a specific T-cell immune response induced in the heart may combine the activation of a specific T-cell subset expressing the certain adhesion molecules and chemokine receptors and a unique cardiac local environment with a specific pattern of chemokine ligands and receptors to enable recruitment to the heart. However, it remains challenging to identify unique T-cell heart-trafficking signatures. CXCR3 and CCR4 define T-cell cardiotropism in cardiac rejection (41). More recently, we have learned that hepatocyte growth factor can be produced by the myocardium, transported to the draining lymph nodes passively or bound to DCs, and bind to c-Met, its receptor on T cells. This process induces a heart-trafficking specific signature that guides CD4+CXCR3+CCR4+ T cells to the inflamed heart (41). These data support a direct connection between T-cell activation and specific recruitment to the heart in an experimental model of LPS-induced heart inflammation.
Thus the initial insult that triggers myocardial inflammation will be critical in inducing a specific T-cell response. Whether new activated T-cell clones that escaped thymic selection are expanded or T cells with a TCR repertoire for neoantigens proliferate remains unknown and is an important area of investigation. The mechanisms of T-cell trafficking between the thymus and the secondary lymphoid organs as well as the trafficking of DCs from the heart to the lymph nodes remain also largely unexplored. The heart is irrigated by lymph vessels, implying that such trafficking must occur to maintain heart homeostasis. Indeed, the lymphatic vasculature has been reported to play a protective antifibrotic role post-myocardial infarction (post-MI) (29). However, the unique and potential novel roles of lymphatics in cardiac diseases and repair have only recently begun to be investigated. Given that T-cell activation occurs predominantly in the lymph nodes, cross talk between the thymus, the secondary lymph nodes, the vascular endothelium, and the heart is warranted during homeostasis and heart inflammation. Overall, the T-cell immune response may be regulated at different stages and locations that include T-cell selection in the thymus or in secondary lymphoid organs through central or peripheral tolerance mechanisms, in T-cell activation in secondary lymphoid organs, and/or in specific patterns that guide T-cell recruitment the heart (Fig. 1).
HF, CLINICAL DEFINITION, ASSOCIATION WITH IMMUNE SYSTEM ACTIVATION
While this review focuses on the mechanisms of T-cell recruitment and subsequent effects on different components of the cardiac remodeling process, these mechanistic studies must be considered in the context of clinical HF. Although multiple definitions of HF exist, the American College of Cardiology defines it as “… a complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood” (American College of Cardiology website: https://www.acc.org/education-and-meetings/products-and-resources/guideline-education/heart-failure). Other commonly accepted definitions include the notion that HF represents an inability to maintain an adequate cardiac output at a normal cardiac filling pressure. HF has reached epidemic proportions and currently represents the most common reason for hospitalization in the United States, as well as a major cause of mortality worldwide. Aside from the poor prognosis, HF syndrome also includes significant symptoms including dyspnea (shortness of breath), exercise intolerance, and fluid accumulation resulting in peripheral and central edema. The current paradigm holds that HF occurs generally as a result of cardiac remodeling, the structural and functional abnormalities that occur in response to pathophysiological stress. Experimental studies in animals demonstrate a direct role for T cells in several aspects of cardiac remodeling. However, this is not so obvious in humans, as discussed here. Clinicians typically divide HF into two broad categories: HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF). These two entities share similar symptoms, and each have poor prognoses but differ in terms of the standard medical therapy options. Because clinical studies typically divide these entities, we will discuss these conditions separately as well, in the context of systemic inflammation and T-cell effects.
Immune Activation and T-Cell Phenotypes
HFrEF.
Certain forms of HF such as myocarditis and heart transplant rejection represent conditions in which circulating immune cells and T cells in particular home to the myocardium and acutely reduce left ventricular (LV) function. These conditions often respond, as expected, to acute immunomodulating therapies. The mechanisms of T-cell homing to the LV in these conditions have been studied in a number of models, which we will discuss below. However, the majority of cases of HF do not arise from these primary autoimmune disorders but rather from other more common pathophysiological stresses such as pressure overload (hypertension, valve disease) or ischemic disease.
Over the past several decades, HF arising from these highly prevalent conditions has also been correlated with increased immune system activation. Seminal observations in the late 20th century identified that circulating cytokines, such as TNF-α, increased in patients with HF compared with controls (46). This led to the detection of multiple proinflammatory cytokines as markers of HF (93). Furthermore, a large body of literature has now correlated cytokine concentrations to outcomes and symptom severity in HF. Extensive experimental studies (31) demonstrated that cytokines such as IL-6 and TNF-α or their receptors could promote cardiac remodeling, whereas inhibition of these molecules could oppose pathologic remodeling (42, 53, 93). These and other findings led to the generally accepted concept that HF represents a proinflammatory state. However, the negative results of a number of clinical trials of anticytokine agents (13, 53, 54) in HFrEF has suggested the need for 1) further understanding and refining the mechanisms of cytokine generation and effects in the human cardiovascular system; and 2) defining the roles of specific immune cells in HF.
In addition to the general role of cytokines in HF, other work has identified the adaptive immune response as correlated with HF in certain conditions. For example, the presence of autoantibodies to cell surface receptors in patients with idiopathic cardiomyopathy (84) suggests mechanisms through which the immune response could promote myocardial dysfunction and chronic remodeling. The detection of circulating antibodies against cardiac myocyte contractile and sarcomeric proteins in patients after MI (16, 52), in some cases correlates with HF outcome. Given that T-helper cells direct B-cell production of antibodies, these data further suggest that T cells may play a causative role in HF development even in disorders that do not arise from primary immune conditions.
The well-established correlation of cytokines and autoantibodies with HF therefore suggests that other circulating immune cells, besides T cells, may also become altered in HFrEF patients, although this review focuses on alterations in T cells that accompany HFrEF and its associated risk factors. For example, multiple observational studies also support that an increased neutrophil to lymphocyte ratio in HF patients correlates with adverse outcomes and mortality in HF patients (94). The role of T cells in proremodeling processes such as hypertension and atherosclerosis has been established in experimental models and identified in humans (104). Initial evidence implicating T-cell activation specifically in HFrEF came from studies of patients with dilated cardiomyopathy, who demonstrated increased circulating levels of T-cell-generated cytokines IL-2 and IL-10 compared with asymptomatic relatives. IL-2 concentrations also increased in patients with LV remodeling without overt HF (55). Additionally, peripheral T cells isolated from patients with HF demonstrated increased mRNA for Tnfa, Ifng, Il18, and Il10 as well as increased surface expression of the T-cell activation markers (CD25 and CD69) compared with healthy controls (103), thus providing further evidence in humans that T-cell alterations accompany HFrEF and cardiac remodeling.
The relative proportion of T-cell subsets has also been examined in HFrEF patients and appears to shift toward a general proinflammatory T-cell activation state. For example, in patients specifically with dilated cardiomyopathy, most of whom had evidence of circulating autoantibodies, FACS sorting of circulating T cells identified reduced proportion of Tregs (85). Several other studies have made similar observations but in more general forms of HFrEF, in which Tregs again decreased (47, 65, 85, 86, 88) in HFrEF patients, compared with non-HF patients. Along the same lines, others reported that the relative proportion of Th17 (proinflammatory) T cells increased (47) in HFrEF. These combined data suggest a shift toward a proinflammatory T-cell state thata cannot be suppressed by the reduced Treg levels present in HFrEF. In addition, investigations in HFrEF patients have also detected differential T-cell phenotypes and function. Tregs isolated from HFrEF patients had less suppressive activity when coincubated CD4+ effector T cells (85, 86). Furthermore, Treg from HFrEF patients displayed increased susceptibility to apoptosis (88) providing further mechanistic support for reduction of Treg inhibitory potency as a component of the HFrEF phenotype.
In humans with HFrEF, T-cell measures correlate with quantitative parameters of LV remodeling and dysfunction, in addition to simply associating categorically with the presence or absence of HF. The relative proportion of circulating Treg negatively correlates with BNP (47, 88), nt-proBNP, LV chamber remodeling (65, 86), and C-reactive protein (65), indicating that the reduction of Treg-mediated immunosuppression may promote worsening of these parameters. Reduction in Treg proportion is also associated with reduced LV systolic function and survival (65). On the other hand, increased Th17-cell proportion also correlates with NT-proBNP, further supporting the association of increased T-cell axis activity with myocardial abnormalities. Taken together, these observed relations between human T-cell subsets and markers of myocardial dysfunction and remodeling provide more compelling evidence that T cells may directly affect cardiac structure and function.
Direct T-cell migration into the failing LV in HFrEF patients has been less extensively studied, however. Biopsy specimens from hearts of patients with dilated cardiomyopathy from presumed viral myocarditis unexpectedly demonstrated reduced presence of antigen presenting dendritic cells compared with control healthy specimens (71), directly implicating alterations in T-cell axis cell components within the heart. Mediastinal lymph nodes of HFrEF patients had decreased Tregs (88), further identifying abnormalities of T cells within cardiac-associated tissue. More recently, we identified increased CD3+ T infiltration in LV specimens from end-stage nonischemic cardiomyopathy patients compared with nonfailing controls (61). CD3+ T cells isolated from patients with severe HFrEF demonstrated increased adhesion to activated ECs, supporting that T cells from HF patients are prone to be recruited to the myocardial tissue. These data, combined with the aforementioned associative observations of Treg/effector T-cell imbalance and markers of myocardial dysfunction and remodeling in HFrEF patients, provide further evidence that T-cell recruitment to the heart may directly alter cardiac structure and function as a plausible mechanism contributing to HFrEF.
HFpEF.
Unlike HFrEF, HFpEF has only more recently been identified and accepted as a distinct clinical entity. As such, the potential dysregulation of T cells in this phenotype remain less well explored. However, understanding the functions of T cells in promoting this condition in humans has significant clinical relevance, since currently no effective medical treatments for HFpEF exist. Importantly, most of the main predisposing factors for HFpEF, including diabetes, adiposity, metabolic syndrome, hypertension, and aging represent proinflammatory phenotypes, with T-cell abnormalities having been implicated in the pathogenesis of a number of these risk factors (49, 57). In fact, the current prevailing model holds that chronic systemic inflammation from these conditions drives endothelial dysfunction and subsequent derangements in myocardial intracellular signaling, thus promoting HFpEF pathophysiology. As in HFrEF, the ratio of Th17/Treg cells increases in patients with HFpEF, compared with non-HF patients (47). Compared with hearts from patients with HFrEF or with asymptomatic LV hypertrophy, HFpEF hearts display increased ICAM-1 expression (24). Since ICAM-1 directly recruits T cells into the LV, this finding suggests a mechanism of T-cell recruitment in human HFpEF. Aside from these observations, the majority of studies to date have focused on T cells in HFrEF, rather than in HFpEF. By definition HFpEF encompasses normal LV systolic function, and typically does not display LV chamber dilation. However, other remodeling processes such as LV hypertrophy, fibrosis, and fetal gene reexpression mark the HFpEF phenotype. As such, understanding the mechanisms, described later in this review, through which T-cell infiltration to the LV modulates these phenotypes likely will have relevance to HFpEF in addition to HFrEF pathogenesis.
