Abstract
Dietary nitrate () supplementation has been shown to reduce resting blood pressure. However, the mechanism responsible for the reduction in blood pressure has not been identified. Dietary supplementation may increase nitric oxide (NO) bioavailability, and NO has been shown to inhibit sympathetic vasoconstriction in resting and contracting skeletal muscle. Therefore, the purpose of this study was to investigate the hypothesis that acute dietary supplementation would attenuate sympathetic vasoconstrictor responsiveness at rest and during exercise. In a double-blind randomized crossover design, 12 men (23 ± 5 yr) performed a cold-pressor test (CPT) at rest and during moderate- and heavy-intensity alternate-leg knee-extension exercise after consumption of rich beetroot juice (~12.9 mmol ) or a -depleted placebo (~0.13 mmol ). Venous blood was sampled before and 2.5 h after the consumption of beetroot juice for the measurement of total plasma nitrite/ [NOx]. Beat-by-beat blood pressure was measured by Finometer. Leg blood flow was measured at the femoral artery via Doppler ultrasound, and leg vascular conductance (LVC) was calculated. Sympathetic vasoconstrictor responsiveness was calculated as the percentage decrease in LVC in response to the CPT. Total plasma [NOx] was greater (P < 0.001) in the (285 ± 120 µM) compared with the placebo (65 ± 30 µM) condition. However, mean arterial blood pressure and plasma catecholamines were not different (P > 0.05) between and placebo conditions at rest or during moderate- and heavy-intensity exercise. Sympathetic vasoconstrictor responsiveness (Δ% LVC) was not different (P > 0.05) between and placebo conditions at rest (: −33 ± 10%; placebo: −35 ± 11%) or during moderate (: −18 ± 8%; placebo: −20 ± 10%)- and heavy (: −12 ± 8%; placebo: −11 ± 9%)-intensity exercise. These data demonstrate that acute dietary supplementation does not alter sympathetic vasoconstrictor responsiveness at rest or during exercise in young healthy males.
NEW & NOTEWORTHY Dietary nitrate may increase nitric oxide bioavailability, and nitric oxide has been shown to attenuate sympathetic vasoconstriction in resting and contracting skeletal muscle and enhance functional sympatholysis. However, the effect of dietary nitrate on sympathetic vasoconstrictor responsiveness is unknown. Acute dietary nitrate supplementation did not alter blood pressure or sympathetic vasoconstrictor responsiveness at rest or during exercise in young healthy males.
Keywords: autonomic nervous system, beetroot, blood flow, conductance, vascular
INTRODUCTION
Dietary nitrate () supplementation with beetroot juice has been shown to reduce resting blood pressure (BP) in sedentary and exercise-trained men and women (26, 35, 66, 67), and in patient populations (36, 56). Dietary appears to increase nitric oxide (NO) bioavailability and enhance brachial artery flow-mediated dilation (5, 24, 67, 70). NO has also been shown to inhibit sympathetic vasoconstriction in resting and contracting skeletal muscle (sympatholysis) (30–32, 34, 59, 63). Therefore, dietary supplementation may attenuate sympathetic vasoconstriction at rest and during exercise and alter sympathetic control of BP. Consistent with this notion, our laboratory has recently demonstrated that acute treatment with the NO cofactor tetrahydrobiopterin attenuated sympathetic vasoconstrictor responsiveness at rest and during exercise in rats (33).
An increase in NO bioavailability may also alter efferent sympathetic outflow. Indeed, Notay et al. (46) recently reported that acute dietary supplementation decreased muscle sympathetic nerve activity (MSNA) at rest and blunted the MSNA response to sympathoexcitation via static handgrip exercise in young adults. However, the effect of dietary supplementation on sympathetic nervous system activity during large muscle mass dynamic exercise has not been investigated.
Therefore, the purpose of this study was to investigate the effect of acute dietary supplementation on sympathetic vasoconstrictor responsiveness, BP, and plasma norepinephrine (NE) and epinephrine (Epi) (an index of sympathetic outflow) at rest and during large muscle mass dynamic exercise in humans. It was hypothesized that acute dietary supplementation would: 1) attenuate sympathetic vasoconstrictor responsiveness at rest and during exercise and enhance sympatholysis; 2) reduce plasma catecholamines at rest and during dynamic large-muscle mass exercise and in response to sympathoexcitation, and 3) reduce BP at rest and blunt the BP response to exercise and sympathoexcitation.
METHODS
Subjects.
Twelve healthy young males (23 ± 5 yr) volunteered and provided written informed consent to participate in the study. All subjects were nonobese nonsmokers and did not have respiratory, cardiovascular, metabolic, neurological, or musculoskeletal disease. Subjects were not taking any medications known to alter the cardiovascular, respiratory, or metabolic response to exercise. Subjects were recreationally active but were not engaged in an exercise-training program during the project. The study was approved by the University of Alberta Health Research Ethics Board and was conducted according to the Declaration of Helsinki (2008).
Experimental protocol.
Subjects reported to the Integrative Human Exercise Physiology Laboratory at the University of Alberta on four separate occasions. Subjects reported to the laboratory in a rested state (no exercise 24 h prior), after having consumed a light meal 2 h before testing and having abstained from caffeine, alcohol, and ibuprofen for 12 h before testing. Subjects also refrained from using antibacterial mouthwash, toothpaste, or chewing gum before testing.
On day 1, subjects completed an incremental exercise test to volitional exhaustion (V̇o2peak) on a cycle ergometer (Ergoselect 200 K; Ergoline, Bitz, Germany) for determination of maximal aerobic capacity. Testing began with 2 min of resting data collection, after which the work rate was progressively incremented (30 W/min) in a ramp-like fashion. Criteria used to establish a maximal test included a plateau in O2 consumption (V̇o2) despite an increase in work rate, a respiratory exchange ratio >1.10, achievement of >90% of age-predicted maximal heart rate (HR), and volitional exhaustion.
On day 2, subjects performed an alternate-leg incremental exercise test to volitional exhaustion on a knee-extensor ergometer, as previously described (2, 11, 42). Briefly, subject legs were attached to padded bars attached to a lever arm on a Monark cycle ergometer (model 814E) to allow for alternating two-legged knee extension. Following a resting baseline, knee-extension exercise was performed at a cadence of 30 contractions/min for each leg in alternating pattern. Exercise was initiated from a baseline work rate of 18 watts and was increased by 3 watts every minute until volitional exhaustion or the subject was unable to maintain the required cadence. Criteria used to establish a maximal test included a plateau in V̇o2 despite an increase in work rate, a respiratory exchange ratio >1.10, and volitional exhaustion. The maximum work rate achieved was used to calculate the work rates for constant-load knee-extensor exercise on testing days 3 and 4.
