Abstract
Hepatic energy metabolism is a key element in many metabolic diseases. Hepatic anaplerosis provides carbons for gluconeogenesis (GNG) and triglyceride (TG) synthesis. We aimed to optimize a protocol that measures hepatic anaplerotic contribution for GNG, TG synthesis, and hepatic pentose phosphate pathway (PPP) activity using a single dose of oral [U−13C3]glycerol paired with an oral sugar tolerance test (OSTT) in a population with significant insulin resistance. The OSTT (75 g glucose + 25 g fructose) was administered to eight obese adolescents with polycystic ovarian syndrome (PCOS) followed by ingestion of [U-13C3]glycerol at t = 180 or t = 210 min. 13C-labeling patterns of serum glucose and TG-glycerol were determined by nuclear magnetic resonance. 13C enrichment in plasma TG-glycerol was detectable and stable from 240 to 390 min with the [U-13C3]glycerol drink at t = 180 min(3.65 ± 2.3 to 4.47 ± 1.4%; P > 0.4), but the enrichment was undetectable at 240 min with the glycerol drink at t = 210 min. The relative contribution from anaplerosis was determined at the end of the OSTT [18.5 ±3.4% (t = 180 min) vs. 16.0 ± 3.5% (t = 210 min); P = 0.27]. [U-13C3]glycerol was incorporated into GNG 390 min after the OSTT with an enrichment of 7.5–12.5%. Glucose derived from TCA cycle activity was 0.3–1%, and the PPP activity was 2.8–4.7%. In conclusion, it is possible to obtain relative measurements of hepatic anaplerotic contribution to both GNG and TG esterification following an OSTT in a highly insulin-resistant population using a minimally invasive technique. Tracer administration should be timed to allow enough de novo TG esterification and endogenous glucose release after the sugar drink.
Keywords: anaplerosis, gluconeogenesis, insulin resistance, liver metabolism, mitochondria, pentose phosphate pathway, polycystic ovarian syndrome
INTRODUCTION
Hepatic energy metabolism is altered in many disease states, and it changes through the life spectrum. Understanding alternations in hepatic metabolism may be key to prevention and treatment of metabolic disease, including nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes. Several new classes of medications are being developed to treat NAFLD, and there is a need to understand how these will affect hepatic substrate metabolism (13). However, current methods to study liver-specific Tricarboxylic acid (TCA) energetics in vivo in humans are few, complex to administer, and restricted to the fasting state (2). Here we describe a simple method with oral tracer administration using glucose and triglyceride (TG) plasma isotopomer analysis to study TCA cycling contribution to gluconeogenesis (GNG) and glyceroneogenesis adapted for use in an insulin-resistant population and following an oral sugar challenge.
Glycerol can provide carbons to multiple pathways in the liver. The process of hepatic GNG necessitates the entry of extra carbons into the TCA cycle through anaplerosis (i.e., nonoxidative pathways replenishing the TCA cycle alternative to acetyl-coA) and exiting the TCA cycle through cataplerosis via oxaloacetate conversion to phosphoenolpyruvate (PEP). Cataplerosis also contributes to the hepatic synthesis of TG as PEP can be converted via glyceroneogenesis to glycerol, which is then used as the backbone for the esterification of free fatty acids (FFAs). As this pathway is nonoxidative, the flux of anaplerosis directly equals the rate of cataplerosis. Moreover, acetyl-coA concentrations also stimulate a higher flux of anaplerosis/cataplerosis to provide the extra carbons necessary to its oxidation (18). Hepatic delivery of FFA can increase anaplerosis and glucose production independent of the effect of insulin on the liver (18, 21, 23). In animal models, as well as human studies, increased hepatic anaplerosis/cataplerosis has been identified as a contributor to GNG and linked to increased TCA cycle activity and oxidative stress in liver (12, 23, 24).
We recently described a novel methodology using an oral bolus of [U-13C3]glycerol and NMR analysis of metabolic products in plasma in healthy human participants during fasting, following a mixed meal tolerance, to measure de novo fatty acid esterification, pentose phosphate pathway (PPP) activity, and GNG (10). We then applied this methodology in adults with high or low visceral fat and with or without nonalcoholic fatty liver disease during both fasting and following a standard meal test to assess the effect of visceral fat on these pathways (17). However, the application under a postprandial condition remains to be optimized due to the concerns of low 13C enrichments especially in plasma glucose, secondary to suppression of endogenous glucose release (EGP) from meal insulin. For example, the pentose phosphate pathway measurement using [U-13C3]glycerol requires active gluconeogenesis. Thus the approach underestimates the PPP activity under a postprandial condition since gluconeogenesis is suppressed. In a postprandial protocol, sample collection must occur several hours after the meal when EGP and de novo TG synthesis rates are high enough to detect tracer enrichment. However, this methodology has not been examined in the setting of a standard sugar only meal challenge such as an oral glucose tolerance test + fructose, which is known to stimulate de novo lipogenesis and is a good setting to simultaneously evaluate dysglycemia and insulin resistance.
In individuals who have considerable insulin resistance, such as pubertal adolescents or those with metabolic disease such as polycystic ovarian syndrome (PCOS) or type 2 diabetes, alterations in the timing of postmeal glucose and TG dynamics are significant (6, 20a). Thus the objective of this study was to make the proof-of-concept and optimization of a minimally invasive method to study in vivo hepatic TCA anaplerosis/cataplerosis contribution to GNG and de novo TG synthesis, as well as PPP activity following a standard sugar load in a population with significant metabolic abnormalities. We selected obese girls with PCOS, known to be highly insulin resistant with a 50% prevalence of hepatic steatosis and impaired glucose tolerance (4, 5). To study the contribution of anaplerosis/cataplerosis to de novo TG synthesis and GNG in this population, we designed a 6.5-h 75-g glucose + 25-g fructose oral sugar tolerance test (OSTT) that stimulates de novo lipogenesis but is long enough to allow sufficient GNG to be quantified. Based on our previous observations with glucose tracers to measure GNG, as well as glucose and insulin concentrations following a similar OSTT in a similar population, we assessed two different drink timings to obtain the optimal enrichment of the glycerol tracer in both pathways simultaneously.
METHODS
Study Participants
Cohort 1.
