Abstract
High-frequency spinal cord stimulation (HF-SCS) applied at the T2 spinal level results in physiologic activation of the inspiratory muscles in C2 spinal-sectioned dogs. Although the bulbo-spinal fibers were cut, they likely survived the duration of acute experiments, and inspiratory muscle activation may have involved stimulation of these fibers. In two anesthetized, C2 paralyzed, intubated, and mechanically ventilated dogs, HF-SCS (300 Hz) was applied at the T2 level. The effectiveness of HF-SCS in generating inspired volume (V) and negative airway pressures (P) was evaluated over a period of 5 days during which time the bulbo-spinal fibers would have degenerated. Because the effectiveness of HF-SCS may be adversely affected by deterioration of these fibers and/or the condition of the animal, low-frequency (50 Hz) SCS (LF-SCS) was also performed and served as a control. All vital signs, oxygen saturation, and end-tidal Pco2 remained stable over the 5-day period. V and P also remained stable over the study period. For example, mean V and P were 771 ± 25 ml and 64 ± 1 cmH2O with HF-SCS (3 mA) during the initial and 674 ± 59 ml and 63 ± 5 cmH2O on the final day. Comparable values during LF-SCS (8 mA) were 467 ± 12 ml and 48 ± 1 cmH2O during the initial and 397 ± 20 ml and 42 ± 2 cmH2O on the final day. Because V and P in response to HF-SCS remained stable over a 5-day period following which the bulbo-spinal fibers would have degenerated, the mechanism of HF-SCS does not depend upon the viability of these tracts. HF-SCS therefore may be a useful method to restore ventilation in chronic ventilator dependent tetraplegics.
NEW & NOTEWORTHY This study indicates that the respiratory responses to high-frequency spinal cord stimulation applied at the T2 level results in activation of the inspiratory motoneuron pools via interneuronal circuits and/or the inspiratory motoneurons directly and does not depend upon activation of long descending inspiratory bulbo-spinal fibers. This method therefore, may provide an alternative method to restore ventilation in ventilator dependent spinal cord injured patients.
Keywords: respiration, respiratory muscles, spinal cord stimulation
INTRODUCTION
In previous studies, high-frequency spinal cord stimulation (HF-SCS) applied on the ventral epidural surface at the T2 level was shown to result in physiologic activation of the both the diaphragm and inspiratory intercostal muscles in C2-sectioned dogs (6–11, 23). Physiologic activation of the inspiratory muscles was demonstrated by the facts that the pattern of inspiratory muscle electromyogram was asynchronous as occurs during spontaneous breathing and motoneuron firing rates were similar to that occurring during normal breathing. Moreover, inspiratory intercostal activity was concentrated in those regions of muscle in which the mechanical advantage was greatest as also occurs during spontaneous breathing (4, 7, 8, 17, 21). The mechanisms by which HF-SCS results in inspiratory muscle activation are unknown. One possibility is that electrical stimulation results in activation of inspiratory neuronal circuits in the spinal cord and/or activates the inspiratory motoneurons directly (6–11). Alternatively, the observed effects of HF-SCS require stimulation of long descending tracts such as the inspiratory bulbo-spinal fibers. Although these fibers were cut at the C2 level, they likely survived given the short duration of the acute experiments and therefore may have been responsive to applied electrical stimulation. We hypothesized that HF-SCS activates the inspiratory muscles at the spinal level without the involvement of bulbo-spinal tracts. To address this issue, we performed HF-SCS in subacute dog experiments over a 5-day period following which the bulbo-spinal fibers would have degenerated (18, 25). Because the efficiency of HF-SCS may be affected by deterioration of these fibers and/or the condition of the animal, low-frequency (50 Hz) SCS (LF-SCS), which involved direct root stimulation (3, 5, 15), was also performed and served as a control. Our results demonstrate that the effects of HF-SCS on inspiratory muscle activation remain intact over this time period and therefore do not require activation of bulbo-spinal fibers.
METHODS
Studies were attempted in 3 adult mongrel dogs weighing 24–34 kg (mean 28.3 ± 2.8kg) with the approval of the Institutional Animal Care and Use Committees of Case Western Reserve University. All procedures were conducted under sterile conditions. Each animal was anesthetized initially with pentobarbital sodium [PB (25 mg/kg)], given intravenously. Additional doses of PB (1–2 mg/kg) were provided, as needed. Each animal was maintained under deep anesthesia throughout the procedure as determined by absence of pupillary response to light, response to noxious stimuli, corneal reflexes, and jaw tone.