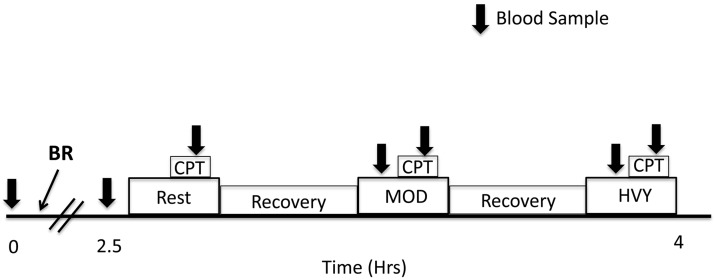
Testing on days 3 and 4 was performed in a randomized double-blind crossover manner and separated by at least 48 h. Subjects were asked to consume the same meal 12 h before and the same breakfast and snacks before testing on days 3 and 4. Subjects were also provided with a list of foods containing and were asked to avoid eating the foods on the list 48 h before testing. Following arrival at the laboratory at 8:00 AM on each day, an indwelling venous catheter was inserted in the antecubital vein of their right arm. Venous blood (10 ml) was drawn for the measurement of resting total plasma [/nitrite ()] (NOx) levels. Subjects then consumed either a placebo -depleted beetroot juice (~0.13 mmol : Beet It, James White Drinks, Ipswich, UK) or a -rich beetroot juice (~12.9 mmol ). Following 2.5 h of rest, the subject was seated and rested quietly on the alternate-leg knee-extension ergometer, and another venous blood sample was withdrawn. Data were collected under resting conditions for 7 min, subjects then performed a cold-pressor test (CPT) where they submerged their hand in −4°C ice water for 3 min, and another venous blood sample was withdrawn at the midway point of the CPT.
Following a recovery period, subjects performed 10 min of constant-load alternate-leg knee extension at 30% (24 ± 6 watts) and at 60% (47 ± 9 watts) of the peak work rate achieved on day 2. Knee-extension exercise was performed over a 2-s duty cycle (1 s contraction, 1 s relaxation), resulting in 30 contractions/min for each leg in an alternating pattern. Following 6 min of exercise, subjects submerged their hand in −4°C ice water for 3 min. Venous blood was sampled during exercise and the CPT. The timeline for testing and blood samples is shown in Fig. 1. Blood draws were attempted during each CPT; however, because of venoconstriction, it was either not possible to withdraw an adequate amount of blood or the blood sample was hemolyzed and unusable. Therefore, catecholamines could not be measured in response to the CPT. Blood samples for four subjects were mishandled postcollection; therefore, the sample size for catecholamine analysis at all other time points is n = 8. Rest and exercise trials were separated by a recovery period of ~30 min.
Fig. 1.
Timeline of testing on days 3 and 4. Following a resting blood sample, beetroot juice (BR) containing dietary nitrate () or placebo was consumed (time 0). After 2.5 h, a resting blood sample was taken while the subject was seated in the alternate-leg knee-extension ergometer. The resting trial (Rest) and moderate (MOD)- and heavy (HVY)-intensity exercise trials were separated by ~30 min of recovery. Subjects performed a 3-min cold pressor test (CPT) at rest and during each exercise bout. Blood samples were taken at rest, during exercise, and during each CPT and are denoted by the arrows.
Measurements.
Oxygen consumption, CO2 production, and ventilation were measured breath-by-breath using a mass flow sensor and metabolic cart (Vmax 229d; Viasys; Healthcare, Palm Springs, CA). The ECG was continuously recorded in a three-lead configuration (Power Laboratory 16/30; AD instruments, Colorado Springs, CO), and HR was derived from the ECG waveform. Beat-by-beat arterial BP was measured by finger photoplethysmography (Finometer, Amsterdam, the Netherlands). BP was also measured by sphygmomanometer, and Finometer BP was corrected to manually measured pressures when pressure differences were observed. Cardiac output (Q) and stroke volume (SV) were derived from the BP waveform via Modelflow. Modelflow estimates stroke volume from a three-component model that includes aortic impedance, arterial compliance, and vascular resistance (68). Studies that have compared echocardiography, dye-densitometry, and thermodilution measures of cardiac output with Modelflow estimates have reported that Modelflow reliably tracks changes in cardiac output in response to exercise and sympathoexcitation (13, 23, 27, 43, 51, 55, 57).
Femoral artery mean blood velocity (MBV) was measured in the right leg using pulsed-Doppler ultrasonography (Vivid I; GE, Waukesha, WI). Data were acquired continuously with a 7.5-MHz probe with a 45° angle of insonation placed on the skin surface 2–3 cm distal to the inguinal ligament. The diameter of the common femoral artery was measured during diastole in triplicate at rest. Previous studies have reported that the common femoral artery does not dilate during exercise; therefore, resting diameters were used to calculate blood flow at rest and during exercise (50, 53). Mean blood velocity was measured on a beat-by-beat basis. Leg blood flow (LBF) was calculated as LBF (ml/min) = MBV (cm/s)·πr2·60, where r is the radius of the femoral artery. Leg vascular conductance (LVC) was calculated as LBF/mean arterial pressure (MAP) (l·min−1·mmHg−1). The percentage change in LVC in response to the CPT was used to assess sympathetic vasoconstrictor responsiveness at rest and during exercise.
After withdrawal, venous blood samples were immediately mixed with EDTA and centrifuged at 2,500 revolutions/min for 10 min. Plasma was separated into aliquots, placed in microcentrifuge tubes, and frozen for subsequent analysis of plasma NE ([NE]) and Epi ([Epi]) concentration and plasma ([]) and concentration ([]). All samples from the same subjects were run in the same assay, and in duplicate. and were analyzed in plasma, using a commercially available colorimetric assay kit (Nitrate/Nitrite Colorimetric Assay Kit ab65328; Abcam, Toronto, Ontario), according to the procedures provided by the manufacturer. Total / was measured after the addition of reductase cofactor to each diluted sample, and the mixture was incubated for 3 h to allow for the full conversion of to . Greiss reagent was then added, which converts to a deep purple azo compound. The absorbance was measured at 540 nm, and quantification of each sample was performed with a calibration curve. The intra-assay coefficient of variation for the duplicate samples was 2.51%. Samples and standard curve were also prepared in the absence of reductase for measurement of plasma . Plasma is then calculated as the difference between total plasma / and plasma . Unfortunately, plasma in our samples is below the detection limit of the assay kit. Therefore, we report total / (NOx).
Plasma [NE] and [Epi] were measured using a commercially available enzyme-linked immunoassay kit using the manufacturer’s recommended procedures (2-CAT Plasma ELISA High Sensitive Enzyme Immunoassay; Rocky Mountain Diagnostics, Colorado Springs, CO). Briefly, NE and Epi were extracted by using a cis-diol-specific affinity gel, acylated, and then modified enzymatically. The absorbance was measured at 540 nm, and quantification of each sample was performed with a calibration curve. The intra-assay coefficient of variation for the duplicate samples was 15.34 and 9.33% for [NE] and [Epi], respectively.
Data analysis.
Data were recorded using a PowerLab 16/30 system and Chart 7 data acquisition software (AD Instruments) at a sampling frequency of 100 Hz.
Absolute values of HR, MAP, systolic BP (SBP), diastolic BP (DBP), LBF, and LVC were measured at rest and during exercise. Changes in HR, MAP, SBP, DBP, LBF, and LVC in response to the CPT at rest and during exercise were calculated. For each variable, the difference between the peak/nadir response (20 s average) and preceding baseline was calculated and was expressed as an absolute and percentage change. The percentage change of LVC (∆%LVC) in response to sympathoexcitation is the accepted metric to assess the magnitude of sympathetic vasoconstrictor responsiveness, since it accurately reflects the change in resistance vessel radius across different baseline levels of vascular conductance (7, 61). The magnitude of functional sympatholysis was calculated as the difference between the vasoconstrictor response at rest and during exercise (∆%LVC).