Eight obese or overweight females [body mass index (BMI) ≥ 90th percentile for age and sex], who were physically inactive (exercising <2.5 h a week), aged between 14 and 21 yr with a diagnosis of PCOS were recruited for this proof-of-concept study (clinicaltrials.gov, NCT03041129).
Cohort 2.
For comparison of the effect of the glycerol tracer per se, seven obese or overweight females recruited for a separate study with an identical protocol without the glycerol tracer and inclusion/exclusion criteria and then matched for age, weight, BMI percentile, and ethnicity to the participants of cohort 1 were included in some analyses (clinicaltrials.gov, NCT03041129). PCOS was defined according to the National Institutes of Health definition as the presence of irregular menstrual cycles at least 2 yr after menarche and either clinical or biochemical hyperandrogenism (14, 26). Exclusion criteria included the use of medications known to affect insulin sensitivity (including metformin, estrogen therapies, atypical antipsychotics, or systemic steroids), antihypertensive medication, diabetes (defined as HbA1c >6.4%), liver disease other than hepatic steatosis, aspartate aminotransferase or alanine aminotransferase >125 IU/l, and other major illness within the past 60 days. The University of Colorado Anschutz Medical Campus Institutional Review Board and scientific advisory review committee approved the study. All participants aged 18 to –21 yr provided written informed consent, and the parents and participants provided consent and assent, respectively, for all participants aged <18 yr.
OSTT With [U-13C3]Glycerol Tracer
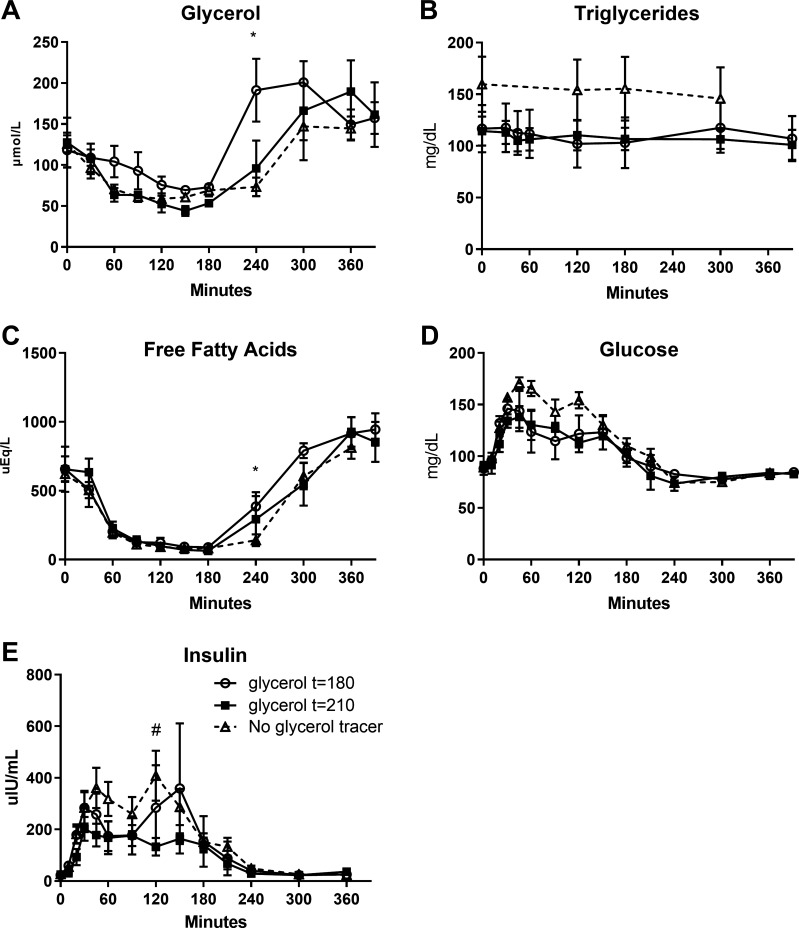
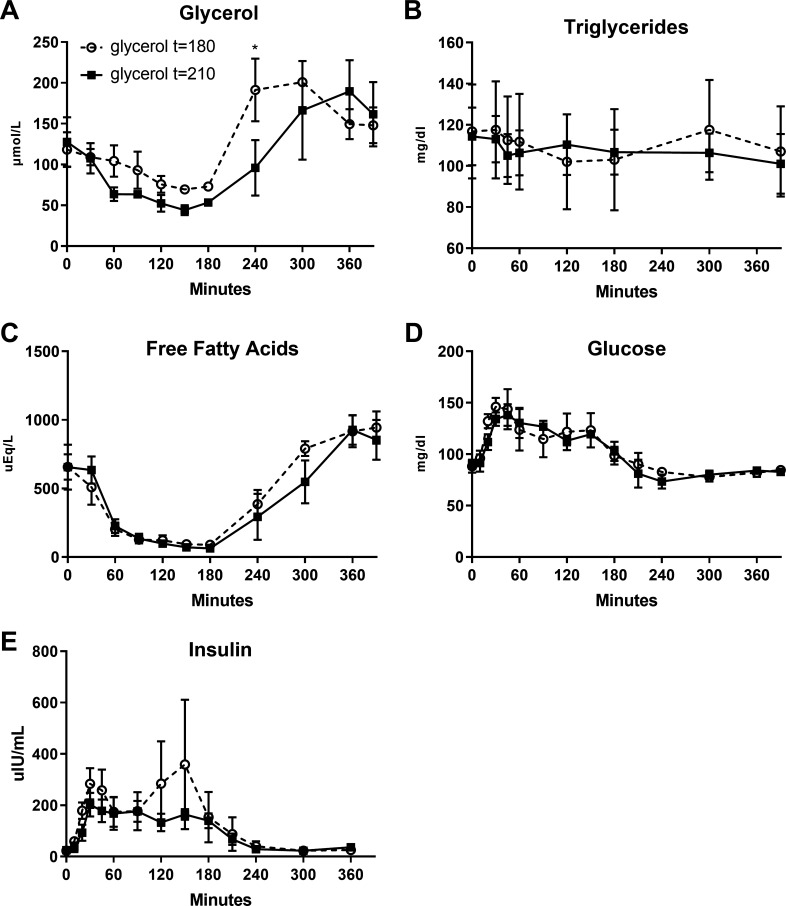
Participants were admitted to the Children’s Hospital Colorado Pediatric Clinical Translational Research Center inpatient unit for an overnight observed fast. Participants were asked to refrain strenuous physical activity for 3 days before the metabolic study and to avoid caffeine consumption for 24 h before the metabolic study. A standard snack and dinner containing 65% carbohydrate, 20% fat, 15% protein, and caloric content corresponding to 1.25 × weight maintenance was provided the night before the OSTT, followed by an overnight 12-h fast. At 8:00 AM (t = 0), an oral load of 75 g glucose+ 25 g fructose in water was given and consumed within 5 min. [U-13C3]glycerol at 50 mg/kg (Cambridge Isotope Laboratories, Tewksbury, MA), dissolved in water and warmed at ≈99°F, was given as a bolus drink at t = 210 min for half of the participants (n = 4) and t = 180 min for the other half (n = 4). Thirty-milliliter blood draws were obtained at 240, 300, 360, and 390 min of the OSTT for isolation of glucose and TGs for NMR analysis. Samples were also drawn for insulin, glucose, glycerol, TG, and FFA concentrations at frequent intervals. The timing of the administration of the oral tracer and blood draws was selected based on previous data demonstrating the beginning of return of EGP toward fasting rates following an OSTT, at the moment where insulin concentration dropped significantly toward baseline values, in insulin resistant and healthy patients (25). In our previous observation in a similar obese adolescent population, included in the cohort 2, this significant drop appears between 180 and 240 min after the OSTT (see Fig. 3E).