Animals were tracheostomized and intubated with a cuffed endotracheal tube (10-mm ID), which was sutured into the trachea in the mid-cervical region. Catheters were placed in the femoral vein to administer fluids and supplemental anesthesia and the femoral artery for continuous monitoring of blood pressure and heart rate (Waveline Pro Multi-Function Monitor, DRE Inc., Louisville, KY). Body temperature was maintained with a heating blanket (Harvard Apparatus, Cambridge, MA) at 38 ± 0.5°C. Animals were mechanically ventilated with a Harvard Ventilator (Harvard Apparatus, Cambridge, MA). End-tidal Pco2 was maintained between 35 and 45 mmHg. Each animal was given intravenous cefazolin (20 mg/kg) every 8 h, intravenous heparin (300 units/kg) and intramuscular atropine (0.04 mg/kg) every 24 h, and intravenous 0.5% dextrose 5 ml·kg−1·h−1.
A laminectomy was performed at the T1 level to allow placement of a stimulation lead with four 4-mm contacts (model AD-TEDH Medical Instrument Corp, Racine, WI), which were inserted onto the ventral epidural surface of the spinal cord and advanced to the region of the T2 spinal level. A separate laminectomy was performed in the high cervical region to section the cervical spinal cord at the C2 level. The completeness of the section was verified by lifting a hook across the area of transection and confirmed postmortem by visual inspection.
Pressure (P) generation during SCS was measured at functional residual capacity under conditions of hyperventilation-induced apnea and airway occlusion with a pressure transducer (Validyne, MP45, Northridge, CA) connected to the airway opening. Volume (V) was recorded by electrical integration of the flow signal from a pneumotachograph (Series 3700, Hans Rudolph, Kansas City, MO). All recordings were monitored and stored for offline analysis on a computer utilizing a data acquisition and analyzing system (Spike2 with 1401 interface, CED Ltd., UK).
P and V were evaluated during HF-SCS following complete C2 section. Measurements of P and V were made every 6 h at stimulus amplitudes of 1, 2, 3, and 4 mA, stimulus frequency of 300 Hz, pulse width 0.2 ms. Based upon our previous studies, HF-SCS in this range of stimulation (plateaus reached at 3–4 mA, see Ref. 9), results in near-maximum P and V generation by activating both the diaphragm and intercostal muscles via spinal cord circuitry.
Because the effectiveness of HF-SCS could be adversely affected by deterioration in the condition of the animal, LF-SCS was also performed every 6 h (just following HF-SCS) at 1, 2, 3, 4, 6, and 8 mA and served as a control. Based upon our previous studies, LF-SCS with this range of stimulation (plateaus reached at 6 mA), results in significant P and V generation by predominantly activating the intercostal muscles via direct root stimulation and therefore would not be adversely affected by degeneration of spinal cord tracts (3, 5, 15). The fact that LF-SCS does not involve spinal cord pathways was determined by the demonstration that P before and after section of the T1–T5 spinal roots was unchanged (3). If we observed reductions in P and V during both LF-SCS and HF-SCS, this would suggest deterioration in the status of the animal rather than an effect of spinal cord section. All measurements were usually completed within a 10-min period during which time the animal was frequently placed back on mechanical ventilation to maintain stability of vital signs.
One animal expired within 2 days of study initiation because of suspected pneumonia, which was manifested by copious thick airway secretions and fever. The other two animals were maintained over a period of 5 full days. Therefore, data are only presented in the two successful experiments. The experiment was concluded in each of these two animals when the predetermined study duration had been achieved at which point each animal was euthanized.
Data analysis.
P and V were monitored over time during HF-SCS and LF-SCS; single measurements were taken four times/day, ~every 6 h. Consequently, the mean ± SE of four measurements was determined over the 5-day period. The control values refer to the initial measurements following the injury. Heart rate, oxygen saturation, respiratory rate, temperature, end-tidal Pco2, and mean blood pressure were also monitored over the 5-day period. Comparisons were made, where applicable, using repeated-measures ANOVA and post hoc Newman-Keuls tests. A P value < 0.05 was accepted as statistically significant. Data are reported as means ± SE.