Statistical analysis.
All data are reported as means ± SD. The effect of dietary supplementation on HR, MAP, SBP, DBP, LBF, LVC, and V̇o2 at rest and during moderate- and heavy-intensity exercise was analyzed by two-way (exercise intensity × treatment) repeated-measures ANOVA. The effect of dietary supplementation on the response (absolute change) of HR, MAP, SBP, DBP, LBF, LVC, and V̇o2 to the CPT at rest and during exercise was analyzed by two-way (exercise intensity × treatment) repeated-measures ANOVA. The effect of dietary supplementation on sympatholysis and sympathetic vasoconstrictor responsiveness (∆%LVC) at rest and during exercise was also analyzed by two-way (exercise intensity × treatment) repeated-measures ANOVA. When significant main effects and/or interactions were identified, Student-Newman-Keuls post hoc analyses were performed (SigmaPlot version 13.0; Systat Software, San Jose, CA). A P value <0.05 was considered statistically significant.
RESULTS
Subject characteristics.
Subject age, height, weight, body mass index, absolute and relative V̇o2peak from the maximal cycling test, and maximal work rate and V̇o2peak achieved during the alternate-leg knee-extension test are reported in Table 1.
Table 1.
Subject characteristics
| Age, yr | 23 ± 5.0 |
| Height, cm | 178 ± 8 |
| Weight, kg | 73.0 ± 9.5 |
| Body mass index | 23 ± 1.6 |
| Absolute V̇o2peak, l/min | 3.34 ± 1.08 |
| Relative V̇o2peak, ml·kg−1·min−1 | 44.8 ± 11.7 |
| Peak KE work rate, watts | 77 ± 15 |
| Peak KE V̇o2, l/min | 1.78 ± 0.08 |
Values are means ± SD. KE, knee extension.
Effect of supplementation at rest.
-rich beetroot juice elevated (P < 0.05) total plasma Nox concentration ([NOx]) levels, whereas the -depleted beetroot juice did not alter (P > 0.05) total plasma [NOx] (Fig. 2). Resting HR, cardiac output, MAP, SBP, DBP, LBF, and LVC were not different between placebo and -supplemented conditions (Table 2) (P > 0.05). Dietary did not alter resting plasma [NE] or [Epi] (Fig. 3) (P > 0.05).
Fig. 2.
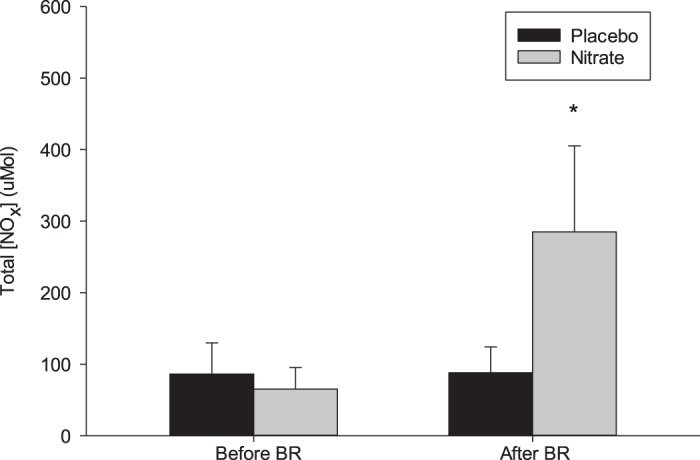
Total plasma /nitrite () at rest and 2.5 h following consumption of beetroot juice containing (n = 12) or -depleted (n = 12) beetroot juice. [NOx], total / concentration. Values are means ± SD. *Significant difference from placebo trial, P < 0.001.
Table 2.
Effect of dietary at rest and during moderate- and heavy-intensity alternate-leg knee-extension exercise
| Rest |
Moderate-Intensity Exercise |
Heavy-Intensity Exercise |
||||
|---|---|---|---|---|---|---|
| Placebo | Placebo | Placebo | ||||
| Heart rate, beats/min | 63 ± 10 | 61 ± 9 | 88 ± 11* | 85 ± 7* | 108 ± 12*# | 105 ± 10*# |
| Stroke volume, ml/beat | 103 ± 10 | 97 ± 16 | 127 ± 21* | 125 ± 26* | 125 ± 22* | 131 ± 29* |
| Cardiac output, l/min | 6.5 ± 1.0 | 5.9 ± 0.9 | 11.1 ± 1.5* | 10.5 ± 2.1* | 13.3 ± 2.0*# | 14.1 ± 2.6*# |
| Mean arterial pressure, mmHg | 84 ± 8 | 82 ± 5 | 97 ± 9* | 98 ± 7* | 116 ± 13*# | 110 ± 5*# |
| Systolic blood pressure, mmHg | 121 ± 12 | 117 ± 7 | 152 ± 14 | 151 ± 13 | 180 ± 18*# | 169 ± 15*# |
| Diastolic blood pressure, mmHg | 66 ± 6 | 64 ± 6 | 69 ± 9* | 73 ± 6* | 84 ± 11*# | 81 ± 4*# |
| Leg blood flow, l/min | 0.346 ± 0.086 | 0.324 ± 0.054 | 1.676 ± 0.365* | 1.722 ± 0.310* | 2.158 ± 0.366*# | 2.266 ± 0.377*# |
| Leg vascular conductance, l·min−1·mmHg−1 | 0.004 ± 0.001 | 0.004 ± 0.001 | 0.017 ± 0.004* | 0.018 ± 0.003* | 0.019 ± 0.004*# | 0.021 ± 0.003*# |
| Oxygen consumption, l/min | 0.257 ± 0.022 | 0.270 ± 0.031 | 0.802 ± 0.130* | 0.788 ± 0.086* | 1.161 ± 0.146*# | 1.169 ± 0.138*# |
Values are means ± SD. A significant (P < 0.05) main effect of exercise intensity was observed.
Significant post hoc test difference from rest.
Significant post hoc test difference from moderate-intensity exercise.
Fig. 3.