Fig. 3.
Plasma concentration of glycerol (A), triglycerides (B), free fatty acids (C), glucose (D), and insulin (E) in comparison to control participants (n = 7) who did not receive glycerol tracer (oral sugar tolerance test duration: 360 min). Control participants (n = 7) have been matched according to age (±1 yr), weight (±10 kg), body mass index percentile (±2%), and ethnicity to each participant with glycerol drink. Data are presented as means (SD). *P < 0.05 vs. control participants with administration of the tracer at t = 180. #P < 0.05 vs. control participants with administration of the tracer at t = 210.
Laboratory Analysis and Calculations
Analyses were performed by the University of Colorado Anschutz Research Core Laboratory or the Children’s Hospital Colorado clinical laboratory, except as noted otherwise. Plasma glycerol (R-Biopharm, Marshall, MI), FFAs (Wako Chemicals, Richmond, VA), and TGs, total cholesterol, and high-density lipoprotein cholesterol (Hitachi 917 autoanalyzer; Boehringer Mannheim Diagnostics, Indianapolis, IN) were analyzed enzymatically. Insulin was analyzed with radioimmunoassay (Millipore, Billerica, MA). Plasma glucose was measured at the bedside using a StatStrip Hospital Glucose Monitoring System (Novo Biomedical, Waltham, MA). Insulin resistance was measured using 1) homeostatic model assessment of insulin resistance (HOMA-IR) and calculated as fasting plasma glucose (mg/dl) × fasting plasma insulin (µIU/ml)/405; and 2) (16) the Matsuda index was calculated as 10,0000/√fasting plasma glucose × fasting plasma insulin × means plasma glucose (0–120 min) × mean plasma insulin (0–120 min) (15).
Statistics
Data are presented as means (SD) or proportions, as appropriate. Group comparisons were made using two-way repeated measures ANOVA (for balanced data) or repeated measures mixed models (for unbalanced data), and the Sidak’s method was used to adjust for multiple comparisons. P < 0.05 was considered significant. All statistical analyses were performed with SAS Software, version 9.4 (Cary, NC) and GraphPad Software, version 7 (La Jolla, CA).
Sample Processing for NMR Analysis
Triglycerides.
Lipids were extracted from 3 ml of plasma using previously described methods (10). In summary, plasma was mixed with 12 ml of chloroform-methanol (2:1), stirred for 30 min, and centrifuged at 1,500 rpm for 15 min. The bottom layer containing the lipids was transferred to another glass vial. Then, 8 ml of chloroform were added to the first plasma mixture, mixed for 30 min, and centrifuged again for 15 min at low rpm. The second lipid layer was extracted and added to the first lipid extract. The lipid extract was dried using a nitrogen evaporator. The dried lipids were dissolved in deuterated chloroform (CDCl3, 170 μl; Cambridge Isotopes, Andover, MA) for 13C NMR acquisition.
Glucose and monoacetone glucose conversion.
Glucose was extracted and purified from 6 ml of plasma using 600 µl of 70% perchloric acid (PCA). The mixture was centrifuged at 13,000 rpm for 10 min, and the supernatant was transferred. Six milliliters of 10% PCA was added to the remaining plasma and centrifuged at high revolutions per minute for 10 min, and the supernatant added to the first extract. PCA extracts were neutralized using KOH for a pH of 7.5–8.0. Purified glucose extracts were dried and converted to monoacetone glucose (MAG) as previously described (8).
NMR Spectroscopy and Pathway Analysis
13C-labeled glycerol backbones of TG and plasma MAG were analyzed using NMR spectroscopy. NMR spectra were collected as previously described (10) using a Varian Inova 14.1 T spectrometer (Agilent, Santa Clara, CA) equipped with a 3-mm broadband probe with the observe coil tuned to 13C (150 MHz) and were analyzed using ACD/Laboratories NMR spectral analysis program (Advanced Chemistry Development, Toronto, Canada). An explanation of the complex pathway considerations was previously described by Jin et al. (10), but a simplified explanation of measured used for TG and glucose pathway analysis is described below.