Additional Systemic Factors: Aging, Immunodeficiency, and Environment
The above studies correlate circulating and myocardial T-cell alterations with HFrEF and HFpEF phenotypes. However, HF represents a systemic disease rather than a myocardial-specific condition and thus arises through complex interactions between environmental factors, concomitant cardiovascular disorders, and additional risk factors. For example, hypertension and atherosclerosis both contribute to the pathogenesis of HF, and alterations in T-cell function and localization have been detected in humans in each of these conditions. The roles of T cells in these conditions have been established experimentally as well. We refer the reader to several in-depth reviews on the role of T cells in blood pressure regulation and pathophysiology of atherosclerosis (92, 101, 105).
Advanced age represents a strong risk factor for HF and predicts HF risk independently of other cardiovascular risks (17). In fact, a global phenotype of T-cell senescence accompanies advancing age and has been described (104). Briefly, advanced age leads to reductions in thymic output of T cells, T-cell proliferative capacity, and molecular diversity of the TCR (104). Furthermore, T cells of older adults display increased inhibitory receptor expression, as well as increased levels of memory T cells. As described further in heart t cells and cardiac hypertrophy, experimental evidence also implicates T cells in directly promoting myocardial aging (72).
While accumulation of T cells into the myocardium appears to correlate with HF, immunocompromised states do not generally predict reduced HF risk. In fact, many immunocompromised states lead to systemic inflammation, opportunistic infections, or even autoimmunity, each of which may promote cardiac remodeling and HF. HIV infection, the most prevalent condition of acquired immunodeficiency, represents an important example of immune compromise promoting HF. In this condition, although T cells become infected and reduced, the resultant cardiomyopathy actually arises from direct viral effects on the myocardium, secondary systemic inflammation, opportunistic infections, and even from direct effects of antiviral therapies (74).
Although multiple environmental stressors may alter T-cell function and contribute to HF, recent findings in humans specifically identify derangements in the gut microflora as a potential proinflammatory stimulus that could contribute to the observed alterations in T-cell biology described above. Multiple recent studies in HF patients have detected alterations in gut microbial composition (38, 50, 68, 77), which may influence susceptibility to autoimmune and proinflammatory conditions (2, 8). Furthermore, this pathologic gut dysbiosis, as well as associated changes in gut permeability, leads in humans to altered circulating metabolites, which could directly promote HF risk factors and even directly alter myocardial substrate utilization. Although a specific link between gut dysbiosis, T cells, and HF remains unclear, studies in other cardiac conditions such as atherosclerosis have identified changes in gut microflora composition which appear to affect T-cell function (15). Thus pathologic alterations in gut microflora may represent a key mechanism promoting T-cell and general systemic inflammatory alterations predisposing patients to HF.
Although this review focuses on mechanisms within the myocardium through which T cells promote cardiac remodeling and HF, we note that T-cell derangements have been identified multiple systemic conditions that predispose to HF. Therefore, other cardiovascular diseases, general aging, and chronic alterations in immune function and/or microbiome composition remain important areas for study.
Summary: Inflammation and T-Cell Phenotype in Human HF
In summary, increased systemic inflammation represents an established hallmark of both HFrEF and HFpEF. Furthermore, corresponding alterations in T-cell activation, T-cell population distribution, endothelial adhesion, and myocardial infiltration occur in HFrEF and possibly in HFpEF patients. Importantly, due to the observational nature of these studies in humans, the degree to which this T-cell infiltration actually promotes HF and remodeling, as opposed to simply serving as a correlative biomarker of the process, remains unknown. However, the presence of T-cell alterations in humans with HF justifies the mechanistic investigations of T-cell infiltration of the myocardium described below. Moreover, basic and translational studies in preclinical models permit investigation of the effects of T cells on specific aspects of the cardiac remodeling process.
HEART T CELLS AND CARDIAC FIBROSIS
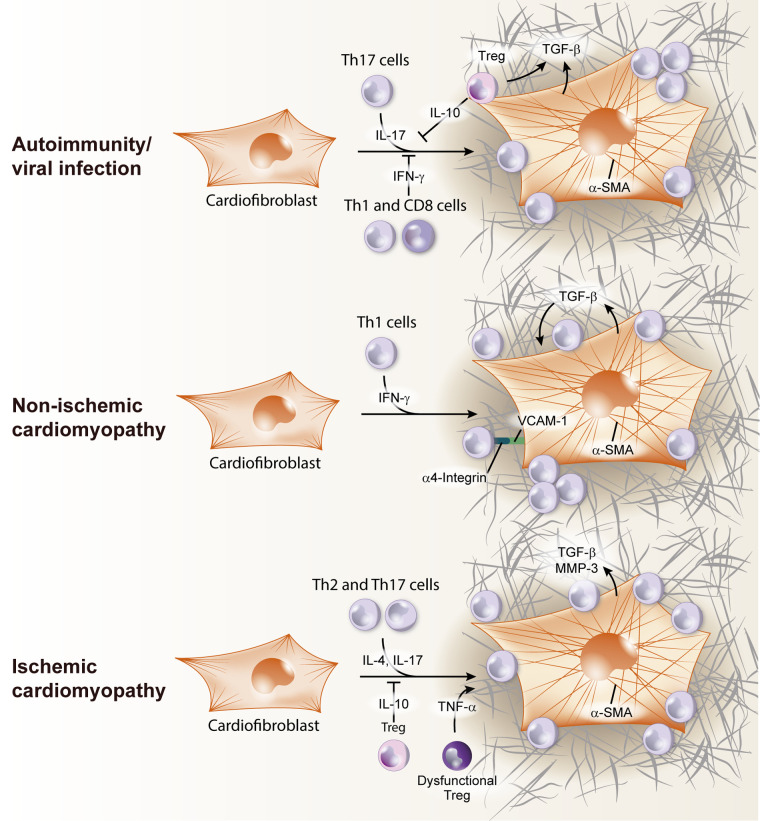
Cardiac fibrosis is key feature of adverse cardiac remodeling and typically represents the accumulation of extracellular matrix proteins such as collagen into the interstitial space as well as the perivascular space (23). Cardiac fibroblasts differentiate into activated myofibroblasts to fill in the space left after cardiomyocyte loss due to infection or ischemia. Although originally thought to represent only a marker of adverse remodeling, cardiac fibrosis has increasingly been identified to contribute to further LV functional deterioration during cardiac remodeling. Additionally, in humans, cardiac fibrosis promotes reentrant arrhythmias, which further contribute to mortality in cardiac remodeling (35). Cardiac fibrosis arises when cardiac fibroblasts, a prevalent resident cell type in the heart, become activated and transform into myofibroblasts, which in turn deposit fibrillary extracellular matrix proteins in the myocardium, to fill in the space left after cardiomyocyte loss. The mechanisms that induce cardiac fibroblast transformation to myofibroblasts are complex and are under active investigation due to the potential therapeutic relevance of opposing this process (see Fig. 3).
Fig. 3.
T-cell induction of cardiac fibroblast transition to myofibroblast in the heart. In viral infection/autoimmunity, Th17 cells produce IL-17, that lead to cardiac fibroblast activation and transforming growth factor-β (TGF-β) production. Treg can also release TGF-β and contribute to extracellular matrix remodeling, but T regulatory cells (Tregs) have a dual role, inhibiting Th17 response by IL-10 secretion. Cardiofibroblast activation can be inhibited by Th1/CD8 T-cell-produced IFN-γ. During nonischemic heart failure (HF), Th1 cells use α4-integrin to adhere to cardiac fibroblasts and contact is required for the transition to TGF-β producing myofibroblasts. In ischemic HF, Th2 and Th17 cells activate cardiac fibroblast by IL-17 and IL-4 release, stimulating TGF-β production and metalloproteinase 3 (MMP-3). This process was reported to be inhibited by IL-10 produced by Tregs. α-SMA, α-smooth muscle actin.
Heart Recruited T Cells and Myocardial Fibrosis in Viral and Autoimmune Myocarditis
Myocarditis is a spectrum of conditions in humans with symptoms that can range from asymptomatic, to chest pain with little or no overt cardiac damage, to acute and severe cardiac dysfunction with associated high mortality if untreated. In myocarditis, unlike other stimuli for cardiac remodeling, the immune system represents the primary abnormality and the first step of the pathophysiologic process (14). Viruses can infect cardiac cells and induce the acute immune response and inflammation necessary to clear infection. Often, the initial acute inflammatory response resolves the infection but also induces off target/damaging effects on surrounding cardiac cells, which can lead to HF and eventually to dilated cardiomyopathy. Coxsackieviruses of group B have high tropism for the heart in humans and mice and induce T-cell immune responses that result in high T-cell tropism for the heart (12). How the balance tilts between immune-mediated viral killing and cardiac damage defines the severity of myocarditis, cardiac damage and repair by fibrosis, and cardiac dysfunction. ICAM-1 has been reported not only as an entry point for Coxsackieviruses of group B to propagate infection but also as an entry point for T-cell extravasation (83). Early studies in T-cell-deficient, athymic nude (nu/nu) mice, in heterozygote (nu/+) mice with normal T-cell function, and in nu/nu mice reconstituted with spleen cells from CB3- or EMC-infected nu/+ mice demonstrated that immature but antigen-specific T cells play a role in the pathogenesis of myocarditis (40). These were followed by many other studies that teased out the role of specific subsets of T cells in this and other models of viral infection and autoimmunity reviewed here.
