Abstract
Based on previous studies suggesting a role of renal nerves in renal inflammation, the present studies were performed to test the hypothesis that renal nerves mediate renal damage in Dahl salt-sensitive (SS) hypertension by increasing renal leukocyte infiltration. Experiments were performed in Dahl SS rats with bilateral renal denervation (RDN) and bilateral sham operation (n = 10 or 11 per group) and with unilateral RDN and contralateral sham operation (n = 10). After denervation, rats were switched from a low-salt 0.4% NaCl (LS) diet to a high-salt 4% NaCl (HS) diet and maintained on HS diet for 21 days. Bilateral RDN reduced the magnitude of hypertension assessed by radiotelemetry in Dahl SS rats compared with sham-operated rats (mean arterial pressure 140.9 ±4.8 mmHg and 159.7 ± 3.5 mmHg, respectively) and reduced proteinuria at day 21 of HS diet. However, assessment of renal leukocyte infiltration demonstrated no significant effect of bilateral RDN on the number of infiltrating leukocytes (RDN 3.6 ± 0.5 × 106 vs. sham operated 4.3 ± 0.3 × 106 CD45+ cells) or any of the subsets examined by flow cytometry. The unilateral RDN experiment showed no effect of RDN on the renal infiltration of leukocytes (RDN 6.5 ± 0.9 × 106 vs. sham operated 6.1 ± 1.1 × 106 CD45+ cells/kidney) or renal damage in RDN vs. sham-operated kidney after 21 days of HS diet. This work investigated the relationship between renal nerves and renal inflammation during Dahl SS hypertension. Contrary to our hypothesis, the results of this work suggest that immune cell infiltration in the kidney of Dahl SS rats is not mediated by the renal nerves.
Keywords: Dahl salt-sensitive rat, hypertension, leukocytes, renal damage, renal nerves
INTRODUCTION
Based on the latest American College of Cardiology/American Heart Association high blood pressure guidelines, it is estimated that ~103 million adults in the United States have hypertension, with 82 million of them requiring antihypertensive medications (23). Despite the availability of many classes of antihypertensive medications, hypertension remains the most important modifiable risk factor for cardiovascular disease (12). Approximately 95% of human hypertension is essential, where no single identifiable cause can be determined. Among the host of factors contributing to essential hypertension, sodium intake, which leads to salt-sensitive hypertension, is arguably the most important environmental factor (15). The Dahl salt-sensitive (SS) rat is a widely accepted animal model used to study salt-sensitive hypertension. It recapitulates many of the clinical and pathophysiological features of human hypertension (20). Studies on Dahl SS rats have elucidated several factors, pathways, and mechanisms that contribute to salt-sensitive hypertension (19, 27, 29). Previous studies have shown, by using both pharmacological and genetic methods, the important role that the immune system plays in several animal models of hypertension, including the Dahl SS rat (34), and clinical studies have demonstrated the role of immunity in human hypertension (13, 14, 32, 33). Of importance are a subset of immune cells that infiltrate the kidneys and amplify renal damage and hypertension (4, 5, 28). The mechanisms leading to the infiltration and activation of immune cells in the kidney, however, are largely unknown (21).
Potential mediators of immune cell infiltration in the kidney are the renal sympathetic nerves, where it is known that their actions in the kidney promote hypertension (18). Renal sympathetic denervation (RDN) is currently in clinical trials for treatment of resistant hypertension in humans (3, 7, 11). Experiments using RDN in different experimental animal models of hypertension show varying effects on hypertension (1, 10, 18, 24, 37). In the Dahl SS rat, RDN before the development of hypertension does not alter blood pressure response to high salt. However, RDN after development of hypertension blunts the hypertension (9, 10). Interestingly, recent studies demonstrated that RDN can reduce immune cell infiltration in the kidneys of angiotensin II as well as DOCA-salt hypertensive animals, with differential effects of bilateral and unilateral RDN (1, 2, 37). Given that immune mechanisms have been shown to be important to amplify salt-sensitive hypertension and renal damage in the Dahl SS rat and renal nerves have been reported to elicit renal inflammation, the present studies interrogated the role of renal nerves as mediators of renal immune cell infiltration in Dahl SS rats placed on a high-salt (HS) diet. Experiments were performed to test the hypothesis that renal nerves mediate renal damage in Dahl SS hypertension by increasing renal infiltrating immune cells.
Initial studies were performed to examine the influence of bilateral RDN or sham surgery on the development of salt-sensitive hypertension and renal damage in Dahl SS rats. Given that systemic factors including blood pressure have been shown recently to modulate renal immune cell infiltration (8), additional experiments were performed with unilateral RDN and contralateral sham surgery to eliminate systemic factors and blood pressure as variables. In each case, the infiltration of immune cells into the kidney was assessed.
MATERIALS AND METHODS
Experimental animals.
All experiments were performed on 7-wk-old male Dahl SS rats from a colony maintained at the Medical College of Wisconsin (SS/JrHsdMcwi). All animals were maintained on a low-salt (LS) diet containing 0.4% NaCl (no. 113755; Dyets, Bethlehem, PA). The rats were housed in pairs or triplets when possible in temperature- and humidity-controlled rooms, with 12:12-h dark-light cycles. All animals were provided food and water ad libitum, and protocols were reviewed and approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee and conducted in accordance with institutional and National Institutes of Health guidelines.