TG-Glycerol Isotopomer Analysis
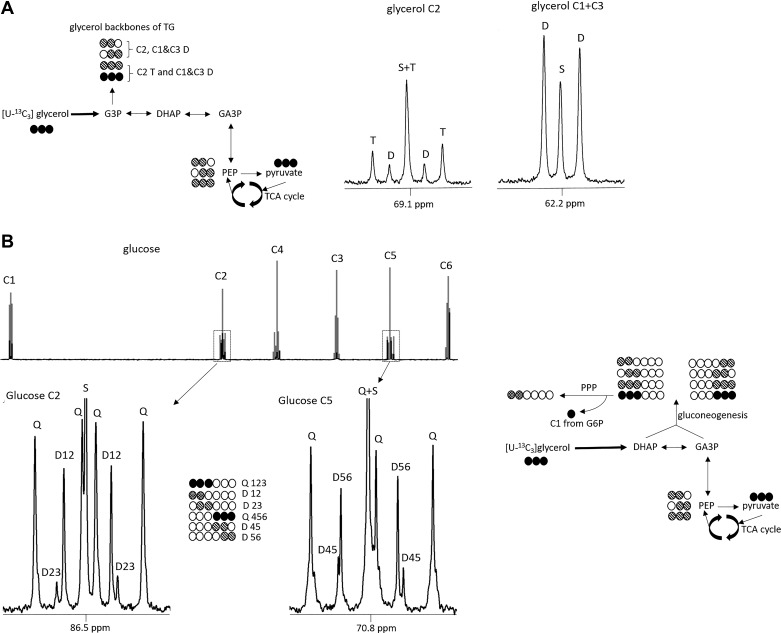
The fraction of the glycerol backbone that comprises TG derived from [U-13C3]glycerol was determined by measuring the area of a doublet at the glycerol backbone C1 and C3 region, referenced to the natural abundance of the 13C singlet signals. Direct contribution from [U-13C3]glycerol to de novo TG synthesis (direct pathway) through glycerol-3-phosphate (G3P), without entering mitochondria, leads to an intact tripled-labeled glycerol TG backbone (position 1,2,3). In contrast, incorporation of 13C glycerol after conversion to pyruvate and anaplerotic TCA cycle activity before glyceroneogenesis will lead to the formation of doubly labeled glycerol TG (position 1,2 or position 2,3) (Fig. 1A). The percentage of TG synthesis attributable to the direct pathway was measured as the ratio of triple-labeled glycerol over the sum of triple- and double-labeled glycerol that represent the total of newly synthetized TG from [U-13C3]glycerol. The indirect contribution through TCA cycle activity was measured as 100% direct contribution.
Fig. 1.
Metabolic pathways assessed by isotopomer analysis after [U-13C3]glycerol administration. De novo triglycerides (TGs) esterification pathways (A). [U-13C3]glycerol direct incorporation to fatty acid esterification leads to [U-13C3]glycerol backbones of triglycerides that are detected as a triplet in C2 region or a doublet in C1 and C3 region. Indirect pathway (transformation to pyruvate before anaplerosis to tricarboxylic (TCA) cycle and then incorporation as glycerol backbones of triglycerides) leads to the formation of double-labeled, [13C2]glycerol backbones that are detected as a doublet in C2 region. Gluconeogenesis from glycerol pathways (B). [U-13C3]glycerol direct incorporation to gluconeogenesis produces [1,2,3-13C3]- or [4,5,6-13C3]glucose that is detected as a quartet in glucose C2 or C5 region, respectively. Labeling after cycling through TCA cycle produces double-labeled glucose that can be detected in glucose C2 or C5 region. Pentose phosphate pathway (PPP) leads to an increased production of double-labeled, [1,2-13C2]glucose carbon rearrangement after C1 decarboxylation from glucose-6-phosphate. C, carbon; S, singlet; D, doublet; T, triplet; Q, quartet; G3P, glucose-3-phosphate; DHAP, dihydroxyacetone phosphate; GA3P, glyceraldehyde-3-phosphate; G6P, glucose-6-phosphate; PEP, phosphoenolpyruvate.
Glucose Isotopomer Analysis
Similarly, the fraction of de novo plasma glucose derived from [U-13C3]glycerol was determined by measuring the areas of multiplet versus singlet 13C signals from the two methyl groups of MAG, the latter representing natural abundance (9). Gluconeogenesis derived from [U-13C3]glycerol was measured as the sum of all glucose isotopomers with 13C in excess and is referred to as 13C enrichment in glucose (Fig. 1B). Direct incorporation of [U-13C3]glycerol to glucose by condensation of dihydroxyacetone phosphate and glyceraldehyde-3-phosphate leads to [1,2,3-13C3]glucose and [4,5,6-13C3]glucose. In contrast, [U-13C3]glycerol conversion to [U-13C3]pyruvate, followed by anaplerotic flux through TCA cycle before gluconeogenesis, leads to the formation of doubly labeled glucose (Fig. 1B). Because the incorporation of 13C tracer in position 4,5,6 is not affected by other pathways (such as the PPP), the labeling of [4,5-13C2]- or [5,6-13C2]glucose indicates metabolism of [U-13C3]glycerol through the TCA cycle before gluconeogenesis. Therefore, indirect gluconeogenesis through the TCA cycle from glycerol was measured by [5,6 13C2]glucose (%total enrichment). The PPP activity was measured by the difference between the ratios of [1,2-13C2]/[2,3-13C2] versus [5,6-13C2]/[4,5-13C2] in glucose, as this pathway produces [1,2-13C2]glucose and leads to an increased ratio of [1,2-13C2]/[2,3-13C2].
RESULTS
Participants Characteristics
A loss of intravenous access occurred in one of the participants in the group t = 210 such that NMR analysis was available in seven out of eight girls with PCOS. Detailed participant characteristics included in final analysis are shown in Table 1. The seven participants who completed the study were between 14 and 21 yr old; had a BMI ranging from 27.8 to 45.5 kg/m2 (BMI percentile for age and gender: 94.9–99.3%ile); and had a HOMA-IR from 2.2 to 10.4 and MATSUDA from 0.65 to 5.35. None was diagnosed with diabetes based on HbA1c or fasting glucose during the OSTT.
Table 1.
Individual characteristics of study participants
| Participant | Time Glycerol Drink, min | Age, yr | BMI (%tile) | Ethnicity | HOMA-IR | Matsuda Index |
|---|---|---|---|---|---|---|
| 1 | 210 | 16 | 33.3 (97.7) | Hispanic | 3.2 | 2.36 |
| 2 (excluded) | 210 | 17 | 47.7 (99.5) | Black | 13.3 | 0.97 |
| 3 | 210 | 16 | 36.0 (98.7) | Hispanic | 9.6 | 0.99 |
| 4 | 210 | 14 | 27.8 (94.9) | Caucasian | 4 | 2.35 |
| 5 | 180 | 19 | 34.9 (97.3) | Hispanic | 10.3 | 0.65 |
| 6 | 180 | 16 | 31.3 (96.7) | Asian | 3.2 | 1.95 |
| 7 | 180 | 17 | 44.6 (99.2) | Black | 5.0 | 1.58 |
| 8 | 180 | 21 | 30.3 (N/A) | Asian | 2.2 | 5.35 |
BMI, body mass index; HOMA-IR, homeostatic model assessment of insulin resistance.