Myocarditis can emerge as a result of a T-cell response to self-antigens that escape the mechanisms of central and peripheral tolerance, as well as molecular mimicry in viral infections towards these host antigens. For instance, α-myosin heavy chain peptides can induce T-cell clones that escape selection in the thymus and become activated when reencountering antigen in response to infection or cardiac damage (51). The PD-1:PD-L1 pathway works together with other control mechanisms to exclude T cells from the heart, and combined impairment of this pathway along with other regulatory mechanisms can synergize to cause myocarditis (89). Myocarditis can indeed be induced experimentally by immunization with cardiac myosin in the well-established model of experimental autoimmune myocarditis (EAM). Deficiency of PD-1 alone is sufficient to induce significant T-cell infiltration in the heart and lethal myocarditis, confirming a critical role for peripheral tolerance to α-myosin heavy chain peptides. α-Myosin heavy chain-reactive T cells are present in NOD DQ8+ mice that spontaneously develop myocarditis (51). In both cases these cells produce IFN-γ, supporting a role for Th1 cells in the pathogenesis of myocarditis. Furthermore, IFN-γ transgenic mice on a C57Bl/6 background develop a chronic active myocarditis with no pathology observed in other inflammatory organs. However, there is also robust evidence that IFN-γ also serves a protective role against myocarditis. For example, BALB/c mice with IFN-γ or IFN-γ receptor deficiencies, and T-bet-deficient mice, develop markedly more severe and lethal EAM than wild-type controls (20, 73). Another example of the protective role for IFN-γ in experimental myocarditis comes from observations in the cMy-ovalbumin peptide (OVA) model of CD8+ T-cell-mediated myocarditis. CD8+ T-cell-derived IFN-γ is essential for induction of PDL-1 in the heart, which is required to prevent lethal disease (26). Thus it is likely that this tolerance mechanism, together with the prevention of exaggerated Th17 responses balanced by IFN-γ in EAM, is at work in both EAM and the cMy-mOva models. Therefore, a plausible model is that T cells that respond to myocardial antigens and initiate myocarditis produce IFN-γ, which contributes to the initial inflammatory pathology. However, IFN-γ is also required to limit further Th17 cell activation and induced damage to the heart.
While acute lymphocytic myocarditis is characterized by a predominant myocardial patchy infiltration of T lymphocytes but minimal fibrosis, if not lethal, it develops into chronic lymphocytic myocarditis, pathologically characterized by existence of extensive myocardial fibrosis. Studies in RORγT-deficient mice (Rorc−/−) demonstrated resistance to myocarditis, implying that Th17 cells are pathogenic in this model (100). A second study further demonstrated that IL-17A is responsible for the induction of heart fibrosis in this model and that Th17 cells are required for progression from myocarditis to dilated cardiomyopathy, characterized by excessive fibrosis (5, 60, 99). These studies globally demonstrated that T-cell-derived IL-17A contributes to cardiac fibrosis in chronic myocarditis by mechanisms that involve cardiac fibrosis derived granulocyte-monocyte colony-stimulating factor (Figs. 2 and 3).
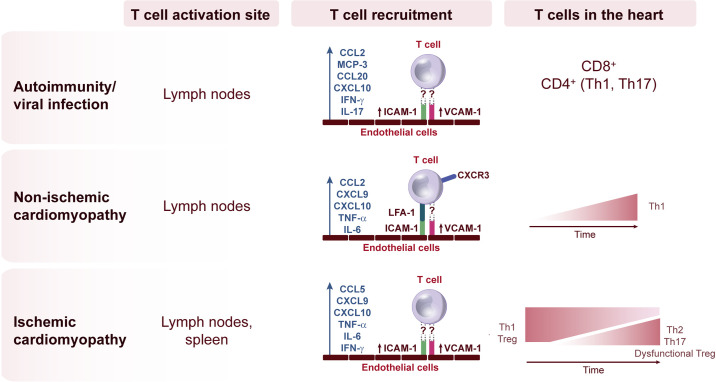
Fig. 2.
Diversity of T-cell activation and recruitment signals in different etiologies of heart failure (HF). The location of T-cell activation and the signals that induce T-cell adhesion to the intramyocardial endothelial cells during recruitment to the heart is dependent on the inflammatory insult in the heart and shapes the progression to HF.
Tregs have also been reported in the heart in autoimmune myocarditis and to exert dual roles in the fibrotic response, due to their production of profibrotic TGF-β, but also of IL-10, which has been shown to be a potent antifibrotic agent in a mouse myocarditis model through the inhibition of collagen synthesis by cardiac fibroblasts (82). As discussed earlier, Tregs can contribute to peripheral tolerance in conjunction with the PD-1/PD-L1/2 pathway in place in effector cells. When any of these are disrupted, autoreactive clones emerge, are recruited to the heart and induce cardiac damage. α-Myosin heavy chain-reactive T cells are found in blood of myocarditis patients, suggesting tolerance to this cardiac protein is lost (51). More recently, cancer patients treated with the PD-1 inhibitor pembrolizumab to achieve T-cell overactivation and antitumor activity have been reported to develop lethal myocarditis, with the presence of robust numbers of both CD4+ and CD8+ T cells (36). As the fields of cardio-oncology and antitumor immunology develop, we are rapidly learning that the immunogenic cell death induced to kill cancer cells likely similarly induces cardiac cell death through cytotoxic activity of CD8+ T cells. The cardiac toxic effect of targeted cancer therapies, including immune check point inhibitors, has recently been reviewed in detail (59).
In summary, both the human syndrome of myocarditis as well as preclinical models of the disease are marked by early and significant infiltration of leukocytes, including T cells, which likely induce much of the cardiac effects. Importantly, the above studies support that even in this primary immune-mediated group of conditions, the specific T-cell subtypes and effects likely differ between various forms of the disease. The significant fibrosis observed in these models also suggests a key role of T-cell infiltration to the myocardium as a profibrotic stimulus. Despite the differences in experimental models, however, several themes emerge. First, T-cell infiltration represents a hallmark of each model. Second, the associated cytokines regulated by T cells in this condition have complex and potentially opposing effects on the process, depending of the timing of secretion and on the cell types present. Third, the resultant fibrosis induced by T-cell infiltration points to a central role of T-cell infiltration in the pathogenesis of cardiac fibrosis. Even though primary myocarditis represents a small proportion of causes of HF in humans, the mechanisms of T-cell infiltration in this condition may provide insights into the broader role of T cells in promoting cardiac fibrosis and failure in the more common forms of HF. Finally, immune tolerance to heart antigens is critical, and patients treated with anti-PD-1 appear to be at increased risk for myocarditis, further highlighting the importance of immune checkpoint regulation in cardiac physiology and pathophysiology.
Heart Recruited T Cells in Myocardial Fibrosis Postischemia and Chronic Ischemic HF
HF often occurs in the setting of an acute MI. Alternatively, the cardiac remodeling occurring after one or more prior MIs can lead to HFrEF and is termed ischemic cardiomyopathy. We will discuss each condition separately below.
MI is defined as myocardial cell death triggered by prolonged ischemia. MI most commonly arises after rupture of an atherosclerotic coronary artery plaque, resulting in subtotal or total coronary occlusion, downstream myocardial ischemia, and eventual myocardial death (infarction). Although contemporary advances in coronary revascularization and acute medical therapy have reduced the acute mortality of MI, the surviving patients still often develop long-term complications such as adverse remodeling and HF. Cardiac repair after MI results from finely orchestrated events initiated by intense sterile inflammation and immune cell infiltration that include T cells in response to cardiomyocyte necrotic death. This proinflammatory phase is followed by a reparative phase in which a different set of immune cells, including T cells, promote wound healing in the heart, but at the expense of excessive fibrosis that can be an early trigger for adverse LV remodeling and ischemic HF. In clinical practice, timely coronary reperfusion can limit the infarction extent; however, reperfusion itself also induces robust tissue inflammation, termed reperfusion injury, and thus therapeutic manipulation is very challenging. Moreover, MI typically occurs as a result of advanced atherosclerosis, which is also characterized by EC activation and plaque inflammation mediated in part by Th1 cells. Thus this partially dependent T-cell inflammatory stage also determines the proinflammatory outcome post-MI (3). It remains unclear what triggers T-cell activation and recruitment to the heart post-MI, as well as the specific role for T cells in the different phases post-MI. Antigens such as cardiac-specific myosin heavy chain isoform or troponin-I, released by dying myocytes post-MI and not expressed in the thymus, may be reactive to T cells that escaped central and peripheral tolerance.
In mouse models of permanent coronary occlusion, infiltrating CD4+ T cells and CD8+ T cells gradually infiltrate the heart and peak on day 7 after MI. Th1 and Treg cells are the predominant subsets also found expanded in the mediastinal lymph nodes and the spleen. Studies using CD4+ T-cell-deficient mice (Cd4−/− or MhcII−/−) concluded that CD4+ T cells are necessary to promote wound healing (30). Tregs were shown to modulate cardiac fibroblast phenotype and function to promote healing and tissue repair: Treg-depleted mice had reduced survival rates, reduced fractional shortening, and accentuated myocardial dilation upon MI (96). Conversely, increasing the frequency of Tregs either by adoptive transfer or by treatment with a CD28 superagonist antibody, positively impacted healing post-MI (81, 87). In contrast to the reparative role postischemia, Tregs exhibit functional perturbations and acquire a proinflammatory phenotype in mice with chronic ischemic HF, contributing to the progression of fibrosis. In this setting, Treg depletion using the DTR (Foxp3-diphtheria toxin receptor transgenic mice) was indeed beneficial and able to reverse cardiac fibrosis and improve cardiac function in ischemic chronic HF, likely due to the elimination of “proinflammatory and dysfunctional” Tregs (7). In contrast to the findings in the experimental model of permanent ischemia, in the ischemia-reperfusion (I/R) model, CD4+ T cells seem to have detrimental effects by exacerbating inflammation and inducing myocardial damage in the early stage of postischemic reperfusion. Notably, this I/R model may be more analogous to contemporary human disease, as the majority of acute infarctions in the United States receive primary revascularization and reperfusion. The extent of myocardial injury 60 min post-I/R was decreased in Rag1−/− mice, which lack T cells and B cells, compared with wild-type mice. Additionally, antibody depletion of CD4+ T cells, but not CD8+ T cells, before I/R in wild-type mice demonstrated that CD4+ T cells induce this pathology. Conversely, reconstitution of CD4+ T-cell-deficient mice with CD4+ T cells was sufficient to induce pathology post-I/R (56). The role of Tregs in this model is also not clear, since there are studies reporting that Treg contribute to protection conferred by rosuvastatin post-I/R injury (39) but also reports indicating that specific ablation of Tregs resulted in decreased infarct size in this model (56). The presence of T cells in the myocardium of patients with acute MI is in agreement with the above findings in experimental models (1).