Bilateral renal denervation and telemetry protocol.
At 7 wk of age, animals were randomly assigned to experimental groups (n = 10 or 11 per group) to undergo either bilateral RDN or sham operation (Sham) as has been described in the literature, with some modifications (17). Briefly, rats were deeply anesthetized with inhalation of 2% isoflurane balanced with O2. Denervation was performed by applying 10% (vol/vol) phenol in ethanol (or saline in the sham operation) to the renal artery for 1 min. After a week of recovery, animals were surgically instrumented with HD-S10 radiotelemeters (Data Sciences International, Minneapolis, MN) in the right carotid artery, with the body of the transmitter placed subcutaneously on the back of the animal, for the continuous 24-h measurement of blood pressure throughout the study as we have described previously (5). The animals were treated with analgesia (Buprenorphine SR, 0.3 mg/kg) and antibiotics (cefazolin, 25 mg/kg) by subcutaneous injection for control of pain and infection. After recovery, baseline blood pressure was recorded for 4 days and then the animals were switched to a HS diet containing 4% NaCl (no. 113756; Dyets) and maintained on the HS diet for 21 days. Overnight urine collections were performed in metabolic cages for the assessment of urinary excretion of albumin, protein, and electrolytes on the LS diet and on days 7, 14, and 21 of the HS diet.
Unilateral renal denervation protocol.
At 7 wk of age, animals (n = 10) underwent unilateral RDN and contralateral sham operation as described above. The rats were then allowed to recover and at 9 wk of age were switched to the HS diet for 21 days. At the end of the HS period, kidneys were extracted and the rats euthanized. The kidneys were divided for 1) renal norepinephrine measurement, 2) immune cell isolation and flow cytometry, and 3) histological assessment of renal damage.
Renal immune cell isolation and flow cytometry.
At the end of 21 days of the HS diet, animals were deeply anesthetized and kidneys were flushed with heparin phosphate-buffered saline (PBS) solution and extracted. Immune cell isolation, staining, and flow cytometry were performed to assess immune cells in the kidney as we have described in detail previously (8). Briefly, the whole kidney was used from the bilateral denervation groups and half of each kidney from the unilateral RDN group. After the kidney was flushed of blood and collected, the renal tissue was minced in a petri dish and passed through a 100-μm strainer in RPMI-1640 medium containing collagenase and DNase; the contents were then incubated at 37°C for digestion for 30 min. The kidney tissue was then passed through serial strainers (70 μm, 40 μm). Mononuclear cells were isolated by centrifugation in a Percoll gradient and washed. The cells were then counted with a hemocytometer. In unilateral RDN experiments, half of each kidney was obtained, and the mass of the slice and of the full kidney was used to calculate immune cell number per kidney. Approximately 1 × 106 cells were stained with fluorochrome-bound antibody cocktail containing anti-CD45 PE-Cy7 (BioLegend, catalog no. 202214, clone OX-1, 0.062 µg), anti-CD3 PerCP eFluor710 (eBioscience, catalog no. 46-0030-82, clone G4.18, 0.062 µg), anti-CD8a FITC (BioLegend, catalog no. 201703, clone OX-8, 0.125 µg), anti-CD4 APC-Cy7 (BioLegend, catalog no. 201518, clone W3/25, 0.062 µg), anti-CD11b/c Alexa eFluor 660 (eBioscience, catalog no. 50-0110-82, clone OX-42, 0.062 µg), and anti-CD45R PE (BD Bioscience, catalog no. 554881, clone HIS24, 0.125 µg) in 100 µl of wash buffer [PBS (1×) without Ca2+ and Mg2+, 2% FBS, and 2 mM EDTA). Excess antibody was washed and cells resuspended in 300 µl of wash buffer. DAPI was added for quantification of dead cells 10 min before samples were run on the flow cytometer. Flow cytometry was then done with a LSR II flow cytometer (BD Biosciences, San Jose, CA) and data analysis with FlowJo Software (FlowJo, Ashland, OR).
Renal tissue norepinephrine content measurement.
For confirmation of RDN, measurement of norepinephrine content in the kidneys was performed by high-performance liquid chromatography (HPLC) with electrochemical detection as previously described (22). Briefly, renal tissue homogenates were extracted with acid-washed alumina, and 2,5-dihydroxybenzylamine hydrobromide was introduced as an internal standard. The samples were then separated by reverse-phase HPLC with a Waters μBondapak C18 column (300 × 3.9 mm), mobile phase consisting of 0.1 M Na, 0.1 mM Na2EDTA, 3% acetonitrile, and 54 mg/l sodium octyl sulfate. The flow rate was constant at 0.9 ml/min (isocratic), and the samples were quantified with an electrochemical detector (BAS LC4C). The results are expressed in picograms per milligram of wet weight.
Renal histology.
For renal damage assessment, a histological analysis was performed as previously described (4, 5, 28); a portion of each kidney was fixed in a 10% natural buffered formalin solution, paraffin embedded, cut in 3-μm sections, mounted, and stained with Masson’s one-step trichrome stain. Individual glomeruli were scored semiquantitatively as described previously (4) and assigned an injury score of 0–4. At least 40 individual glomeruli were scored by a scorer blinded to the treatment group, and averaged data from each kidney are presented. With the same trichrome-stained kidney slices tubular cast formation was assessed; the percentage of tubular casts was quantified as cast area/renal outer medulla total area with ImageJ image analysis software (31).