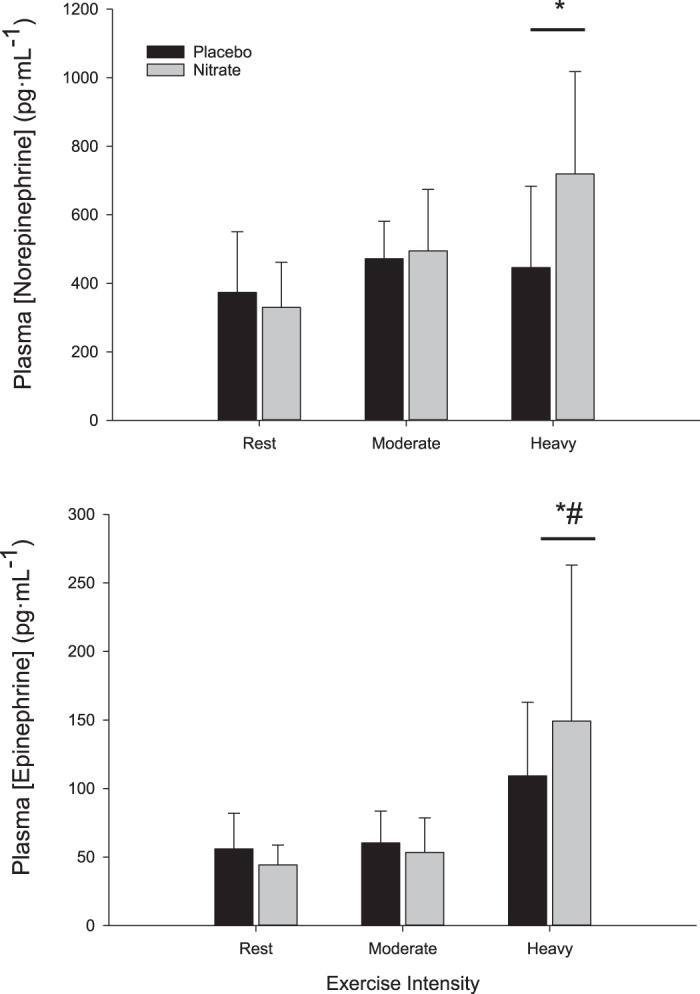
Plasma norepinephrine ([norepinephrine]) and epinephrine ([epinephrine]) concentration at rest and during moderate- and heavy-intensity exercise in the placebo (n = 8) and (n = 8) conditions. Values are means ± SD. A main effect of exercise intensity was observed. P < 0.05, significant post hoc test difference from rest (*) and significant post hoc test difference from moderate-intensity exercise (#).
Effect of supplementation during exercise.
MAP, SBP, DBP, HR, Q, LVC, V̇o2, and LBF increased (P < 0.05) in response to exercise and were not different between the placebo and supplementation conditions during moderate- and heavy-intensity exercise (Table 2) (P > 0.05). A main effect of exercise intensity (P < 0.05) on plasma [NE] and [Epi] was observed. Post hoc testing demonstrated that plasma [NE] was greater (P < 0.05) during heavy-intensity exercise compared with rest, and [Epi] was greater (P < 0.05) during heavy-intensity exercise compared with rest and moderate-intensity exercise (Fig. 3). Plasma [NE] or [Epi] were not different (P > 0.05) between placebo (n = 8) and -supplemented (n = 8) conditions during moderate- and heavy-intensity exercise (Fig. 3).
Effect of supplementation on the response to the CPT.
The responses of HR, SV, Q, MAP, SBP, DBP, LBF, LVC, and V̇o2 to the CPT at rest and during moderate- and heavy-intensity exercise are reported in Table 3. Sympathetic vasoconstrictor responsiveness (decrease in LVC in response to CPT) was not different (P > 0.05) between the supplementation and placebo conditions at rest or during moderate- and heavy-intensity exercise (Fig. 4). The magnitude of sympatholysis was also not different (P > 0.05) in the and placebo conditions (Fig. 5).
Table 3.
Effect of dietary on the response to cold-pressor test at rest, and during moderate- and heavy-intensity knee-extension exercise
| Rest |
Moderate Exercise |
Heavy Exercise |
||||
|---|---|---|---|---|---|---|
| Placebo | Placebo | Placebo | ||||
| ΔHeart rate, beats/min | 5 ± 8 | 6 ± 10 | −2 ± 6* | −4 ± 7* | −6 ± 6* | −5 ± 5* |
| ΔStroke volume, ml/beat | 10 ± 11 | 15 ± 17 | 2 ± 7* | 5 ± 7* | 1 ± 6* | −3 ± 14* |
| ΔCardiac output, l/min | 1.14 ± 0.8 | 1.63 ± 1.3 | 0.43 ± 0.8* | 0.85 ± 0.9* | 0.80 ± 0.8* | 0.33 ± 1.6* |
| ΔMean arterial pressure, mmHg | 22 ± 8 | 23 ± 11 | 17 ± 10* | 18 ± 8* | 9 ± 10*# | 10 ± 8*# |
| ΔSystolic blood pressure, mmHg | 29 ± 10 | 23 ± 11 | 23 ± 14 | 25 ± 10 | 14 ± 16*# | 18 ± 14*# |
| ΔDiastolic blood pressure, mmHg | 19 ± 7 | 19 ± 9 | 15 ± 9* | 15 ± 7* | 6 ± 7*# | 6 ± 6*# |
| ΔLeg blood flow, l/min | −0.063 ± 0.06 | −0.044 ± 0.03 | −0.115 ± 0.14 | −0.079 ± 0.13 | −0.107 ± 0.12 | −0.144 ± 0.10 |
| ΔLeg vascular conductance, l·min−1·mmHg−1 | −0.0015 ± 0.001 | −0.0013 ± 0.001 | −0.0037 ± 0.0028* | −0.0033 ± 0.0028* | −0.0023 ± 0.003* | −0.0029 ± 0.002* |
| ΔOxygen consumption, l/min | 0.053 ± 0.06 | 0.061 ± 0.10 | −0.020 ± 0.07* | −0.006 ± 0.07* | 0.006 ± 0.05 | 0.027 ± 0.10 |
Values are means ± SD. No effect of was observed on any variable at any exercise intensity. A significant (P < 0.05) main effect of exercise intensity was observed for all variables except leg blood flow.
Significant post hoc test difference from rest.
Significant post hoc test difference from moderate intensity.
Fig. 4.
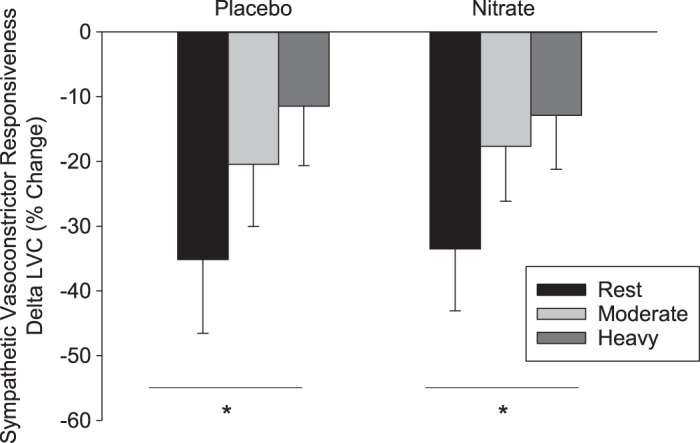
Percentage change in leg vascular conductance (LVC) at rest and during moderate- and heavy-intensity exercise in the placebo (n = 12) and (n = 12) conditions in response to a cold pressor test. Values are means ± SD. *Significant difference across all exercise conditions, P < 0.001.
Fig. 5.