The specific mechanisms underlying T-cell recruitment to the heart postischemia continue to be studied in different experimental models. Initial findings to support T-cell extravasation from the blood stream to the heart come from observations of a decrease in peripheral blood T cells 60 min after reperfusion post-MI, in parallel with increased T cells in the myocardium within 2 min of reperfusion, suggesting rapid T-cell recruitment (102). Disrupting the CCR5-CCL5 axis by genetic deletion of CCR5 increased inflammation and exacerbated dilated cardiomyopathy. This was in part related to decreased Treg infiltration, supporting that the early recruitment of Treg is protective in the infarcted myocardium (18). Because CCR5 is also expressed in EC progenitors that are critical for wound healing (33), and Tregs have been described to induce CCL5 in ECs (7), the worsened function of Ccr5−/− mice may result from a combination of impaired Treg recruitment and function, as well as EC dysfunction (33). The CXCR3 ligands CXCL9 and CXCL10, which are predominantly in Th1 cells, were found upregulated in the heart post-MI. In addition, CXCL10 was reported to protect the cardiac tissue from disproportionate fibrotic remodeling by inhibiting growth factor-induced fibroblast migration (10). However, the antifibrotic effects of CXCL10 in the infarcted heart were independent of CXCR3 and Th1 cells were not evaluated in this context (10, 78) (Fig. 2).
Thus it is evident that Th1 cells and Tregs contribute to fibrosis early post-MI to promote healing and scar formation. More recent studies have demonstrated a spatiotemporal divergence in Th subsets during the development of ischemic HF and reported both CD4+ and CD8+ T-cell expansion predominantly in the spleen. All Th cell subsets are globally expanded in chronic ischemic HF, 8 wk post-MI and increased in the failing myocardium. Furthermore, a marked reduction of the Th1/Th2 ratio, an augmentation of the Th17/Treg ratio, and upregulation of Th2 cytokines were reported, and further work demonstrated dysfunctional Tregs being present in the ischemic heart. DCs are also robustly expanded in the ischemic heart (34). Altogether, the presence of DCs and several CD4+ T-cell subsets occurring in the ischemic failing heart supports a CD4+ T-cell immune response that contributes to cardiac fibrosis and dysfunction, possibly through release of Th2 profibrotic cytokines such as IL-4 and IL-13 (6, 7). The mechanisms of how T cells regulate healing versus pathological fibrosis have been explored in vitro. Coculture experiments of Tregs with macrophages support that Tregs induce the expression of genes associated with healing such as osteopontin and arginase 1, also upregulated in the scar of mice with preferential expansion of Tregs induced by anti-CD8 superagonist (79, 96). Tregs cocultured with cardiac fibroblasts, on the other hand, reduced cardiac fibroblast transformation to myofibroblasts, suggesting Tregs attenuate cardiac fibrosis through direct modulation of cardiac fibroblasts (79). Understanding whether these act in conjunction in vivo requires further studies that unveil the mechanistic differences in fibrosis during healing or in established HF and how the special temporal recruitment of T cells to the myocardium contributes to the different fibrotic processes. (Figs. 2 and 3).
In summary, the mechanisms of T-cell recruitment to and infiltration of the myocardium in MI appear time dependent and, as in myocarditis, likely involve differential roles depending on the time postischemia. Several important conclusions can be made. First, in contrast to the traditional view that myocardial scarring after infarction simply represents replacement fibrosis of necrotic tissue with extracellular matrix protein, it is now very evident that T cells play a central role in coordinating this process and that T-cell infiltration to the myocardium likely directly modulates fibroblast phenotype and function at all stages of the process. Furthermore, in addition to their effects on the early phases of infarct scar formation, T cells within the heart appear to direct the remote fibrosis and scarring present throughout the LV during the chronic remodeling process. Further defining the complex roles of different T-cell subsets, as well as putative self-antigens that promote this process, has the potential to suggest therapeutic or even preventative strategies for ischemic cardiomyopathy.
It should be noted that, in addition to T-cell recruitment into the heart during and after MI, the process of leukocyte exit from the LV is also a highly regulated process. Furthermore, the resolution of inflammation and leukocyte presence in the postinfarcted myocardium corresponds with the degree of healing, whereas lack of sufficient inflammation resolution likely contributes to the transition from acute injury to chronic remodeling (91). Importantly, while myocardial CD4 T cells promote increased infarct size, experimental studies also support a requirement of CD4 cells for healing postinfarct; however, the exact role T cells play coordinating the resolution phase has not been explored in depth to date (91).
Heart Recruited T Cells in Cardiac Fibrosis in Nonischemic Cardiomyopathy
Hypertension is a common risk factor for HF, accounting for ~25% of HF cases (9). Hypertensive heart disease is commonly associated with LV hypertrophy and perivascular and interstitial cardiac fibrosis and manifests clinically as HFpEF or HFrEF (19). Angiotensin II (ANG II) infusion increases blood pressure, and transverse-aortic constriction (TAC) produces an abrupt LV pressure overload that mimics LV pressure that can lead to HF in patients (>140 mmHg). These are the two most commonly used experimental models of nonischemic HF. ANG II-driven cardiac fibrosis was reduced in IFN-γ-deficient mice, which showed decreased infiltration of T cells, supporting a profibrotic role for IFN-γ in the hypertensive heart (28). This contrasts the antifibrotic role described for IFN-γ in models of viral infection and autoimmunity, as described earlier (21, 22). Transfer of Tregs to hypertensive mouse hearts also attenuated cardiac fibrosis, reducing TGF-β and the presence of myofibroblasts. This antifibrotic role of Tregs may be attributed to IL-10 (58).
TAC induces compensatory maladaptive cardiac hypertrophy followed by the slow progression of cardiac inflammation, cardiac fibrosis, and progressive cardiac dysfunction (75). Pressure overload-induced HF is characterized by T-cell activation mainly occurring in the mediastinal lymph nodes that drain the heart and T-cell trafficking to the heart that results in adverse cardiac remodeling and cardiac dysfunction (44, 61). Remarkably, splenic remodeling does not appear to occur in response to ANG II or TAC (70), in sharp contrast with ischemic HF. Several studies support that CD4+ T cells dominate the pathological immune response in nonischemic HF. Specifically, experimental studies reveal the absence of cardiac fibrosis and preserved cardiac function in several knockout mice with an altered B/T-cell response, such as Rag2−/− mice, MhcII−/− mice (44), and Tcra−/− mice, and by pharmacologically depleting T cells with an anti-CD3 antibody (61). In addition, adoptive transfer of Th1 cells into Tcra−/− mice partially reconstituted cardiac fibrosis and cardiac dysfunction, supporting a central profibrotic role for Th1 cells (62). Furthermore, IFN-γ−/− T cells were unable to do so, further supporting a role for Th1 cells in cardiac fibrosis. Antigen recognition by T cells seems to be critical to induce the pathology associated with TAC, since blockade of T-cell costimulatory molecules on antigen-presenting cells and depletion of bone marrow-derived CD11c+ DCs significantly attenuate LV fibrosis and hypertrophy induced by TAC (37, 95). Along the same lines, OTII mice, which express a transgenic CD4+ TCR specific for exogenous OVA, are protected from adverse cardiac remodeling in response to TAC (44), although a different study in the cMy-mOVA mice subjected to TAC demonstrated some pathology (27). Thus both cardiac and noncardiac antigens may induce cardiac fibrosis in the pressure overloaded heart.
With the use of REX3 mice, reporters for CXCL9 and CXCL10, we recently demonstrated that cardiac fibroblasts and myeloid cells are sources of CXCL10 and, to a minor extent, CXCL9 in response to TAC. These signals attract CXCR3+ Th1 cells to the heart in an LFA-1-ICAM-1-dependent manner. Interestingly, Cxcr3−/− mice did not develop cardiac fibrosis and dysfunction in response to TAC. While Cxcr3−/− mice had reduced T-cell infiltration in the heart, CCR2+ macrophages, which precede T-cell infiltration to the heart in this model (69), were detectable in the heart (64). These data also demonstrated that CCR2+ macrophages produce CXCL10 and CXCL9 and support that early recruitment of CCR2+ macrophages post-TAC triggers subsequent T-cell recruitment to the heart (64). Because Th1 cells are drivers of cardiac fibrosis associated with TAC, highly express the chemokine receptor CXCR3, and do not infiltrate the heart in Cxcr3−/− mice, this CXCR3-CXCL9/10 pathway is thought to dominate T-cell infiltration in response to cardiac pressure overload. Th1 cells express LFA-1, the ligand of endothelial ICAM-1, which is upregulated on the vascular endothelium of the LV (76). Moreover, Icam1−/− mice show decreased T cells in the LV in response to TAC and do not develop cardiac fibrosis or dysfunction after pressure overload. These findings further suggest that ICAM-1 normally mediates the ability of activated T cells to bind the endothelium and infiltrate into the pressure overloaded LV to induce functional and structural abnormalities (76). Interestingly, CXCL9 and CXCL10 positively correlate with Th1 type cytokines in the circulation of patients with symptomatic HF (4), and we found that CXCR3+ T cells are infiltrated in the hearts of end-stage nonischemic HF patients (64) (Fig. 2).
While the above studies strongly support that T-cell infiltration into the pressure overloaded LV promotes pathological fibrosis and remodeling, additional lines of evidence have further identified primary changes in the T-cell activation state as well as effects on cardiac cell types. For example, T cells isolated from mice after TAC display enhanced adhesion to ECs in ex vivo adhesion assays under flow conditions, directly identifying that pressure overload induces primary changes in T-cell activation state and infiltration potential (61). Furthermore, T cells isolated from mediastinal lymph nodes of pressure-overloaded mice, when cocultured with normal cardiac fibroblasts, induced cardiac fibroblast to myofibroblast transition in vitro in a TGF-β-dependent manner (62). When compared with T cells from sham mediastinal lymph nodes, the TAC T cells alone produced this transition and also displayed increased direct interaction with cardiac fibroblasts and their transformation to myofibroblasts. These observations provide evidence that pressure overload induces systemic changes in T cells rendering them more able to adhere to cardiac endothelium and to modulate directly cardiac fibroblast transformation (62) (Fig. 3).
In summary, while the precise mechanisms through which T cells home to the myocardium after pressure overload remain unclear, multiple lines of evidence and experimental models now support that T-cell infiltration to the myocardium is both necessary and sufficient to produce LV fibrosis and dysfunction. These studies support CD4+ T cells as the primary cell type involved in this process through direct adhesion and actions on cardiac fibroblasts. Thus one working model holds that, in response to pressure overload, peripheral T cells become activated (through as yet unclear mechanisms), which enables their recruitment to the LV through cell surface interaction with ICAM-1 and myocardial CXCR3 ligands. T-cell contact with and modulation of cardiac fibrosiss then promotes pathologic interstitial fibrosis and cardiac remodeling. Furthermore, it appears from these combined studies that the mechanisms of T-cell infiltration and promotion of fibrosis may differ between pressure overload and ischemia/infarction. Future investigations to define the precise mechanisms of T-cell recruitment to the LV in pressure overload may therefore offer phenotype-specific therapeutic strategies to test in patients with nonischemic cardiomyopathy.