Urinalysis.
Urine electrolytes were measured by a flame photometer (model 2655-10; Cole Parmer, Vernon Hills, IL) coupled with a dilutor (model 805; Sherwood Scientific) and an autosampler (model 860; Sherwood Scientific). Urine albumin was quantified with a fluorescent assay that utilized albumin blue 580 dye (Molecular Probes, Eugene, OR) and a fluorescent plate reader (FL600; Bio-Tek, Winooski, VT). Urine protein values were measured with Weichselbaum biuret reagent and an autoanalyzer (no. 402900-1ACE; Alfa Wasserman, Fairfield, NJ). Electrolyte results are expressed as excretion rates in millimoles per 24 h and albumin and protein as milligrams or grams per 24 h.
Statistical analysis.
Results are expressed as means ± SE. A two-way repeated-measures ANOVA with all pairwise multiple comparisons (Holm-Sidak method) was used for analysis of blood pressure and urine data, and a two-sided Student’s t-test was used for norepinephrine, flow cytometry, and histology data. A difference was considered statistically significant at P < 0.05. Exact P values are listed in results when they are >0.001.
RESULTS
Effect of bilateral RDN on hypertension in Dahl SS rats.
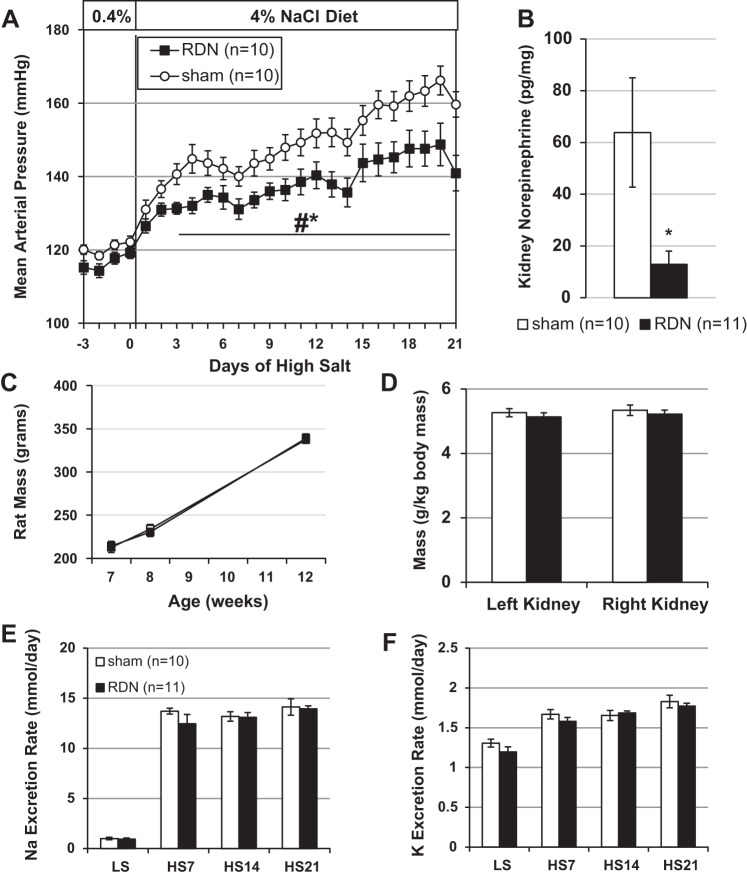
No differences were detected in mean arterial pressure (MAP), measured 24 h per day, between RDN and Sham rats when maintained on the 0.4% NaCl diet (Fig. 1A). When sodium content in the diet was increased, MAP rapidly and progressively increased in the Sham rats (from 121.4 ± 1.3 mmHg on LS diet to 159.7 ± 3.5 mmHg after 21 days of HS diet). Although the RDN rats also demonstrated an increase in MAP when fed the 4.0% NaCl diet (from 119.3 ± 1.6 mmHg on LS diet to 140.9 ± 4.8 mmHg after 21 days of HS diet), the absolute increase in MAP in the RDN group was significantly lower than observed in the Sham group (P < 0.001). At the end of the experiment renal norepinephrine concentrations were measured to confirm the RDN, and Fig. 1B shows significant reduction of ~80% in norepinephrine content in the kidneys of the RDN rats (P = 0.02). No differences were observed in body weights or kidney weights between the groups (Fig. 1, C and D), and the steady-state urinary excretion rates of sodium and potassium were not different between the groups over the time course of the experiment (Fig. 1, E and F).
Fig. 1.
Bilateral renal denervation (RDN) in Dahl SS rats blunts hypertension after 3 wk of high-salt diet. A: daily mean arterial pressure over the duration of the study. B: kidney norepinephrine concentration for confirmation of RDN. C: rat mass average at surgeries and euthanasia time points. D: kidney mass at euthanasia. E: urinary sodium excretion rate. F: urinary potassium excretion rate. LS, low-salt diet (0.4% NaCl); HS7, HS14, HS21, days on high-salt diet (4.0% NaCl). *P < 0.05 vs. sham operated (Sham); #P < 0.05 vs. LS. Two-way ANOVA used for A, C, E, and F. Two-sided t-test used for B and D.