RESULTS
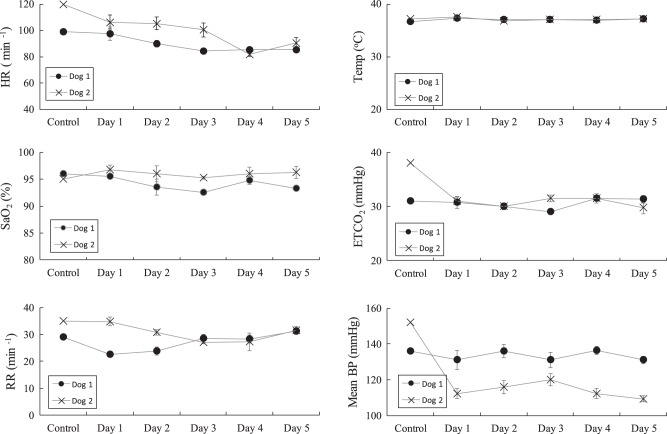
Mean values of heart rate, oxygen saturation, respiratory rate, body temperature, end-tidal Pco2, and blood pressure for each of the 5 days is shown in Fig. 1. Each parameter remained stable and in the physiologic range throughout the study period. There were no significant differences in either of the parameters over the 5-day period. However, in Dog 2, mean blood pressure decreased after the control measurements most likely secondary to a deepening level of anesthesia.
Fig. 1.
Mean values ± SE of vital signs over the course of the 5-day study period in each animal. Each of these values remained stable and in the physiologic range throughout the study period. Mean BP during control compared with subsequent days was different, likely secondary to a deeper level of anesthesia in Dog 2. ETCO2 end-tidal carbon dioxide; HR, heart rate; mean BP, mean arterial blood pressure; RR, respiratory rate; SaO2, oxygen saturation; Temp, body temperature.
The raw tracings of P and V during HF-SCS (1 mA, 300 Hz) for each animal are shown in Fig. 2 (top). Over the 5-day period, there were only small changes in P and V. In Dog 1, P and V were 795 ml and 63 cmH2O with HF-SCS during the initial day (control) and 715 ml and 70 cmH2O during the final day. In Dog 2, P and V were 773 ml and 65 cmH2O with HF-SCS during the initial day (control) and 623ml and 60 cmH2O during the final day.
Fig. 2.
Inspired volumes and airway pressure generation during HF-SCS (3 mA, 300 Hz) under control conditions and each day of the study period, for Dog 1 (left) and Dog 2 (right). Raw data of inspired volumes and airway pressure is shown in the upper panels. Mean inspired volume (middle) and airway pressure generation (bottom) under control conditions and each day of the study period, for each animal over a range of stimulus amplitudes during HF-SCS. There were no significant changes in these parameters over the duration of the study period. HF-SCS, high-frequency spinal cord stimulation.
The mean values (for each day) of P and V during HF-SCS over a range of stimulus amplitudes (1.0–4.0 mA) over the 5-day period for each animal are shown in Fig. 2 (middle and bottom). Over the course of the 5-day period, there were no significant differences in either parameter. For example, in Dog 1, the HF-SCS at 3 mA resulted in mean P and V during the initial and final day 5 of 795 ± 25 ml and 63 ± 2 cmH2O and 733 ± 23 ml and 68 ± 1 cmH2O, respectively (P < 0.05). In Dog 2, the HF-SCS at 3 mA resulted in mean P and V during the initial and final day 5 of 746 ± 32 ml and 65 ± 2 cmH2O and 615 ± 16 ml and 58 ± 2 cmH2O, respectively (P < 0.05).
The mean values of P and V during LF-SCS (50 Hz) over a range of stimulus amplitudes (1–8 mA) over the 5-day period are shown in Fig. 3. Over the course of the 5-day period, there were no significant differences in either parameter. For example, in Dog 1, the LF-SCS at 6 mA resulted in mean P and V during the initial and final day 5 of 415 ± 11 ml and 43 ± 3 cmH2O and 390 ± 29 ml and 42 ± 3 cmH2O, respectively (P < 0.05). In Dog 2, the LF-SCS at 6 mA resulted in mean P and V during the initial and final day 5 of 385 ± 13 ml and 39 ± 2 cmH2O and 306 ± 18 ml and 32 ± 3 cmH2O, respectively (P < 0.05).
Fig. 3.
Mean inspired volume and airway pressure generation under control conditions and each day of the study period, for each animal over a range of stimulus amplitudes during LF-SCS. Mean inspired volume (top) and airway pressure generation (bottom) under control conditions and each day of the study period, for each animal over a range of stimulus amplitudes during LF-SCS. There were no significant changes in these parameters over the duration of the study period. LF-SCS, low-frequency spinal cord stimulation.