HEART T CELLS AND CARDIAC HYPERTROPHY
In addition to fibrosis and LV dysfunction, LV hypertrophy represents another key component of the cardiac remodeling process. LV hypertrophy involves a gross increase in chamber mass, as well as cardiac myocyte size. The pathologic hypertrophic response is marked early on by dedifferentiating abnormalities of gene expression in which fetal isoforms replace a number of normal adult gene products (61, 76). LV hypertrophy predicts increased mortality in hypertensive patients, and its regression correlates with improved outcomes and survival in epidemiologic studies and clinical trials (66). The TAC pressure overload model has provided valuable information about the functions of T cells in the development of hypertrophy, especially because the fixed afterload induced by TAC allows interpretation of afterload-independent effects of experimental conditions. In response to TAC, Tcra−/− and MhcII−/− mice display nearly complete inhibition of LV hypertrophy and normalization of cardiac myocyte area (44, 61). Furthermore, in the case of Tcra−/− mice, the maladaptive fetal gene expression pattern also reverted to normal. Adoptive transfer of either naïve T cells or Th1 T cells to TAC Tcra−/− mice restored LV hypertrophy after TAC (61). Icam1−/− mice, which also display reduced T-cell infiltration into the LV after TAC, have reduced TAC-induced LV hypertrophy and cardiomyocyte dimensions, directly supporting that the T-cell infiltration to the LV mediates the hypertrophic development (76). Interestingly, whereas genetic ablation of T cells in the above models reduced TAC-induced pathological LV hypertrophy, pharmacological T-cell depletion after TAC had no effect on LV mass, although it significantly improved wild-type fibrosis and LV function. These combined findings indicate that T-cell infiltration into the LV likely directly promotes cardiac myocyte and LV hypertrophy, although the specific subsets involved and timing of these effects may be quite complicated. Along these lines, the direct effects of Tregs subsets on cocultured cardiomyocytes, including Treg inhibition of LPS-induced cardiomyocyte death, have been observed (87). These observations suggest that some T-cell subsets may play a protective role in the LV through direct effects on cardiomyocytes and that subset-specific effects of different T-cell populations may explain the different effects observed between genetic disruption of T cells versus pharmacological depletion in pressure overload.
In addition to experimentally induced models of HF described above, recent work also implicates T cells in the pathogenesis of myocardial dysfunction during aging, even in the absence of additional external stimuli. For example, Ramos et al. (72) observed that aging-associated increases in T cells within heart draining mediastinal lymph nodes accompanied pathological fibrosis, gene expression, and remodeling in hearts of aged (12–15 mo) mice. Furthermore, the elderly mouse mediastinal lymph nodes displayed relatively higher proportions of antigen-experienced conventional T cells compared with mediastinal lymph nodes of younger mice. Remarkably, genetic depletion of CD4 T cells in three different models, MhcII−/−, Cd4−/−, and TCR mutant to irrelevant MHC peptide, rescued age-dependent pathologic gene expression and remodeling changes, indicating the likely role of T cells in normally mediating these myocardial aging effects. These experimental findings therefore suggest that T cells associated with cardiac draining lymph nodes may represent primary drivers of pathophysiological changes in LV structure and function in aging.
HEART T CELLS AND INTRAMYOCARDIAL VASCULAR ENDOTHELIAL PHYSIOLOGY
As reviewed in previous sections, T cells are in constant contact with the vascular endothelium, and this cross talk is critical for leukocyte recruitment to the heart through protein-protein interactions described above. Additionally, it is likely that during such intimate contact, T cells have direct and indirect effects on different aspects of cardiac EC physiology during homeostasis and in response to several inflammatory insults to the heart. EC dysfunction in the coronary arteries is an important contributor to atherosclerotic plaques, so it is the accumulation of effector T cells in the atherosclerotic lesions. This is thought to predispose plaque rupture and, if occurring in the coronary artery, results in MI with the above mentioned consequences in HF. Increased Th1 cells and decreased Tregs are associated with vascular inflammation in this context (25, 98). IL-17 has been demonstrated to increase blood pressure by decreasing endothelial nitric oxide production. Given the pathogenic role T cells play in hypertension, it is likely that Th17 cells induce vascular dysfunction in this context, which could have effects in hypertensive HF (63). Besides the well-established role of T cells in vascular inflammation, recent evidence indicates a role for T cells in endothelial angiogenesis, a critical process in the healing process post-MI. Disrupting T-cell activation using Cd28−/− mice resulted in a significant reduction in postischemic vessel growth, although these studies did not involve heart ischemia (107). The specific T-cell subset regulating this process and the molecular mechanism involved in the context of cardiac ischemia was recently investigated and reported to require Treg contact with ECs. Moreover, dysfunctional Tregs generated during ischemic HF exerted antiangiogenic effects through TNF-R1 that resulted in impaired mobilization of proangiogenic endothelial progenitors to the heart (7). Taken together, heart-infiltrated T cells exert effects on intramyocardial ECs through direct contact as well as by releasing cytokines that impair vascular function. This likely represents an important pathogenic mechanism in ischemic cardiomyopathy.
CLOSING REMARKS AND SUGGESTIONS FOR FUTURE INVESTIGATION
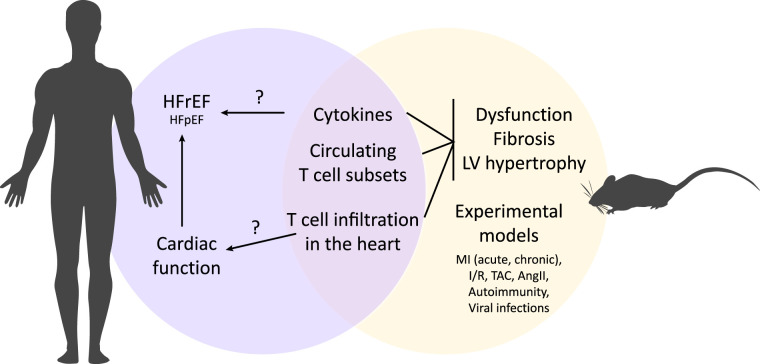
Over the past several decades, HF has become recognized as a generally proinflammatory state. As discussed in this review, growing evidence now supports that dysregulation of T cells represents an important component of the HF syndrome. These studies further identify T cells as important not only in autoimmune or postinfectious HF and cardiac remodeling but in the more prevalent cases of HF arising from pathophysiological stimuli such as ischemia, hypertension, and pressure overload. Experimental studies strongly support this notion and reveal a specific role for T-cell recruitment and infiltration into the myocardium in the pathogenesis of cardiac fibrosis, dysfunction, and hypertrophy. These studies also suggest that the maladaptive effects of T cells during cardiac remodeling not only involve T-cell regulation of traditional immune cells but likely also involve direct T-cell effects on resident cardiac fibroblasts and possibly even cardiac myocytes. A further emerging paradigm holds that the complex mechanisms of T-cell recruitment and infiltration into the myocardium differ depending on the nature of the cardiac remodeling stimulus. Although these precise mechanisms remain under investigation, T-cell recruitment to the myocardium is becoming established as a novel pathophysiologic mechanism in cardiac remodeling and thus in HF (Fig. 4). Yet, most of our knowledge stems from studies in well-established experimental models of myocarditis, ischemic, and nonischemic HF using genetic and pharmacological approaches (Table 1). Although these have provided a wealth of information, they do not fully mimic human disease: mice live in clean environments, with a uniform microbiome and defined controlled diet, and their immune system does not fully mimic the immune system in humans (Fig. 4).
Fig. 4.
Potential shared mechanisms of T-cell dysregulation and immune system activation between experimental models and human heart failure (HF). Observations from human studies and from experimental models identify dysregulation of cytokines, circulating T-cell subsets, and T-cell infiltration in the heart as hallmarks of the HF (human) or pathological remodeling (preclinical) phenotypes. In humans, these abnormalities correlate with cardiac dysfunction and the HF syndrome. In experimental models, these mechanisms are necessary and sufficient to induce pathophysiological abnormalities. The degree to which T-cell dysregulation causes the HF syndrome or cardiac dysfunction, and the efficacy in modulating T cells in most forms of HF in humans, remains open for further investigation. MI, myocardial infarction; HFrEF, HF with reduced ejection fraction; TAC, transverse-aortic constriction; I/R, ischemia-reperfusion; LV, left ventricle.
Table 1.
Genes associated with T-cell immune responses in HF
| Experimental Animal Models | Disease Model Phenotype |
|||||
|---|---|---|---|---|---|---|
| Ischemic HF |
||||||
| I/R | Acute | Chronic | Nonischemic HF | Experimental autoimmunity | References | |
| Ccr2−/− or Ab depletion | Protected | (69) | ||||
| Ccr5 −/− | More damage | (18) | ||||
| Cd28 −/− | More damage | (107) | ||||
| Cd4−/−, Ab depletion, MhcII−/− | Protected | More damage | Protected | (30, 44, 56) | ||
| Cxcr3 −/− | Similar to WT | Protected | (64, 78) | |||
| Cxcl10−/− | Protected | (10) | ||||
| Cd11c−/− or Ab depletion | More damage | Protected | (37, 95) | |||
| Icam1 −/− | Protected | (76) | ||||
| Ifng−/−, Ifngr−/− | Protected | Protected/more damage | (20–22, 28) | |||
| Il17a−/−, Il17ra−/− | Protected | (5, 99) | ||||
| NOD DQ8+ | More damage | (51) | ||||
| OTII | Protected | (44) | ||||
| Pdcd1 (PD-1)−/− | More damage | (89) | ||||
| Cd274 (PD-L1/2)−/− | More damage | (26, 89) | ||||
| Rag2 −/− | Protected | (44) | ||||
| Rorc −/− | Protected | (100) | ||||
| Tbx21 −/− | More damage | (73) | ||||
| Tcra −/− | Protected | (44, 61) | ||||
| Treg DTR or Ab depletion | Protected | More damage | Protected | (7, 30, 56) | ||
A protected phenotype frequently shows low inflammatory responses, which usually lead to a reduction of adverse cardiac remodeling. In contrast, a “more damage” phenotype is based on an increase in T-cell immune responses that mainly contributes to cardiac fibrosis and therefore to dilated cardiomyopathy. I/R, ischemic-reperfusion; Ab, antibody; HF, heart failure; WT, wild type.