Effect of bilateral RDN on renal damage and immune cell infiltration.
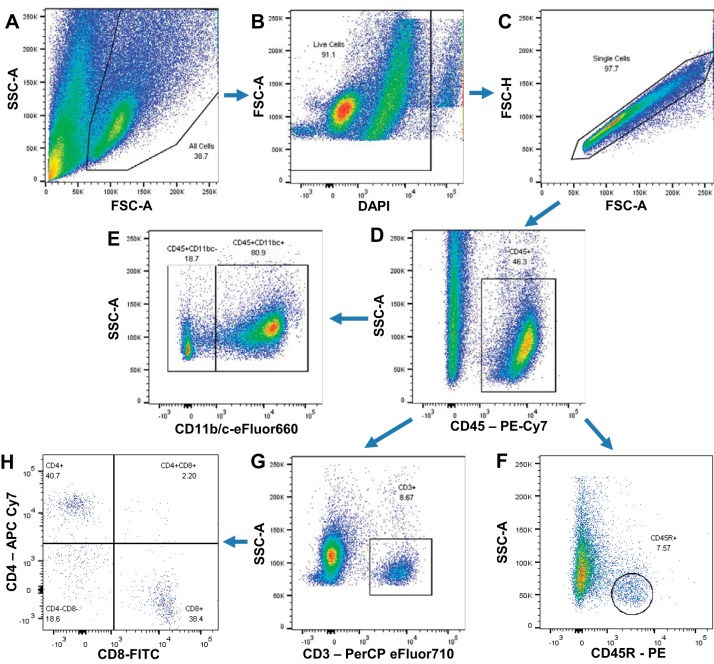
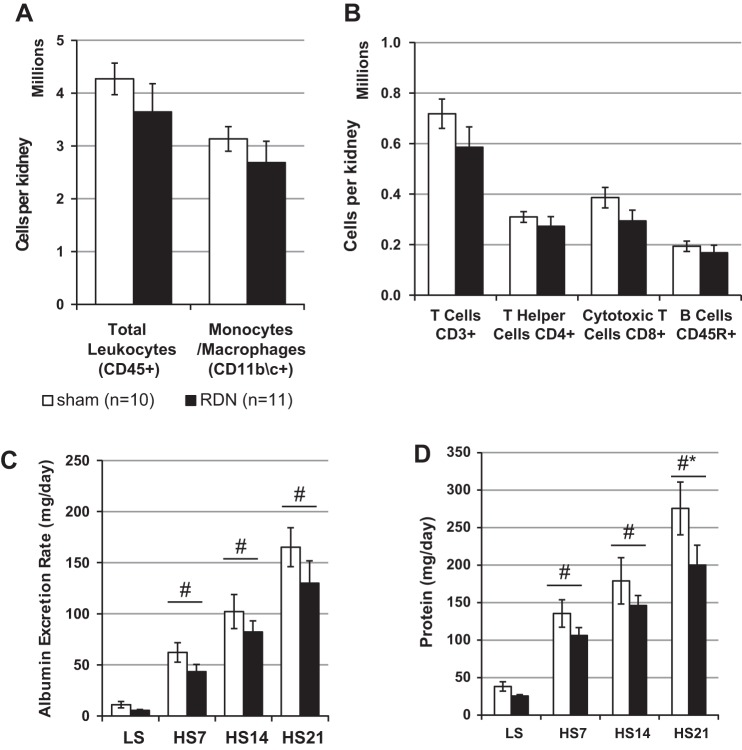
The infiltration of immune cells in the kidney was evaluated at the end of the study by a flow cytometry-based approach. The gating strategy that was used to assess leukocyte subpopulations is shown in Fig. 2. Approximately 74% of the total CD45+ leukocytes in the kidney were monocytes/macrophages (CD11b/c+) (Fig. 3A), with fewer numbers of CD3+ T cells, both CD3+CD4+ T helper and CD3+CD8+ cytotoxic T cells, and CD45R+ B cells (Fig. 3B). Despite a tendency for reduction in immune cells in the kidneys of the RDN SS rats (3.6 ± 0.5 × 106 vs. 4.3 ± 0.3 × 106 CD45+ cells in Sham group), no significant differences were observed in the number of immune cells isolated from the kidneys of RDN or Sham rats in any of the subtypes examined. Assessment of renal damage by urinary protein and albumin excretion demonstrated a progressive increase in albumin excretion rate with HS diet in both groups, with no significant difference between the groups at any of the time points (Fig. 3C). No differences in urinary protein excretion rates were observed with LS diet (25 ± 2 mg/day in RDN vs. 38 ± 6 mg/day in Sham). Protein excretion rate increased after the switch to HS diet in both groups, but the RDN group had a significantly blunted protein excretion rate at 21 days of HS diet (200 ± 26 mg/day vs. 276 ± 35 mg/day in Sham group) (Fig. 3D).
Fig. 2.