DISCUSSION
The results of this investigation demonstrate that P and V generation in response to HF-SCS remains essentially unchanged over a period of 5 days. Because previous studies have demonstrated that the bulbo-spinal fibers would have fully degenerated over this time, these results provide strong evidence that activation of the inspiratory motoneuron pools via HF-SCS activates inspiratory neuronal circuits or the inspiratory motoneurons directly (25). Stated differently, the respiratory responses to HF-SCS are not dependent upon bulbo-spinal fibers. The respiratory responses to LF-SCS also remained stable throughout the duration of the experiment.
Study limitations.
Although these studies provided useful data in only two of the three animals, the results in each were very similar demonstrating that the effects of HF-SCS on P and V generation remained completely intact. The effects of LF-SCS, monitored as a control, also remained unchanged. It is also important to emphasize that in each of the two experiments, which lasted 5 days, the study was concluded when the predetermined study duration had been achieved. In fact, each animal was in stable condition, as determined by measurements of blood pressure, heart rate, oxygen saturation and end-tidal Pco2 at the end of these studies. Given the high cost and labor-intensive nature of this study and consistency of results in both animals therefore, we felt it was reasonable to forego additional studies.
There are only few electrophysiological studies which have evaluated the rate of degeneration of bulbo-spinal fibers over time. Perhaps most notable, McDonald (25) evaluated the time course of Wallerian degeneration in adult cats. In that study, the posterior columns were transected at the L1–L2 level. Conduction in the posterior columns was studied 24 h to 14 days after transection. The time course of degeneration was determined by measuring the amplitude of the posterior column compound action potential. The compound action potential was of normal amplitude at 24 h after section. Although many fibers ceased to conduct within 48 h, the great majority failed by the fourth day with no further declines beyond 1 wk. Large fibers failed at the same rate as medium sized fibers. McDonald also found that the time course of axonal disruption as determined by histological analysis correlated with the time course of physiological changes. Of interest, other electrophysiological studies have found that conduction failed at 54–101 h in peripheral nerves (16, 20, 24, 26, 30, 31). Although the bulbo-spinal tracts may not have fully degenerated over the 5-day period in the present investigation, these prior studies provide strong evidence that there was substantial loss of function over the 120 h of observation. Consequently, if bulbo-spinal fibers were involved in the responses to HF-SCS, a significant decline in physiologic outcome parameters would have been observed.
Clinical implications.
Current methods of diaphragm pacing are successful in achieving full-time ventilatory support in ventilator dependent tetraplegics in only ~50% of patients (12–14). There are several potential explanations for lack of greater success of this method including the fact that the intercostal muscles, which are responsible for ~40% of the vital capacity are not activated (2, 15, 22, 32). In addition, the pattern of diaphragm activation results in the conversion of the diaphragm to predominantly slow twitch muscle fibers which have high endurance capacity but reduced strength, resulting in smaller V generation (1, 19, 27–29). Finally, current electrode technology does not result in complete activation of the diaphragm, which also reduces V. In contrast, HF-SCS activates both the inspiratory intercostal muscles and diaphragm, results in more physiologic activation, which is not likely to alter muscle fiber composition and, based upon previous animal studies, generates ~90% of the inspiratory capacity (6–11). HF-SCS to restore ventilation, therefore, may have a significantly higher success rate compared with current technology.
For purposes of clinical application, the results of the present study are reassuring because the effects of HF-SCS are maintained in the subacute preparation and therefore not dependent on viable bulbo-spinal tracts. It is therefore likely that this technique would be successful in restoring ventilation in chronic ventilator dependent tetraplegics and provide an additional alternative to mechanical ventilation. Clinical trials will be necessary to fully evaluate the potential usefulness of this technique.
GRANTS
This work was supported by the National Institutes of Health grant no. R01 NS105785 and the MetroHeath Foundation.
DISCLOSURES
A. DiMarco is a founder of and has a significant financial interest in Synapse BioMedical, a manufacturer of diaphragm pacing systems and holds patents for spinal cord stimulation to restore cough and respiration (nos. 5,678,535; 5,911,218; 5,999,855; and 8,751,004).
A. DiMarco and K. Kowalski hold the U.S. patents for technology related to the content of this manuscript, Respiratory Muscle Activation by Spinal Cord Stimulation (no. 8,352,036).