We suggest several central questions for future investigation. From the basic perspective, studies to define the complete mechanisms of T-cell activation during HF will be important. It will be useful to identify, as well, the exact differential effects of various T-cell subtypes on the separate components of the cardiac remodeling process. Along these same lines, understanding the direct effects induced on resident cardiac cells, such as cardiac fibroblast and cardiomyocytes, will likely prove informative. The basic studies performed to date also raise the question of the extent to which drugs currently used to treat HF potentially exert their effects through modulation of T cells. Conversely, the degree to which T cells mediate the pathologies of known cardiotoxic agents should be examined.
A number of important clinical and translational questions also emerge from the novel role of T cells identified in HF. First, compared with HFrEF, the pattern of T-cell function and surface marker expression in HFpEF remains relatively unexplored. Because no disease-modifying medical therapies exist currently for HFpEF, and further because T cells appear to modulate a number of HFpEF risk factors such as hypertension, identifying the potential roles of T cells specifically in HFpEF may offer new insights into managing this condition. Understanding the role of T-cell activation or subtype distribution as a predictor for HF risk, prognosis, or even a response to specific therapies might improve the clinical management of this disease.
Finally, can therapies to modulate T-cell infiltration into the LV be safely devised to improve outcomes in HF? While cytokine targeted therapies did not improve outcomes in HF, perhaps specific targeting of one or more proremodeling T-cell subsets, or the pathways they use to interact with cardiac cells, could offer more refined therapeutic options. The idea of broad immunosuppression to reduce T-cell number does not currently appear attractive for most forms of HF and will likely remain limited to obvious autoimmune cases of myocarditis and acute HF. However, one intriguing possibility could be to identify compounds to prevent T-cell infiltration into the heart to oppose T-cell cardiotoxic effects but avoid the systemic effects of chronic systemic immunosuppression. This therapeutic strategy will require further exploration of the mechanisms underlying T-cell recruitment to the myocardium.
GRANTS
This work is supported by National Heart, Lung, and Blood Institute Grants HL-131831 (to R. M. Blanton) and HL-123658 and HL-144477 (to P. Alcaide) and American Heart Association Grant 19POST34430075/2019 (to F. J. Carrillo-Salinas).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.M.B. and P.A. analyzed data; F.J.C.-S. and P.A. prepared figures; R.M.B. and P.A. drafted manuscript; R.M.B., F.J.C.-S., and P.A. edited and revised manuscript; R.M.B., F.J.C.-S., and P.A. approved final version of manuscript.
REFERENCES
- 1.Abbate A, Bonanno E, Mauriello A, Bussani R, Biondi-Zoccai GG, Liuzzo G, Leone AM, Silvestri F, Dobrina A, Baldi F, Pandolfi F, Biasucci LM, Baldi A, Spagnoli LG, Crea F. Widespread myocardial inflammation and infarct-related artery patency. Circulation 110: 46–50, 2004. doi: 10.1161/01.CIR.0000133316.92316.81. [DOI] [PubMed] [Google Scholar]
- 2.Abdollahi-Roodsaz S, Abramson SB, Scher JU. The metabolic role of the gut microbiota in health and rheumatic disease: mechanisms and interventions. Nat Rev Rheumatol 12: 446–455, 2016. doi: 10.1038/nrrheum.2016.68. [DOI] [PubMed] [Google Scholar]
- 3.Abdolmaleki F, Gheibi Hayat SM, Bianconi V, Johnston TP, Sahebkar A. Atherosclerosis and immunity: a perspective. Trends Cardiovasc Med 29: 363–371, 2018. doi: 10.1016/j.tcm.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Altara R, Manca M, Hessel MH, Gu Y, van Vark LC, Akkerhuis KM, Staessen JA, Struijker-Boudier HA, Booz GW, Blankesteijn WM. CXCL10 is a circulating inflammatory marker in patients with advanced heart failure: a pilot study. J Cardiovasc Transl Res 9: 302–314, 2016. doi: 10.1007/s12265-016-9703-3. [DOI] [PubMed] [Google Scholar]
- 5.Baldeviano GC, Barin JG, Talor MV, Srinivasan S, Bedja D, Zheng D, Gabrielson K, Iwakura Y, Rose NR, Cihakova D. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ Res 106: 1646–1655, 2010. doi: 10.1161/CIRCRESAHA.109.213157. [DOI] [PubMed] [Google Scholar]
- 6.Bansal SS, Ismahil MA, Goel M, Patel B, Hamid T, Rokosh G, Prabhu SD. Activated T lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circ Heart Fail 10: e003688, 2017. doi: 10.1161/CIRCHEARTFAILURE.116.003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bansal SS, Ismahil MA, Goel M, Zhou G, Rokosh G, Hamid T, Prabhu SD. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation 139: 206–221, 2019. doi: 10.1161/CIRCULATIONAHA.118.036065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barin JG, Tobias LD, Peterson DA. The microbiome and autoimmune disease: report from a Noel R. Rose Colloquium. Clin Immunol 159: 183–188, 2015. doi: 10.1016/j.clim.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UK, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 137: e67–e492, 2018. [Erratum in Circulation 137: e493, 2018]. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 10.Bujak M, Dobaczewski M, Gonzalez-Quesada C, Xia Y, Leucker T, Zymek P, Veeranna V, Tager AM, Luster AD, Frangogiannis NG. Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res 105: 973–983, 2009. doi: 10.1161/CIRCRESAHA.109.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 27: 111–122, 2007. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung PK, Yuan J, Zhang HM, Chau D, Yanagawa B, Suarez A, McManus B, Yang D. Specific interactions of mouse organ proteins with the 5′untranslated region of coxsackievirus B3: potential determinants of viral tissue tropism. J Med Virol 77: 414–424, 2005. doi: 10.1002/jmv.20470. [DOI] [PubMed] [Google Scholar]
- 13.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT; Anti-TNF Therapy Against Congestive Heart Failure Investigators . Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 107: 3133–3140, 2003. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 14.Cihakova D, Rose NR. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv Immunol 99: 95–114, 2008. doi: 10.1016/S0065-2776(08)00604-4. [DOI] [PubMed] [Google Scholar]
- 15.Cui L, Zhao T, Hu H, Zhang W, Hua X. Association study of gut flora in coronary heart disease through high-throughput sequencing. BioMed Res Int 2017: 3796359, 2017. doi: 10.1155/2017/3796359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dangas G, Konstadoulakis MM, Epstein SE, Stefanadis CI, Kymionis GD, Toutouza MG, Liakos C, Sadaniantz A, Cohen AM, Chesebro JH, Toutouzas PK. Prevalence of autoantibodies against contractile proteins in coronary artery disease and their clinical implications. Am J Cardiol 85: 870–872, 2000. doi: 10.1016/S0002-9149(99)00883-8. [DOI] [PubMed] [Google Scholar]
- 17.Dhingra R, Vasan RS. Age as a risk factor. Med Clin North Am 96: 87–91, 2012. doi: 10.1016/j.mcna.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol 176: 2177–2187, 2010. doi: 10.2353/ajpath.2010.090759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drazner MH. The progression of hypertensive heart disease. Circulation 123: 327–334, 2011. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson U, Kurrer MO, Bingisser R, Eugster HP, Saremaslani P, Follath F, Marsch S, Widmer U. Lethal autoimmune myocarditis in interferon-gamma receptor-deficient mice: enhanced disease severity by impaired inducible nitric oxide synthase induction. Circulation 103: 18–21, 2001. doi: 10.1161/01.CIR.103.1.18. [DOI] [PubMed] [Google Scholar]
- 21.Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Gatewood SJ, Njoku DB, Rose NR. Interferon-gamma protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-beta 1, interleukin-1 beta, and interleukin-4 in the heart. Am J Pathol 165: 1883–1894, 2004. doi: 10.1016/S0002-9440(10)63241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Steele RA, Gatewood SJ, Rose NR. IL-12 protects against coxsackievirus B3-induced myocarditis by increasing IFN-gamma and macrophage and neutrophil populations in the heart. J Immunol 174: 261–269, 2005. doi: 10.4049/jimmunol.174.1.261. [DOI] [PubMed] [Google Scholar]
- 23.Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair 5: 15, 2012. doi: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschöpe C, Leite-Moreira AF, Musters R, Niessen HW, Linke WA, Paulus WJ, Hamdani N. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail 4: 312–324, 2016. doi: 10.1016/j.jchf.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Gimbrone MA Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 118: 620–636, 2016. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grabie N, Gotsman I, DaCosta R, Pang H, Stavrakis G, Butte MJ, Keir ME, Freeman GJ, Sharpe AH, Lichtman AH. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation 116: 2062–2071, 2007. doi: 10.1161/CIRCULATIONAHA.107.709360. [DOI] [PubMed] [Google Scholar]