Flow cytometry gating strategy for identification of leukocytes and their subsets from kidney tissue. A: 2-parameter dot plots of forward vs. side scatter were used to identify cells from debris. SSC-A, side scatter area; FSC-A, forward scatter area. B: live cells were gated as DAPI negative. C: the correlation between FSC-A and forward scatter height (FSC-H) identified single cells from doublets. D: from that, CD45+ total leukocytes were gated. Of the CD45+ cells, subpopulations were gated. E: CD11b/c+ monocytes/macrophages. F: CD45R+ B lymphocytes. G: CD3+ T lymphocytes. H: subpopulations of the CD3+ T lymphocytes of CD3+CD4+ T helper cells and CD3+CD8+ cytotoxic T cells.
Fig. 3.
Bilateral renal denervation (RDN) in Dahl SS rats reduces renal damage but does not modulate renal leukocyte infiltration after 3 wk of high-salt diet. A and B: flow cytometry results of infiltrated kidney leukocytes. C and D: urinary albumin and protein excretion rates at low-salt (LS; 0.4% NaCl) diet and days 7, 14, and 21 of high-salt (HS; 4.0% NaCl) diet. *P < 0.05 vs. sham operated (Sham); #P < 0.05 vs. LS. Two-way ANOVA used for C and D. Two-sided t-test used for A and B.
Effect of unilateral RDN on renal damage and immune cell infiltration.
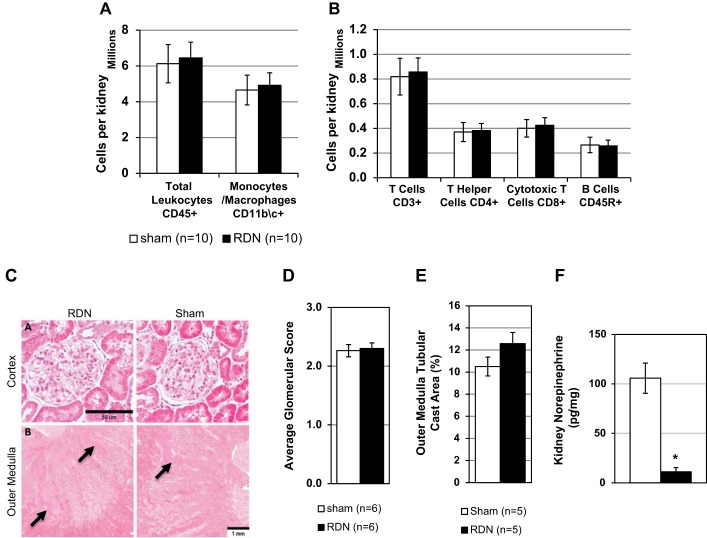
To test the direct effects of the renal nerves on renal immune cell infiltration independent of blood pressure or other circulating systemic factors, a unilateral RDN with a contralateral sham operation was performed. After recovery the rats were fed the 4.0% NaCl diet for 21 days, and renal damage, as assessed histologically, and immune cell infiltration in the kidney were compared in the RDN and Sham kidneys in paired comparisons within each rat at the conclusion of the experiment. Using the same approach and gating strategy to assess renal leukocyte infiltration, we observed no significant differences between RDN and Sham kidneys in the number of total leukocytes (6.5 ± 0.9 × 106 vs. 6.1 ± 1.1 × 106 CD45+ cells in Sham kidney) or monocytes/macrophages (CD11b/c+) (Fig. 4A). We also observed no differences in CD3+ T cells, both CD3+CD4+ T helper and CD3+CD8+ cytotoxic T cells, and CD45R+ B cells (Fig. 4B) in the RDN and Sham kidneys. Renal damage was assessed histologically, and no differences were observed in the average glomerular damage scoring or outer medullary tubular cast area formation. Figure 4C shows representative histological images of glomeruli in the renal cortex and of tubular protein casts in the renal outer medulla. Figure 4, D and E, show quantification of the data from multiple animals. Finally, we confirmed the denervation by measuring renal norepinephrine content, which showed significant reduction of ~90% in RDN compared with Sham kidney (P < 0.001; Fig. 4F).
Fig. 4.
Unilateral renal denervation (RDN) does not modulate renal damage or renal leukocyte infiltration after 3 wk of high-salt diet. A and B: flow cytometry results from infiltrated kidney leukocytes. C, top: light microscopy of trichrome-stained kidney sections showing glomerulus, ×10. Bottom: renal outer medulla at ×4 with protein casts (arrows). D and E: quantification of renal histological damage by glomerular scoring and outer medulla tubular cast area. F: kidney norepinephrine concentration. *P < 0.05 vs. sham operated (Sham). Two-sided t-test used for all panels.
DISCUSSION
The role of the renal nerves in hypertension has been an area of great interest over the past few decades (7, 18). Previously published studies in the Dahl SS rat suggest a role of the renal nerves in the development of renal damage without a significant effect on the development of hypertension (24). One potential mechanism whereby the renal nerves may mediate renal damage is by mediating infiltration of immune cells into the kidney (2, 37). More recently, in studies performed in the DOCA-salt hypertension model, RDN was found to modulate inflammation in the kidney, with differential effects of renal leukocyte infiltration with unilateral and bilateral denervation approaches (1). This is supported by data demonstrating a role of immune cells in the renal interstitium that increase oxidative stress and contribute to renal damage (4). The present studies were therefore designed to investigate the potential role of renal nerves to mediate renal immune cell infiltration and subsequent renal damage in kidneys of Dahl SS rats during the development of hypertension. Two complementary approaches were used: the first utilized bilateral RDN to examine the role of the renal nerves on the development of salt-sensitive hypertension and renal damage with an accompanying analysis of immune cells in the kidneys at the conclusion of the experiment. Subsequent studies were performed using unilateral RDN to assess the direct effect of renal nerves on the kidneys independent of systemic factors such as hormones or other circulating factors including components of the renin-angiotensin-aldosterone axis.