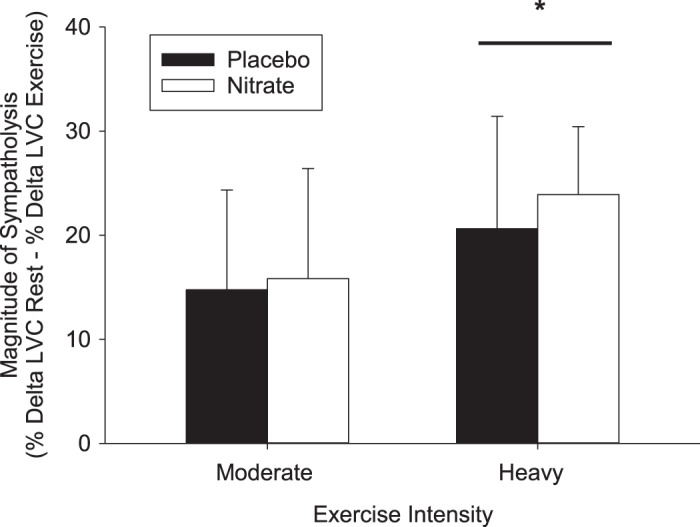
Magnitude of sympatholysis during moderate- and heavy-intensity exercise in the placebo (n = 12) and (n = 12) trial. LVC, leg vascular conductance. Values are means ± SD. *Significant difference from moderate-intensity exercise, P < 0.01.
DISCUSSION
The purpose of the present study was to investigate the effect of acute dietary supplementation on the control of sympathetic vasoconstriction at rest and in response to exercise. Consistent with previous studies, acute consumption of -rich beetroot juice increased total plasma [NOx], suggesting that NO bioavailability was enhanced following consumption of beetroot juice (26, 35, 41, 66, 67, 69). However, BP, plasma catecholamines, and sympathetic vasoconstrictor responsiveness were not different between placebo and supplementation conditions at rest or during exercise, indicating that dietary supplementation did not alter sympathetic nervous system-mediated vascular control at rest or during exercise in young healthy men.
Effect of supplementation on sympathetic vasoconstrictor responsiveness.
Dietary supplementation did not alter sympathetic vasoconstrictor responsiveness at rest or during moderate- or heavy-intensity exercise in the present study. Several studies in humans and rodents have reported that NO inhibits sympathetic vasoconstriction at rest (21, 44, 58) and during muscle contraction (9, 15, 21, 22, 31, 44, 47, 49, 54, 60, 63). Indeed, NO derived from both endothelial nitric oxide synthase (29, 47) and neuronal nitric oxide synthase (29, 54, 60, 62) has been shown to inhibit sympathetic vasoconstriction in human and animal preparations. In contrast, Dinenno and Joyner (12) reported that NOS inhibition did not alter tyramine-evoked vasoconstrictor responses in the forearm at rest or during handgrip exercise. Although the collective results from these studies are inconclusive, overall the accumulated evidence indicates that inhibition of NO production impairs the inhibition of sympathetic vasoconstriction in resting and contracting skeletal muscle of humans and rodents.
Fewer studies have investigated the effect of upregulating NO bioavailability on the inhibition of sympathetic vasoconstriction. The present findings suggest that enhanced NO bioavailability via beetroot juice supplementation does not alter sympathetic vasoconstrictor responsiveness to sympathoexcitation at rest or during exercise. Consistent with the present findings, oral sodium did not alter sympatholysis measured by near-infrared spectroscopy during small-muscle-mass mild-intensity (20% maximal voluntary contractile force) intermittent isometric handgrip exercise (45). Rosenmeier et al. (52) have also reported that infusion of the NO donor sodium nitroprusside did not alter vasoconstrictor responsiveness to tyramine or selective α-adrenergic receptor agonists. In contrast, our laboratory has shown that acute supplementation with the NO cofactor tetrahydrobiopterin reduced sympathetic vasoconstrictor responsiveness in resting and contracting skeletal muscle of rats (33).
Although the available scientific evidence is not conclusive, the present findings and other studies in humans indicate that enhanced NO bioavailability does not alter sympathetic vasoconstrictor responsiveness to sympathoexcitation at rest or during exercise.
An increase in NO bioavailability following dietary supplementation may augment the scavenging of reactive O2 species (ROS). Several studies have reported that treatment with superoxide scavenging anions enhanced the inhibition of sympathetic vasoconstriction in resting and contracting muscle (14, 17, 28, 64). In contrast, treatment with exogenous antioxidants has been shown to impair vasodilation (65). Although ROS were not measured in the present study, the lack of difference in sympathetic vasoconstrictor responsiveness and skeletal muscle blood flow between dietary and placebo conditions suggests that ROS levels were not different between placebo and conditions.
Effect of on blood pressure at rest and during exercise.
Resting MAP, SBP, and DBP were not different between placebo and supplementation conditions in the present study. Consistent with the present findings, other laboratories have also reported no effect of acute dietary supplementation on resting BP in young normotensive adults (8, 37). In contrast, several studies have reported a reduction of resting BP following acute dietary supplementation with beetroot juice (4, 26, 35, 38, 41, 66, 67, 69). The dose of dietary used in present study (12.9 mmol) was similar or above the amount of dietary used in other studies (26, 69), suggesting that the lack of effect of dietary on BP in the present study was not related to the dose of dietary . Subjects in the present study also had V̇o2peak values (~45 ± 12 ml·kg−1·min−1) similar to those in other studies that have reported reduced BP following acute dietary supplementation (38, 41, 66, 69), suggesting that the lack of effect of dietary on resting BP in the present study was not a function of aerobic fitness. Ghosh et al. (19) have reported reductions in SBP and DBP following acute dietary supplementation in hypertensive adults, suggesting that resting BP may influence the effect of dietary on BP. However, chronic dietary supplementation has been shown to have minimal effects on resting BP in hypertensive diabetics (20). Furthermore, Lansley et al. (38) and Vanhatalo et al. (66) have reported reduced SBP following supplementation in subjects with BPs similar to those in the present study. Collectively, these studies suggest that the effect of dietary supplementation on the control of BP is not a function of resting BP. Further investigation is required to fully understand the effect of dietary on the regulation of BP.
Effect of supplementation on plasma catecholamines.
Plasma catecholamines were not different between the placebo or condition at rest or during exercise in the present study. Local brain stem inhibition of NOS has been shown to increase sympathetic nerve activity, and studies have reported a decrease in BP following injection of NO donors in the brain stem, suggesting that brain stem NO bioavailability may inhibit efferent sympathetic nerve activity (6, 25, 48). Whether dietary treatment results in increased NO bioavailability in the cardiovascular control centers of the brain stem has not been established. However, in a group of healthy young African-American females, Bond et al. (4) reported increased heart rate variability with an unchanged low- to high-frequency spectral power ratio following acute dietary supplementation, suggesting that supplementation may alter autonomic control of heart rate. Moreover, Notay et al. (46) recently reported that acute dietary supplementation decreased resting MSNA and increased MSNA (burst incidence) in response to static handgrip exercise. Interestingly, these changes in MSNA occurred despite dietary supplementation having no effect on resting blood pressure and the blood pressure response to handgrip exercise. Differences in the mode and intensity of exercise, muscle mass involved in the exercise, method of sympathoexcitation, and index used to assess sympathetic outflow between these studies make it difficult to determine the reason for these divergent findings. Furthermore, the inability to measure catecholamines in response to the CPT in the present study limits our ability to compare responses to sympathoexcitation. We would not, however, expect acute dietary supplementation to alter the expression of postsynaptic α-adrenergic receptors; therefore, similar levels of circulating catecholamines appear consistent with similar vasoconstrictor responsiveness at rest and during exercise in the present study.
Effect of dietary on leg blood flow and V̇o2 at rest and during exercise.