- 27.Gröschel C, Sasse A, Röhrborn C, Monecke S, Didié M, Elsner L, Kruse V, Bunt G, Lichtman AH, Toischer K, Zimmermann WH, Hasenfuß G, Dressel R. T helper cells with specificity for an antigen in cardiomyocytes promote pressure overload-induced progression from hypertrophy to heart failure. Sci Rep 7: 15998, 2017. doi: 10.1038/s41598-017-16147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henri O, Pouehe C, Houssari M, Galas L, Nicol L, Edwards-Lévy F, Henry JP, Dumesnil A, Boukhalfa I, Banquet S, Schapman D, Thuillez C, Richard V, Mulder P, Brakenhielm E. Selective stimulation of cardiac lymphangiogenesis reduces myocardial edema and fibrosis leading to improved cardiac function following myocardial infarction. Circulation 133: 1484–1497, 2016. doi: 10.1161/CIRCULATIONAHA.115.020143. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, Kerkau T, Frantz S. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 125: 1652–1663, 2012. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann U, Frantz S. How can we cure a heart “in flame”? A translational view on inflammation in heart failure. Basic Res Cardiol 108: 356, 2013. doi: 10.1007/s00395-013-0356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science 307: 430–433, 2005. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 33.Ishida Y, Kimura A, Kuninaka Y, Inui M, Matsushima K, Mukaida N, Kondo T. Pivotal role of the CCL5/CCR5 interaction for recruitment of endothelial progenitor cells in mouse wound healing. J Clin Invest 122: 711–721, 2012. doi: 10.1172/JCI43027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res 114: 266–282, 2014. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jalife J, Kaur K. Atrial remodeling, fibrosis, and atrial fibrillation. Trends Cardiovasc Med 25: 475–484, 2015. doi: 10.1016/j.tcm.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA Jr, Anders RA, Sosman JA, Moslehi JJ. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 375: 1749–1755, 2016. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kallikourdis M, Martini E, Carullo P, Sardi C, Roselli G, Greco CM, Vignali D, Riva F, Ormbostad Berre AM, Stølen TO, Fumero A, Faggian G, Di Pasquale E, Elia L, Rumio C, Catalucci D, Papait R, Condorelli G. T cell costimulation blockade blunts pressure overload-induced heart failure. Nat Commun 8: 14680, 2017. doi: 10.1038/ncomms14680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamo T, Akazawa H, Suda W, Saga-Kamo A, Shimizu Y, Yagi H, Liu Q, Nomura S, Naito AT, Takeda N, Harada M, Toko H, Kumagai H, Ikeda Y, Takimoto E, Suzuki JI, Honda K, Morita H, Hattori M, Komuro I. Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PLoS One 12: e0174099, 2017. doi: 10.1371/journal.pone.0174099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ke D, Fang J, Fan L, Chen Z, Chen L. Regulatory T cells contribute to rosuvastatin-induced cardioprotection against ischemia-reperfusion injury. Coron Artery Dis 24: 334–341, 2013. doi: 10.1097/MCA.0b013e3283608c12. [DOI] [PubMed] [Google Scholar]
- 40.Kishimoto C, Abelmann WH. In vivo significance of T cells in the development of Coxsackievirus B3 myocarditis in mice. Immature but antigen-specific T cells aggravate cardiac injury. Circ Res 67: 589–598, 1990. doi: 10.1161/01.RES.67.3.589. [DOI] [PubMed] [Google Scholar]
- 41.Komarowska I, Coe D, Wang G, Haas R, Mauro C, Kishore M, Cooper D, Nadkarni S, Fu H, Steinbruchel DA, Pitzalis C, Anderson G, Bucy P, Lombardi G, Breckenridge R, Marelli-Berg FM. Hepatocyte growth factor receptor c-met instructs T cell cardiotropism and promotes t cell migration to the heart via autocrine chemokine release. Immunity 42: 1087–1099, 2015. doi: 10.1016/j.immuni.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, Demetris AJ, Feldman AM. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res 81: 627–635, 1997. doi: 10.1161/01.RES.81.4.627. [DOI] [PubMed] [Google Scholar]
- 43.Kunicki MA, Amaya Hernandez LC, Davis KL, Bacchetta R, Roncarolo MG. Identity and diversity of human peripheral Th and T regulatory cells defined by single-cell mass cytometry. J Immunol 200: 336–346, 2018. doi: 10.4049/jimmunol.1701025. [DOI] [PubMed] [Google Scholar]
- 44.Laroumanie F, Douin-Echinard V, Pozzo J, Lairez O, Tortosa F, Vinel C, Delage C, Calise D, Dutaur M, Parini A, Pizzinat N. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation 129: 2111–2124, 2014. doi: 10.1161/CIRCULATIONAHA.113.007101. [DOI] [PubMed] [Google Scholar]
- 45.Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, Oukka M, Kuchroo VK, Glimcher LH. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORγt. Nat Immunol 12: 96–104, 2011. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 323: 236–241, 1990. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 47.Li N, Bian H, Zhang J, Li X, Ji X, Zhang Y. The Th17/Treg imbalance exists in patients with heart failure with normal ejection fraction and heart failure with reduced ejection fraction. Clin Chim Acta 411: 1963–1968, 2010. doi: 10.1016/j.cca.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 48.Lichtman AH. The heart of the matter: protection of the myocardium from T cells. J Autoimmun 45: 90–96, 2013. doi: 10.1016/j.jaut.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu X, Crowley SD. Inflammation in salt-sensitive hypertension and renal damage. Curr Hypertens Rep 20: 103, 2018. doi: 10.1007/s11906-018-0903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luedde M, Winkler T, Heinsen FA, Rühlemann MC, Spehlmann ME, Bajrovic A, Lieb W, Franke A, Ott SJ, Frey N. Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Fail 4: 282–290, 2017. doi: 10.1002/ehf2.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lv H, Havari E, Pinto S, Gottumukkala RV, Cornivelli L, Raddassi K, Matsui T, Rosenzweig A, Bronson RT, Smith R, Fletcher AL, Turley SJ, Wucherpfennig K, Kyewski B, Lipes MA. Impaired thymic tolerance to α-myosin directs autoimmunity to the heart in mice and humans. J Clin Invest 121: 1561–1573, 2011. doi: 10.1172/JCI44583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lynch TL 4th, Kuster DW, Gonzalez B, Balasubramanian N, Nair N, Day S, Calvino JE, Tan Y, Liebetrau C, Troidl C, Hamm CW, Güçlü A, McDonough B, Marian AJ, van der Velden J, Seidman CE, Huggins GS, Sadayappan S. Cardiac myosin binding protein-C autoantibodies are potential early indicators of cardiac dysfunction and patient outcome in acute coronary syndrome. JACC Basic Transl Sci 2: 122–131, 2017. doi: 10.1016/j.jacbts.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res 116: 1254–1268, 2015. doi: 10.1161/CIRCRESAHA.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 109: 1594–1602, 2004. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 55.Marriott JB, Goldman JH, Keeling PJ, Baig MK, Dalgleish AG, McKenna WJ. Abnormal cytokine profiles in patients with idiopathic dilated cardiomyopathy and their asymptomatic relatives. Heart 75: 287–290, 1996. doi: 10.1136/hrt.75.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathes D, Weirather J, Nordbeck P, Arias-Loza AP, Burkard M, Pachel C, Kerkau T, Beyersdorf N, Frantz S, Hofmann U. CD4+ Foxp3+ T-cells contribute to myocardial ischemia-reperfusion injury. J Mol Cell Cardiol 101: 99–105, 2016. doi: 10.1016/j.yjmcc.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Meng X, Yang J, Dong M, Zhang K, Tu E, Gao Q, Chen W, Zhang C, Zhang Y. Regulatory T cells in cardiovascular diseases. Nat Rev Cardiol 13: 167–179, 2016. doi: 10.1038/nrcardio.2015.169. [DOI] [PubMed] [Google Scholar]
- 58.Mian MO, Barhoumi T, Briet M, Paradis P, Schiffrin EL. Deficiency of T-regulatory cells exaggerates angiotensin II-induced microvascular injury by enhancing immune responses. J Hypertens 34: 97–108, 2016. doi: 10.1097/HJH.0000000000000761. [DOI] [PubMed] [Google Scholar]
- 59.Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med 375: 1457–1467, 2016. doi: 10.1056/NEJMra1100265. [DOI] [PubMed] [Google Scholar]
- 60.Myers JM, Cooper LT, Kem DC, Stavrakis S, Kosanke SD, Shevach EM, Fairweather D, Stoner JA, Cox CJ, Cunningham MW. Cardiac myosin-Th17 responses promote heart failure in human myocarditis. JCI Insight 1: 85851, 2016. doi: 10.1172/jci.insight.85851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nevers T, Salvador AM, Grodecki-Pena A, Knapp A, Velázquez F, Aronovitz M, Kapur NK, Karas RH, Blanton RM, Alcaide P. Left ventricular T-cell recruitment contributes to the pathogenesis of heart failure. Circ Heart Fail 8: 776–787, 2015. doi: 10.1161/CIRCHEARTFAILURE.115.002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nevers T, Salvador AM, Velazquez F, Ngwenyama N, Carrillo-Salinas FJ, Aronovitz M, Blanton RM, Alcaide P. Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J Exp Med 214: 3311–3329, 2017. doi: 10.1084/jem.20161791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc Res 97: 696–704, 2013. doi: 10.1093/cvr/cvs422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ngwenyama N, Salvador AM, Velázquez F, Nevers T, Levy A, Aronovitz M, Luster AD, Huggins GS, Alcaide P. CXCR3 regulates CD4+ T cell cardiotropism in pressure overload-induced cardiac dysfunction. JCI Insight 4: 125527, 2019. doi: 10.1172/jci.insight.125527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okamoto N, Noma T, Ishihara Y, Miyauchi Y, Takabatake W, Oomizu S, Yamaoka G, Ishizawa M, Namba T, Murakami K, Iwado Y, Ohmori K, Kohno M. Prognostic value of circulating regulatory T cells for worsening heart failure in heart failure patients with reduced ejection fraction. Int Heart J 55: 271–277, 2014. doi: 10.1536/ihj.13-343. [DOI] [PubMed] [Google Scholar]
- 66.Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, Snapinn S, Harris KE, Aurup P, Edelman JM, Wedel H, Lindholm LH, Dahlöf B; LIFE Study Investigators . Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA 292: 2343–2349, 2004. doi: 10.1001/jama.292.19.2343. [DOI] [PubMed] [Google Scholar]
- 67.Pankuweit S, Klingel K. Viral myocarditis: from experimental models to molecular diagnosis in patients. Heart Fail Rev 18: 683–702, 2013. doi: 10.1007/s10741-012-9357-4. [DOI] [PubMed] [Google Scholar]
- 68.Pasini E, Aquilani R, Testa C, Baiardi P, Angioletti S, Boschi F, Verri M, Dioguardi F. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail 4: 220–227, 2016. doi: 10.1016/j.jchf.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 69.Patel B, Bansal SS, Ismahil MA, Hamid T, Rokosh G, Mack M, Prabhu SD. CCR2+ monocyte-derived infiltrating macrophages are required for adverse cardiac remodeling during pressure overload. JACC Basic Transl Sci 3: 230–244, 2018. doi: 10.1016/j.jacbts.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patel B, Ismahil MA, Hamid T, Bansal SS, Prabhu SD. Mononuclear phagocytes are dispensable for cardiac remodeling in established pressure-overload heart failure. PLoS One 12: e0170781, 2017. doi: 10.1371/journal.pone.0170781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pistulli R, König S, Drobnik S, Kretzschmar D, Rohm I, Lichtenauer M, Fritzenwanger M, Mall G, Mall G, Figulla HR, Yilmaz A. Decrease in dendritic cells in endomyocardial biopsies of human dilated cardiomyopathy. Eur J Heart Fail 15: 974–985, 2013. doi: 10.1093/eurjhf/hft054. [DOI] [PubMed] [Google Scholar]