Initial experiments were performed with bilateral RDN followed by 21 days of HS diet. Although RDN did not alter blood pressure when the rats were fed a LS diet, there was a significant blunting of the hypertension that developed on the HS diet in the RDN group compared with the Sham group. This was contrary to our expectations based upon the literature (16, 24, 25, 36). Although we do not have a definitive answer as to why we observed an attenuation of salt-sensitive hypertension in the Dahl SS rat while others did not, we speculate that differences in study design contributed to the observed differences in experimental outcome. These differences included the method of measuring blood pressure, which included tail plethysmography in two studies (16, 36) and femoral indwelling catheters in a third (25) whereas we employed telemetry and thus report the mean of 24 h of blood pressure recording. Also, although all studies performed RDN using a combination of 10% phenol application ± stripping, two studies performed uninephrectomy in addition to contralateral RDN (24, 25) rather than bilateral RDN. Additionally, the percentage of NaCl in the HS diet was 8% in the prior studies cited (16, 24, 25, 36), whereas we used 4% NaCl in the present study. Moreover, one study was performed on female rats (25), whereas we studied male rats. Further examination assessed urinary albumin and protein excretion rates from overnight urinary collections as indexes of renal damage. In the RDN group, we observed a significant reduction in protein excretion rate and a nonsignificant trend to blunt the salt-sensitive increase in albumin excretion rate at the end of the study. We recognize the potential limitation of the telemetry catheter placement in the carotid artery, which interrupts blood flow to the carotid sinus unilaterally with potential baroreceptor unloading; however, the blood pressures measured in our rats after recovery from the surgery were similar to blood pressures measured through the femoral artery with telemetry and with chronic indwelling catheters (26, 35). Additionally, in these studies we have not performed any selective afferent RDN; therefore we cannot dissect any potential differential effects of afferent and efferent nerves on development of hypertension.
Interestingly, despite the blunting of the hypertension phenotype, we observed no significant differences in the number of infiltrating immune cells in the kidneys of rats from the bilaterally denervated group compared with the Sham group. Given recent data demonstrating an important role of perfusion pressure in renal immune cell infiltration (8), it was intriguing that differences were not observed given the reductions in MAP that occurred in the RDN group. One potential factor that could help explain these findings relates to the effect of renal nerves on hemodynamics in the kidney (6, 18), where activation of renal sympathetic nerves causes intrarenal vasoconstriction and reduces renal blood flow. Given that Dahl SS rats have been shown to have an increased renal nerve activity on a HS diet (6), it is possible that denervation caused a net effect to increase the transmission of pressure to the kidneys by causing vasodilation during hypertension development that partially offset the protective effects of a reduced systemic blood pressure. Indeed, the minimal protection from renal damage observed in the RDN kidneys and the lack of a difference in immune cell infiltration of the Sham and RDN kidneys support this notion. Although we did not directly measure intrarenal vascular pressures, we did perform additional studies on rats with a unilateral RDN to control for differences in systemic factors. Another possibility is that there is a threshold of pressure difference necessary to influence immune cell infiltration into the kidney; again, this remains to be determined.
Given the effects of the bilateral RDN to reduce systemic blood pressure in the present studies, we examined the direct effect of the renal nerves on the kidneys in Dahl SS hypertension by performing additional studies in rats with a unilateral RDN. These experiments allowed us to perform paired comparisons within individual animals while eliminating systemic factors as variables. We assessed renal immune cell infiltration after 21 days of high salt and observed no difference in any of the immune cell subtypes examined between the RDN and sham-operated kidneys. Evaluation of histological renal damage of the renal cortex and medulla also demonstrated no effect of the denervation on the renal damage. One potential limitation of this set of experiments is that blood pressure was not measured in the unilateral denervation experiment. Despite this, we do not expect it to have a major impact, as the pressure would be expected to fall somewhere between that of the Sham and bilateral denervation groups as depicted in Fig. 1. A second limitation is the inclusion of only male rats in the present study. It has been demonstrated that there are sex differences in the immune system’s involvement in hypertension. Specifically, renal immune cell infiltration has been demonstrated to have sex-dependent effects, with males, in general, demonstrating a greater hypertensive response with a greater extent of immune cell infiltration in the kidney. The mechanisms mediating these differences are currently under investigation (30).