Consistent with previous studies of forearm blood flow (8, 16, 37), acute dietary supplementation did not alter resting leg blood flow in the present study. Ferguson et al. (16) also reported no difference in resting hindlimb muscle blood flow between rats that received 5 days of dietary supplementation and controls. Collectively, the results of these studies suggest that enhanced NO bioavailability via dietary does not alter the regulation of resting skeletal muscle blood flow.
Dietary did not alter blood flow during exercise in the present study. Consistent with the present findings, several other studies have also reported no difference in blood flow during handgrip exercise in dietary and placebo conditions (8, 10, 44). In contrast, Ferguson et al. (16) reported increased total hindlimb blood flow during exercise in rats given 5 days of supplemental dietary . The findings of Ferguson et al. (16) may be specific to rats and may also reflect a difference between chronic versus acute supplementation. Furthermore, muscle blood flow was primarily increased to fast-twitch muscles of the rat hindlimb, suggesting that the effect of dietary on vascular control may be fiber-type specific (16).
Acute dietary supplementation did not alter V̇o2 during moderate- or heavy-intensity knee extension exercise in the present study. Other studies have also reported no effect of acute dietary supplementation on the O2 cost of exercise (3, 18). In contrast, chronic (3–5 days) dietary supplementation has been shown to reduce the O2 cost of exercise (1, 39, 40), suggesting that chronic supplementation may be necessary to alter metabolic efficiency.
In conclusion, acute dietary supplementation increased total plasma [NOx], suggesting that NO bioavailability was enhanced. However, blood pressure, blood flow, plasma catecholamines, and sympathetic vasoconstrictor responsiveness were not different between placebo and supplementation conditions at rest or during exercise. These results suggest that enhanced NO bioavailability following dietary supplementation did not alter sympathetic nervous system-mediated vascular control at rest or during exercise in young healthy males.
GRANTS
This project was supported by grants from the Natural Sciences and Engineering Research Council of Canada, the Canadian Foundation for Innovation, and the Sport Science Association of Alberta.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.J.d.V. and D.S.D. conceived and designed research; C.J.d.V. and D.S.D. performed experiments; C.J.d.V. and D.S.D. analyzed data; C.J.d.V. and D.S.D. interpreted results of experiments; C.J.d.V. and D.S.D. prepared figures; C.J.d.V. and D.S.D. drafted manuscript; C.J.d.V. and D.S.D. edited and revised manuscript; C.J.d.V. and D.S.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Rhonda DeLorey and Michelle Gonzalez for assistance with intravenous catheterization and blood sampling. We also thank Ian MacLean for assistance with the measurement of catecholamines.
REFERENCES
- 1.Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol (1985) 109: 135–148, 2010. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 2.Bell C, Paterson DH, Kowalchuk JM, Moy AP, Thorp DB, Noble EG, Taylor AW, Cunningham DA. Determinants of oxygen uptake kinetics in older humans following single-limb endurance exercise training. Exp Physiol 86: 659–665, 2001. doi: 10.1113/eph8602209. [DOI] [PubMed] [Google Scholar]
- 3.Betteridge S, Bescos R, Martorell M, Pons A, Garnham AP, Stathis CG, McConell GK. No effect of acute beetroot juice ingestion on oxygen consumption, glucose kinetics or skeletal muscle metabolism during submaximal exercise in males. J Appl Physiol (1985) 120: 391–398, 2015. doi: 10.1152/japplphysiol.00658.2015. [DOI] [PubMed] [Google Scholar]
- 4.Bond V, Curry BH, Adams RG, Asadi MS, Stancil KA, Millis RM, Haddad GE. Effects of nitrate supplementation on cardiovascular and autonomic reactivity in African-American females. ISRN Physiol pii: 676235, 2014. doi: 10.1155/2014/676235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bondonno CP, Yang X, Croft KD, Considine MJ, Ward NC, Rich L, Puddey IB, Swinny E, Mubarak A, Hodgson JM. Flavonoid-rich apples and nitrate-rich spinach augment nitric oxide status and improve endothelial function in healthy men and women: a randomized controlled trial. Free Radic Biol Med 52: 95–102, 2012. doi: 10.1016/j.freeradbiomed.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Bruno RM, Ghiadoni L, Seravalle G, Dell’oro R, Taddei S, Grassi G. Sympathetic regulation of vascular function in health and disease. Front Physiol 3: 284, 2012. doi: 10.3389/fphys.2012.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckwalter JB, Clifford PS. The paradox of sympathetic vasoconstriction in exercising skeletal muscle. Exerc Sport Sci Rev 29: 159–163, 2001. doi: 10.1097/00003677-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Casey DP, Treichler DP, Ganger CT IV, Schneider AC, Ueda K. Acute dietary nitrate supplementation enhances compensatory vasodilation during hypoxic exercise in older adults. J Appl Physiol (1985) 118: 178–186, 2015. doi: 10.1152/japplphysiol.00662.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG, Thomas GD. Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol 540: 377–386, 2002. doi: 10.1113/jphysiol.2001.013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig JC, Broxterman RM, Smith JR, Allen JD, Barstow TJ. Effect of dietary nitrate supplementation on conduit artery blood flow, muscle oxygenation, and metabolic rate during handgrip exercise. J Appl Physiol (1985) 125: 254–262, 2018. doi: 10.1152/japplphysiol.00772.2017. [DOI] [PubMed] [Google Scholar]
- 11.DeLorey DS, Shaw CN, Shoemaker JK, Kowalchuk JM, Paterson DH. The effect of hypoxia on pulmonary O2 uptake, leg blood flow and muscle deoxygenation during single-leg knee-extension exercise. Exp Physiol 89: 293–302, 2004. doi: 10.1113/expphysiol.2003.026864. [DOI] [PubMed] [Google Scholar]
- 12.Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments alpha-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol 287: H2576–H2584, 2004. doi: 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- 13.Dyson KS, Shoemaker JK, Arbeille P, Hughson RL. Modelflow estimates of cardiac output compared with Doppler ultrasound during acute changes in vascular resistance in women. Exp Physiol 95: 561–568, 2010. doi: 10.1113/expphysiol.2009.050815. [DOI] [PubMed] [Google Scholar]