AUTHOR CONTRIBUTIONS
A.F.D. and K.E.K. conceived and designed research; A.F.D. and K.E.K. performed experiments; A.F.D. and K.E.K. analyzed data; A.F.D. and K.E.K. interpreted results of experiments; A.F.D. and K.E.K. prepared figures; A.F.D. and K.E.K. drafted manuscript; A.F.D. and K.E.K. edited and revised manuscript; A.F.D. and K.E.K. approved final version of manuscript.
REFERENCES
- 1.Acker MA, Mannion JD, Brown WE, Salmons S, Henriksson J, Bitto T, Gale DR, Hammond R, Stephenson LW. Canine diaphragm muscle after 1 yr of continuous electrical stimulation: its potential as a myocardial substitute. J Appl Physiol (1985) 62: 1264–1270, 1987. doi: 10.1152/jappl.1987.62.3.1264. [DOI] [PubMed] [Google Scholar]
- 2.Agostoni E, Mognoni P, Torri G, Agostoni AF. Static features of the passive rib cage and abdomen-diaphragm. J Appl Physiol 20: 1187–1193, 1965. doi: 10.1152/jappl.1965.20.6.1187. [DOI] [Google Scholar]
- 3.Budzinska K, Supinski G, DiMarco AF. Inspiratory action of separate external and parasternal intercostal muscle contraction. J Appl Physiol (1985) 67: 1395–1400, 1989. doi: 10.1152/jappl.1989.67.4.1395. [DOI] [PubMed] [Google Scholar]
- 4.De Troyer A, Kirkwood PA, Wilson TA. Respiratory action of the intercostal muscles. Physiol Rev 85: 717–756, 2005. doi: 10.1152/physrev.00007.2004. [DOI] [PubMed] [Google Scholar]
- 5.DiMarco AF, Altose MD, Cropp A, Durand D. Activation of the inspiratory intercostal muscles by electrical stimulation of the spinal cord. Am Rev Respir Dis 136: 1385–1390, 1987. doi: 10.1164/ajrccm/136.6.1385. [DOI] [PubMed] [Google Scholar]
- 6.DiMarco AF, Kowalski KE. Activation of inspiratory muscles via spinal cord stimulation. Respir Physiol Neurobiol 189: 438–449, 2013. doi: 10.1016/j.resp.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiMarco AF, Kowalski KE. Distribution of electrical activation to the external intercostal muscles during high frequency spinal cord stimulation in dogs. J Physiol 589: 1383–1395, 2011. doi: 10.1113/jphysiol.2010.199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiMarco AF, Kowalski KE. Electrical activation to the parasternal intercostal muscles during high-frequency spinal cord stimulation in dogs. J Appl Physiol (1985) 118: 148–155, 2015. doi: 10.1152/japplphysiol.01321.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiMarco AF, Kowalski KE. High-frequency spinal cord stimulation of inspiratory muscles in dogs: a new method of inspiratory muscle pacing. J Appl Physiol (1985) 107: 662–669, 2009. doi: 10.1152/japplphysiol.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiMarco AF, Kowalski KE. Intercostal muscle pacing with high frequency spinal cord stimulation in dogs. Respir Physiol Neurobiol 171: 218–224, 2010. doi: 10.1016/j.resp.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiMarco AF, Kowalski KE. Spinal pathways mediating phrenic activation during high frequency spinal cord stimulation. Respir Physiol Neurobiol 186: 1–6, 2013. doi: 10.1016/j.resp.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiMarco AF, Onders RP, Ignagni A, Kowalski KE. Inspiratory muscle pacing in spinal cord injury: case report and clinical commentary. J Spinal Cord Med 29: 95–108, 2006. doi: 10.1080/10790268.2006.11753863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiMarco AF, Onders RP, Ignagni A, Kowalski KE, Mortimer JT. Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest 127: 671–678, 2005. doi: 10.1378/chest.127.2.671. [DOI] [PubMed] [Google Scholar]
- 14.DiMarco AF, Onders RP, Kowalski KE, Miller ME, Ferek S, Mortimer JT. Phrenic nerve pacing in a tetraplegic patient via intramuscular diaphragm electrodes. Am J Respir Crit Care Med 166: 1604–1606, 2002. doi: 10.1164/rccm.200203-175CR. [DOI] [PubMed] [Google Scholar]