Although the role of renal nerves in the development of hypertension in Dahl SS rats has been investigated (16, 25, 36), the role of renal nerves in mediating renal immune cell infiltration has not been examined in the Dahl SS rat. Immune mechanisms have been shown to be important in the development of renal disease in this model (4, 5, 28); also, the data suggest a potential role of the renal nerves in mediating immune infiltration in the kidney (37). Given that, and the similarities of hypertension in the Dahl SS rat and humans (16), the findings presented in this work are relevant to understanding mechanisms of salt-sensitive hypertension and novel. Results from bilateral RDN showed no difference in the number of infiltrated total leukocytes in the kidneys of HS diet-fed animals between the groups. Unilateral RDN experiments also demonstrated no role of RDN on immune cell infiltration compared with sham-operated kidney. These results do not support a role of the renal nerves in the infiltration of immune cells into the kidneys of Dahl SS rats. We conclude that other pathways, perhaps related to the absolute level of renal perfusion pressure (8), are more important to mediate renal immune cell infiltration in salt-sensitive hypertension.
Perspectives and Significance
Several animal studies have investigated the role of immune cells in the development of hypertension and end-organ damage, and data from our laboratory and others demonstrate an important role of renal infiltrating immune cells to amplify Dahl SS rat hypertension. The mechanisms mediating the renal immune cell infiltration remain incompletely understood. The present studies prompted by recent findings in the literature examined the role of renal sympathetic nerves in this process with two complementary approaches. The findings do not support an important role of the renal nerves in renal infiltration of immune cells during salt-sensitive hypertension in the Dahl SS rat. Further studies are needed to understand the mechanisms involved in the infiltration of immune cells into the kidney during the development of hypertension and associated renal damage. Such findings may provide important new avenues of treatment for hypertension and end-organ damage.
GRANTS
The studies were supported by National Heart, Lung, and Blood Institute Grants HL-137748 and HL-116264.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.A., H.L., J.H.D., J.M.A.-B., D.J.F., and D.L.M. conceived and designed research; A.J.A. and H.L. performed experiments; A.J.A., H.L., J.H.D., J.M.A.-B., and D.L.M. analyzed data; A.J.A., H.L., J.H.D., J.M.A.-B., D.J.F., and D.L.M. interpreted results of experiments; A.J.A. prepared figures; A.J.A. drafted manuscript; A.J.A., H.L., J.H.D., J.M.A.-B., D.J.F., and D.L.M. edited and revised manuscript; A.J.A., H.L., J.H.D., J.M.A.-B., D.J.F., and D.L.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Lisa Henderson, Jenifer Phillips, and Camille Taylor for performing the urinalysis and tissue norepinephrine measurements. We also thank the Children’s Research Institute histology core for performing sectioning and staining of kidneys, Galina Petrova and the Children’s Research Institute flow cytometry core for assistance with flow cytometry, and Glenn Slocum for assistance with microscopy.
REFERENCES
- 1.Banek CT, Gauthier MM, Van Helden DA, Fink GD, Osborn JW. Renal inflammation in DOCA-salt hypertension. Hypertension 73: 1079–1086, 2019. doi: 10.1161/HYPERTENSIONAHA.119.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, Shimizu Y, Osborn JW. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension 68: 1415–1423, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C, Upadhyay A. Renal denervation for uncontrolled hypertension: critical review of the evidence. Curr Opin Nephrol Hypertens 26: 114–122, 2017. doi: 10.1097/MNH.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 4.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298: R1136–R1142, 2010. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Miguel C, Guo C, Lund H, Feng D, Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol 300: F734–F742, 2011. doi: 10.1152/ajprenal.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 77: 75–197, 1997. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 7.Esler M, Guo L. The future of renal denervation. Auton Neurosci 204: 131–138, 2017. doi: 10.1016/j.autneu.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Evans LC, Petrova G, Kurth T, Yang C, Bukowy JD, Mattson DL, Cowley AW Jr. Increased perfusion pressure drives renal T-cell infiltration in the Dahl salt-sensitive rat. Hypertension 70: 543–551, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foss JD, Fink GD, Osborn JW. Differential role of afferent and efferent renal nerves in the maintenance of early- and late-phase Dahl S hypertension. Am J Physiol Regul Integr Comp Physiol 310: R262–R267, 2016. doi: 10.1152/ajpregu.00408.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foss JD, Fink GD, Osborn JW. Reversal of genetic salt-sensitive hypertension by targeted sympathetic ablation. Hypertension 61: 806–811, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frame AA, Carmichael CY, Wainford RD. Renal afferents. Curr Hypertens Rep 18: 69, 2016. doi: 10.1007/s11906-016-0676-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall JE, Granger JP, do Carmo JM, da Silva AA, Dubinion J, George E, Hamza S, Speed J, Hall ME. Hypertension: physiology and pathophysiology. Compr Physiol 2: 2393–2442, 2012. doi: 10.1002/cphy.c110058. [DOI] [PubMed] [Google Scholar]