- 14.Fadel PJ, Farias Iii M, Gallagher KM, Wang Z, Thomas GD. Oxidative stress and enhanced sympathetic vasoconstriction in contracting muscles of nitrate-tolerant rats and humans. J Physiol 590: 395–407, 2012. doi: 10.1113/jphysiol.2011.218917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fadel PJ, Zhao W, Thomas GD. Impaired vasomodulation is associated with reduced neuronal nitric oxide synthase in skeletal muscle of ovariectomized rats. J Physiol 549: 243–253, 2003. doi: 10.1113/jphysiol.2003.038828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J Physiol 591: 547–557, 2013. doi: 10.1113/jphysiol.2012.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao J, Zhao WX, Zhou LJ, Zeng BX, Yao SL, Liu D, Chen ZQ. Protective effects of propofol on lipopolysaccharide-activated endothelial cell barrier dysfunction. Inflamm Res 55: 385–392, 2006. doi: 10.1007/s00011-006-5116-0. [DOI] [PubMed] [Google Scholar]
- 18.Ghiarone T, Ataide-Silva T, Bertuzzi R, McConell GK, Lima-Silva AE. Effect of acute nitrate ingestion on VO2 response at different exercise intensity domains. Appl Physiol Nutr Metab 42: 1127–1134, 2017. doi: 10.1139/apnm-2017-0198. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh SM, Kapil V, Fuentes-Calvo I, Bubb KJ, Pearl V, Milsom AB, Khambata R, Maleki-Toyserkani S, Yousuf M, Benjamin N, Webb AJ, Caulfield MJ, Hobbs AJ, Ahluwalia A. Enhanced vasodilator activity of nitrite in hypertension: critical role for erythrocytic xanthine oxidoreductase and translational potential. Hypertension 61: 1091–1102, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00933. [DOI] [PubMed] [Google Scholar]
- 20.Gilchrist M, Winyard PG, Aizawa K, Anning C, Shore A, Benjamin N. Effect of dietary nitrate on blood pressure, endothelial function, and insulin sensitivity in type 2 diabetes. Free Radic Biol Med 60: 89–97, 2013. doi: 10.1016/j.freeradbiomed.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 21.Häbler HJ, Wasner G, Jänig W. Attenuation of neurogenic vasoconstriction by nitric oxide in hindlimb microvascular beds of the rat in vivo. Hypertension 30: 957–961, 1997. doi: 10.1161/01.HYP.30.4.957. [DOI] [PubMed] [Google Scholar]
- 22.Hansen J, Jacobsen TN, Victor RG. Is nitric oxide involved in the tonic inhibition of central sympathetic outflow in humans? Hypertension 24: 439–444, 1994. doi: 10.1161/01.HYP.24.4.439. [DOI] [PubMed] [Google Scholar]
- 23.Harms MP, Wesseling KH, Pott F, Jenstrup M, Van Goudoever J, Secher NH, Van Lieshout JJ. Continuous stroke volume monitoring by modelling flow from non-invasive measurement of arterial pressure in humans under orthostatic stress. Clin Sci (Lond) 97: 291–301, 1999. doi: 10.1042/cs0970291. [DOI] [PubMed] [Google Scholar]
- 24.Heiss C, Meyer C, Totzeck M, Hendgen-Cotta UB, Heinen Y, Luedike P, Keymel S, Ayoub N, Lundberg JO, Weitzberg E, Kelm M, Rassaf T. Dietary inorganic nitrate mobilizes circulating angiogenic cells. Free Radic Biol Med 52: 1767–1772, 2012. doi: 10.1016/j.freeradbiomed.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 25.Hirooka Y, Kishi T, Sakai K, Takeshita A, Sunagawa K. Imbalance of central nitric oxide and reactive oxygen species in the regulation of sympathetic activity and neural mechanisms of hypertension. Am J Physiol Regul Integr Comp Physiol 300: R818–R826, 2011. doi: 10.1152/ajpregu.00426.2010. [DOI] [PubMed] [Google Scholar]
- 26.Hobbs DA, Kaffa N, George TW, Methven L, Lovegrove JA. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Br J Nutr 108: 2066–2074, 2012. doi: 10.1017/S0007114512000190. [DOI] [PubMed] [Google Scholar]
- 27.Jansen JR, Schreuder JJ, Mulier JP, Smith NT, Settels JJ, Wesseling KH. A comparison of cardiac output derived from the arterial pressure wave against thermodilution in cardiac surgery patients. Br J Anaesth 87: 212–222, 2001. doi: 10.1093/bja/87.2.212. [DOI] [PubMed] [Google Scholar]
- 28.Jendzjowsky NG, DeLorey DS. Acute superoxide scavenging reduces sympathetic vasoconstrictor responsiveness in short-term exercise-trained rats. J Appl Physiol (1985) 114: 1511–1518, 2013. doi: 10.1152/japplphysiol.00131.2013. [DOI] [PubMed] [Google Scholar]
- 29.Jendzjowsky NG, DeLorey DS. Role of neuronal nitric oxide in the inhibition of sympathetic vasoconstriction in resting and contracting skeletal muscle of healthy rats. J Appl Physiol (1985) 115: 97–106, 2013. doi: 10.1152/japplphysiol.00250.2013. [DOI] [PubMed] [Google Scholar]
- 30.Jendzjowsky NG, DeLorey DS. Short-term exercise training augments 2-adrenoreceptor-mediated sympathetic vasoconstriction in resting and contracting skeletal muscle. J Physiol 591: 5221–5233, 2013. doi: 10.1113/jphysiol.2013.257626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jendzjowsky NG, DeLorey DS. Short-term exercise training enhances functional sympatholysis through a nitric oxide-dependent mechanism. J Physiol 591: 1535–1549, 2013. doi: 10.1113/jphysiol.2012.238998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jendzjowsky NG, Just TP, DeLorey DS. Exercise training augments neuronal nitric oxide synthase-mediated inhibition of sympathetic vasoconstriction in contracting skeletal muscle of rats. J Physiol 592: 4789–4802, 2014. doi: 10.1113/jphysiol.2014.278846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jendzjowsky NG, Just TP, Jones KE, DeLorey DS. Acute tetrahydrobiopterin supplementation attenuates sympathetic vasoconstrictor responsiveness in resting and contracting skeletal muscle of healthy rats. Physiol Rep 2: e12164, 2014. doi: 10.14814/phy2.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson JM. Physical training and the control of skin blood flow. Med Sci Sports Exerc 30: 382–386, 1998. doi: 10.1097/00005768-199803000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, Macallister R, Hobbs AJ, Webb AJ, Ahluwalia A. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension 56: 274–281, 2010. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 36.Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson JL, Vanbruggen M, Privette G, Yim E, Kraus WE, Allen JD. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol (1985) 110: 1582–1591, 2011. doi: 10.1152/japplphysiol.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JK, Moore DJ, Maurer DG, Kim-Shapiro DB, Basu S, Flanagan MP, Skulas-Ray AC, Kris-Etherton P, Proctor DN. Acute dietary nitrate supplementation does not augment submaximal forearm exercise hyperemia in healthy young men. Appl Physiol Nutr Metab 40: 122–128, 2015. doi: 10.1139/apnm-2014-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Gilchrist M, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol (1985) 110: 591–600, 2011. doi: 10.1152/japplphysiol.01070.2010. [DOI] [PubMed] [Google Scholar]
- 39.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic Biol Med 48: 342–347, 2010. doi: 10.1016/j.freeradbiomed.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 191: 59–66, 2007. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee JS, Stebbins CL, Jung E, Nho H, Kim JK, Chang MJ, Choi HM. Effects of chronic dietary nitrate supplementation on the hemodynamic response to dynamic exercise. Am J Physiol Regul Integr Comp Physiol 309: R459–R466, 2015. doi: 10.1152/ajpregu.00099.2015. [DOI] [PubMed] [Google Scholar]