Plasma Concentration of Glycerol, TGs, FFAs, and Glucose After [U-13C]Glycerol Drink
Glycerol, TG, FFA, and glucose plasma concentrations were not different between the two timing conditions (condition effect: P > 0.64), except for the glycerol concentration that was higher at 240 min in the group who received the glycerol tracer at t = 180 min (Fig. 2, A–D). To assess the effect of the glycerol tracer drink per se, we compared seven female participants with PCOS previously studied in the same conditions, listed in cohort 2 (Fig. 3). We detected a slight increase in glycerol and FFA concentrations at t = 240 min for those who received the glycerol drink at t = 180 min in comparison to those who did not received a glycerol drink, with no difference in those who received the drink at t = 210 min (Fig. 3, A and C). Nevertheless, there were no changes in TG or glucose concentrations induced by the glycerol tracer drink in both timing groups in comparison to those without tracers (Fig. 3, B–D).
Fig. 2.
Plasma concentrations of glycerol (A), triglycerides (B), free fatty acids (C), glucose (D), and insulin (E) following oral sugar tolerance test, according to administration of the glycerol tracer at the timing t = 180 min (open circles) and t = 210 min (closed circles). Data are presented as means (SD). *P < 0.05.
Contribution of [U-13C]Glycerol to De Novo TG Synthesis According to the Different Timing of Tracer Drink
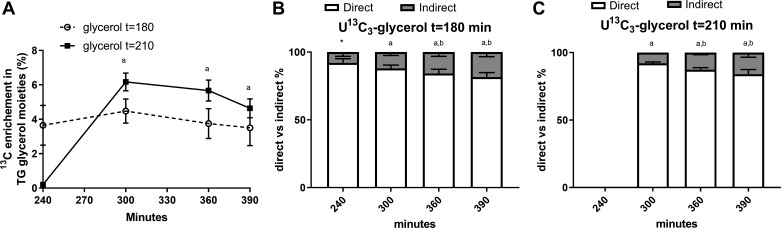
Figure 4 shows the enrichment in glycerol moieties of TG and the contribution from the direct versus indirect pathways to hepatic de novo esterification of TG. Excess 13C enrichment in TG glycerol moieties was negligible at 240 min when the glycerol drink was given at t = 210 min. The enrichment increased from 240 to 300 min and then stabilized. However, the enrichment was detectable and stable from t = 240–390 min when given at t = 180 min (P > 0.41 between time points, Fig. 4A). There was no difference in the relative contribution of the direct versus indirect (via the TCA cycle before incorporation in TG) glycerol contribution to hepatic de novo esterification of TG, according to the condition of glycerol drink, apart for the 240 min where the enrichment was not detectable with the tracer drink given at t = 210 min (P > 0.18, Fig. 4, B and C).
Fig. 4.
Contribution of [U-13C]glycerol to de novo triglyceride (TG) synthesis according to the different timing of tracer ingestion. Excess 13C enrichment in TG glycerol moieties was negligible at 240 min when the glycerol drink was given at t = 210 min. However, the enrichment was detectable and stable from t = 240–390 min when given at t = 180 min (A). Contribution from direct vs. indirect pathway to de novo TG synthesis when the glycerol tracer is given at 180 min (B). Contribution from direct vs. indirect pathway to de novo TG synthesis when the glycerol tracer is given at 210 min (C). Data are presented as means (SD). aP < 0.05 compared with t = 240 min within the same glycerol drink timing condition; bP < 0.05 compared with t = 300 within the same glycerol drink timing condition. *P < 0.05 compared with the same time point between glycerol drink t = 180 vs. t = 210 min.
Contribution of [U-13C]Glycerol to Gluconeogenesis and Pentose Phosphate Pathway According to the Timing of Tracer Drink
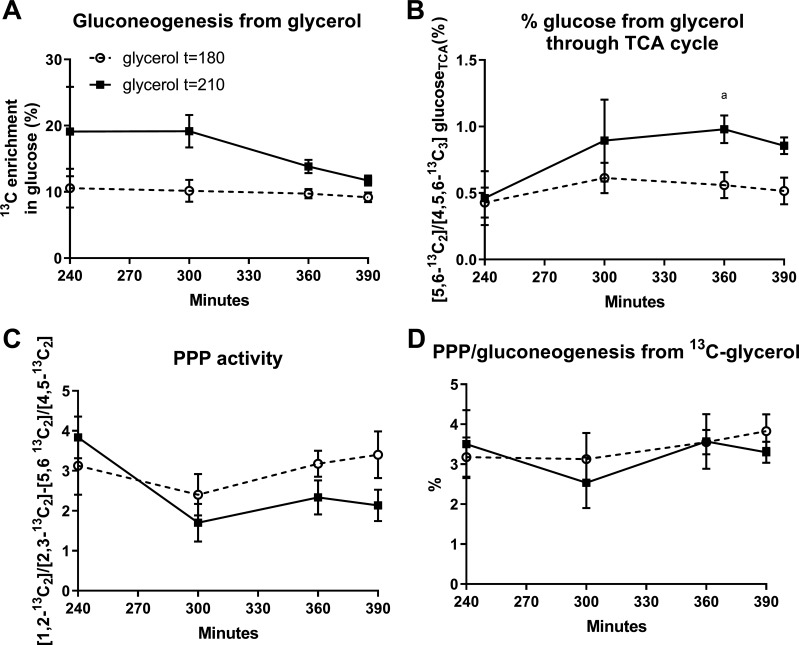
Figure 5 shows GNG from glycerol, measured by total 13C enrichment in glucose. The total enrichment was detectable from t = 240–390 and plateaued for both timing conditions of the glycerol drink. There was a trend to a decreased total 13C enrichment in glucose at t = 240 and 300 min in the group who received the glycerol tracer at t = 180 (P = 0.09), but there was no difference for t = 360 and t = 390 min (P > 0.48, Fig. 5A). In both glycerol drink conditions, the GNG from glycerol through the TCA cycle and PPP activity was not different from one drink timing condition to the other (Fig. 5, B–D).