- 15.DiMarco AF, Supinski GS, Budzinska K. Inspiratory muscle interaction in the generation of changes in airway pressure. J Appl Physiol (1985) 66: 2573–2578, 1989. doi: 10.1152/jappl.1989.66.6.2573. [DOI] [PubMed] [Google Scholar]
- 16.Erlanger J, Schoepfle GM. A study of nerve degeneration and regeneration. Am J Physiol 147: 550–581, 1946. doi: 10.1152/ajplegacy.1946.147.3.550. [DOI] [PubMed] [Google Scholar]
- 17.Gandevia SC, Hudson AL, Gorman RB, Butler JE, De Troyer A. Spatial distribution of inspiratory drive to the parasternal intercostal muscles in humans. J Physiol 573: 263–275, 2006. doi: 10.1113/jphysiol.2005.101915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandevia SC, Kirkwood PA. Spinal breathing: stimulation and surprises. J Physiol 589: 2661–2662, 2011. doi: 10.1113/jphysiol.2011.210476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glenn WW, Hogan JF, Phelps ML. Ventilatory support of the quadriplegic patient with respiratory paralysis by diaphragm pacing. Surg Clin North Am 60: 1055–1078, 1980. doi: 10.1016/S0039-6109(16)42233-4. [DOI] [PubMed] [Google Scholar]
- 20.Gutmann E, Holubar J. The degeneration of peripheral nerve fibers. J Neurol Neurosurg Psychiatry 13: 89–105, 1950. doi: 10.1136/jnnp.13.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hudson AL, Gandevia SC, Butler JE. Common rostrocaudal gradient of output from human intercostal motoneurones during voluntary and automatic breathing. Respir Physiol Neurobiol 175: 20–28, 2011. doi: 10.1016/j.resp.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 22.Konno K, Mead J. Measurement of the separate volume changes of rib cage and abdomen during breathing. J Appl Physiol 22: 407–422, 1967. doi: 10.1152/jappl.1967.22.3.407. [DOI] [PubMed] [Google Scholar]
- 23.Kowalski KE, Hsieh YH, Dick TE, DiMarco AF. Diaphragm activation via high frequency spinal cord stimulation in a rodent model of spinal cord injury. Exp Neurol 247: 689–693, 2013. doi: 10.1016/j.expneurol.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lissák K, Dempsey EW, Rosenblueth A. The failure of transmission of motor nerve impulses in the course of Wallerian degeneration. Am J Physiol 128: 45–56, 1939. doi: 10.1152/ajplegacy.1939.128.1.45. [DOI] [Google Scholar]
- 25.McDonald WI. The time course of conduction failure during degeneration of a central tract. Exp Brain Res 14: 550–556, 1972. doi: 10.1007/BF00236596. [DOI] [PubMed] [Google Scholar]
- 26.Novak J, Salafsky B. Early electrophysiological changes after denervation of slow skeletal muscle. Exp Neurol 19: 388–400, 1967. doi: 10.1016/0014-4886(67)90034-9. [DOI] [PubMed] [Google Scholar]
- 27.Peterson DK, Nochomovitz M, DiMarco AF, Mortimer JT. Intramuscular electrical activation of the phrenic nerve. IEEE Trans Biomed Eng BME-33: 342–351, 1986. doi: 10.1109/TBME.1986.325720. [DOI] [PubMed] [Google Scholar]
- 28.Peterson DK, Nochomovitz ML, Stellato TA, Mortimer JT. Long-term intramuscular electrical activation of the phrenic nerve: efficacy as a ventilatory prosthesis. IEEE Trans Biomed Eng 41: 1127–1135, 1994. doi: 10.1109/10.335861. [DOI] [PubMed] [Google Scholar]
- 29.Pette D, Vrbová G. What does chronic electrical stimulation teach us about muscle plasticity? Muscle Nerve 22: 666–677, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 30.Rosenblueth A, Dempsey EW. A study of Wallerian degeneration. Am J Physiol 128: 19–30, 1939. doi: 10.1152/ajplegacy.1939.128.1.19. [DOI] [Google Scholar]
- 31.Salafsky B, Jasinski D. Early electrophysiological changes after denervation of fast skeletal muscle. Exp Neurol 19: 375–387, 1967. doi: 10.1016/0014-4886(67)90033-7. [DOI] [PubMed] [Google Scholar]
- 32.Warner DO, Warner MA, Ritman EL. Mechanical significance of respiratory muscle activity in humans during halothane anesthesia. Anesthesiology 84: 309–321, 1996. doi: 10.1097/00000542-199602000-00008. [DOI] [PubMed] [Google Scholar]