- 42.MacPhee SL, Shoemaker JK, Paterson DH, Kowalchuk JM. Kinetics of O2 uptake, leg blood flow, and muscle deoxygenation are slowed in the upper compared with lower region of the moderate-intensity exercise domain. J Appl Physiol (1985) 99: 1822–1834, 2005. doi: 10.1152/japplphysiol.01183.2004. [DOI] [PubMed] [Google Scholar]
- 43.Matsukawa K, Kobayashi T, Nakamoto T, Murata J, Komine H, Noso M. Noninvasive evaluation of cardiac output during postural change and exercise in humans: comparison between the modelflow and pulse dye-densitometry. Jpn J Physiol 54: 153–160, 2004. doi: 10.2170/jjphysiol.54.153. [DOI] [PubMed] [Google Scholar]
- 44.Nase GP, Boegehold MA. Endothelium-derived nitric oxide limits sympathetic neurogenic constriction in intestinal microcirculation. Am J Physiol Heart Circ Physiol 273: H426–H433, 1997. doi: 10.1152/ajpheart.1997.273.1.H426. [DOI] [PubMed] [Google Scholar]
- 45.Nelson MD, Rosenberry R, Barresi R, Tsimerinov EI, Rader F, Tang X, Mason O, Schwartz A, Stabler T, Shidban S, Mobaligh N, Hogan S, Elashoff R, Allen JD, Victor RG. Sodium nitrate alleviates functional muscle ischaemia in patients with Becker muscular dystrophy. J Physiol 593: 5183–5200, 2015. doi: 10.1113/JP271252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Notay K, Incognito AV, Millar PJ. Acute beetroot juice supplementation on sympathetic nerve activity: a randomized, double-blind, placebo-controlled proof-of-concept study. Am J Physiol Heart Circ Physiol 313: H59–H65, 2017. doi: 10.1152/ajpheart.00163.2017. [DOI] [PubMed] [Google Scholar]
- 47.Ohyanagi M, Nishigaki K, Faber JE. Interaction between microvascular alpha 1- and alpha 2-adrenoceptors and endothelium-derived relaxing factor. Circ Res 71: 188–200, 1992. doi: 10.1161/01.RES.71.1.188. [DOI] [PubMed] [Google Scholar]
- 48.Patel KP, Li YF, Hirooka Y. Role of nitric oxide in central sympathetic outflow. Exp Biol Med (Maywood) 226: 814–824, 2001. doi: 10.1177/153537020122600902. [DOI] [PubMed] [Google Scholar]
- 49.Rabelo E, De Angelis K, Bock P, Gatelli Fernandes T, Cervo F, Belló Klein A, Clausell N, Cláudia Irigoyen M. Baroreflex sensitivity and oxidative stress in adriamycin-induced heart failure. Hypertension 38: 576–580, 2001. doi: 10.1161/hy09t1.096185. [DOI] [PubMed] [Google Scholar]
- 50.Rådegran G, Saltin B. Human femoral artery diameter in relation to knee extensor muscle mass, peak blood flow, and oxygen uptake. Am J Physiol Heart Circ Physiol 278: H162–H167, 2000. doi: 10.1152/ajpheart.2000.278.1.H162. [DOI] [PubMed] [Google Scholar]
- 51.Rang S, de Pablo Lapiedra B, van Montfrans GA, Bouma BJ, Wesseling KH, Wolf H. Modelflow: a new method for noninvasive assessment of cardiac output in pregnant women. Am J Obstet Gynecol 196: 235.e1–238, 2007. doi: 10.1016/j.ajog.2006.10.896. [DOI] [PubMed] [Google Scholar]
- 52.Rosenmeier JB, Fritzlar SJ, Dinenno FA, Joyner MJ. Exogenous NO administration and alpha-adrenergic vasoconstriction in human limbs. J Appl Physiol (1985) 95: 2370–2374, 2003. doi: 10.1152/japplphysiol.00634.2003. [DOI] [PubMed] [Google Scholar]
- 53.Saltin B, Rådegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand 162: 421–436, 1998. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- 54.Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci USA 97: 13818–13823, 2000. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Senay S, Toraman F, Gelmez S, Dağdelen S, Karabulut H, Alhan C. Continuous arterial pressure waveform analysis accurately detects cardiac output in cardiac surgery: a prospective comparison with thermodilution, echocardiography, and magnetic resonance techniques. Heart Surg Forum 12: E75–E78, 2009. doi: 10.1532/HSF98.20081142. [DOI] [PubMed] [Google Scholar]
- 56.Siervo M, Lara J, Ogbonmwan I, Mathers JC. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: a systematic review and meta-analysis. J Nutr 143: 818–826, 2013. doi: 10.3945/jn.112.170233. [DOI] [PubMed] [Google Scholar]
- 57.Sugawara J, Tanabe T, Miyachi M, Yamamoto K, Takahashi K, Iemitsu M, Otsuki T, Homma S, Maeda S, Ajisaka R, Matsuda M. Non-invasive assessment of cardiac output during exercise in healthy young humans: comparison between Modelflow method and Doppler echocardiography method. Acta Physiol Scand 179: 361–366, 2003. doi: 10.1046/j.0001-6772.2003.01211.x. [DOI] [PubMed] [Google Scholar]
- 58.Tesfamariam B, Weisbrod RM, Cohen RA. Endothelium inhibits responses of rabbit carotid artery to adrenergic nerve stimulation. Am J Physiol Heart Circ Physiol 253: H792–H798, 1987. doi: 10.1152/ajpheart.1987.253.4.H792. [DOI] [PubMed] [Google Scholar]
- 59.Thomas GD, Hansen J, Victor RG. ATP-sensitive potassium channels mediate contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Clin Invest 99: 2602–2609, 1997. doi: 10.1172/JCI119448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci USA 95: 15090–15095, 1998. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol (1985) 97: 731–738, 2004. doi: 10.1152/japplphysiol.00076.2004. [DOI] [PubMed] [Google Scholar]
- 62.Thomas GD, Shaul PW, Yuhanna IS, Froehner SC, Adams ME. Vasomodulation by skeletal muscle-derived nitric oxide requires alpha-syntrophin-mediated sarcolemmal localization of neuronal nitric oxide synthase. Circ Res 92: 554–560, 2003. doi: 10.1161/01.RES.0000061570.83105.52. [DOI] [PubMed] [Google Scholar]
- 63.Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol 506: 817–826, 1998. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomas GD, Zhang W, Victor RG. Impaired modulation of sympathetic vasoconstriction in contracting skeletal muscle of rats with chronic myocardial infarctions: role of oxidative stress. Circ Res 88: 816–823, 2001. doi: 10.1161/hh0801.089341. [DOI] [PubMed] [Google Scholar]
- 65.Trinity JD, Broxterman RM, Richardson RS. Regulation of exercise blood flow: role of free radicals. Free Radic Biol Med 98: 90–102, 2016. doi: 10.1016/j.freeradbiomed.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG, Jones AM. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol 299: R1121–R1131, 2010. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- 67.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 51: 784–790, 2008. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol (1985) 74: 2566–2573, 1993. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]
- 69.Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A, Jones AM. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol (1985) 115: 325–336, 2013. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]
- 70.Wylie LJ, Mohr M, Krustrup P, Jackman SR, Ermιdis G, Kelly J, Black MI, Bailey SJ, Vanhatalo A, Jones AM. Dietary nitrate supplementation improves team sport-specific intense intermittent exercise performance. Eur J Appl Physiol 113: 1673–1684, 2013. doi: 10.1007/s00421-013-2589-8. [DOI] [PubMed] [Google Scholar]