Fig. 5.
Contribution of [U-13C]glycerol to gluconeogenesis and pentose phosphate pathway activity according to the different timing of tracer ingestion. Total excess 13C enrichment in glucose (A), contribution of [U-13C3]glycerol to GNG through TCA cycle (B), the difference of ratio between [1,213C2]/[2,313C2] and [5,613C2]/[4,513C2] in glucose (C), and pentose phosphate pathway (PPP) flux relative to gluconeogenesis (GNG) from glycerol (%) (D).
DISCUSSION
Understanding alterations in postprandial hepatic substrate metabolism is key to the comprehension of several metabolic conditions, especially those related to obesity and insulin resistance. Provocative tests, such as an oral glucose challenge, are often utilized to understand key physiological and pathological variations with disease. We thus optimized a minimally invasive method to study post-OSTT hepatic mitochondrial dynamics in a highly insulin-resistant obese youth population as an example of feasibility even in a population with significant metabolic disease. Furthermore, we demonstrated in a relatively racially diverse, female, adolescent sample that it is possible to simultaneously measure the direct and indirect contributions of glycerol to hepatic GNG and TG synthesis in a post-OSTT condition, allowing us to both stimulate de novo lipogenesis and appropriately study gluconeogenesis. Moreover, by integrating an OSTT with an oral [U-13C3]glycerol tracer, we considerably reduced the burden of metabolic studies to several drinks and blood draws via one peripheral intravenous line, especially important in humans, in comparison to studies using in vivo carbon spectroscopy or intravenous tracer infusions (1, 24).
We were able to detect adequate enrichments in glucose and TG-glycerol representative of both the indirect and direct incorporation of [U-13C3]glycerol, despite the postprandial suppression of EGP. Even after an oral load of glucose, [U-13C3]glycerol was incorporated into GNG 390 min after the OSTT with an enrichment of 7.5–12.5% and between 0.3 and 1% of glucose ([5,6 13C2]glucose) was derived from TCA cycle activity before GNG. The PPP contributed to 2.8–4.7% of GNG. These ranges are similar to what we previously found in healthy adults during the fasting state (10). Furthermore, we detected a 3.3–6.6% 13C enrichment in glycerol-TG and 14–23% of the glycerol incorporated in de novo TG synthesis was derived from anaplerosis through the TCA cycle. This is also comparable to what has been reported in adults 240 min after a [U-13C3]glycerol bolus in the fasted or fed condition (10). However, the post-OSTT has the advantage to study liver metabolism in a condition where de novo lipogenesis is stimulated, and we have shown that it was also possible to have enough enrichment in GNG despite the post-OGTT condition.
The [U-13C3]glycerol drink and subsequent blood sample collection must be timed to coincide with the decline of hepatic glucose release and nascent TG secretion back to fasting concentrations following the glucose load. In a population with significant insulin resistance, it was appropriate to administer the [U-13C3]glycerol tracer at t = 180 min after the OSTT, allowing a stable enrichment in TGs and glucose pathways from t = 240 to t = 390 min after OSTT. The t = 390 min time point after OSTT seems to be the optimal timing to interpret the contribution of direct and indirect pathways to GNG during steady-state condition. In addition, at this time point, direct/indirect pathway contribution to glucose and TG synthesis reached a plateau and glycerol, FFA, glucose, and insulin concentrations were stable. Furthermore, the volume of blood sampling for future studies would be considerably reduced by having identified the steady-state time point optimal for interpretation of data. We based our initial timing on data suggesting that de novo lipogenesis peaks from t = 240 to 390 min after a OGTT in obese youth and that EGP starts to increase in the same time interval in adolescents with PCOS (22, 23a). Furthermore, glucose and insulin concentrations were close to baseline 240 min after the OSTT, allowing the EGP to resume after the initial inhibition by the glucose load. In translation of this methodology to other patient populations, the timing of blood draws for steady state should be timed to the return of insulin and glucose close to baseline conditions, allowing return of EGP to a level allowing enrichment in GNG.
Whereas the [U-13C3]glycerol tracer drink did not impact the concentrations of glucose, insulin, or TGs, there was a trend, early after the 180-min glycerol tracer drink, of a short and slight increase in glycerol plasma concentration. The glycerol concentrations were back to preglycerol tracer drink and similar to control participant concentrations by the next blood draw. In the first description of this method in adults (10), the plasma glycerol concentration also increased in the first 30 min and then came back to baseline, when given in fasting state. With the 180-min time administration in this patient population, glycerol is still suppressed by the post-OSTT hyperinsulinemia, and thus a minute amount of tracer is detectable. However, as the insulin and glycerol concentrations start to return to fasting values, the glycerol tracer no longer is apparent. With the 210-min drink administration, the endogenous concentrations are already rising, and thus the tracer concentration is not detectable.
We have now extended our previous methodology into an OSTT model and optimized timing for an insulin-resistant population to allow quantification of the contribution of hepatic cataplerosis to both de novo TG synthesis and GNG simultaneously. This is of importance in the context of comprehensively evaluating hepatic energy metabolism, as cataplerosis contributes to both the TG and GNG synthetic pathways. Furthermore, the hepatic PPP has a major contribution in the formation of NADPH involved in FFA synthesis during de novo lipogenesis, which makes it an important pathway to assess in cardiometabolic diseases. The PPP is also involved in glutathione reduction, an important antioxidant mechanism preventing damages from reactive oxygen species.
We have optimized an existing protocol (10, 17) to comprehensively study the metabolic pathways in a postsugar load setting that triggers de novo lipogenesis as well as simultaneously allowing adequate quantification of GNG. Healthy adults have been previously studied with the same methodology while fasting, with the [U-13C3]glycerol tracer 60 min following a meal, and along with a glucose load 60 min following a meal. The utilization of an OSTT, which includes both glucose and fructose, not only allows measurement of glucose metabolism itself but also stimulates de novo lipogenesis, an important factor implicated in NAFLD. This protocol can be adjusted to different populations by estimating the timing of administration of the glycerol tracer according to the timing of a significant drop in plasma insulin following OSTT, allowing EGP to increase. This allows the detection enough enrichment in the direct and indirect pathways.