- 72.Ramos GC, van den Berg A, Nunes-Silva V, Weirather J, Peters L, Burkard M, Friedrich M, Pinnecker J, Abeßer M, Heinze KG, Schuh K, Beyersdorf N, Kerkau T, Demengeot J, Frantz S, Hofmann U. Myocardial aging as a T-cell-mediated phenomenon. Proc Natl Acad Sci USA 114: E2420–E2429, 2017. doi: 10.1073/pnas.1621047114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rangachari M, Mauermann N, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, Penninger JM, Eriksson U. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med 203: 2009–2019, 2006. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Remick J, Georgiopoulou V, Marti C, Ofotokun I, Kalogeropoulos A, Lewis W, Butler J. Heart failure in patients with human immunodeficiency virus infection: epidemiology, pathophysiology, treatment, and future research. Circulation 129: 1781–1789, 2014. doi: 10.1161/CIRCULATIONAHA.113.004574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J Jr, Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci USA 88: 8277–8281, 1991. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salvador AM, Nevers T, Velázquez F, Aronovitz M, Wang B, Abadía Molina A, Jaffe IZ, Karas RH, Blanton RM, Alcaide P. Intercellular adhesion molecule 1 regulates left ventricular leukocyte infiltration, cardiac remodeling, and function in pressure overload-induced heart failure. J Am Heart Assoc 5: e003126, 2016. doi: 10.1161/JAHA.115.003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, Schroedl W, Karhausen T, Doehner W, Rauchhaus M, Poole-Wilson P, Volk HD, Lochs H, Anker SD. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol 50: 1561–1569, 2007. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 78.Saxena A, Bujak M, Frunza O, Dobaczewski M, Gonzalez-Quesada C, Lu B, Gerard C, Frangogiannis NG. CXCR3-independent actions of the CXC chemokine CXCL10 in the infarcted myocardium and in isolated cardiac fibroblasts are mediated through proteoglycans. Cardiovasc Res 103: 217–227, 2014. doi: 10.1093/cvr/cvu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saxena A, Dobaczewski M, Rai V, Haque Z, Chen W, Li N, Frangogiannis NG. Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function. Am J Physiol Heart Circ Physiol 307: H1233–H1242, 2014. doi: 10.1152/ajpheart.00328.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schnoor M, Alcaide P, Voisin MB, van Buul JD. Crossing the vascular wall: common and unique mechanisms exploited by different leukocyte subsets during extravasation. Mediators Inflamm 2015: 946509, 2015. doi: 10.1155/2015/946509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharir R, Semo J, Shimoni S, Ben-Mordechai T, Landa-Rouben N, Maysel-Auslender S, Shaish A, Entin-Meer M, Keren G, George J. Experimental myocardial infarction induces altered regulatory T cell hemostasis, and adoptive transfer attenuates subsequent remodeling. PLoS One 9: e113653, 2014. doi: 10.1371/journal.pone.0113653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shi Y, Fukuoka M, Li G, Liu Y, Chen M, Konviser M, Chen X, Opavsky MA, Liu PP. Regulatory T cells protect mice against coxsackievirus-induced myocarditis through the transforming growth factor beta-coxsackie-adenovirus receptor pathway. Circulation 121: 2624–2634, 2010. doi: 10.1161/CIRCULATIONAHA.109.893248. [DOI] [PubMed] [Google Scholar]
- 83.Shim SH, Kim DS, Cho W, Nam JH. Coxsackievirus B3 regulates T-cell infiltration into the heart by lymphocyte function-associated antigen-1 activation via the cAMP/Rap1 axis. J Gen Virol 95: 2010–2018, 2014. doi: 10.1099/vir.0.065755-0. [DOI] [PubMed] [Google Scholar]
- 84.Störk S, Boivin V, Horf R, Hein L, Lohse MJ, Angermann CE, Jahns R. Stimulating autoantibodies directed against the cardiac beta1-adrenergic receptor predict increased mortality in idiopathic cardiomyopathy. Am Heart J 152: 697–704, 2006. doi: 10.1016/j.ahj.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 85.Tang H, Zhong Y, Zhu Y, Zhao F, Cui X, Wang Z. Low responder T cell susceptibility to the suppressive function of regulatory T cells in patients with dilated cardiomyopathy. Heart 96: 765–771, 2010. doi: 10.1136/hrt.2009.184945. [DOI] [PubMed] [Google Scholar]
- 86.Tang TT, Ding YJ, Liao YH, Yu X, Xiao H, Xie JJ, Yuan J, Zhou ZH, Liao MY, Yao R, Cheng Y, Cheng X. Defective circulating CD4CD25+Foxp3+CD127(low) regulatory T-cells in patients with chronic heart failure. Cell Physiol Biochem 25: 451–458, 2010. doi: 10.1159/000303050. [DOI] [PubMed] [Google Scholar]
- 87.Tang TT, Yuan J, Zhu ZF, Zhang WC, Xiao H, Xia N, Yan XX, Nie SF, Liu J, Zhou SF, Li JJ, Yao R, Liao MY, Tu X, Liao YH, Cheng X. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol 107: 232, 2012. doi: 10.1007/s00395-011-0232-6. [DOI] [PubMed] [Google Scholar]
- 88.Tang TT, Zhu ZF, Wang J, Zhang WC, Tu X, Xiao H, Du XL, Xia JH, Dong NG, Su W, Xia N, Yan XX, Nie SF, Liu J, Zhou SF, Yao R, Xie JJ, Jevallee H, Wang X, Liao MY, Shi GP, Fu M, Liao YH, Cheng X. Impaired thymic export and apoptosis contribute to regulatory T-cell defects in patients with chronic heart failure. PLoS One 6: e24272, 2011. [Erratum in PLoS ONE 6: 10.1371/annotation/bc0b9ac0-b1d4-44ff-aa8c-cb27e199c1b1]. doi: 10.1371/journal.pone.0024272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol 188: 4876–4884, 2012. doi: 10.4049/jimmunol.1200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3: 541–547, 1995. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 91.Tourki B, Halade G. Leukocyte diversity in resolving and nonresolving mechanisms of cardiac remodeling. FASEB J 31: 4226–4239, 2017. doi: 10.1096/fj.201700109R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tse K, Tse H, Sidney J, Sette A, Ley K. T cells in atherosclerosis. Int Immunol 25: 615–622, 2013. doi: 10.1093/intimm/dxt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ueland T, Gullestad L, Nymo SH, Yndestad A, Aukrust P, Askevold ET. Inflammatory cytokines as biomarkers in heart failure. Clin Chim Acta 443: 71–77, 2015. doi: 10.1016/j.cca.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 94.Vulesevic B, Sirois MG, Allen BG, de Denus S, White M. Subclinical inflammation in heart failure: a neutrophil perspective. Can J Cardiol 34: 717–725, 2018. doi: 10.1016/j.cjca.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 95.Wang H, Kwak D, Fassett J, Liu X, Yao W, Weng X, Xu X, Xu Y, Bache RJ, Mueller DL, Chen Y. Role of bone marrow-derived CD11c+ dendritic cells in systolic overload-induced left ventricular inflammation, fibrosis and hypertrophy. Basic Res Cardiol 112: 25, 2017. doi: 10.1007/s00395-017-0615-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res 115: 55–67, 2014. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- 97.Wiedle G, Dunon D, Imhof BA. Current concepts in lymphocyte homing and recirculation. Crit Rev Clin Lab Sci 38: 1–31, 2001. doi: 10.1080/20014091084164. [DOI] [PubMed] [Google Scholar]
- 98.Witztum JL, Lichtman AH. The influence of innate and adaptive immune responses on atherosclerosis. Annu Rev Pathol 9: 73–102, 2014. doi: 10.1146/annurev-pathol-020712-163936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu L, Ong S, Talor MV, Barin JG, Baldeviano GC, Kass DA, Bedja D, Zhang H, Sheikh A, Margolick JB, Iwakura Y, Rose NR, Ciháková D. Cardiac fibroblasts mediate IL-17A-driven inflammatory dilated cardiomyopathy. J Exp Med 211: 1449–1464, 2014. doi: 10.1084/jem.20132126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yamashita T, Iwakura T, Matsui K, Kawaguchi H, Obana M, Hayama A, Maeda M, Izumi Y, Komuro I, Ohsugi Y, Fujimoto M, Naka T, Kishimoto T, Nakayama H, Fujio Y. IL-6-mediated Th17 differentiation through RORγt is essential for the initiation of experimental autoimmune myocarditis. Cardiovasc Res 91: 640–648, 2011. doi: 10.1093/cvr/cvr148. [DOI] [PubMed] [Google Scholar]
- 101.Yamashita T, Sasaki N, Kasahara K, Hirata K. Anti-inflammatory and immune-modulatory therapies for preventing atherosclerotic cardiovascular disease. J Cardiol 66: 1–8, 2015. doi: 10.1016/j.jjcc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 102.Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, Marshall MA, French BA, Linden J. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation 114: 2056–2064, 2006. doi: 10.1161/CIRCULATIONAHA.106.649244. [DOI] [PubMed] [Google Scholar]
- 103.Yndestad A, Holm AM, Müller F, Simonsen S, Frøland SS, Gullestad L, Aukrust P. Enhanced expression of inflammatory cytokines and activation markers in T-cells from patients with chronic heart failure. Cardiovasc Res 60: 141–146, 2003. doi: 10.1016/S0008-6363(03)00362-6. [DOI] [PubMed] [Google Scholar]
- 104.Yu HT, Park S, Shin EC, Lee WW. T cell senescence and cardiovascular diseases. Clin Exp Med 16: 257–263, 2016. doi: 10.1007/s10238-015-0376-z. [DOI] [PubMed] [Google Scholar]
- 105.Zhang J, Crowley SD. Role of T lymphocytes in hypertension. Curr Opin Pharmacol 21: 14–19, 2015. doi: 10.1016/j.coph.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu J, Jankovic D, Oler AJ, Wei G, Sharma S, Hu G, Guo L, Yagi R, Yamane H, Punkosdy G, Feigenbaum L, Zhao K, Paul WE. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity 37: 660–673, 2012. doi: 10.1016/j.immuni.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zouggari Y, Ait-Oufella H, Waeckel L, Vilar J, Loinard C, Cochain C, Récalde A, Duriez M, Levy BI, Lutgens E, Mallat Z, Silvestre JS. Regulatory T cells modulate postischemic neovascularization. Circulation 120: 1415–1425, 2009. [Erratum in Circulation 121: e31, 2010]. doi: 10.1161/CIRCULATIONAHA.109.875583. [DOI] [PubMed] [Google Scholar]