- 13.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol 17, Suppl 3: S218–S225, 2006. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 14.Hughson MD, Gobe GC, Hoy WE, Manning RD Jr, Douglas-Denton R, Bertram JF. Associations of glomerular number and birth weight with clinicopathological features of African Americans and whites. Am J Kidney Dis 52: 18–28, 2008. doi: 10.1053/j.ajkd.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Iatrino R, Manunta P, Zagato L. Salt sensitivity: challenging and controversial phenotype of primary hypertension. Curr Hypertens Rep 18: 70, 2016. doi: 10.1007/s11906-016-0677-y. [DOI] [PubMed] [Google Scholar]
- 16.Iwata T, Muneta S, Kitami Y, Okura T, Ii Y, Murakami E, Hiwada K. Effect of renal denervation on the development of hypertension in Dahl-Iwai salt-sensitive rats. Nihon Jinzo Gakkai Shi 33: 867–871, 1991. [PubMed] [Google Scholar]
- 17.Jacob F, Ariza P, Osborn JW. Renal denervation chronically lowers arterial pressure independent of dietary sodium intake in normal rats. Am J Physiol Heart Circ Physiol 284: H2302–H2310, 2003. doi: 10.1152/ajpheart.01029.2002. [DOI] [PubMed] [Google Scholar]
- 18.Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol 1: 731–767, 2011. doi: 10.1002/cphy.c100043. [DOI] [PubMed] [Google Scholar]
- 19.Johnson RJ, Gordon KL, Giachelli C, Kurth T, Skelton MM, Cowley AW Jr. Tubulointerstitial injury and loss of nitric oxide synthases parallel the development of hypertension in the Dahl-SS rat. J Hypertens 18: 1497–1505, 2000. doi: 10.1097/00004872-200018100-00019. [DOI] [PubMed] [Google Scholar]
- 20.Lerman LO, Chade AR, Sica V, Napoli C. Animal models of hypertension: an overview. J Lab Clin Med 146: 160–173, 2005. doi: 10.1016/j.lab.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Mattson DL. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. Am J Physiol Renal Physiol 307: F499–F508, 2014. doi: 10.1152/ajprenal.00258.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muere C, Neumueller S, Miller J, Olesiak S, Hodges MR, Pan L, Forster HV. Atropine microdialysis within or near the pre-Botzinger complex increases breathing frequency more during wakefulness than during NREM sleep. J Appl Physiol (1985) 114: 694–704, 2013. doi: 10.1152/japplphysiol.00634.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, Whelton PK. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 137: 109–118, 2018. doi: 10.1161/CIRCULATIONAHA.117.032582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagasu H, Satoh M, Kuwabara A, Yorimitsu D, Sakuta T, Tomita N, Kashihara N. Renal denervation reduces glomerular injury by suppressing NAD(P)H oxidase activity in Dahl salt-sensitive rats. Nephrol Dial Transplant 25: 2889–2898, 2010. doi: 10.1093/ndt/gfq139. [DOI] [PubMed] [Google Scholar]
- 25.Osborn JL, Roman RJ, Ewens JD. Renal nerves and the development of Dahl salt-sensitive hypertension. Hypertension 11: 523–528, 1988. doi: 10.1161/01.HYP.11.6.523. [DOI] [PubMed] [Google Scholar]
- 26.Pavlov TS, Levchenko V, Ilatovskaya DV, Li H, Palygin O, Pastor-Soler NM, Hallows KR, Staruschenko A. Lack of effects of metformin and AICAR chronic infusion on the development of hypertension in Dahl salt-sensitive rats. Front Physiol 8: 227, 2017. doi: 10.3389/fphys.2017.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pavlov TS, Staruschenko A. Involvement of ENaC in the development of salt-sensitive hypertension. Am J Physiol Renal Physiol 313: F135–F140, 2017. doi: 10.1152/ajprenal.00427.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL; PhysGen Knockout Program . CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension 63: 559–564, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudemiller NP, Mattson DL. Candidate genes for hypertension: insights from the Dahl S rat. Am J Physiol Renal Physiol 309: F993–F995, 2015. doi: 10.1152/ajprenal.00092.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandberg K, Ji H, Hay M. Sex-specific immune modulation of primary hypertension. Cell Immunol 294: 95–101, 2015. doi: 10.1016/j.cellimm.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seaberg EC, Muñoz A, Lu M, Detels R, Margolick JB, Riddler SA, Williams CM, Phair JP; Multicenter AIDS Cohort Study . Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS 19: 953–960, 2005. doi: 10.1097/01.aids.0000171410.76607.f8. [DOI] [PubMed] [Google Scholar]
- 33.Sommers SC, Relman AS, Smithwick RH. Histologic studies of kidney biopsy specimens from patients with hypertension. Am J Pathol 34: 685–715, 1958. [PMC free article] [PubMed] [Google Scholar]
- 34.Wade B, Abais-Battad JM, Mattson DL. Role of immune cells in salt-sensitive hypertension and renal injury. Curr Opin Nephrol Hypertens 25: 22–27, 2016. doi: 10.1097/MNH.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wade B, Petrova G, Mattson DL. Role of immune factors in angiotensin II-induced hypertension and renal damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 314: R323–R333, 2018. doi: 10.1152/ajpregu.00044.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wyss JM, Sripairojthikoon W, Oparil S. Failure of renal denervation to attenuate hypertension in Dahl NaCl-sensitive rats. Can J Physiol Pharmacol 65: 2428–2432, 1987. doi: 10.1139/y87-385. [DOI] [PubMed] [Google Scholar]
- 37.Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, Takahashi T, Loperena R, Foss JD, Mernaugh RL, Chen W, Roberts J 2nd, Osborn JW, Itani HA, Harrison DG. Renal denervation prevents immune cell activation and renal inflammation in angiotensin II-induced hypertension. Circ Res 117: 547–557, 2015. doi: 10.1161/CIRCRESAHA.115.306010. [DOI] [PMC free article] [PubMed] [Google Scholar]