Other in vivo methods have been used to quantify hepatic anaplerotic fluxes in humans and provide valuable information on liver metabolism (20). Briefly, the first method uses oral [U-13C]propionate and plasma glucose samples to examine positional isotopomers to assess the relative contributions from glycogen, glycerol, and the TCA cycle into glucose production. It is usually combined with administration of oral deuterated water (2H2O) and a primed-constant intravenous [3,4-13C2]glucose infusion to measure EGP and relative contributions of glycogenolysis and GNG to EGP (9, 11, 24). This method allows quantification of absolute rates of phosphoenolpyruvate carboxykinase flux, pyruvate cycling, and the rate of GNG relative to citrate synthase flux in the TCA cycle. More recently, an intraveous infusion of [3-13C]lactate combined with plasma ex vivo positional isotopomer NMR analysis allowed quantification of citrate synthase flux and pyruvate carboxylase flux in humans (19). Another option would be the direct measurement of the incorporation of [1-13C]acetate in hepatic glutamate using in vivo MR spectroscopy (1). This technique necessitates intravenous infusion of 13C tracer and simultaneous 13C-MR spectroscopy, only available in a few highly specialized imaging centers, with high operational costs. Furthermore, the study burden may be higher for the participants due to the time required for MR data acquisition. However, none of these techniques allows the simultaneous quantification of anaplerotic pathways involved in de novo TG synthesis and GNG and all require bilateral intravenous access, in comparison to our comprehensive approach with only unilateral intravenous access required.
Limitations
The proposed method possesses some limitations. First, our approach was not designed to quantitate absolute rates of TG or GNG fluxes but rather a relative contribution from direct and indirect pathways. As shown by the comparison between adults with high visceral fat and those with low visceral fat assessed previously with this method (17), the total 13C enrichment may be influenced by the precursor pool of glycerol and absolute GNG measured by excess 13C glucose may complicate the interpretation of absolute fluxes of GNG. However, the relative contribution from each pathway to GNG from [U-13C3]glycerol is not affected by the precursor pool and is highly informative of the hepatic mitochondrial function and contribution of anaplerosis to GNG. To assess absolute fluxes, this protocol could be combined with 2H2O administration and a [6,6-2H2]glucose intravenous tracer, similarly to what have been used in the [13C]propionate protocols (9, 11, 24). Also, multiplication of the ratio of PPP or indirect [U-13C3]glycerol to GNG by plasma glucose concentration allows the measurement an absolute contribution of the pathway to the plasma glucose pool in micromoles per liter at the different time point measured (17).
Conclusions
In summary, we have shown that it was possible to measure the anaplerotic/cataplerotic contribution of glycerol to GNG and de novo TG synthesis in a context of postoral sugar load, designed to stimulate de novo lipogenesis in a highly insulin-resistant population. Furthermore, we were able to quantify PPP activity at the same time. The oral administration of a [U-13C3]glycerol tracer 180 min after the OSTT and blood collection up to 390 min after an OSTT allows an optimal steady state for the evaluation of multiple pathways currently thought to be abnormal in insulin-resistant populations.
Perspectives and Signifiance
This minimally invasive technique will now be applicable to obese, insulin resistant populations, especially in youth, to understand the link between hepatic metabolic pathways and metabolic disease across the lifespan.
GRANTS
This work was supported by the National Institutes of Health Grants K23-DK-107871 (to M. Cree-Green), DK-099289 (to E. S. Jin), and P41-EB-015908 (to C. R. Malloy; Children’s Hospital Colorado (to M. Cree-Green); Doris Duke Foundation Fund to Retain Clinical Scientists (to M. Cree-Green); Diabetes Canada Postdoctoral Fellowship Award (to A. M. Carreau); and Endocrine Fellow Foundation Early Career Award (to A. M. Carreau). It was also supported by NIH/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Award Grant UL1-TR-002535.
DISCLAIMERS
Contents are the authors’ sole responsibility and do not necessarily represent official National Institutes of Health views.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.-M.C., E.S.J., C.R.M., and M.C.-G. conceived and designed research; A.-M.C., E.S.J., Y.G.-R., H.R., and M.C.-G. performed experiments; A.-M.C., E.S.J., and M.C.-G. analyzed data; A.-M.C., E.S.J., C.R.M., and M.C.-G. interpreted results of experiments; A.-M.C. prepared figures; A.-M.C. drafted manuscript; A.-M.C., E.S.J., Y.G.-R., H.R., K.J.N., C.R.M., and M.C.-G. edited and revised manuscript; A.-M.C., E.S.J., Y.G.-R., H.R., K.J.N., C.R.M., and M.C.-G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the participants, their families, Clinical and Translational Research Center nurses, and staff and Laura Pyle for statistical analysis expertise.
REFERENCES
- 1.Befroy DE, Perry RJ, Jain N, Dufour S, Cline GW, Trimmer JK, Brosnan J, Rothman DL, Petersen KF, Shulman GI. Direct assessment of hepatic mitochondrial oxidative and anaplerotic fluxes in humans using dynamic 13C magnetic resonance spectroscopy. Nat Med 20: 98–102, 2014. doi: 10.1038/nm.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Browning JD, Weis B, Davis J, Satapati S, Merritt M, Malloy CR, Burgess SC. Alterations in hepatic glucose and energy metabolism as a result of calorie and carbohydrate restriction. Hepatology 48: 1487–1496, 2008. doi: 10.1002/hep.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cree-Green M, Bergman BC, Coe GV, Newnes L, Baumgartner AD, Bacon S, Sherzinger A, Pyle L, Nadeau KJ. Hepatic steatosis is common in adolescents with obesity and PCOS and relates to de novo lipogenesis but not insulin resistance. Obesity (Silver Spring) 24: 2399–2406, 2016. doi: 10.1002/oby.21651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cree-Green M, Rahat H, Newcomer BR, Bergman BC, Brown MS, Coe GV, Newnes L, Garcia-Reyes Y, Bacon S, Thurston JE, Pyle L, Scherzinger A, Nadeau KJ. Insulin resistance, hyperinsulinemia, and mitochondria dysfunction in nonobese girls with polycystic ovarian syndrome. J Endocr Soc 1: 931–944, 2017. doi: 10.1210/js.2017-00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cree-Green M, Xie D, Rahat H, Garcia-Reyes Y, Bergman BC, Scherzinger A, Diniz Behn C, Chan CL, Kelsey MM, Pyle L, Nadeau KJ. Oral glucose tolerance test glucose peak time is most predictive of prediabetes and hepatic steatosis in obese girls. J Endocr Soc 2: 547–562, 2018. doi: 10.1210/js.2018-00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin ES, Burgess SC, Merritt ME, Sherry AD, Malloy CR. Differing mechanisms of hepatic glucose overproduction in triiodothyronine-treated rats vs. Zucker diabetic fatty rats by NMR analysis of plasma glucose. Am J Physiol Endocrinol Metab 288: E654–E662, 2005. doi: 10.1152/ajpendo.00365.2004. [DOI] [PubMed] [Google Scholar]
- 9.Jin ES, Jones JG, Merritt M, Burgess SC, Malloy CR, Sherry AD. Glucose production, gluconeogenesis, and hepatic tricarboxylic acid cycle fluxes measured by nuclear magnetic resonance analysis of a single glucose derivative. Anal Biochem 327: 149–155, 2004. doi: 10.1016/j.ab.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 10.Jin ES, Sherry AD, Malloy CR. An oral load of [13C3]glycerol and blood nmr analysis detect fatty acid esterification, pentose phosphate pathway, and glycerol metabolism through the tricarboxylic acid cycle in human liver. J Biol Chem 291: 19031–19041, 2016. doi: 10.1074/jbc.M116.742262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin ES, Szuszkiewicz-Garcia M, Browning JD, Baxter JD, Abate N, Malloy CR. Influence of liver triglycerides on suppression of glucose production by insulin in men. J Clin Endocrinol Metab 100: 235–243, 2015. doi: 10.1210/jc.2014-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koliaki C, Roden M. Hepatic energy metabolism in human diabetes mellitus, obesity and non-alcoholic fatty liver disease. Mol Cell Endocrinol 379: 35–42, 2013. doi: 10.1016/j.mce.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Konerman MA, Jones JC, Harrison SA. Pharmacotherapy for NASH: current and emerging. J Hepatol 68: 362–375, 2018. [Erratum in: J Hepatol 68: 1337, 2018. 10.1016/j.jhep.2018.03.002. 29567301] 10.1016/j.jhep.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK; Endocrine Society . Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 98: 4565–4592, 2013. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Neeland IJ, Hughes C, Ayers CR, Malloy CR, Jin ES. Effects of visceral adiposity on glycerol pathways in gluconeogenesis. Metabolism 67: 80–89, 2017. doi: 10.1016/j.metabol.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perry RJ, Camporez JG, Kursawe R, Titchenell PM, Zhang D, Perry CJ, Jurczak MJ, Abudukadier A, Han MS, Zhang XM, Ruan HB, Yang X, Caprio S, Kaech SM, Sul HS, Birnbaum MJ, Davis RJ, Cline GW, Petersen KF, Shulman GI. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160: 745–758, 2015. doi: 10.1016/j.cell.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perry RJ, Peng L, Cline GW, Butrico GM, Wang Y, Zhang XM, Rothman DL, Petersen KF, Shulman GI. Non-invasive assessment of hepatic mitochondrial metabolism by positional isotopomer NMR tracer analysis (PINTA). Nat Commun 8: 798, 2017. [Erratum in: Nat Commun 31: 498, 2018. 10.1038/s41467-018-03023-3. 29386503] doi: 10.1038/s41467-017-01143-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Previs SF, Kelley DE. Tracer-based assessments of hepatic anaplerotic and TCA cycle flux: practicality, stoichiometry, and hidden assumptions. Am J Physiol Endocrinol Metab 309: E727–E735, 2015. doi: 10.1152/ajpendo.00216.2015. [DOI] [PubMed] [Google Scholar]
- 20a.RISE Consortium Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: II. Observations using the oral glucose tolerance test. Diabetes Care 41: 1707–1716, 2018. doi: 10.2337/dc18-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roden M, Stingl H, Chandramouli V, Schumann WC, Hofer A, Landau BR, Nowotny P, Waldhäusl W, Shulman GI. Effects of free fatty acid elevation on postabsorptive endogenous glucose production and gluconeogenesis in humans. Diabetes 49: 701–707, 2000. doi: 10.2337/diabetes.49.5.701. [DOI] [PubMed] [Google Scholar]
- 22.Santoro N, Caprio S, Pierpont B, Van Name M, Savoye M, Parks EJ. hepatic de novo lipogenesis in obese youth is modulated by a common variant in the GCKR gene. J Clin Endocrinol Metab 100: E1125–E1132, 2015. doi: 10.1210/jc.2015-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satapati S, Kucejova B, Duarte JA, Fletcher JA, Reynolds L, Sunny NE, He T, Nair LA, Livingston KA, Fu X, Merritt ME, Sherry AD, Malloy CR, Shelton JM, Lambert J, Parks EJ, Corbin I, Magnuson MA, Browning JD, Burgess SC. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J Clin Invest 125: 4447–4462, 2015. doi: 10.1172/JCI82204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.Simens JL, Cree-Green M, Bergman BC, Nadeau KJ, Diniz-Behn C. Structural Identifiability Analysis of a Labeled Oral Minimal Model for Quantifying Hepatic Insulin Resistance. New York: Springer, 2018. [Google Scholar]
- 24.Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab 14: 804–810, 2011. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Visentin R, Dalla Man C, Basu R, Basu A, Rizza RA, Cobelli C. Hepatic insulin sensitivity in healthy and prediabetic subjects: from a dual- to a single-tracer oral minimal model. Am J Physiol Endocrinol Metab 309: E161–E167, 2015. doi: 10.1152/ajpendo.00358.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witchel SF, Oberfield S, Rosenfield RL, Codner E, Bonny A, Ibáñez L, Pena A, Horikawa R, Gomez-Lobo V, Joel D, Tfayli H, Arslanian S, Dabadghao P, Garcia Rudaz C, Lee PA. The Diagnosis of Polycystic Ovary Syndrome during Adolescence. Horm Res Paediatr 83: 376–389, 2015. doi: 10.1159/000375530. [DOI] [PubMed] [Google Scholar]